Abstract
In humans and other mammals, the unexpected loss of a resource can lead to emotional conflict. Consummatory successive negative contrast (cSNC) is a laboratory model of reward devaluation meant to capture that conflict. In this paradigm, animals are exposed to a sharp reduction in the sucrose concentration of a solution after several days of access. This downshift in sucrose content leads to behavioral responses such as the suppression of consumption and physiologic responses including elevation of corticosterone levels. However, response heterogeneity in cSNC has yet to be explored and may be relevant for increasing the validity of this model, as humans demonstrate clinically meaningful heterogeneity in response to resource loss. The current analysis applied latent growth mixture modeling to test for and characterize heterogeneity in recovery from cSNC among rats (N = 262). Although most animals exhibited recovery of consummatory behavior after a sharp drop in consumption in the first postshift trial (Recovery class; 83%), two additional classes were identified including animals that did not change their consumption levels after downshift (No Contrast class; 6%), and animals that exhibited an initial response similar to that of the Recovery class did not recover to preshift consumption levels (No Recovery class; 11%). These results indicate heterogeneity in recovery from reward loss among rats, which may increase the translatability of this animal model to understand diverse responses to loss among humans.
Keywords: Reward loss, consummatory successive negative contrast, individual differences, animal models, latent growth mixture modeling
The loss or reduction of key resources such as food, mates, and shelter results in physiological and behavioral responses crucial for survival [42,44]. Neurocircuitry associated with survival functions involve evolutionarily conserved homologous mechanisms, allowing for animal research into functional similarities across several key levels of analysis [27,43]. For example, in the case of threat detection and response, important connections between environmental danger, memory, and fear that are highly relevant to the understanding of stress and anxiety have been extensively characterized at genetic [23], neurochemical [36], neurocircuitry [56], and behavioral [8] levels through the use of Pavlovian threat (fear) conditioning paradigms. However, the contribution of reward or resource loss to the understanding of anxiety, depression, and resilience, has received relatively little attention [26].
Human responses to loss of resources are complex. For example, significant financial and material losses are associated with stress and life dissatisfaction [21,61]. Even in the context of significant trauma exposure, loss of resources can outweigh event-specific predictors of clinical outcome such as the nature of the trauma itself [20,30]. Similarly, distress and depression following the loss of a significant other is influenced by loss of access to resources previously facilitated by one’s spouse [4,15]. Importantly, significant heterogeneity has been observed in response to resource loss. In the context of bereavement, for example, only a minority of individuals develop clinically relevant depression symptoms, and among those many are able to recover; the majority, on the other hand, demonstrate resilience characterized by a healthy psychological adaptation to resource loss [3,15].
While humans follow diverse and clinically distinct patterns of response to loss, a limitation of animal models of human phenomena is that they typically examine mean-level responses based on the assumption of population homogeneity. Recent research utilizing data analytic methods for identifying heterogeneous populations has characterized divergent behavior patterns in human psychiatric populations as well as in animal models of such conditions. For example, patterns of extinction after threat (fear) conditioning in animals are similar to observed trajectories in the symptoms of posttraumatic stress disorder (PTSD) in recently traumatized individuals [14,16]. This approach helped increased translatability of such models for studying neurobiological causes and correlates of stress and anxiety across distinct subpopulations [29]. However, response heterogeneity in an animal model of surprising reward loss has yet to be explored with the same statistical methods that are being applied in other animal models. The data presented here provide a key step in the translation of animal models of reward loss to human phenomena.
Consummatory successive negative contrast (cSNC) is a model of reward devaluation in which animals are exposed to a sharp decrease in the concentration of a sucrose solution (typically from 32% to 4% sucrose, an 8-to-1 reduction in magnitude) after several days of access to the higher reward [10]. The performance of downshifted animals is compared to that of unshifted controls always given the lower sucrose concentration. Although various emotional terms have been applied to describe the reaction of nonhuman animals to reward decrement, including anxiety [11], anger [58], depression [5], disappointment [57], frustration [1], and psychological pain [49], it is important to acknowledge that no animal model can fully capture the complexity of emotions experienced by humans who have endured a meaningful loss. However, LeDoux [27] recently suggested that much of the neurocircuitry underlying responses to significant environmental stressors, such as loss of access to key resources, is highly conserved across species. As such, common neurobiological responses are both accessible for research across species and provide information directly translatable for understanding human responses to stressors brought on by threats to survival. The cSNC effect has potential to provide information on basic processes related to reward loss. For example, cSNC leads to consummatory suppression [60], raises levels of stress hormones [31,50], and can be manipulated by targeting opioid [7,55], cannabinoid [17], serotonergic [37], and GABAergic [12,39] neurotransmitter systems. Reward devaluation has also been shown to affect sexual behavior [13], aggressive behavior [33], and to induce hypoalgesia [34]. In turn, peripheral pain [38] and restraint stress [41] were shown to enhance the effects of reward devaluation on behavior. Taken together, these results are consistent with an emotional interpretation of the cSNC effect that posits a significant weight on the role of brain circuits relevant to anxiety, depression, and stress responses [22,51]. Since individual variability plays a role in these diverse responses, as shown by selective breeding studies [10,40], mean-level analyses of the cSNC paradigm may not provide a complete picture.
The current study applied latent growth mixture modeling (LGMM) to test for heterogeneity in recovery from reward reduction in rats. This statistical method identifies heterogeneous latent classes displaying qualitatively distinct patterns of change by determining both the quantity and shape of trajectories that best characterize the data [9]. The LGMM technique provides data on individual differences complementing research using selective breeding. This approach was applied to a pooled sample of a substantial number of untreated (i.e., control) animals from experiments applying very similar conditions to test the hypothesis that rats exposed to reward devaluation in the cSNC situation would exhibit heterogeneous profiles of recovery.
Method
Subjects
Data from adult male and female rats (Long-Evans and Wistar) from 21 experiments completed between 2004 and 2014 under very similar conditions were included in the analysis. Extensive research with these strains and published comparisons among rat strains [10] show no detectable differences in terms of the cSNC effect. Therefore, animals were pooled together for the purpose of the present analyses. One set of animals was exposed to a 32-to-4% sucrose downshift, with 32% sucrose on Trials 1-10 and 4% sucrose on Trials 11-15 (175 males; 87 females), whereas other animals were unshifted controls exposed to 4% sucrose throughout the 15 trials (140 males; 53 females). Unequal sample sizes by contrast condition were caused mainly by a single experiment involving only the downshifted condition; the unequal sample sizes by sex were caused by the majority of selected experiments involving only males. Typically, these animals were those assigned to the control conditions of experiments involving psychobiological or behavioral manipulation (e.g., saline controls in psychopharmacological experiments). Rats were either purchased from Harlan Labs or bred in the TCU vivarium from adults purchased at Harlan Labs (Indianapolis, IN), and housed in individual, wire-bottom cages at around 40 days of age until the end of the experiment. In more recent experiments, a dark red Plexiglas rodent retreat (BioServ, Frenchtown, NJ) measuring 15×9×9 cm (L×H×W) was placed in each cage for enrichment. Animals were deprived to 81-84% of their ad libitum weight; food deprivation started when rats were at least 90 days old. Training started when animals were 95-100 days of age. Water was continuously available throughout the experiment. The colony room was kept at a constant temperature (around 22-23 °C) and humidity (40-60%), and under a 12:12 light:dark cycle (lights on at 07:00 h).
Apparatus
Behavioral training was conducted in conditioning boxes (MED Associates, VT) made of aluminum and Plexiglas (29.4×28.9×24.7 cm, L×H×W). The floor of each box consisted of steel rods. A tray with corncob bedding was placed below the floor to collect feces and urine. A hole in the feeder wall (1 cm wide, 2 cm high, and 4 cm from the floor) allowed the insertion of a sipper tube (1 cm in diameter). When fully inserted, the sipper tube was 1 cm inside the box (early experiments) or flush against the wall (later experiments). Diffuse light was provided by a house light located in the upper part of a wall opposite to the sipper tube (early experiments) or in the back wall. A computer located in an adjacent room controlled the presentation and retraction of the sipper tube, and recorded the rat’s contact with it. When the rats made contact with the sipper tube, a circuit involving the steel rods in the floor and the sipper was closed, and the signal was recorded by the computer. This provided a measure of cumulative contact with the sipper tube, called goal-tracking time, measured in 0.05-s or 0.01-s units in different experiments, and transformed to seconds for the current analysis. Goal-tracking time correlates positively and significantly with fluid intake for both 32% and 4% sucrose concentrations [32], and it leads to essentially the same results as lick frequency [53] and amount of fluid intake [47]. Each conditioning box was placed in a sound-attenuating chamber that contained a speaker to deliver white noise and a fan for ventilation (combined noise: 80.1 dB, scale C).
Procedure
There were some variations in training procedures. A minority of the animals (approximately 10% of the total sample) received two 5-min trials of habituation to the conditioning boxes before training started; no solution was provided during these trials. Most other animals started directly with the first training trial. Training involved one trial per day for 15 consecutive days. The preshift phase involved 10 trials (Trials 1-10) during which animals had access to a 32% sucrose solution; the postshift phase involved 5 trials (Trials 11-15) during which the solution was downshifted to 4% sucrose. In most experiments, for each 32-to-4% sucrose downshift group there was an unshifted control exposed to 4% sucrose in all trials. Solutions were prepared weight/weight by mixing 32 g (or 4 g) of sucrose for every 68 g (or 96 g) of distilled water. Animals were transported from the colony room to a waiting room in a transport rack in groups of 4-8, in their own cages, before being placed in the conditioning box. At the start of each trial, the house light was turned on and a variable 30-s pretrial interval (range: 15-45 s) was initiated before the presentation of the sipper tube. Each trial lasted 5 min counting from the rat’s first recorded contact with the sipper tube. At the end of the 5 min, the sipper tube was withdrawn and a variable 30-s posttrial interval (range: 15-45 s) was introduced before relocating the animal to its cage. Animals were then moved to the waiting room or directly to the colony room. In all the experiments, animals were given additional food in their cages at least 15 min after the end of the training trial. Conditioning boxes were wiped with a damp paper towel after each trial. All inputs (e.g., sipper-tube contacts) and outputs (e.g., sipper presentation and withdrawal) were controlled by a computer located in an adjacent room via MED interface with a program written in MED Notation (MED Associates, VT).
Data Analysis
To characterize the cSNC effect, the goal-tracking times for the sample of 32-to-4% (downshifted) animals were compared to the sample of animals exposed to 4% sucrose through the entire procedure (unshifted controls). A mixed within-between analysis of variance (ANOVA) was used to examine differences between groups across trials in the preshift stage (Trials 1-10) and in the postshift stage (Trials 11-15). Post-hot tests derived from the main analysis and based on Bonferroni adjustments were used for pairwise comparisons. The effects of the main analysis and post-hoc tests were considered significant at α < 0.05 level.
Trajectories of recovery from cSNC were identified with LGMM using Mplus 6.12 [35]. To facilitate model convergence given the relatively small sample size for this modeling technique, the number of parameters for estimating trajectories was reduced to only the five postshift trials (i.e., Trials 11-15, the recovery phase). The goal-tracking time for each subject, in each postshift trial, was subtracted from the goal-tracking time in the last preshift trial (Trial 10). This adjustment allowed for the modeling of trajectories of change in goal-tracking behavior relative to the last preshift trial. It also served to standardize measurements across animals from several experiments where systematic differences may have influenced raw measurements. Individuals were allowed to vary in their slope and intercept (random effects). Three indices of information criteria (i.e., Akaike, Bayesian, and sample size adjusted Bayesian), the Lo-Mendel-Rubin likelihood ratio test (LRT), and entropy were all considered in the evaluation of model fit, as well as interpretability and parsimoniousness [24]. Improvement in model fit is characterized by lower values of information criteria, significant LRT, high entropy, and classes containing no fewer than 2% of the sample. Posterior probabilities of class assignments were exported to SPSS 22 (IBM) for additional post-hoc analyses examining differences in acquisition and recovery between the three classes. All analyses were considered significant at α < 0.05 level after Bonferroni adjustment for multiple comparisons.
Results
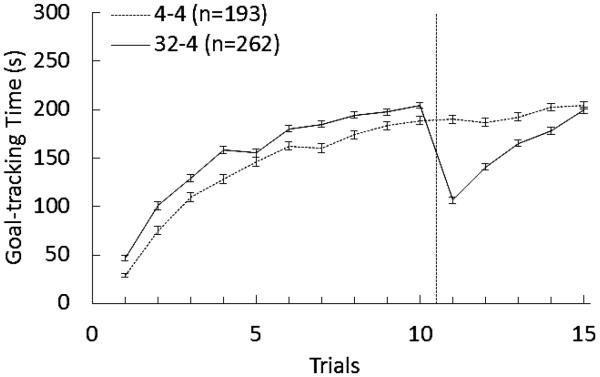
Figure 1 shows the performance of the combined set of animals in the 32-to-4% sucrose downshift condition and in the 4% sucrose unshifted controls. Both groups increased their consummatory behavior across trials, but animals with access to 32% sucrose produced consistently higher goal-tracking times than those with access to 4% sucrose. These data were analyzed with Group (32-4%, 4-4%) × Trial ANOVA separately for the preshift (Trials 1-10) and postshift (Trials 11-15) phases. For the preshift, there were significant main effects for both factors and for their interaction, Fs > 2.45 ps < 0.009, partial η2s ≥ 0.005. Bonferroni adjusted pairwise tests derived from the main analysis indicated that rats with access to 32% sucrose displayed higher goal-tracking times than those with access to 4% sucrose on all trials, Fs(1, 447) > 7.24, ps < 0.008, except Trial 5, F(1, 471) = 2.48, p > 0.11. The phenomenon of interest is illustrated by the difference between groups during postshift Trials 11-15. On these trials, all the animals receive access to 4% sucrose, thus any differences in behavior reflect differential history with the reward. The cSNC effect is defined in terms of the distinct behavioral trajectories during these postshift trials of downshifted vs. unshifted groups. The effect is transient, as animals recover from the initial disruption to reach a level of performance similar to that of unshifted controls. The statistical analysis indicated that both main effects and their interaction were significant for postshift trials, Fs > 67.74, ps < 0.001, partial η2s ≥ 0.13. Bonferroni pairwise comparisons indicated that downshifted animals contacted the sipper tube significantly less than unshifted controls on Trials 11-14, Fs(1, 477) > 19.09, ps < 0.001, but not on Trial 15, F < 1. Therefore, the sample used in the following analysis, aimed at identifying how behavioral profiles during recovery from reward devaluation, corresponds to a set of animals that had shown evidence of the cSNC effect after a 32-to-4% sucrose downshift.
Figure 1.
Consummatory performance measure in terms of cumulative contact with the sipper tube (goal-tracking time, in seconds) in two groups of rats exposed to a 32-to-4% sucrose downshift (32-4) or to unshifted 4% sucrose (4-4). The cSNC effect is illustrated by the difference in consummatory behavior during Trials 11-15, when animals in both groups receive access to 4% sucrose, but differ in terms of prior reward history.
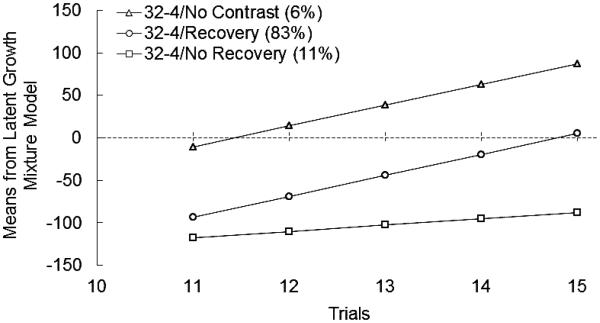
A single-solution unconditional LGMM was identified to compare the relative fit of successive models. The 3-class model provided the best fit, with a significant LRT (p = 0.05), lower scores on two of the three information criteria, an increase in entropy relative to the 2- class solution (0.75 vs. 0.65), and an advantage in interpretability. The 4-class solution provided a poorer overall fit, with increases across all information indices, lower entropy (0.52), and a nonsignificant LRT (p = 0.56); thus, the 3-class solution was selected. An examination of the plot shown in Figure 2 revealed three distinct trajectories of recovery from cSNC (i.e., Recovery, No Recovery, No Contrast). In this figure, the performance of each animal on each postshift trial was subtracted by its performance on Trial 10 (the last preshift trial). Most subjects (83%) were characterized by a sharp drop in goal-tracking time in the first postshift trial relative to the last preshift trial (ESTintercept = −93.72, SE = 4.68, p < 0.001) followed by increases (i.e., recovery) in subsequent trials (ESTslope = 24.74, SE = 1.23, p < 0.001). A second class (11%) was identified that also decreased their consummatory behavior in the first postshift trial (ESTintercept = −117.19, SE = 12.82, p < 0.001). However, their slope was less than one third of the Recovery class’s slope, indicating slower rate of recovery (ESTslope = 7.24, SE = 2.59, p = 0.01). Compared to the other two trajectories, the No Contrast trajectory (6%) was characterized by a nonsignificant change in consummatory behavior in the first postshift trial (ESTintercept = −10.37, SE = 21.90, p = 0.64) and continued increase in the remaining trials (ESTslope = 24.34, SE = 2.66, p < 0.001).
Figure 2.
Goal-tracking time (s) during each postshift trial (4% sucrose) was subtracted from the goal-tracking time (s) of each animal in its last preshift trial (32% sucrose) in downshifted animals from the three classes of recovery identified. The classes (percentage of total sample) were labeled according to their postshift performance: Recovery (83%), No Recovery (11%), and No Contrast (6%). The sample size for each class was 217, 30, and 15 animals, respectively. Change scores are estimated means derived from the latent growth mixture model.
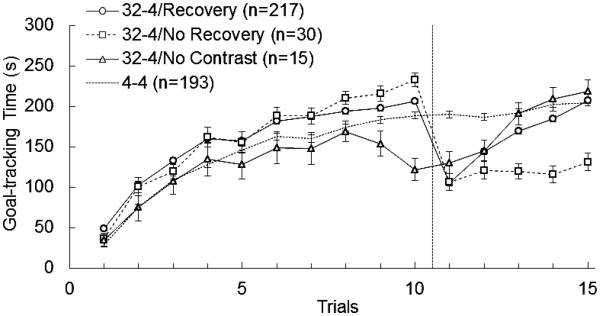
Raw data depicting the 3-class model over the complete procedure are plotted in Figure 3. The performance of the group of animals given always access to 4% sucrose (Group 4) is added as a reference. A post-hoc mixed within-between ANOVA was conducted to examine differences between the three classes in the preshift phase, Trials 1-10. Six cases (< 2.3%) with missing data on some of the acquisition trials could not be included. There was a main effect of trial F(9, 253) = 110.76, p < 0.001, partial η2 = 0.30, and class, F(2, 253) = 5.75, p = 0.004, partial η2 = 0.04, as well as a trial-by-class interaction, F(18, 253) = 2.73, p < 0.001, partial η2 = 0.02. Bonferroni adjusted post-hoc pairwise comparisons of acquisition trials demonstrated lower mean goal-tracking time in the No Contrast class compared to the Recovery class on Trials 7, 9, and 10 (all M differences < −39.77, ps < 0.03), between the No Contrast and No Recovery classes on Trials 9 and 10 (all M differences < −60.59, ps < 0.001), and between the Recovery and No Recovery classes on Trial 10 (M differences = −27.45, p = 0.01). Thus, in the final preshift trial, animals in the No Recovery class displayed greater goal-tracking time relative to the Recovery class, whereas animals in the No Contrast class displayed reduced goal-tracking time relative to the Recovery class.
Figure 3.
Three-class solution of mean goal-tracking time (s) for 10 preshift trials and 5 postshift trials in downshifted animals (32-4) and unshifted controls (4-4). The classes were labeled according to their postshift performance: Recovery, No Recovery, and No Contrast. The performance of unshifted controls exposed always to 4% sucrose (4-4) was added as reference.
Examination of the first postshift trial (Trial 11) with one-way ANOVA suggests the three classes were not significantly different in their goal-tracking behavior at the onset of the recovery stage, F(2, 259) = 1.72, p = 0.18. To better characterize differences between the three classes at the end of the recovery stage, two independent analyses were calculated. First, a within-subject analysis comparing the final postshift trial (Trial 15) to the final preshift trial (Trial 10) with a post-hoc mixed within-between ANOVA. This analysis examines the degree of change for each animal in the three recovery classes, but compares conditions that differ in the concentration of sucrose available (4 vs. 32%) and in the amount of training (15 vs. 10 sessions). There was no main effect of Trial, F < 1, but an effect of Class, F(2, 259) = 7.74, p < 0.001, partial η2 = 0.06), as well as a Trial-by-Class interaction, F(2, 259) = 159.48, p < 0.001, partial η2 = 0.55). Bonferroni adjusted matched-pairs t-tests (Trial 15 vs. Trial 10) demonstrate that by the final recovery trial the Recovery group’s goal-tracking time on the 4% solution was not significantly different than goal-tracking time on the final acquisition trial (i.e., 32%) prior to the downshift, t < 1, indicating full recovery. The No Contrast class, which did not exhibit a reduction in goal-tracking time after the downshift in sucrose concentration, also had significantly greater goal-tracking time by the end of the recovery stage, M difference = 97.42, t(14) = 12.83, p < 0.001, indicating continued increase in consumption beyond preshift levels, despite the decrease in sucrose concentration. Although the No Recovery trajectory was characterized by a significant positive slope in the LGMM, this class maintained significantly lower goal-tracking time at the end of the recovery stage, M difference = −101.53, t(29) = −15.29, p < 0.001, demonstrating that they did not recover to preshift levels of goal-tracking behavior.
Second, a one way ANOVA comparing the last postshift trial (Trial 15) between the three recovery classes of downshifted rats as well as the unshifted sample revealed significant differences between these four groups, F(3, 475) = 17.46, p < .001. This comparison equates sucrose concentration and the number of trials across the four groups irrespective of behavioral change. Post hoc analyses with Bonferroni adjustment indicate that in the last trial of the recovery phase, the No Recovery class had significantly lower goal-tracking time than each of the three other groups (Recovery class: M difference = −79.09, SE = 11.20, p < 0.001; No Contrast class: M difference = −87.72, SE = 18.19, p < 0.001; unshifted controls: M difference = −76.07, SE = 11.20, p < 0.001). No other differences were observed between groups.
Finally, sex distributions between the three classes were examined. Male rats (n = 175) were primarily in the Recovery class (84%), with 10% and 6% in the No Recovery and No Contrast classes, respectively. Female rats (n = 87) were also primarily in the Recovery class (81%), followed by the No Recovery class (14%), and the No Contrast class (6%). A chi-square test suggests that class distribution within each sex is not significantly different, Χ2(2) = 0.71, p = 0.70.
Discussion
Incentive devaluation as studied in the SNC and related situations continues to play a significant role in the development of learning theory [1], in the comparative analysis of learning [2], and in the neurobiological [19] and pharmacological [10] bases of anxiety [22,45,46,59]. Here the emphasis has been on detecting and characterizing profiles of behavioral recovery after reward devaluation, a problem that tends to be overlooked in experimental research emphasizing average performance of relatively small samples.
Applying LGMM to a large (N = 262) sample of rats subjected to reward devaluation in the cSNC situation yielded different profiles of behavioral recovery. There was some indication that the preshift and postshift profiles were associated. For example, the preshift profile of the Recovery and No Recovery classes diverged toward the end of this phase, with the No Recovery class performing at the highest level. Thus, the class of animals that showed the poorest recovery from a 32-to-4% sucrose devaluation also demonstrated the highest level of goal tracking for 32% sucrose. The level of consummatory suppression is known to be a function of the disparity between the magnitude of the preshift and postshift rewards [48], so it is plausible that there is a subjective correlate to this discrepancy. Thus, individuals that tag the preshift reward with a high incentive value would then respond with extreme levels of suppression after the devaluation, recovering slowly or not at all (at least during the typical length of a cSNC experiment), whereas animals that confer relatively lower valuation to the reward respond to its reduction with less or no behavioral suppression.
Moreover, animals in the No Contrast class exhibited the lowest levels of preshift consumption, did not suppress their goal-tracking behavior after the downshift, and behavior continued to increase through the postshift, eventually reaching a level comparable to that of the Recovery class. Although it is possible that there are other reasons for the preshift performance of these animals, a parsimonious account would suggest a connection with satiation levels. The performance of animals receiving access to 32% sucrose sometimes is depressed toward the end of the 5-min session, thus yielding lower session average scores [51]. This might be a subgroup of animals that are either more sensitive to the satiating effects of sucrose or that happened to be deprived relatively less than the others. Deprivation levels are kept constant between 81 and 84% of the animal’s ad lib weight, but is it possible that these animals were at the upper end of the deprivation scale. Nondeprived animals and animals fed before the session tend to respond at lower levels than deprived animals [6].
The identification of distinct profiles of recovery from cSNC may facilitate further exploration of how individual differences observed in this model relate to other behavioral and biological correlates of anxiety and stress. For example, Pellegrini et al. [52] reported that the exploratory behavior of animals that recovered slowly from the downshift was more disrupted by the nonselective opioid-receptor antagonist naloxone than the behavior of animals that recovered relatively faster. Moreover, rats artificially selected for high recovery rates exhibited enhanced opioid receptor efficiency during reward downshift, attenuated emotional reactivity to partial reinforcement, and increased ultrasound vocalization to infant separation relative to low and random lines [40]. Untreated animals that deviate from the typically observed pattern of recovery, whether by a lack of recovery or a lack of emotional conflict after the reward devaluation, may represent behavioral phenotypes of vulnerability or resilience, respectively, to resource loss. Animal experiments that assume population homogeneity may fail to accurately measure how their manipulations affect these abnormal phenotypes, which may be more relevant to the understanding of risk, maintenance, and treatment of humans with anxiety and stress disorders.
Individual differences in emotional reactivity to resource loss may also be predictive of subsequent behaviors related to the regulation of stress and anxiety. For example, although Roman high- and low-avoidance inbred rat strains exhibit similar levels of initial suppression in cSNC, high-avoidance rats recovered faster than low-avoidance rats from reward devaluation [18]. Moreover, low-avoidance rats were also observed to consume larger amounts of ethanol immediately after appetitive extinction in consummatory and instrumental situations [28]. Because ethanol has been shown to have anxiolytic properties in reward devaluation situations [25], this effect was interpreted as anti-anxiety self-medication [28]. In humans, high comorbidity of anxiety and substance use disorders is well documented [54], but there may be diverse neurobiological mechanisms underlying this co-occurrence which may be better understood through the use of animal models that identify and incorporate heterogeneous subpopulations in their analyses.
One limitation of the LGMM approach is that large sample sizes are typically required to identify meaningful subpopulations, especially when some of the proportions of subjects that follow abnormal response patterns are relatively low. The present analysis capitalized on the availability of control groups from several experiments of cSNC, but this approach has its own set of limitations. First, although the procedure was substantially similar across experiments, there were a number of variations across the years. It is possible that some of these changes could influence the outcome of cSNC experiments. Even under objectively similar conditions, the cSNC effect exhibits variation from one experiment to another. It is a very reliable effect, but in some experiments contrast is completely eliminated after a single devaluation trial (e.g., Experiment 2 in [41]), whereas in others it lasts 3-5 trials (e.g., Experiment 3 in [41]). it seems possible that these variations may result from the random assignment of different proportion of animals in the No Recovery subpopulation. However, most experiments (N = 16, 76%) had at least one animal that followed one of the two abnormal trajectories. This suggests that these profiles were not produced by an isolated experiment having a different procedure or a biased sample. Though future research is unlikely to adopt large samples to identify heterogonous populations, especially those involving complex methods for neurobiological specimen collection, the current work is a highly beneficial intermediate step. The identification of the parameters associated with heterogeneous cSNC trajectories can be utilized to characterize animal populations using Bayesian LGMM methods [55]. As such, the current and related work can help characterize populations that can then be studied in future research that has accompanying neurobiology or re-analysis of other datasets that have not examined heterogeneity.
It may seem inappropriate to postulate that rats experiencing a devaluation of sucrose solutions could ever be an animal model for human suffering in the face of loss. To a casual observer, nothing compares to losing a spouse or suffering a salary reduction. As in every instance in which nonhuman animals are used to model human conditions, there are some significant limitations. Typically, animal models only simulate a fraction of human phenomena; but that fraction may be important to unravel a complex process. Importantly, independently of what seems intuitively obvious, the key is to compare the effects before dismissing the model. A substantial amount of evidence supports the conclusion that a reduction in sucrose concentration, no matter how trivial a loss may seem to a human observer, triggers a stress response in rats that shares many important components with a typical state of emotional distress observed in humans [10,46,51]. In turn, these common mechanisms suggest a degree of evolutionary homology in brain processes—the bottom line that validates any animal model of a human condition. It is suggested here that the characterization of the heterogeneous subpopulations identified in this research will provide new insights into the role of reward devaluation in anxiety, depression, and maladaptive emotional self-medication that can lead to drug and alcohol addiction.
Highlights.
Rats reject a devalued reward, but recover from such negative contrast
Latent growth mixture modeling (LGMM) detected three distinct recovery profiles
The recovery, no recovery, and no contrast profiles were detected in a large sample
These subpopulations enhance the validity and translatability of negative contrast Negative contrast relates to anxiety, depression, and emotional self-medication
Table 1.
Model fit indices for the 1- to 4-class unconditional LGMM of goal-tracking time (s) change in postshift trials relative to the last preshift trial (N = 262)
| Classes | AIC | BIC | SSBIC | Entropy | p-value |
|---|---|---|---|---|---|
| 1 | 13803 | 13825 | 13806 | - | - |
| 2 | 13801 | 13834 | 13805 | 0.65 | 0.04 |
| 3 | 13799 | 13842 | 13804 | 0.75 | 0.05 |
| 4 | 13801 | 13855 | 13807 | 0.56 | 0.52 |
Note. P-values reflect comparison of k class model with k - 1 class model with the Lo-Mendel-Rubin likelihood ratio test. LGMM = latent growth mixture modeling; AIC = Akaike information criterion; BIC = Bayesian information criterion; SSBIC = sample size adjusted Bayesian information criterion.
Acknowledgements
The research reported in this article was supported in part by generous funding from the TCU/Research and Creative Activities Fund and by Science and Engineering Research Center grants to MRP. S. Papini’s participation in this project was made possible by grant R25DA035161-01 from NIDA and I. Galatzer-Levy’s participation by grant K01MH102415 from NIMH. Correspondence about this article can be addressed to M. R. Papini (m.papini@tcu.edu).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Isaac Galatzer-Levy, New York University.
Mauricio R. Papini, Texas Christian University
References
- [1].Amsel A. Frustration theory: An analysis of dispositional learning and memory. Cambridge University Press; Cambridge, UK: 1992. [Google Scholar]
- [2].Bitterman ME. The comparative analysis of learning. Science. 1975;188:699–709. doi: 10.1126/science.188.4189.699. [DOI] [PubMed] [Google Scholar]
- [3].Bonanno GA. Loss, trauma, and human resilience: Have we underestimated the human capacity to thrive after extremely aversive events? Amer Psychol. 2004;59:20–28. doi: 10.1037/0003-066X.59.1.20. [DOI] [PubMed] [Google Scholar]
- [4].Bonanno GA, Moskowitz JT, Papa A, Folkman S. Resilience to loss in bereaved spouses, bereaved parents, and bereaved gay men. J Pers Soc Psychol. 2005;88:827–843. doi: 10.1037/0022-3514.88.5.827. [DOI] [PubMed] [Google Scholar]
- [5].Crespi LP. Quantitative variation of incentive and performance in the white rat. Amer J Psychol. 1942;40:467–517. [Google Scholar]
- [6].Cuenya L, Annicchiarico I, Serafini M, Glueck AC, Mustaca AE, Papini MR. Consummatory successive negative contrast in rats: Memory interference versus incentive learning. In preparation. [Google Scholar]
- [7].Daniel AM, Ortega LA, Papini MR. Role of the opioid system in incentive downshift situations. Neurobiol Learn Mem. 2009;92:439–50. doi: 10.1016/j.nlm.2009.06.003. [DOI] [PubMed] [Google Scholar]
- [8].Delamater AR. Experimental extinction in Pavlovian conditioning: behavioural and neuroscience perspectives. Quart J Exp Psychol. 2004;57B:97–132. doi: 10.1080/02724990344000097. [DOI] [PubMed] [Google Scholar]
- [9].Del Boca FK, Darkes J, Greenbaum PE, Goldman MS. Up close and personal: Temporal variability in the drinking of individual college students during their first year. J Cons Clin Psychol. 2004;72:155–164. doi: 10.1037/0022-006X.72.2.155. [DOI] [PubMed] [Google Scholar]
- [10].Flaherty CF. Incentive relativity. Cambridge University Press; Cambridge, UK: 1996. [Google Scholar]
- [11].Flaherty CF, Greenwood A, Martin J, Leszczuk M. Relationship of negative contrast to animal models of fear and anxiety. Anim Learn Behav. 1998;26:397–407. [Google Scholar]
- [12].Flaherty CF, Grigson PS, Rowan GA. Chlordiazepoxide and the determinants of contrast. Anim Learn Behav. 1986;14:315–21. [Google Scholar]
- [13].Freidín E, Mustaca AE. Frustration and sexual behavior in male rats. Learn Behav. 2004;32:311–20. doi: 10.3758/bf03196030. [DOI] [PubMed] [Google Scholar]
- [14].Galatzer-Levy IR, Ankri Y, Freedman S, Israeli-Shalev Y, Roitman P, Gilad M, Shalev AY. Early PTSD symptom trajectories: Persistence, recovery, and response to treatment: Results from the Jerusalem Trauma Outreach and Prevention Study (J-TOPS) PloS One. 2013;8:1–9. doi: 10.1371/journal.pone.0070084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Galatzer-Levy IR, Bonanno GA. Beyond normality in the study of bereavement: Heterogeneity in depression outcomes following loss in older adults. Soc Sci Med. 2012;74:1987–94. doi: 10.1016/j.socscimed.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Galatzer-Levy IR, Bonanno GA, Bush DE, LeDoux JE. Heterogeneity in threat extinction learning: substantive and methodological considerations for identifying individual difference in response to stress. Front Behav Neurosci. 2013;7:1–7. doi: 10.3389/fnbeh.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Genn RF, Tucci S, Parikh S, File SE. Effects of nicotine and a cannabinoid receptor agonist on negative contrast: distinction between anxiety and disappointment? Psychopharmacology. 2004;177:93–9. doi: 10.1007/s00213-004-1932-5. [DOI] [PubMed] [Google Scholar]
- [18].Gómez MJ, Escarabajal MD, de la Torre L, Tobeña A, Fernández-Teruel A, Torres C. Consummatory successive negative and anticipatory contrast effects in inbred roman rats. Physiol Behav. 2009;97:374–80. doi: 10.1016/j.physbeh.2009.03.003. [DOI] [PubMed] [Google Scholar]
- [19].Gray JA, McNaughton N. An enquiry into the functions of the septo-hippocampal system. 2nd Oxford University Press; Oxford, UK: 2000. The neuropsychology of anxiety. [Google Scholar]
- [20].Hobfoll SE. Conservation of resources: A new attempt at conceptualizing stress. Amer Psychol. 1989;44:513–524. doi: 10.1037//0003-066x.44.3.513. [DOI] [PubMed] [Google Scholar]
- [21].Hobson CJ, Delunas L. National norms and life-event frequencies for the Revised Social Readjustment Rating Scale. Int J Stress Manag. 2001;8:299–314. [Google Scholar]
- [22].Huston JP, Silva MA, Komorowski M, Schulz D, Topic B. Animal models of extinction-induced depression: Loss of reward and its consequences. Neurosci Biobehav Rev. 2013;37:2059–70. doi: 10.1016/j.neubiorev.2013.02.016. [DOI] [PubMed] [Google Scholar]
- [23].Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Amer J Psychiat. 2010;167:648–62. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jung T, Wickrama KAS. An introduction to latent class growth analysis and growth mixture modeling. Soc Pers Psychol Comp. 2008;2:302–17. [Google Scholar]
- [25].Kamenetzky GV, Mustaca AE, Papini MR. An analysis of the anxiolytic effects of ethanol on consummatory successive negative contrast. Adv Latin Amer Psychol. 2008;26:135–44. [Google Scholar]
- [26].Kumar V, Bhat ZA, Kumar D. Animal models of anxiety: A comprehensive review. J Pharmacol Toxicol Meth. 2013;68:175–83. doi: 10.1016/j.vascn.2013.05.003. [DOI] [PubMed] [Google Scholar]
- [27].LeDoux J. Rethinking the emotional brain. Neuron. 2012;73:653–76. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Manzo L, Gómez MJ, Callejas-Aguilera JE, Fernández-Teruel A, Papini MR, Torres C. Antianxiety self-medication induced by incentive loss in rats. Physiol Behav. 2014;123:86–92. doi: 10.1016/j.physbeh.2013.10.002. [DOI] [PubMed] [Google Scholar]
- [29].McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: Structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012;62:3–12. doi: 10.1016/j.neuropharm.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].McLaughlin KA, Berglund P, Gruber MJ, Kessler RC, Sampson NA, Zaslavsky AM. Recovery from PTSD following hurricane Katrina. Depres Anx. 2011;28:439–46. doi: 10.1002/da.20790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mitchell C, Flaherty CF. Temporal dynamics of corticosterone elevation in successive negative contrast. Physiol Behav. 1998;64:287–92. doi: 10.1016/s0031-9384(98)00072-9. [DOI] [PubMed] [Google Scholar]
- [32].Mustaca AE, Freidin E, Papini MR. Extinction of consummatory behavior in rats. Int J Comp Psychol. 2002;15:1–10. [Google Scholar]
- [33].Mustaca AE, Martínez C, Papini MR. Surprising nonreward reduces aggressive behavior in rats. Int J Comp Psychol. 2000;13:91–100. [Google Scholar]
- [34].Mustaca AE, Papini MR. Consummatory successive negative contrast induces hypoalgesia. Int J Comp Psychol. 2005;18:255–62. [Google Scholar]
- [35].Muthen LK, Muthen B. Mplus user's guide. 4th Muthen and Muthen; Los Angeles, CA: 2006. [Google Scholar]
- [36].Myers K, Davis M. Mechanisms of fear extinction. Molec Psychi. 2007;12:120–50. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- [37].Nikiforuk A, Popik P. Antidepressants alleviate the impact of reinforcer downshift. Eur Neuropsychopharmacol. 2009;19:41–8. doi: 10.1016/j.euroneuro.2008.08.002. [DOI] [PubMed] [Google Scholar]
- [38].Ortega LA, Daniel AM, Davis JB, Fuchs PN, Papini MR. Peripheral pain enhances the effects of incentive downshifts. Learn Mot. 2011;42:203–9. [Google Scholar]
- [39].Ortega LA, Glueck AC, Daniel AM, Prado-Rivera MA, White MM, Papini MR. Memory interfering effects of chlordiazepoxide on consummatory successive negative contrast. Pharmacol Biochem Behav. 2014;116:96–106. doi: 10.1016/j.pbb.2013.11.031. [DOI] [PubMed] [Google Scholar]
- [40].Ortega LA, Norris JN, Lopez-Seal MF, Ramos T, Papini MR. Correlates of recovery from incentive downshift: A preliminary selective breeding study. Int J Comp Psychol. 2014;27:18–44. [Google Scholar]
- [41].Ortega LA, Prado-Rivera MA, Cardenas-Poveda DC, McLinden KA, Glueck AC, Gutierrez G, Lamprea MR, Papini MR. Tests of the aversive summation hypothesis in rats: Effects of restraint stress on consummatory successive negative contrast and extinction in the Barnes maze. Learn Mot. 2013;44:159–73. [Google Scholar]
- [42].Papini MR. Pattern and process in the evolution of learning. Psychol Rev. 2002;109:186–201. doi: 10.1037/0033-295x.109.1.186. [DOI] [PubMed] [Google Scholar]
- [43].Papini MR. Comparative psychology of surprising nonreward. Brain Behav Evol. 2003;62:83–95. doi: 10.1159/000072439. [DOI] [PubMed] [Google Scholar]
- [44].Papini MR. Role of surprising nonreward in associative learning. Jap J Anim Psychol. 2006;56:35–54. [Google Scholar]
- [45].Papini MR. Diversity of adjustments to reward downshift. Int J Comp Psychol. in press. [Google Scholar]
- [46].Papini MR, Fuchs PN, Torres C. Behavioral neuroscience of psychological pain. doi: 10.1016/j.neubiorev.2014.11.012. Submitted. [DOI] [PubMed] [Google Scholar]
- [47].Papini MR, Mustaca AE, Bitterman MB. Successive negative contrast in the consummatory responding of didelphid marsupials. Anim Learn Behav. 1988;16:53–7. [Google Scholar]
- [48].Papini MR, Pellegrini S. Scaling relative incentive value in consummatory behavior. Learn Mot. 2006;37:357–78. [Google Scholar]
- [49].Papini MR, Wood M, Daniel AM, Norris JN. Reward loss as psychological pain. Int J Psychol Psychologic Ther. 2006;6:189–213. [Google Scholar]
- [50].Pecoraro N, de Jong H, Dallman MR. An unexpected reduction in sucrose concentration activates the HPA axis on successive post shift days without attenuation by discriminative contextual stimuli. Physiol Behav. 2009;96:651–61. doi: 10.1016/j.physbeh.2008.12.018. [DOI] [PubMed] [Google Scholar]
- [51].Pellegrini S, Muzio RN, Mustaca AE, Papini MR. Successive negative contrast after partial reinforcement in the consummatory behavior of rats. Learn Mot. 2004;35:303–21. [Google Scholar]
- [52].Pellegrini S, Wood M, Daniel AM, Papini MR. Opioid receptors modulate recovery from consummatory successive negative contrast. Behav Brain Res. 2005;164:239–49. doi: 10.1016/j.bbr.2005.06.035. [DOI] [PubMed] [Google Scholar]
- [53].Riley EA, Dunlap WP. Successive negative contrast as a function of deprivation condition following shifts in sucrose concentration. Amer J Psychol. 1979;92:59–70. [Google Scholar]
- [54].Ruglass LM, Lopez-Castro T, Cheref S, Papini S, Hien DA. At the crossroads: The intersection of substance use disorders, anxiety disorders, and posttraumatic stress disorder. Curr Psychiatry Rep. doi: 10.1007/s11920-014-0505-5. in press. [DOI] [PubMed] [Google Scholar]
- [55].Schoot R, Kaplan D, Denissen J, Asendorpf JB, Neyer FJ, Aken MA. A gentle introduction to Bayesian analysis: Applications to developmental research. Child Devel. 2014;85:842–60. doi: 10.1111/cdev.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–91. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Spence KW. Behavior theory and conditioning. Yale University Press; New Haven, CT: 1956. [Google Scholar]
- [58].Tinklepaugh OL. An experimental study of representative factors in monkeys. J Comp Psychol. 1928;8:197–236. [Google Scholar]
- [59].Torres C, Sabariego M. Incentive relativity: Gene-environment interactions. Int J Comp Psychol. in press. [Google Scholar]
- [60].Vogel JR, Mikulka PJ, Spear NE. Effects of shifts in sucrose and saccharine concentrations on licking behavior in the rat. J Comp Physiol Psychol. 1968;66:661–6. doi: 10.1037/h0026556. [DOI] [PubMed] [Google Scholar]
- [61].Winkelmann L, Winkelmann R. Why are the unemployed so unhappy? Evidence from panel data. Economica. 1998;65:1–15. [Google Scholar]