Abstract
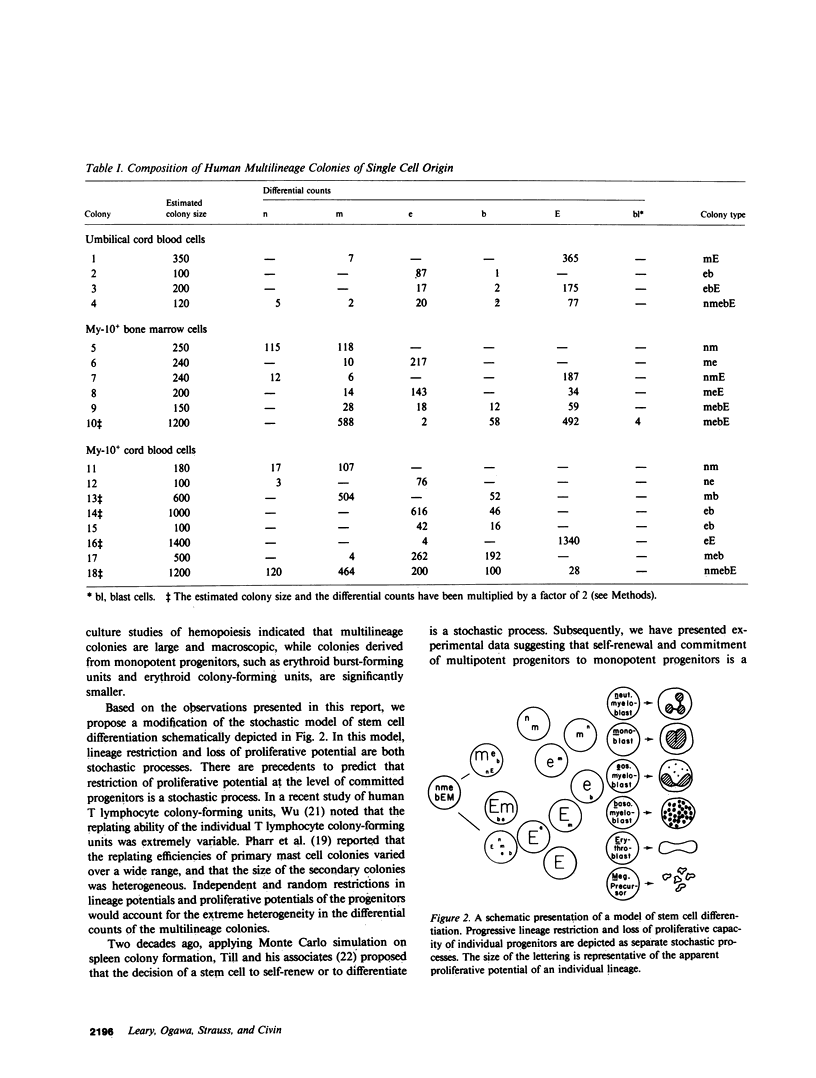
In this paper, we report analysis of differentiation in human hemopoietic colonies derived from a single cell. Cord blood mononulear cells and panned My-10 antigen-positive bone marrow and cord blood cells were plated in methylcellulose medium containing erythropoietin and conditioned medium. Initially, we performed mapping studies to identify candidate colony-forming cells. Subsequently, using a micromanipulator, we transferred single cells individually to 35-mm dishes for analysis of colony formation. Cellular composition of the colony was determined by identifying all of the cells in the May-Grunwald-Giemsa stained preparation. Of 150 single candidate cells replated, 63 produced colonies. The incidences of single lineage colonies included 19 erythroid, 17 monocyte-macrophage, and 9 eosinophil colonies. There were 18 mixed hemopoietic colonies consisting of cells in two, three, four, and five lineages in varying combinations. In some instances, we noted the predominance of one lineage and the presence of very small populations of cells in a second or third lineage. These results provide evidence for the single-cell origin of human multilineage hemopoietic colonies, and are consistent with the stochastic model of stem cell differentiation in man. They also indicate that restriction of the proliferative potential of committed progenitors is a stochastic process.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Civin C. I., Strauss L. C., Brovall C., Fackler M. J., Schwartz J. F., Shaper J. H. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol. 1984 Jul;133(1):157–165. [PubMed] [Google Scholar]
- Engleman E. G., Warnke R., Fox R. I., Dilley J., Benike C. J., Levy R. Studies of a human T lymphocyte antigen recognized by a monoclonal antibody. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1791–1795. doi: 10.1073/pnas.78.3.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauser A. A., Messner H. A. Granuloerythropoietic colonies in human bone marrow, peripheral blood, and cord blood. Blood. 1978 Dec;52(6):1243–1248. [PubMed] [Google Scholar]
- Fauser A. A., Messner H. A. Identification of megakaryocytes, macrophages, and eosinophils in colonies of human bone marrow containing neurtophilic granulocytes and erythroblasts. Blood. 1979 May;53(5):1023–1027. [PubMed] [Google Scholar]
- Iscove N. N., Sieber F., Winterhalter K. H. Erythroid colony formation in cultures of mouse and human bone marrow: analysis of the requirement for erythropoietin by gel filtration and affinity chromatography on agarose-concanavalin A. J Cell Physiol. 1974 Apr;83(2):309–320. doi: 10.1002/jcp.1040830218. [DOI] [PubMed] [Google Scholar]
- Kimura H., Burstein S. A., Thorning D., Powell J. S., Harker L. A., Fialkow P. J., Adamson J. W. Human megakaryocytic progenitors (CFU-M) assayed in methylcellulose: physical characteristics and requirements for growth. J Cell Physiol. 1984 Jan;118(1):87–96. doi: 10.1002/jcp.1041180115. [DOI] [PubMed] [Google Scholar]
- Leary A. G., Ogawa M. Identification of pure and mixed basophil colonies in culture of human peripheral blood and marrow cells. Blood. 1984 Jul;64(1):78–83. [PubMed] [Google Scholar]
- Lim B., Jamal N., Tritchler D., Messner H. A. G6PD isoenzyme analysis of myeloid and lymphoid cells in human multilineage colonies. Blood. 1984 Jun;63(6):1481–1487. [PubMed] [Google Scholar]
- McCulloch E. A. Stem cells in normal and leukemic hemopoiesis (Henry Stratton Lecture, 1982). Blood. 1983 Jul;62(1):1–13. [PubMed] [Google Scholar]
- Messner H. A., Jamal N., Izaguirre C. The growth of large megakaryocyte colonies from human bone marrow. J Cell Physiol Suppl. 1982;1:45–51. doi: 10.1002/jcp.1041130410. [DOI] [PubMed] [Google Scholar]
- Nakahata T., Gross A. J., Ogawa M. A stochastic model of self-renewal and commitment to differentiation of the primitive hemopoietic stem cells in culture. J Cell Physiol. 1982 Dec;113(3):455–458. doi: 10.1002/jcp.1041130314. [DOI] [PubMed] [Google Scholar]
- Nakahata T., Ogawa M. Hemopoietic colony-forming cells in umbilical cord blood with extensive capability to generate mono- and multipotential hemopoietic progenitors. J Clin Invest. 1982 Dec;70(6):1324–1328. doi: 10.1172/JCI110734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata T., Spicer S. S., Ogawa M. Clonal origin of human erythro-eosinophilic colonies in culture. Blood. 1982 Apr;59(4):857–864. [PubMed] [Google Scholar]
- Ogawa M., Porter P. N., Nakahata T. Renewal and commitment to differentiation of hemopoietic stem cells (an interpretive review). Blood. 1983 May;61(5):823–829. [PubMed] [Google Scholar]
- Pharr P. N., Suda T., Bergmann K. L., Avila L. A., Ogawa M. Analysis of pure and mixed murine mast cell colonies. J Cell Physiol. 1984 Jul;120(1):1–12. doi: 10.1002/jcp.1041200102. [DOI] [PubMed] [Google Scholar]
- Strauss L. C., Stuart R. K., Civin C. I. Antigenic analysis of hematopoiesis. I. Expression of the My-1 granulocyte surface antigen on human marrow cells and leukemic cell lines. Blood. 1983 Jun;61(6):1222–1231. [PubMed] [Google Scholar]
- Suda T., Suda J., Ogawa M. Disparate differentiation in mouse hemopoietic colonies derived from paired progenitors. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2520–2524. doi: 10.1073/pnas.81.8.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T., Suda J., Ogawa M. Proliferative kinetics and differentiation of murine blast cell colonies in culture: evidence for variable G0 periods and constant doubling rates of early pluripotent hemopoietic progenitors. J Cell Physiol. 1983 Dec;117(3):308–318. doi: 10.1002/jcp.1041170305. [DOI] [PubMed] [Google Scholar]
- Suda T., Suda J., Ogawa M. Single-cell origin of mouse hemopoietic colonies expressing multiple lineages in variable combinations. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6689–6693. doi: 10.1073/pnas.80.21.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TILL J. E., MCCULLOCH E. A., SIMINOVITCH L. A STOCHASTIC MODEL OF STEM CELL PROLIFERATION, BASED ON THE GROWTH OF SPLEEN COLONY-FORMING CELLS. Proc Natl Acad Sci U S A. 1964 Jan;51:29–36. doi: 10.1073/pnas.51.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. M. Regulation of self-renewal of human T lymphocyte colony-forming units (TL-CFUs). J Cell Physiol. 1983 Oct;117(1):101–108. doi: 10.1002/jcp.1041170114. [DOI] [PubMed] [Google Scholar]