Abstract
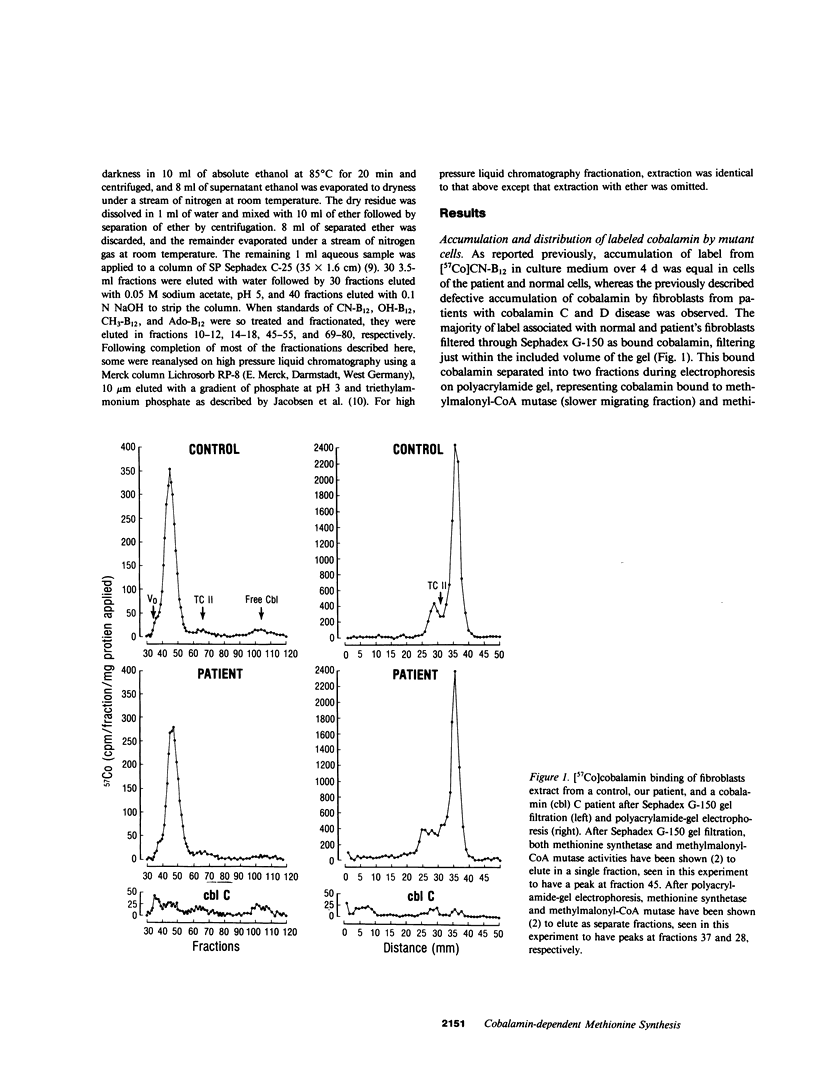
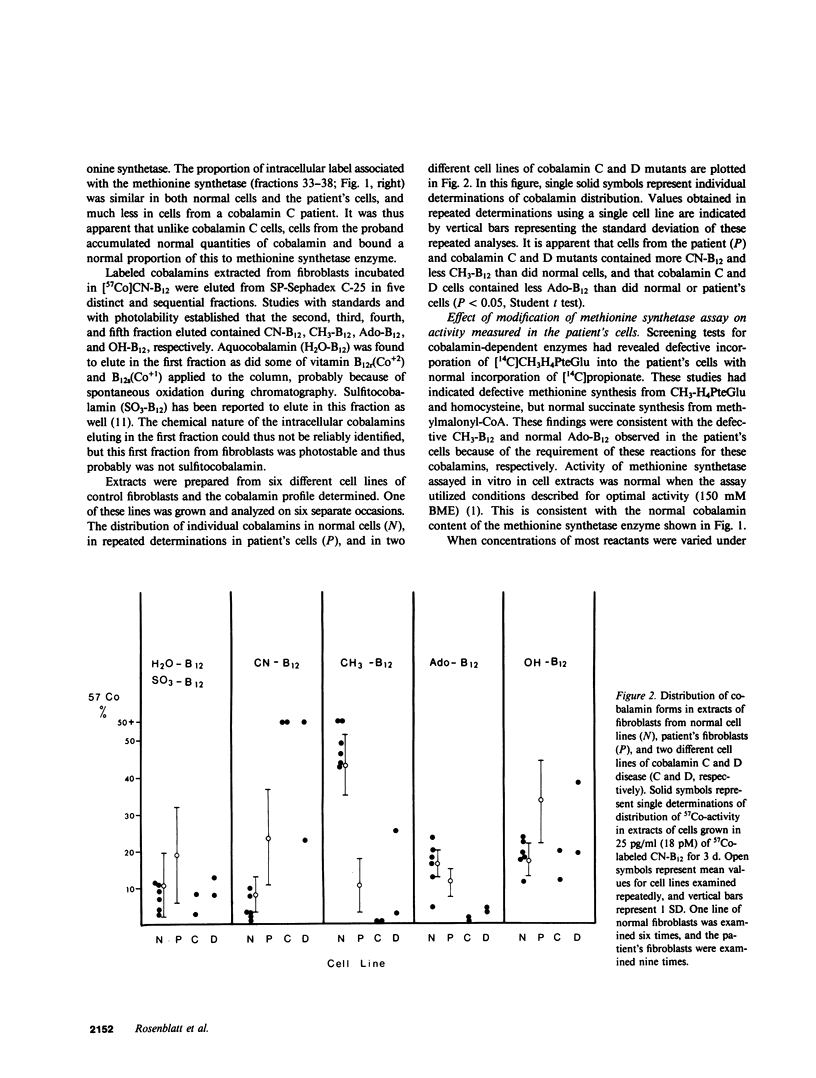
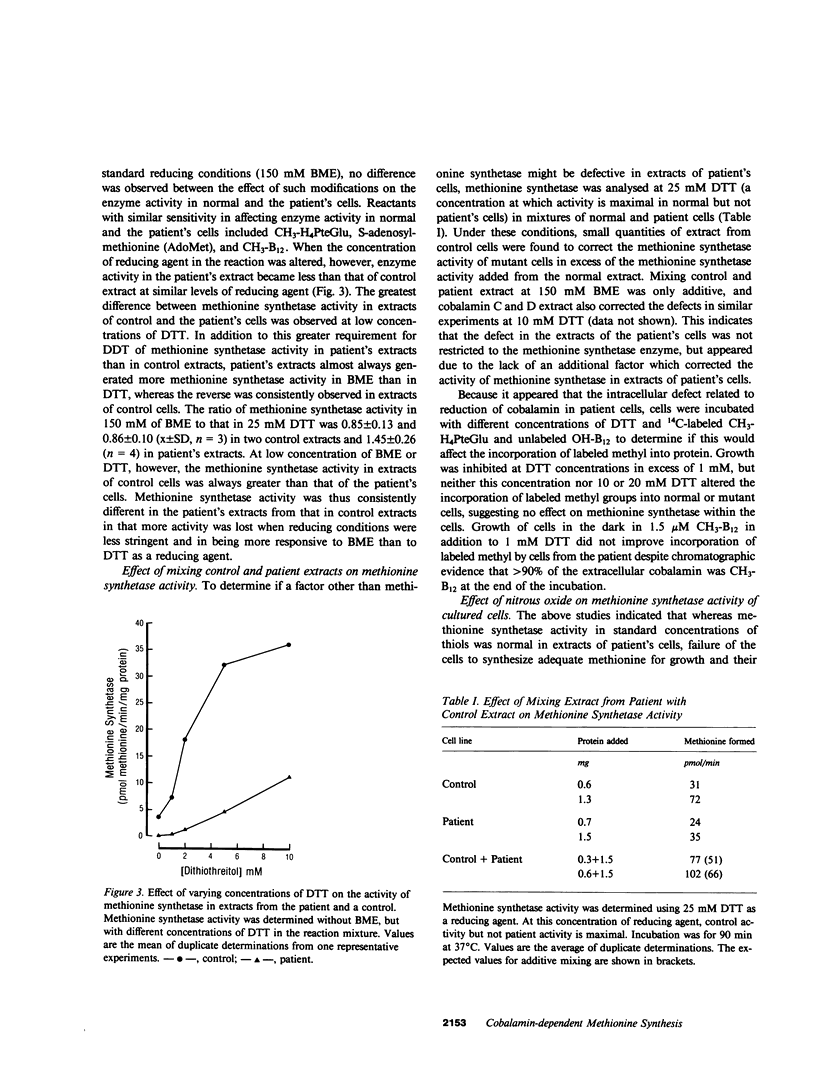
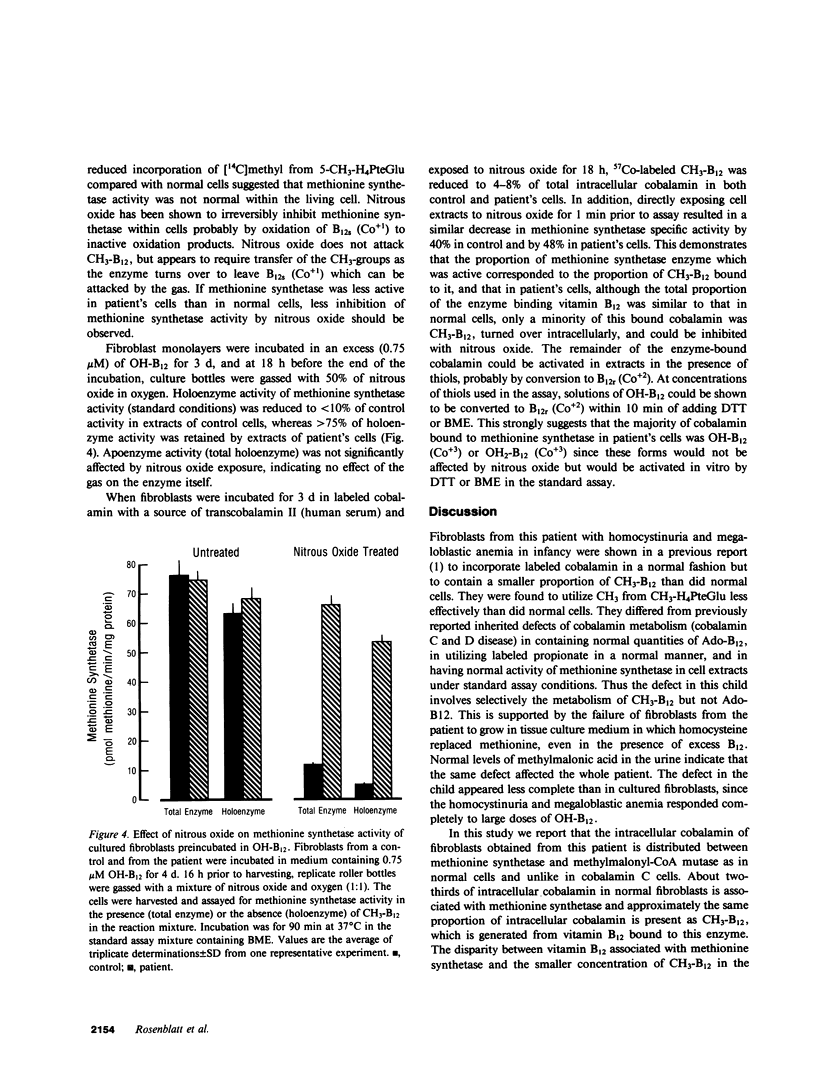
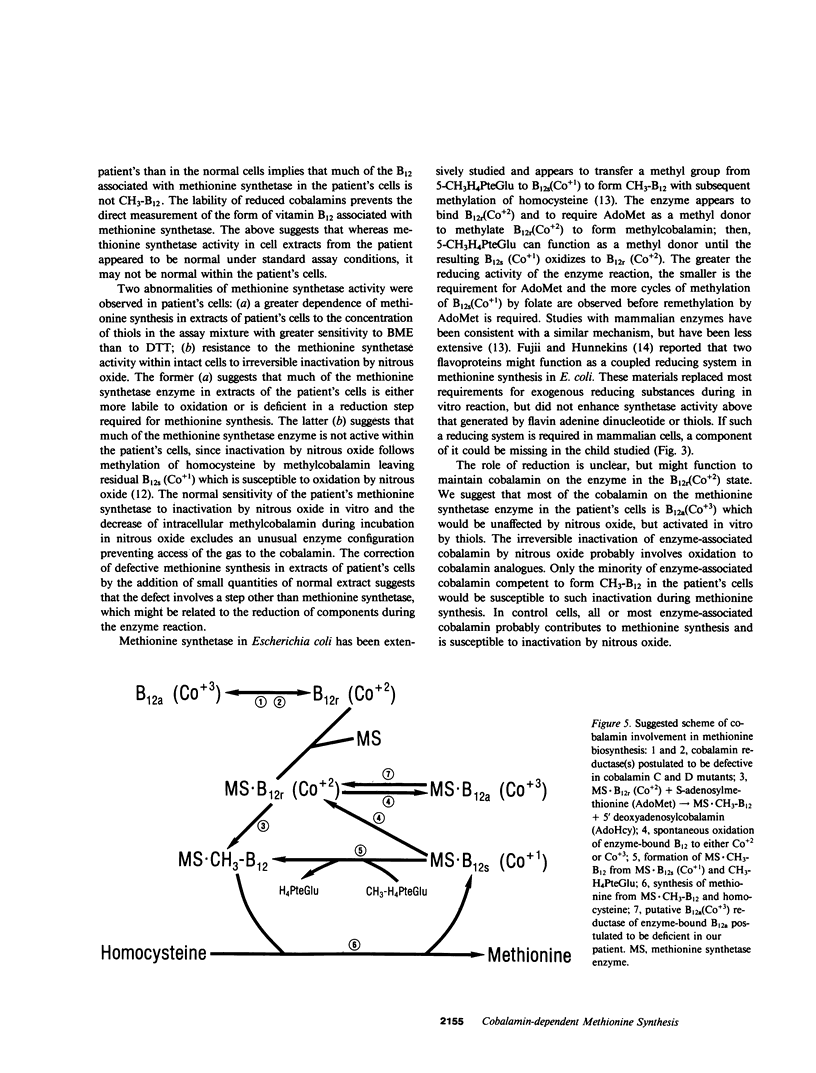
Cultured fibroblasts from a recently described patient with homocystinuria and megaloblastic anemia of infancy without methylmalonic aciduria were previously shown to have normal cobalamin uptake and a specific decrease in the proportion of intracellular methylcobalamin. As in control cells but unlike in those from patients with combined homocystinuria and methylmalonic aciduria (cobalamin C and cobalamin D), accumulated 57Co-labeled cobalamin was bound in appropriate amounts and proportion to intracellular binders which are known to be the two vitamin B12-dependent enzymes, methionine synthetase and methylmalonyl-CoA mutase. Despite the association of a normal quantity of intracellular cobalamin with methionine synthetase, the proportion of intracellular cobalamin which was methyl-B12 was below normal and in the range observed in cobalamin C and D cells. This methyl-B12 was decreased by exposure of fibroblasts in culture to nitrous oxide as was observed with control cells. Exposure of control fibroblasts during culture, but not of fibroblasts from this patient, to nitrous oxide significantly reduced the holoenzyme activity of methionine synthetase assayed in cell extracts. In addition, although methionine synthetase activity in cell extracts of control and cells from the patient were similar in the presence of standard assay concentrations of thiols, at low thiol concentrations, methionine synthetase activity in extracts of cells from the patient was much lower than in control extracts. Mixing of control patient extracts corrected this decreased activity in excess of that explained by addition of the individual activities added. The defect of this patient appears to be in a reducing system required for methionine synthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fujii K., Huennekens F. M. Activation of methionine synthetase by a reduced triphosphopyridine nucleotide-dependent flavoprotein system. J Biol Chem. 1974 Nov 10;249(21):6745–6753. [PubMed] [Google Scholar]
- Jacobsen D. W., Green R., Quadros E. V., Montejano Y. D. Rapid analysis of cobalamin coenzymes and related corrinoid analogs by high-performance liquid chromatography. Anal Biochem. 1982 Mar 1;120(2):394–403. doi: 10.1016/0003-2697(82)90363-3. [DOI] [PubMed] [Google Scholar]
- Linnell J. C., Mackenzie H. M., Wilson J., Matthews D. M. Patterns of plasma cobalamins in control subjects and in cases of vitamin B12 deficiency. J Clin Pathol. 1969 Sep;22(5):545–550. doi: 10.1136/jcp.22.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I., Willard H. F., Rosenberg L. E. Cobalamin binding and cobalamin-dependent enzyme activity in normal and mutant human fibroblasts. J Clin Invest. 1978 Nov;62(5):952–960. doi: 10.1172/JCI109224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I., Willard H. F., Youngdahl-Turner P., Rosenberg L. E. Cobalamin coenzyme synthesis in normal and mutant human fibroblasts. Evidence for a processing enzyme activity deficient in cblC cells. J Biol Chem. 1979 Dec 10;254(23):11847–11853. [PubMed] [Google Scholar]
- Rosenblatt D. S., Cooper B. A., Lue-Shing S., Wong P. W., Berlow S., Narisawa K., Baumgartner R. Folate distribution in cultured human cells. Studies on 5,10-CH2-H4PteGlu reductase deficiency. J Clin Invest. 1979 May;63(5):1019–1025. doi: 10.1172/JCI109370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt D. S., Erbe R. W. Reciprocal changes in the levels of functionally related folate enzymes during the culture cycle in human fibroblasts. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1627–1633. doi: 10.1016/0006-291x(73)91173-x. [DOI] [PubMed] [Google Scholar]
- Schneider E. L., Stanbridge E. J., Epstein C. J. Incorporation of 3H-uridine and 3H-uracil into RNA: a simple technique for the detection of mycoplasma contamination of cultured cells. Exp Cell Res. 1974 Mar 15;84(1):311–318. doi: 10.1016/0014-4827(74)90411-x. [DOI] [PubMed] [Google Scholar]
- Schuh S., Rosenblatt D. S., Cooper B. A., Schroeder M. L., Bishop A. J., Seargeant L. E., Haworth J. C. Homocystinuria and megaloblastic anemia responsive to vitamin B12 therapy. An inborn error of metabolism due to a defect in cobalamin metabolism. N Engl J Med. 1984 Mar 15;310(11):686–690. doi: 10.1056/NEJM198403153101104. [DOI] [PubMed] [Google Scholar]
- Shapiro S. K., Ehninger D. J. Methods for the analysis and preparation of adenosylmethionine and adenosylhomocysteine. Anal Biochem. 1966 May;15(2):323–333. doi: 10.1016/0003-2697(66)90038-8. [DOI] [PubMed] [Google Scholar]
- Tortolani G., Bianchini P., Mantovani V. Separation and determination of cobalamins on an SP-Sephadex column. J Chromatogr. 1971 Dec 23;53(3):577–579. doi: 10.1016/s0021-9673(01)98517-6. [DOI] [PubMed] [Google Scholar]
- Youngdahl-Turner P., Rosenberg L. E., Allen R. H. Binding and uptake of transcobalamin II by human fibroblasts. J Clin Invest. 1978 Jan;61(1):133–141. doi: 10.1172/JCI108911. [DOI] [PMC free article] [PubMed] [Google Scholar]



