Abstract
Understanding the intricate cellular components of the bone marrow microenvironment can lead to the discovery of novel extrinsic factors that are responsible for the initiation and progression of leukemic disease. We have shown that endothelial cells (ECs) provide a fertile niche that allows for the propagation of primitive and aggressive leukemic clones. Activation of the ECs by VEGF-A provides cues that enable leukemic cells to proliferate at higher rates and also increases the adhesion of leukemia to ECs. VEGF-A activated ECs decrease the efficacy of chemotherapeutic agents to target leukemic cells. Inhibiting VEGF-dependent activation of ECs by blocking their signaling through VEGFR2 increases the susceptibility of leukemic cells to chemotherapy. Therefore, the development of drugs that target the activation state of the vascular niche could prove to be an effective adjuvant therapy in combination with chemotherapeutic agents.
Introduction
Acute myelogenous leukemia (AML) is the most common acute leukemia in adults and although it is considered a rare disease, the incidence of AML is predicted to rise with the expected increase in the elderly population. Despite the majority of AML patients achieving complete remission with conventional chemotherapy, 30-70% of patients relapse and ultimately succumb to their disease without a potentially curative transplant [1, 2]. A rare subset of AML cells are capable of self-renewing and differentiating into non-self-renewing, mature AML cells and are accountable for the rapid proliferation and expansion of the leukemia [3]. These leukemia-initiating cells (LICs) exhibit similar properties to normal hematopoietic stem cells (HSCs) and are being widely targeted for potential therapeutic purposes as they are relatively resistant to conventional chemotherapy [4]. Research into the mechanism of LIC self-renewal and expansion has primarily focused on intrinsic signaling pathways that control their development and limitless self-renewal capacity, while the potential extrinsic factors modulating LIC progression remain largely underappreciated [5-7].
Homeostasis of normal HSCs is dependent on both intrinsic signals and extrinsic signals derived from the BM microenvironment (or niche). This interaction is critical in maintaining the quiescence of homeostatic HSCs and the rapid regeneration of the hematopoietic pool following BM injury [8-21]. Modulation of the niche can influence the self-renewal capabilities of the HSC. Although AML is well characterized as a cell autonomous disease in which genetic abnormalities that occur within the transformed hematopoietic cell lead to disease, extrinsic signals may also play a role in promoting the maintenance, survival, and expansion of LICs. Furthermore, the microenvironment may contribute to the maintenance and survival of LICs during and after induction of remission by chemotherapeutic agents [22-26], potentially providing a “safe haven” for LICs to evade treatment and ultimately contribute to relapse.
The aforementioned studies have been restricted to studying the endosteum in in vivo animal models. Similarly, the majority of the in vitro studies have used culture conditions that require supplementation with serum and other growth factors that potentially influence the maintenance and survival of leukemic cells. The BM microenvironment is a complex niche composed of multiple cell types, including and not limited to the endosteum (osteoblast niche), reticular cells (perivascular niche) and the BM vascular niche [27]. Mounting evidence has demonstrated that the endosteum and the vascular network are intimately intertwined, suggesting that both niches could modulate the maintenance of HSCs and leukemic cells [8-10]. Recent data have suggested that ECs are not simply required for the delivery of oxygen, nutrients or waste disposal, but upon proper activation can be considered a specialized vascular niche that supports the maintenance and reconstitution of normal and malignant stem/progenitor cells by secretion of paracrine factors [28]. To define the role of ECs in HSC and leukemic cell self-renewal and maintenance, we have established stable EC cultures by introducing the adenoviral E4ORF1 gene into primary human ECs (VeraVec, Angiocrine Bioscience), enabling their Akt-dependent survival for weeks under serum/cytokine-free conditions [29]. Using this model, we demonstrated that co-culture of HSCs in direct cellular contact with ECs is essential to support the long-term maintenance and self-renewal of HSCs [16, 17].
AML is known to secrete a number of angiogenic factors, including VEGF-A, which has been suggested to support leukemic growth through an autocrine mechanism [30] and can also contribute to the increased bone marrow vascular density (MVD) seen in patients with AML. The concentrations of circulating VEGF-A and degree of MVD are relevant to prognosis, as an increase in each independently is associated with poor clinical outcomes [31, 32]. While the role of VEGF-A on leukemic proliferation is established, the reciprocal role that VEGF-A stimulated ECs play on supporting leukemic growth is not well understood.
Utilizing our in vitro co-culture system, we demonstrate that ECs instruct the expansion of leukemic clones that are phenotypically primitive and elicit an aggressive phenotype when transplanted into recipient mice. Leukemic cells cultured in direct cellular contact with VEGF-A activated VeraVec displayed an increase in their adhesion to ECs, partly through VLA-4, as well as an increase in their proliferative capacity. Furthermore, activation of ECs confers a protective mechanism for the leukemic cells when targeted with the anti-leukemic chemotherapuetic agent Ara-C. When leukemic cells were introduced into recipient mice, activating the BM vascular niche with exogenous VEGF-A lead to the mice succumbing to the leukemic burden at a much greater rate as compared to controls. Activation of the BM vascular niche also protected the leukemia cells from being targeted by Ara-C. However, when blocking the activation of the BM vasculature with an anti-VEGFR2 antibody, we were able to decrease the leukemic burden and prolong the survival of the mice with or without the addition of Ara-C. These data suggest that inhibiting the activation of the BM vascular niche through the VEGF/VEGFR2 axis may serve as an effective adjuvant therapy to standard chemotherapeutic regimens for patients with AML. Further, identification of endothelial cell-derived factors produced in response to VEGFR2 signaling may be informative in the development of treatment strategies targeting LICs.
Materials and Methods
Mice
All animal experiments were performed under the approval of the Weill Medical College of Cornell University’s Institutional Animal Care and Use Committee. C57BL/6J (WT and UBC-GFP) and BALB/C mice were used at 8 weeks of age. C57BL/6J and BALB/C mice were used as transplant recipients of MLL-AF9 cells and were approximately 10 weeks of age.
Generation and Maintenance of VeraVec
The adenoviral E4ORF1 gene from adenovirus (serotype 5) was cloned into the lentiviral vector pCCL [29]. Lentivirus was generated by co-transfecting 15 μg of the pCCL-E4ORF1 with 4 μg pENV/VSV-G, 6 μg pMDL/pRRE, and 3 μg pRSV-REV in subconfluent 293T/17 (ATCC CRL-11268) cells (passage <5) by the calcium precipitation method. Medium was changed 24 hours post-transfection and supernatants were collected at 48 hours. Supernatants were sterile filtered using surfactant-free cellulose acetate membranes, aliquoted and stored at −80°C. Primary HUVECs were transduced with E4ORF1 virus, allowed to recover for 24 hours in media containing serum and growth factors, and then serum starved for 7 days to allow for selection of transduced cells.
Generation of MLL-AF9 Retrovirus
The human MLL-AF9 fusion vector (MigR1) was obtained from Scott Armstrong at Memorial Sloan Kettering. The retrovirus was created using 15μg of MLL-AF9 vector and 15μg of pCL-Eco packaging plasmid in sub confluent 293T/17 cells (passage <5) by the calcium precipitation method. Medium was changed 24 post-transfection and supernatants were collected at 48 hours. Supernatants were sterile filtered using surfactant free cellulose acetate membranes, aliquoted and stored at −80° C.
Isolation of lineage– cells and generation of leukemic cells
Whole BM cells from C57BL/6J (UBC GFP or WT) or BALB/C mice were collected and magnetically depleted of any mature cells (Miltenyi Biotech, CA). The lineage negative (Lin–) cKit+CD34hi+CD16/32+ cells were sorted and stimulated for two days in a cytokine-rich media of OptiMEM, 15% FBS, 25 ng/mL sKitL (SCF), 10 ng/mL Flt3 ligand, 10 ng/mL murine IL6, and 10 ng/mL murine IL3. After two days, approximately 200,000 cells were spinoculated with MLL-AF9 retrovirus and 4 μg/mL polybrene at 2800 rpm for 1 hour. 2 mL of fresh medium was added after infection. Emergent leukemic cells were analyzed by flow cytometry and colony formation, as well as in vivo engraftment studies. In vivo engraftment studies were used to propagate larger numbers of leukemia initiating cells (Lin–cKit+CD34hi+CD16/32+GFP+ cells) for our in vitro co-culture studies as well as to determine the expression of the VEGF-A receptors, VEGFR1 (MF1, Imclone) and VEGFR2 (DC101, Imclone).
Leukemic cell co-culture, isolation, and flow cytometry
50,000 leukemic cells were cultured in 12-well plates with either no feeder layer, co-cultured on VeraVec in serum-free media or no feeder layer in 10% serum-containing media (all conditions supplemented with 50 ng/mL of sKitL). Following 14 days of culture, leukemic cells were separated from VeraVec by magnetically selecting the CD45+ population (Miltenyi Biotech, CA), blocked using 10% rat serum and stained with conjugated antibodies against CD34 (RAM34, BD Pharmingen), CD11b (M1/70, BD Pharmingen), Ly-6G (RB6-8C5, BD PharMingen), CD117 (2B8, BD Pharmingen), CD16/32 (2.4G2, BD Pharmingen). Stained leukemic cells were washed with PBS and analyzed with a LSRII SORP (BD Biosciences). All antibody staining was done at room temperature for 30 minutes. Gating strategies for flow cytometry was determined by single stain and fluorescent minus one controls. Dapi was used to exclude dead cells and use of a “dump” channel was used to exclude unspecific binding of antibodies.
In vitro adhesion and chemoprotection analysis of VeraVec
To determine the biological significance of direct E4+ EC contact with leukemic cells, 100,000 leukemic cells/well (n=6 wells/plate; 4 independent AML clones) were plated on 12-well plates of VeraVec in serum-free media (X-Vivo 20, Lonza) supplemented with 50 ng/mL of sKitL, and separated into two groups. After 24 hours, the medium was collected, isolating the non-adherent leukemic cells from the ECs. The remaining cells were trypsinized and enriched using CD45 magnetic separation (Miltenyi Biotech). This CD45 enriched population represented leukemic cells adhered to VeraVec. Both groups of leukemic cells were counted on a hemocytometer using Trypan Blue exclusion and the adherent leukemic cells that received the mAb to VLA-4 were compared to the control adherent leukemia cells. The adherent and non-adherent leukemic cells of the control group were then stained separately with Annexin-V/PI (BD Pharmigen). To determine the chemoprotective ability of VeraVec, we added 100,000 cells/well (n=6 wells/plate) to three plates: (i) no feeder layer in RPMI + 10% serum; (ii) VeraVec and (iii) VeraVec + transwells in serum-free media (all conditions supplemented with 50 ng/mL of sKitL). Three wells from each plate served as controls, while the remaining three wells on each plate were each administered 500 ng/mL of Cytarabine (Ara-C, Hospira Inc.) 5 days after the initial plating. After 24 hours, cells were collected and stained with Annexin-V/PI (BD Pharmigen). All stained cells were analyzed with a LSRII SORP (BD Biosciences).
In vitro analysis of selective activation of VeraVec on leukemic expansion
To investigate the effect of VEGF-A activation of VeraVec, 100,000 leukemic cells/well (n=6 wells/plate; 4 independent AML clones) were plated onto 12-well plates of pre-stimulated (20 ng/mL VEGF-A 24 hours prior to plating; exogenous VEGF-A was removed by washing pre stimulated ECs 3x with PBS) VeraVec in serum-free media supplemented with 50 ng/mL of sKitL. One plate served as the control, while the other plate was pre-treated with 20 ng/mL of VEGF-A (Peprotech) every day for 7 days prior to co-culture with leukemic cells. One plate of VeraVec and leukemic cells was supplemented with 1 μg/mL of monoclonal antibody (mAb) to VLA-4 (PS/2, Millipore) and the other served as the control. After 7 days, the non-adherent leukemic cells and the EC-adherent leukemic cells were purified by magnetic CD45 enrichment. In a parallel experiment, 500 ng/mL of Ara-C was added to each plate 5 days after the initial plating of leukemia cells with ECs. After 24 hours, Annexin-V/PI was used to quantify the number of adherent and non-adherent leukemia cells.
Leukemia transplantation engraftment/survival and selectively targeting the BM vascular niche
C57BL/6J mice were sublethally irradiated (650 rads) and transplanted with 50,000 GFP+ leukemic cells cultured in 10% serum media via retro-orbital injection. Mice were separated into six groups (n=20/group; 3 independent experiments) and were subjected to the following treatments: (i) PBS (Days 7, 9, 11, 13); (ii) Ara-C (125 mg/kg, Days 9, 10); (iii) VEGF-A (100 ng/mouse, 2X/day, Days 8-14); (iv) Ara-C and VEGF-A (125 mg/kg and 100 ng/mouse respectively); (v) neutralizing monoclonal antibody (mAb) to VEGFR2 (DC101, 800 μg/mouse, Days 7, 9, 11, 13, ImClone); (vi) Ara-C and monoclonal antibodies (mAbs) VEGFR2 (125 mg/kg and 800 μg/mouse respectively). All injections were intraperitoneal. Mice were either sacrificed after 15 days or kept alive to generate survival curves. Cell suspensions from BM and spleens were stained with fluorophore-conjugated antibodies against CD34 (RAM34, BD Pharmingen), CD11b (M1/70, BD Pharmingen), Ly-6G (RB6-8C5, BD Pharmingen), CD117 (2B8, BD Pharmingen), Sca-1 (D7, BD Pharmingen), CD16/32 (2.4G2, BD Pharmingen). Stained BM and spleen cells were analyzed with a LSRII SORP (BD Biosciences). All antibody staining was done at room temperature for 30 minutes. Samples were stained with either Fc Block or 10% rat serum to rule out non-specific Fc receptor binding or other cellular protein interactions. Each sample was analyzed with regards to the height and width in both the forward and side scatter parameters (FSC-HxFSC-W, SSC-HxSSC-W) allowing for the LSRII to discern single cells from adherent populations of two cells or more. Additionally, a “dump” channel (Am-Cyan), in combination with Live/Dead exclusion, was utilized to remove non-specific cell subsets by gating on the negative population. Gating strategies for flow cytometry was determined by single stain and fluorescent minus one controls.
Immunostaining of tissue sections
Freshly obtained liver, spleen, and bone specimens from C57BL/6J mice transplanted with 50,000 leukemic cells following sublethal irradiation (650 Rads) were divided into the six groups described above and tissues were fixed overnight in 4% paraformaldehyde at 4°C for subsequent frozen or paraffin section analysis. For paraffin sections, bones were decalcified in Richard Allan Decalcifying Solution (Thermo) for 1-2 hours. All tissues were then placed in 30% ethanol and shipped to Histoserv Inc. Histoserv Inc. cut 4-6 μm sections and performed hemotoxylin and eosin staining. For imunofluorescence (frozen sections), mice were injected by retro-orbital sinus with 10 μg of αVE-cadherin antibody (BV13, ImClone) conjugated to Alexa Fluor 647 (Alexa 647). After 10 minutes, mice were sacrificed and the femurs, liver, and spleen were removed and fixed overnight in 4% paraformaldehyde at 4°C. Bones were decalcified at room temperature using 10% EDTA (pH 7.2) for three days. All tissues were washed with PBS for 30 minutes and placed in 30% sucrose at 4°C O/N. Tissues were embedded in Tissue-Tek Optimal Cutting Temperature (OCT). 10 μm sections were air dried for 30 minutes, post-fixed with 4% paraformaldehyde for 15 minutes, washed 3X in PBS, and counter stained with DAPI (1 μg/mL) for 20 minutes at room temperature. Sections were mounted with Prolong Gold and viewed with the appropriate filters.
Colony Forming Cell (CFC) Assay
To analyze the ability of leukemic cells cultured in the presence or absence of VeraVec to initiate colony formation, we used a methylcelluose colony assay (MethoCult M3434, Stem Cell Technologies). 20,000 (5,000/well) leukemia cells were added to 4 mL of methylcellulose after each in vitro co-culture time point. After 7 days, colonies were counted and evaluated using an inverted microscope and gridded scoring dishes.
Peripheral Blood Analysis
Retro-orbital blood was collected on the indicated days using capillary pipettes and leukemic burden was analyzed based on cell surface markers and GFP positivity.
Statistical Analysis
Unless otherwise indicated, all data is represented as a mean + s.d., log rank (Survival Curves) and two-tailed Student’s t-test was used to determine statistical significance (*P<0.05, **P<0.001, ***P<0.0001).
Results
ECs support expansion of phenotypic LICs
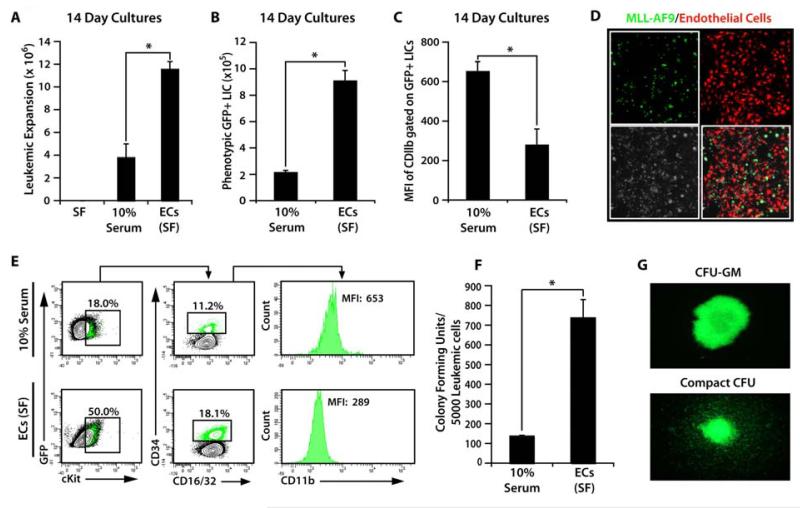
To interrogate the role of ECs in supporting the expansion of leukemic cells, we utilized a genetic model of murine AML generated by the transduction of mouse granulocyte macrophage progenitors with a bicistronic retrovirus expressing the MLL-AF9 fusion protein, encoded by the t(9;11)(p22;q23) translocation, followed by an IRES-driven GFP. We found that leukemic cells co-cultured with VeraVec in serum-free conditions, supplemented only with 50 ng/mL murine soluble kit ligand (sKitL), were able to dramatically expand for up to 14 days, when compared to leukemic cells cultured without feeder layers in media containing 10% serum supplemented with mouse sKitL (Figure 1A). Notably, leukemic cells grown without feeder layers and in serum-free media, supplemented only with mouse sKitL, failed to expand and died within the first 7 days of culturing (Figure 1A). Thus, ECs are able to replace the serum in supporting leukemic cell growth by providing the necessary instructive signals that obviate the need for growth factors contained in serum.
Figure 1. VeraVec support serum/cytokine-free expansion of a primitive population of leukemic cells.
A-C) MLL-AF9 GFP+ leukemic cells cultured for 14 days with or without VeraVec in serum-free conditions and in 10% serum without VeraVec. Expansion of GFP+ leukemic cells were analyzed after 14 days (A). Note that all leukemic cells cultured in serum-free culture conditions without VeraVec died by day 3 in culture. B) Day 14 leukemic cells were separated from the VeraVec and analyzed for phenotypic LIC cell surface markers. VeraVec significantly expanded phenotypic LICs as compared to conditions containing 10% serum. C) Mean fluorescent intensity of CD11b expression. LICs expanded on VeraVec exhibited very little CD11b expression, suggesting that VeraVec maintain and expand a phenotypically primitive LIC by inhibiting differentiation of the LIC population into more mature CD11b+ cells. D) Confocal microscopy of GFP+ leukemic cells co-cultured with RFP+E4+ECs at day 7, demonstrating intimate interaction of the leukemic cells with ECs. E) Representative contour plots demonstrating the phenotypic analysis of leukemic cells cultured without feeder layers in 10% serum compared to those co-cultured with VeraVec after 14 days. Leukemic cells co-cultured on VeraVec display a drastic increase in the number of phenotypically marked primitive LICs. F) Quantification of the leukemic colony formation (CFU) of the leukemic population co-cultured on VeraVec and EC-free 10% serum. There is a six-fold expansion of the CFU cells on cells co-cultured with ECs as compared to those cultured on 10% serum. G) GFP+ leukemic colonies ranged from the larger and more diffuse CFU-GM to the smaller, more compact CFU. Data was generated with 10 individual MLL-AF9 clones and each clone tested in technical triplicates in 3 independent experiments. All data represents mean + s.d. (*P<0.05).
Analysis of the cell surface phenotype of expanded leukemic cells revealed ECs fostered the expansion of subsets of leukemic cells that had higher expression of cKit, CD34, and CD16/32, markers associated with primitive LICs (Figure 1B-E) [33]. We assessed the potential of the expanded leukemic cells to initiate colony formation in a methylcellulose assay. Leukemic cells that were co-cultured with VeraVec showed far greater clonogenic potential (Figure 1F). Methylcellulose colonies consisted of large CFU-GM, as well as small CFU-GM, that were tightly packed, with morphology consistent with CFU-blast (Figure 1G). Taken together, the in vitro data suggests that the leukemic cells cultured with VeraVec are more highly enriched for LICs as evidenced by their cell surface phenotype, clonogenic potential, and increased quiescence compared to conventional culture conditions that contain 10% serum.
LICs cultured on ECs establish an aggressive leukemia in vivo
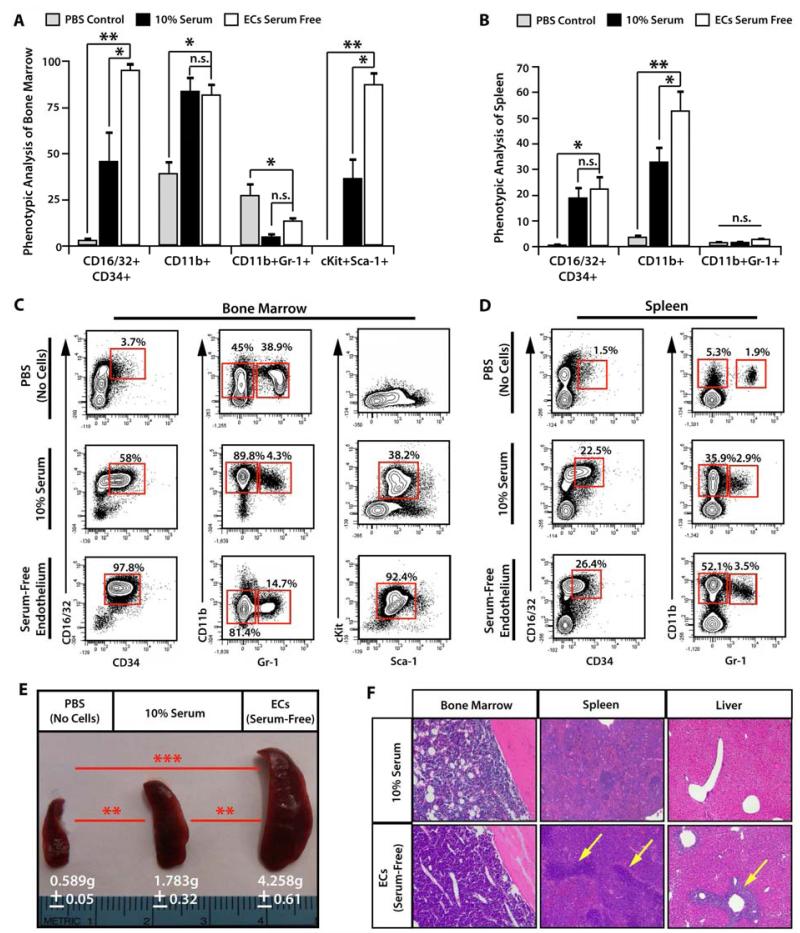
To assess the in vivo potential of the cultured leukemic cells to engraft and establish a de novo leukemia, we sublethally irradiated mice (650 Rads) and transplanted 50,000 leukemic cells from each surving culture conditions (n=10/cohort, 2 independent experiments). Mice transplanted with PBS alone were allowed to recover and served as non-leukemic controls. Additional cohorts of mice were used to generate survival curves (Supplemental Figure 1). By day 20, all the mice transplanted with leukemic cells co-cultured with VeraVec became moribund, with the non-transplanted control mice and mice transplanted with leukemic cells culture in 10% serum showing no signs of leukemic burden. Initial analysis of the mice showed a striking difference in spleen size, with VeraVec co-cultured leukemic cell transplants exhibiting the largest spleens (Figure 2E). Flow cytometric analysis of whole BM showed dramatic differences in the cell surface phenotype of the engrafted leukemic cells, while the spleens in both transplanted cohorts were very similar (Figure 2A-D). Transplanted leukemic cells initiated in co-culture with VeraVec possessed a more primitive phenotype consistent with LICs, exhibiting higher expression of CD16/32, CD34, cKit, and Sca-1. Although the leukemic cells initiated in 10% serum also exhibited cell surface phenotypes indicative of AML disease, the percentage of cells expressing LIC markers was significantly lower than those grown in direct cellular contact with ECs (Figure 2A-D). Histology of the BM, spleen, and liver confirmed that leukemic cells co-cultured on VeraVec were more aggressive and were able to establish disease at an accelerated pace. When analyzing the BM and spleen, we noticed that the cellularity of both organs was greater in the VeraVec cohort (Figure 2F). This is readily evident in the BM of 10% serum cohort, in which there were areas devoid of leukemic cells, while in the E4+ EC cohort the BM parenchyma was congested with leukemic cells (Figure 2F). Strikingly, we found multiple sites of chloroma formation within the livers of the mice injected with leukemic cells co-cultured VeraVec, and very little evidence of chloromas in mice that were transplanted with leukemic cells grown in 10% serum (Figure 2F). The in vivo data confirms the in vitro data shown in Figure 1, further demonstrating that leukemic cells co-cultured with VeraVec exhibit a more aggressive AML phenotype and that mice transplanted with these leukemic cells undergo extensive leukemic engraftment, expansion, and disease progression, which ultimately results in the mice rapidly succumbing to disease.
Figure 2. Leukemic cells co-cultured on VeraVec manifest increased engraftment and progression of disease.
50,000 leukemic cells cultured either without feeder layers in 10% serum or cocultured with VeraVec in serum free + 50 ng/mL of sKitL were injected into sublethally irradiated mice (650 Rads) and after 20 days, bone marrow (BM) and spleen were collected and analyzed by flow cytometry (n=10 per cohort, 3 independent experiments from 3 different clones used for Figure 1). A) The BM revealed the most striking difference, as leukemic cells co-cultured with VeraVec are overwhelmingly CD16/32+CD34+cKit+, indicative of a more primitive, aggressive AML disease. B) The spleens display similar numbers of CD16/32+CD34+ cells, but a marked increase in the number of CD11b+Gr-1+ cells among the leukemic cells cocultured with VeraVec. C, D) Representative contour plots of the data generated in the A) bone marrow and B) spleen. E) Spleen size was analyzed in the mice injected with leukemic cells. Note the larger size of the spleen in the group of mice injected leukemic cells co-cultured with VeraVec. F) Histology of the BM and spleen demonstrating that BM of mice injected with leukemic cells cocultured with VeraVec was packed with leukemic cells, while mice injected with leukemic cells grown in 10% serum displayed a scant population of leukemic cells. The liver of mice injected with leukemic cells cocultured on VeraVec exhibited multiple regions of chloroma formation, whereas the liver of leukemic cells cultured in 10% serum displayed little to no evidence of any chloroma formation. All data represents mean + s.d. (*P<0.05. **P<0.001, n.s.=not significant).
Direct cellular contact with activated ECs enhances leukemic adhesion and chemoprotection
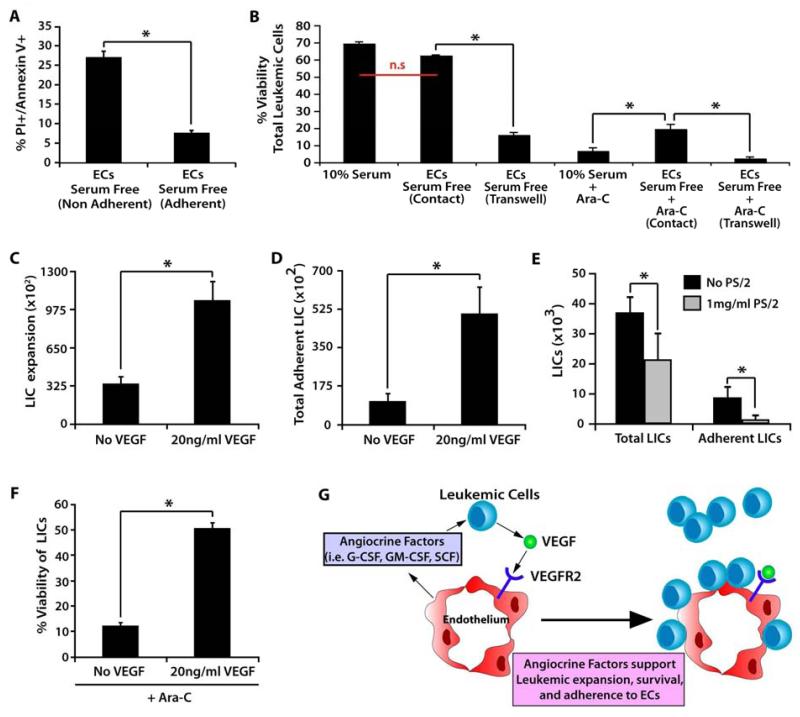
Recent data have suggested that stromal feeder layers and the endosteum can provide a microenvironment that enables leukemic cells to evade chemotherapeutic therapies [23, 24]. To determine if direct cellular contact to ECs confers similar chemo-protection for leukemic cells, we analyzed the frequency of viable leukemic cells (Annexin-V/PI) within the non-adherent and adherent leukemic cells populations. We found a higher percentage of apoptotic cells within the non-adherent leukemic population when compared to VeraVec adherent cells (Figure 3A). To test if ECs serve as a niche that protects leukemic cells from chemotherapeutic agents, we assessed the viability of leukemic cells when grown in 10% serum, in direct cellular contact with serum-free cultures of VeraVec, or physically separated from serum-free cultures of VeraVec by a microporous transwell. We found that treatment of leukemic cells expanded in transwell with Ara-C resulted in a significant decrease in leukemic cell viability (Figure 3B). When 500 ng/mL of Ara-C was added to all the aforementioned conditions, we found that leukemic cells co-cultured in direct cellular contact with VeraVec had a significant survival advantage, whereas the leukemic cells cultured in serum or those leukemic cells physically separated from VeraVec showed a significant decrease in cell viability (Figure 3B).
Figure 3. VEGF-A activated ECs stimulate proliferation and adhesion of leukemic cells and confer chemotherapeutic protection.
A) Annexin/PI staining to determine the number of non-viable cells present among the adherent and non-adherent populations of leukemic cells co-cultured on VeraVec. Note the increase in the percentage of live cells in the EC-adherent population. B) To determine if VeraVec might also provide a chemoprotective environment for adherent leukemic cells, leukemic cells were cultured with no feeder layer in 10% serumor co-cultured with VeraVec in either direct contact separated by transwells in serum-free conditions. These conditions were analyzed alone and with the chemotherapeutic agent Ara-C. Of those with Ara-C, only leukemic cells with direct contact to VeraVec were protected. C-E) 20 ng of VEGF-A was used to pre-stimulate VeraVec for 48 hours prior to coculture with LICs fostering a rapid increase in expansion of both the C) total and D) adherent populations of LICs after 7 days in coculture. E) VLA-4 neutralizing antibodies were added to the coculture conditions and total LIC expansion and the number of adherent LICs were analyzed after 7 days of coculture. F) 20 ng of VEGF-A increased the cell viability of LICs co-cultured on VeraVec in the presence of Ara-C, enhancing the chemoprotective properties of the VeraVec. Data was generated with 6 individual MLL-AF9 clones and each clone tested in technical triplicates in 3 independent experiments. All data represents mean + s.d. (*P<0.05, n.s.=not significant).
Angiogenic factors secreted from human leukemia cells have been shown to stimulate an increase in BM microvascular density in patients [34]. However, whether VEGF-A activated ECs support leukemic progression and maintenance of LICs in vivo is not known. Most studies examining the effect of VEGF-A on AML in vivo utilize AML models that are responsive to VEGF-A, making it difficult to determine if the effect of VEGFR2 stimulation is direct or niche-mediated. Therefore, we set out to determine if activation of VeraVec, by prestimulating with exogenous VEGF-A, could enhance the survival and expansion of leukemic cells. Indeed, when 20 ng/mL VEGF-A was used to pre-stimulate the E4+ECs prior to the addition of leukemic cells, we observed an increase in both the total LIC expansion and in the amount of adhesion to the VeraVec (Figure 3C, D). Adhesion was in part mediated by VLA-4. When neutralizing antibodies specific to VLA-4 were added to the culture conditions, it resulted in a significant decrease in VEGF-A-induced leukemic expansion and adhesion to endothelium (Figure 3E). Exogenous VEGF-A added to VeraVec co-culture conditions, along with 500 ng/mL of Ara-C, demonstrated a significant increase in LIC viability (Figure 3F). To address whether VEGF-A could directly affect the expansion of LICs, we generated 4 independent AML clones and cultured them in 10% serum and 50ng/mL of sKitL, supplemented with or without of 20 ng/mL of VEGF-A for 14 days in the absence of VeraVec. We found that the addition of VEGF-A did not have a direct effect on the overall expansion of phenotypic LICs (Supplemental Figure 2). These data demonstrate that VEGF-A activated ECs establish a primed vascular niche that promotes leukemic expansion and confers leukemic cells with a survival advantage when treated with chemotherapeutic agents (Figure 3G).
Activation of the vascular niche in vivo enhances the progression and chemoprotection of AML cells
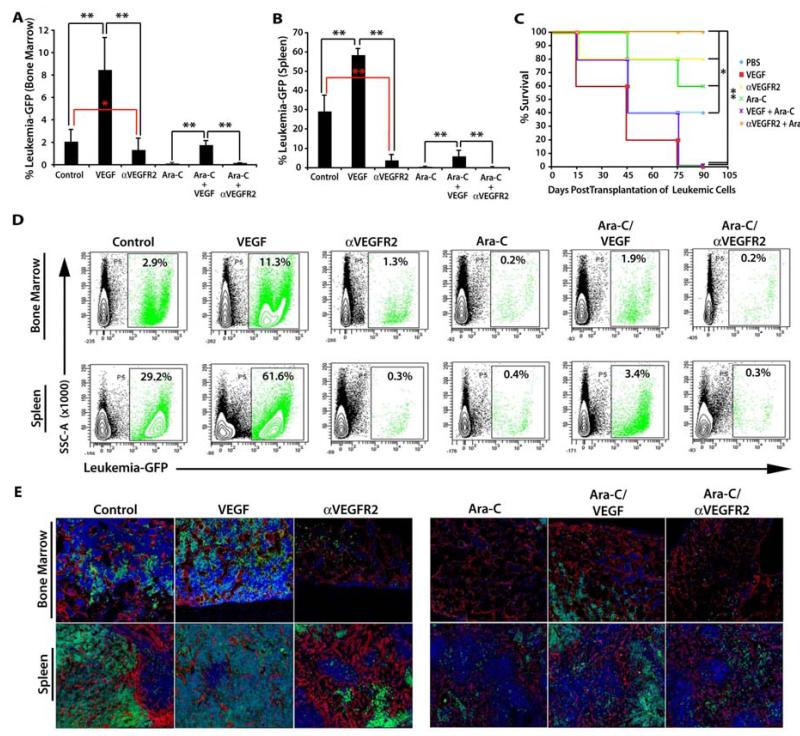
We recently developed in vivo angiogenic models to study the interaction of HSCs with the BM vascular niche [17]. Using a high affinity, neutralizing monoclonal antibody (mAb) to VEGFR2 (αVEGFR2), we found that proper VEGF-A/VEGFR2 signaling was essential for the engraftment and reconstitution of hematopoietic cells that were transplanted into lethally irradiated mice [8]. We next set out to test if modulating the activation state of the vasculature through the VEGF-A/VEGFR2 axis could promote or inhibit the initiation of AML disease by conditioning vascular niches of hematopoietic organs to serve as a safe haven for leukemic cells, protecting them against chemotherapeutic agents. Mice were sublethally irradiated (650 Rads) and transplanted with 5 × 104 GFP+ leukemic cells. All mice were allowed to recover for 7 days and then were split into 6 groups (n=20/cohort; 3 independent experiments). Starting on day 7, two cohorts of mice were given an intraperitoneal injection of 800 μg of αVEGFR2 antibody every other day for a total of 4 injections (day 13). An additional two cohorts were given intraperitoneal injections of 100 ng of VEGF-A twice a day, until time of analysis (day 14). On day 9 post-irradiation, the two aforementioned groups were split into two sub-groups each. One sub-group from both the VEGF-A and αVEGFR2 cohorts were administered Ara-C at a dose of 125 mg/kg on days 9 and 10. One cohort was also treated with an Ara-C regime, but did not receive any VEGF-A or αVEGFR2 injections. The control cohort only received PBS injections (Supplemental Figure 3). On day 15 post-transplant, 5 mice were sacrificed from each cohort and isolated bones and spleen for flow cytometric and histological analysis (Figure 4 A, B and D, E). Mice that received exogenous VEGF-A demonstrated a profound increase in leukemic burden in the BM and spleen, when compared to the control cohort. In particular, the spleen was more than 50% occupied by GFP+ leukemic cells (Figure 4 B, D Bottom Row, E Left Panels). When analyzing the αVEGFR2 group, we found that blocking the VEGF-A/VEGFR2 signaling pathway resulted in a significant decrease in the ability of the GFP+ leukemic cells to initiate and maintain leukemic burden (Figure 4 A, B and D, E). This phenotype was recapitulated in the mice that received the Ara-C regime. Strikingly, the cohort that underwent the Ara-C treatment conditioned with exogenous VEGF-A appeared to be protected against the Ara-C treatment. This observation was clearly evident in flow cytometry and immunofluorescence analysis, in which a significant number of GFP+ leukemic cells survived the chemotherapeutic treatment (Figure 4 D, E). Remaining mice (n=15/cohort; 3 independent experiements) were monitored for survival (Figure 4C). As predicted from the immunofluorescence and flow cytometry, both cohorts that received exogenous VEGF-A succumbed to the leukemic disease by 3 months post-treatment. Approximately half the mice in the control and Ara-C treated cohorts survived 3 months post-treatment, whereas mice treated anti-VEGFR2 had an 80% survival rate and the cohort receiving adjuvant treatment with αVEGFR2 and Ara-C had a 100% survival rate 3 months post transplant.
Figure 4. VEGF-A, through activation of the VEGFR2+ BM vascular niche, enhances engraftment, progression, and chemoprotection of leukemic cells.
A-B) Sublethally irradiated mice (650 Rads) were injected with 1,000 GFP+ MLL-AF9 leukemic cells to interrogate the effect of the VEGF-A/VEGFR2 stimulated vascular niche in leukemic cell progression (n=20 per cohort, 3 independent experiments). A) Mice given 100 ng of VEGF-A showed a remarkable increase in the number of leukemic cells in the BM, while mice treated with 800 μg/injection of mAb to VEGFR2 displayed very low levels of MLL-AF9 engraftment. When treated with 125 mg/kg of Ara-C, mice given exogenous VEGF-A exhibited a marked increase in leukemia survival and leukemic burden, while the control and those treated with mAb to VEGFR2 manifested no chemoprotection. B) These results were mirrored in the spleens, with the only difference being higher initial levels of GFP+ leukemic cells in the control and VEGF-A treated mice. C) Survival graph of mice. Treatment with an αVEGFR2 antibody increases the sensitivity to Ara-C treatment thereby significantly increasing the survival of the treated mice over all the other cohorts (n=15, 3 independent experiments with 3 different clones). D) Representative flow cytometric plots of the leukemic burden in the BM and spleens of VEGF-A mice demonstrating dramatic increased engraftment of GFP+ leukemic cells and resistance to Ara-C. E) Immunofluorescence of the BM and spleen tissues demonstrates the increased leukemic burden in VEGF-A treated mice and the greatly reduced number of GFP+ leukemic cells in those treated with a mAb toVEGFR2. All data represents mean + s.d. Significance of Survival Curve was determined by Log Rank Test (*P<0.05. **P<0.001).
Discussion
Cell autonomous oncogenic alterations play a major role in initiation and progression of leukemic cells. Nevertheless, accumulating evidence suggests that the microenvironment provides necessary extrinsic signals for the progression of leukemia and maintenance of LICs. Recent studies have shown that ECs can be perceived as specialized organ-specific vascular niches that, upon proper activation, could instructively support maintenance and reconstitution of normal and malignant stem/progenitor cells by secretion of angiocrine factors and deposition of extracellular matrix [28]. Here, we demonstrate, by employing in vitro and in vivo angiogenic models, that non-autonomous signals conveyed by activated ECs provide an instructive and fertile niche that supports the expansion of primitive and aggressive leukemic clones that ultimately lead to an increase in leukemic burden and the rapid demise of the transplanted mice. Our in vitro co-culture system demonstrates that direct cellular contact of leukemic cells with the ECs is essential for the expansion of primitive LICs that phenocopy an aggressive myeloid leukemia when transplanted into syngenic mice. Furthermore, activation of the ECs through the VEGF-A/VEGFR2 signaling axis promotes adhesion of the leukemic cells to the ECs. This intimate interaction confers a survival advantage when exposed to a cell-cycle specific chemotherapeutic agent. The data generated from our in vivo models further supports the in vitro data, where VEGF-A activated ECs rapidly increase leukemia initiation, leukemic cell engraftment and expansion, and protects the leukemic cells from chemotherapy. In contrast, inhibiting the VEGF-A/VEGFR2 axis with an antibody raised against VEGFR2 results in significant inhibition of leukemic progression.
The mechanism by which ECs support the expansion of the leukemic cells is likely mediated by the expression of both supportative membrane-bound and secreted angiocrine factors. We have recently shown that the activation state of ECs orchestrate the self-renewal and lineage specific differentiation of hematopoietic stem and progenitor cells [14]. Angiocrine factors expressed by ECs, through Akt and MAPK pathway activation, induced production of pro-hematopoietic cytokines that balance the self-renewal and differentiation of HSC [14]. In this study, we demonstrate that leukemic cells utilize a similar pathway for their expansion and survival. Considering leukemic cells “highjack” and exploit normal survival mechanisms of non-malignant hematopoietic cells and the self-renewal pathways of HSCs, it would not be surprising that many of the same angiocrine factors produced by Akt-activated ECs would support the propagation of primitive and aggressive leukemic clones. One major advancement of our ex vivo vascular niche platform is the ability to cultivate ECs in serum-/growth factor-free conditions. This has allowed for the development of an unbiased co-culture system where we can begin to systematically screen for as of yet unknown angiocrine factors produced by ECs that have been co-cultured with leukemic cells. This screen could have the potential to reveal novel target angiocrine factors that could play a key role in the expansion of leukemia, as well as other factors that are already known to promote the expansion of leukemic cells (i.e. IL-6 and GM-CSF, G-CSF). We are currently evaluating all potential pro-leukemic angiocrine factors that augment adhesion to the ECs and confer chemotherapy resistance.
We show that VEGF-A, through upregulation of VLA-4/VCAM1-mediated adhesion, enhances interaction of the leukemic cells with the ECs. This results in an increase of survival of leukemic clones that are resistant to chemotherapy. In support of these data, we show that inhibition of VEGFR2 results in an increased sensitivity to chemotherapy and a profound increase in the survival of the treated mice. This indicates that activated ECs may sustain minimal residual leukemic burden leading to increase leukemic burden and relapse rate.
Previous studies have shown that functional VEGF-A receptors, including VEGFR2, can potentially be expressed by a subgroup of myeloid leukemias [30, 35]. Therefore, targeting VEGFR2 might not only interfere with the activation of the ECs, but also directly inhibit the growth of the leukemic cells. However, none of the leukemias studied here express VEGFR2 (Supplemental Figure 4). Thus, we surmise that antibodies to VEGFR2 primarily target VEGF-A activated ECs, thereby increasing sensitivity of the leukemic cells to Ara-C and diminishing the leukemic burden. Although our data suggests that in our model system of AML there are no direct effects on the leukemic cells during VEGF-A and α-VEGFR2 administration (Supplemental Figures 2 and 4), given the aforementioned data suggesting that human AML express functional VEGF receptors, as well as non-malignant hematopoietic cells, it is possible that administration of neutralizing antibodies against the VEGF/VEGFR2 axis could have detrimental off target effects. Therefore, it will be necessary to begin developing therapies that specifically target the VEGF/VEGFR2 axis on the endothelium to disrupt the leukemia/endothelial hub to potentially enhance the efficacy of adjuvant therapies.
Collectively, these data suggest that VEGF-A activated ECs establish an instructive vascular niche that support survival and proliferation of the leukemic cells both in vitro and in vivo. Close cellular interaction between activated ECs and leukemic cells confer the latter, in addition to significant resistance to chemotherapeutic agents. As such, targeting VEGFR2+ ECs will not only provide for a potentially effective means to diminish leukemic burden and minimal residual disease, but also increase the sensitivity of leukemic cells to chemotherapeutic agents. Furthermore, interfering with the adhesion of leukemic cells to the ECs may provide an opportunity to increase the sensitivity of the leukemic cells to the chemotherapeutic agents, potentially decreasing the mortality and morbidity associated with refractory leukemias. While treatment strategies targeting angiogenic factors and neovascularization in AML have begun to demonstrate promising results [36, 37], utilizing our novel co-culture system that allows for the maintenance and expansion of LICs promises be an additional tool to help identify and test novel factors that may prove effective in eradicating chemo-resistant LICs that contribute to disease relapse.
Supplementary Material
Acknowledgements
We would like to thank Eva V. George, Mollie R. Goetz, and Bryant Bonner for their technical assistance in obtaining data for the manuscript. J.M.B. and S.R. are supported by Ansary Stem Cell Institute; Empire State Stem Cell Board and New York State Department of Health grants (NYSTEM, C024180, C026438, C026878); NHLBI R01 HL097797; Qatar National Priorities Research Foundation NPRP08-663-3-140. J.M.B is supported by the Tri-Institutional Stem Cell Initiative, American Society of Hematology Scholar Award, and American Federation of Aging Research Award. M.G.P. is supported by a Postdoctoral Tri-Institutional Stem Cell Initiative Fellowship (New York, NY). J.M.B. and C.C.K. are support by a Sponsored Research Agreement from Angiocrine Bioscience.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldstone AH, et al. Attempts to improve treatment outcomes in acute myeloid leukemia (AML) in older patients: the results of the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98(5):1302–11. doi: 10.1182/blood.v98.5.1302. [DOI] [PubMed] [Google Scholar]
- 2.Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341(14):1051–62. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 3.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–7. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 4.Park CY, Tseng D, Weissman IL. Cancer stem cell-directed therapies: recent data from the laboratory and clinic. Mol Ther. 2009;17(2):219–30. doi: 10.1038/mt.2008.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sengupta A, et al. Rac2 GTPase deficiency depletes BCR-ABL+ leukemic stem cells and progenitors in vivo. Blood. doi: 10.1182/blood-2009-10-247437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, et al. The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science. 327(5973):1650–3. doi: 10.1126/science.1186624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao C, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458(7239):776–9. doi: 10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hooper AT, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4(3):263–74. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lo Celso C, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457(7225):92–6. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie Y, et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2009;457(7225):97–101. doi: 10.1038/nature07639. [DOI] [PubMed] [Google Scholar]
- 11.Ding L, et al. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–62. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Himburg HA, et al. Pleiotrophin regulates the retention and self-renewal of hematopoietic stem cells in the bone marrow vascular niche. Cell Rep. 2012;2(4):964–75. doi: 10.1016/j.celrep.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Himburg HA, et al. Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nat Med. 2010;16(4):475–82. doi: 10.1038/nm.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi H, et al. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol. 2010;12(11):1046–56. doi: 10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raaijmakers MH, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464(7290):852–7. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butler JM, et al. Development of a vascular niche platform for expansion of repopulating human cord blood stem and progenitor cells. Blood. 2012;120(6):1344–7. doi: 10.1182/blood-2011-12-398115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butler JM, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6(3):251–64. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendez-Ferrer S, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–34. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–5. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunisaki Y, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502(7473):637–43. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poulos MG, et al. Endothelial jagged-1 is necessary for homeostatic and regenerative hematopoiesis. Cell Rep. 2013;4(5):1022–34. doi: 10.1016/j.celrep.2013.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsunaga T, et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat Med. 2003;9(9):1158–65. doi: 10.1038/nm909. [DOI] [PubMed] [Google Scholar]
- 23.Ishikawa F, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007;25(11):1315–21. doi: 10.1038/nbt1350. [DOI] [PubMed] [Google Scholar]
- 24.Iwamoto S, et al. Mesenchymal cells regulate the response of acute lymphoblastic leukemia cells to asparaginase. J Clin Invest. 2007;117(4):1049–57. doi: 10.1172/JCI30235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cogle CR, et al. Functional integration of acute myeloid leukemia into the vascular niche. Leukemia. 2014 doi: 10.1038/leu.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pezeshkian B, et al. Leukemia Mediated Endothelial Cell Activation Modulates Leukemia Cell Susceptibility to Chemotherapy through a Positive Feedback Loop Mechanism. PLoS One. 2013;8(4):e60823. doi: 10.1371/journal.pone.0060823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunisaki Y, Frenette PS. The secrets of the bone marrow niche: Enigmatic niche brings challenge for HSC expansion. Nat Med. 2012;18(6):864–5. doi: 10.1038/nm.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev Cancer. 2010;10(2):138–46. doi: 10.1038/nrc2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seandel M, et al. Generation of a functional and durable vascular niche by the adenoviral E4ORF1 gene. Proc Natl Acad Sci U S A. 2008;105(49):19288–93. doi: 10.1073/pnas.0805980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dias S, et al. Autocrine stimulation of VEGFR-2 activates human leukemic cell growth and migration. J Clin Invest. 2000;106(4):511–21. doi: 10.1172/JCI8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aguayo A, et al. Plasma vascular endothelial growth factor levels have prognostic significance in patients with acute myeloid leukemia but not in patients with myelodysplastic syndromes. Cancer. 2002;95(9):1923–30. doi: 10.1002/cncr.10900. [DOI] [PubMed] [Google Scholar]
- 32.Kuzu I, et al. Bone marrow microvessel density (MVD) in adult acute myeloid leukemia (AML): therapy induced changes and effects on survival. Leuk Lymphoma. 2004;45(6):1185–90. doi: 10.1080/1042819032000159915. [DOI] [PubMed] [Google Scholar]
- 33.Krivtsov AV, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442(7104):818–22. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- 34.Hussong JW, Rodgers GM, Shami PJ. Evidence of increased angiogenesis in patients with acute myeloid leukemia. Blood. 2000;95(1):309–13. [PubMed] [Google Scholar]
- 35.Dias S, et al. Inhibition of both paracrine and autocrine VEGF/VEGFR-2 signaling pathways is essential to induce long-term remission of xenotransplanted human leukemias. Proc Natl Acad Sci U S A. 2001;98(19):10857–62. doi: 10.1073/pnas.191117498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trujillo A, McGee C, Cogle CR. Angiogenesis in acute myeloid leukemia and opportunities for novel therapies. J Oncol. 2012;2012:128608. doi: 10.1155/2012/128608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madlambayan GJ, et al. Leukemia regression by vascular disruption and antiangiogenic therapy. Blood. 2010;116(9):1539–47. doi: 10.1182/blood-2009-06-230474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.