Abstract
Objectives
Targeted biopsy, using magnetic resonance (MR) – ultrasound (US) fusion, may allow tracking of specific cancer sites in the prostate. We aimed to evaluate initial use of the technique to follow tumor sites in men on active surveillance of prostate cancer.
Methods and Materials
Fifty-three men with prostate cancer (all T1c) underwent re-biopsy of 74 positive biopsy sites, which were tracked and targeted using the Artemis MR-US fusion device (Eigen, Grass Valley, CA, USA) from March 2010 through January 2013. The initial biopsy included 12 cores from a standard template (mapped by software) and directed biopsies from regions of interest seen on MRI. In the repeat biopsy, samples were taken from sites containing cancer at the initial biopsy. Outcomes of interest at second MR-US biopsy included (a) presence of any cancer and (b) presence of clinically significant cancer.
Results
All cancers on initial biopsy were either Gleason score 3+3=6 (N=63) or 3+4=7 (N=11). At initial biopsy, 23 cancers were within an MRI target, and 51 were found on systematic biopsy. Cancer detection rate on repeat biopsy (29/74, 39%) was independent of Gleason score on initial biopsy (p=NS) but directly related to initial cancer core length (CCL) (p<0.02). Repeat sampling of cancerous sites within MRI targets was more likely to show cancer than re-sampling of tumorous systematic sites (61% vs. 29%, p=0.005). When initial CCL was ≥4 mm within an MRI target, over 80% (5/6) of follow-up tracking biopsies were positive. An increase of Gleason score was uncommon (9/74, 12%).
Conclusions
Monitoring of specific prostate cancer-containing sites may be achieved in some men using an electronic tracking system. The chances of finding tumor on repeat specific-site sampling was directly related to the length of tumor in the initial biopsy core and presence of tumor within an MRI target; upgrading of Gleason score was uncommon. Further research is required to evaluate the potential utility of site-specific biopsy tracking for prostate cancer patients on active surveillance.
Keywords: prostatic neoplasms, magnetic resonance imaging, ultrasonography, active surveillance, biopsy
INTRODUCTION
Follow-up biopsy is an important part of active surveillance for prostate cancer.[1–5] Active surveillance biopsies are typically performed using a systematic, 12-core approach guided by trans-rectal ultrasound (TRUS) imaging, with or without increased sampling of prior positive sites. However, TRUS-guided biopsy is not usually tumor-directed. Aside from the occasional hypo-echoic focus, cancerous areas are not visualized and re-sampling can be imprecise. Wide variations in tumor volume and Gleason score on successive biopsies have been reported.[6,7] The extent to which this variation stems from tumor growth and progression versus sampling happenstance is unknown. Accurate re-sampling of specific tumor-bearing sites would be highly desirable.
In a previous validation study, a biopsy-site tracking device (Artemis, Eigen, Grass Valley, CA) was shown to allow repeat biopsy of recorded sites with an error of only 1.2 +/− 1.1mm.[8] The result was independent of prostate volume or biopsy site. We herein report our initial experience with the device to perform follow-up biopsy of prior positive sites in men on active surveillance for prostate cancer.
MATERIALS AND METHODS
Subjects in this study included 53 men on active surveillance for prostate cancer at our institution who underwent an initial Artemis MR-US fusion guided biopsy (biopsy 1) followed by a repeat tracking biopsy of cancerous sites (biopsy 2) from March 2010 through January 2013. The UCLA active surveillance program is an IRB-approved registry with entry criteria restricted to men with low- or intermediate-risk prostate cancer based on D’Amico criteria. [9] The 53 men were culled from a total of 280 men enrolled in the active surveillance protocol at end of study period, of whom 208 underwent fusion biopsy. Excluded were men whose initial Artemis biopsy was negative (N=66) and men whose follow-up Artemis biopsy occurred after the study ended (N=89), leaving 53 men who had both Artemis biopsies during the study period. The protocol was approved in advance by the UCLA Institutional Review Board.
The initial Artemis biopsy session (biopsy 1) was a confirmatory MR-US fusion biopsy performed 6 months after a conventional TRUS-guided diagnostic biopsy. As described previously, we use multiparametric MRI (with T2 weighted imaging, diffusion weighted imaging, and dynamic contrast enhancement). A transabdominal coil is used and imaging is performed on a Siemens TrioTim Somatom 3T (Siemens Medical Solutions, Malvern, PA) magnet with high-performance gradients using a multi-channel external phased-array coil. [8] Biopsy 1 included sampling of 12 systematic sites and targeted biopsy of any regions of interest seen on MRI. MRI regions of interest were graded from 1 (least concerning) to 5 (most concerning) by one of three radiologists using a scoring system that has been described previously. [8] All positive biopsy sites were tracked and mapped to enable specific re-sampling, as described elsewhere. [8] The second Artemis biopsy session (biopsy 2) was performed a median of 11 months later (IQR 7–12 months) and included re-sampling of any sites containing cancer at the first session without systematic sampling. We did not repeat systematic re-sampling because very comprehensive sampling had been achieved only 7–12 months earlier, and the second fusion biopsy was at least the 3rd biopsy overall for all men. Sampling at biopsy 2 was standardized to include 3–4 cores from each prior positive site, aiming at the mid-point of each positive core and at 4-quadrant adjacent areas within 2–3mm of center point (Figure 1). Details of the MRI protocol and fusion biopsy method are available elsewhere. [8]
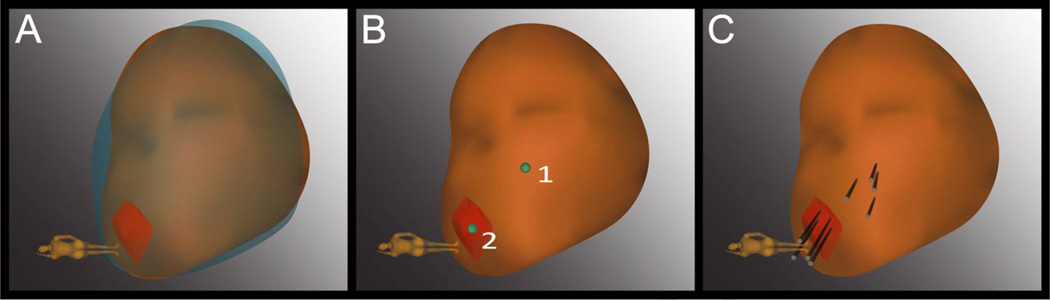
Figure 1. Example of re-sampling of prior positive sites using the Artemis device.
(A) The 3D model of the prostate from the 2nd biopsy (brown) was superimposed on the model from the 1st biopsy (blue), showing a close match in size and shape. The superimposed model was created in real-time at biopsy 2 by the Artemis device. An MRI target (red) was displayed in the model. (B) The location of prior positive sites (1 & 2) was mapped by the device. Site 1 was a systematic site; Site 2 was from the MRI-targeted core. (C) Four cores (black cylinders) were taken from each site.
A single pathologist (J.H.) analyzed all biopsy material. Gleason scoring was based on the 2005 ISUP modifications. [10] In cases of discontinuous cancer foci in a single needle core, each focus was measured individually and the sum was considered to be the CCL for that core. [11] In cases where two cores from a single target had tumor present, we used the maximum CCL from either core. Specific details of the MRI protocol and fusion biopsy method are available elsewhere. [8]
Data Analysis
The primary outcome was the presence of any cancer on biopsy 2 (yes/no). A secondary outcome was detection of clinically significant cancer (defined by Gleason >6 and/or cancer core length (CCL) ≥4mm) on biopsy 2. [12] Bivariate statistics were used to compare cancer characteristics of each positive target on biopsy 1 (n=74) to the corresponding sampled sites on biopsy 2. A multivariate logistic regression model was made to test for associations between patient/tumor characteristics and the biopsy outcomes. Covariates of interest included age, PSA, Gleason score at biopsy 1, prostate volume, maximum cancer length (mm), type of positive site (i.e., MRI region of interest vs. systematic biopsy site), and grade (on five point scale) of MRI region of interest. The covariates that were included in the initial model were maximum cancer length, type of positive site, and grade of MRI region of interest.
To evaluate spatial accuracy of the biopsies, Artemis biopsy locations were visually reviewed after study conclusion to determine if the actual biopsy site on biopsy 2 matched the planned biopsy site within a reasonable margin of error. A “near miss” was a biopsy that sampled the appropriate sextant (e.g., left lateral apex) but missed the intended target.
All analyses were performed in an intent-to-treat fashion by including all results, regardless of targeting accuracy. Statistical analyses included non-parametric Spearman correlation, Fisher exact tests and multivariate logistic regression to evaluate for associations between clinical and tumor factors.
RESULTS
Baseline demographic, clinical, and pathologic variables for the 53 subjects are displayed in Table 1. Of the 74 cancerous sites from biopsy 1, 63 (85%) demonstrated Gleason score 3+3=6 and 11 (15%) were Gleason 3+4=7. On average, CCL for individual targets was 2.2mm (SD 1.4mm). Twenty-three positive biopsy sites (31%) came from MRI regions of interest and 51 (69%) came from systematic sampling.
Table 1.
Demographics and clinical characteristics of 53 patients in active surveillance receiving resampling biopsy using the Artemis biopsy-tracking device.
| Covariate | Patient cohort (n=53) |
|---|---|
| Age1 (years) (median (IQR)) | 64 (59 – 69) |
| Race/ethnicity (n (%)) | |
| White | 44 (83%) |
| Asian | 3 (6%) |
| Hispanic | 6 (11%) |
| Time between diagnostic and 1st targeted biopsy (months) (median (IQR)) | 6 (0 – 8) |
| Time between diagnostic and 2nd targeted biopsy (months) (median (IQR)) | 17 (8 – 21) |
| Prostate volume1 (cc) (median, IQR) | 48 (36 – 60) |
| Prior prostate treatment1 (n (%)) | |
| No prior treatment | 37 (69%) |
| Transurethral resection of prostate | 3 (6%) |
| 5α-reductase inhibitor | 13 (25%) |
| PSA1 (ng/mL) (median, IQR) | 4.3 (2.2 – 6.4) |
| PSA density1 (ng/mL/cc) (median, IQR) | 0.08 (0.05 – 0.12) |
At time of biopsy 1
Table 2 compares the Gleason score on biopsy 1 to the score at biopsy 2. At biopsy 2, a mean of 3.6 (SD=1.4) cores were taken from the each of the 74 positive sites. Cancer was present in 29 sites (39%) from biopsy 2, of which 14 (49%) contained clinically significant cancer. Among biopsy 1 sites with Gleason score 3+3=6, the majority of biopsy 2 samples either did not have cancer (n=39, 62%) or still had Gleason score 3+3=6 (n=16, 25%). The remaining 8 sites (14%) had an upgraded Gleason score on biopsy 2. Most positive sites with Gleason score 3+3=6 at biopsy 1 and with cancer present in both biopsy sessions were not upgraded (16/24, 67%). Rebiopsy of Gleason 3+4=7 sites from biopsy 1 (N=11) showed cancer in 5 sites (44%). The majority of these were downgraded to Gleason 6 (3/5, 60%). Cancer detection rate on repeat biopsy was independent of first biopsy Gleason score (p=NS).
Table 2.
Cross-tabulation of Gleason scores from biopsy 1 and biopsy 2 analyzed by site (n=74).
| Gleason Score from Biopsy 2 (n (%)) |
Gleason Score from Biopsy 1 | |
|---|---|---|
| 3+3=6 (n=63) | 3+4=7 (n=11) | |
| No cancer | 39 (62%) | 6 (55%) |
| 3+3=6 | 16 (25%) | 3 (27%) |
| 3+4=7 | 6 (10%) | 1 (9%) |
| 4+3=7 | 1 (2%) | 0 (0%) |
| 4+4=8 | 1 (2%) | 1 (9%) |
Based on a previously published definition (i.e., Gleason score 3+3=6 and CCL ≤ 4mm) [11] we found that 30/53 (57%) men had clinically insignificant cancer at biopsy 1. Of re-sampled sites containing cancer on biopsy 2 (n=29), CCL increased by half or more in 52%; decreased by half or more in 34%; and was unchanged in 14%. Of the 30 men with insignificant prostate tumors, just 3 (10%) had tumors that were upgraded to significant cancer on biopsy 2. Of the remaining 23 men with clinically significant tumors on biopsy 1, 10 (43%) had a significant tumor again on biopsy 2.
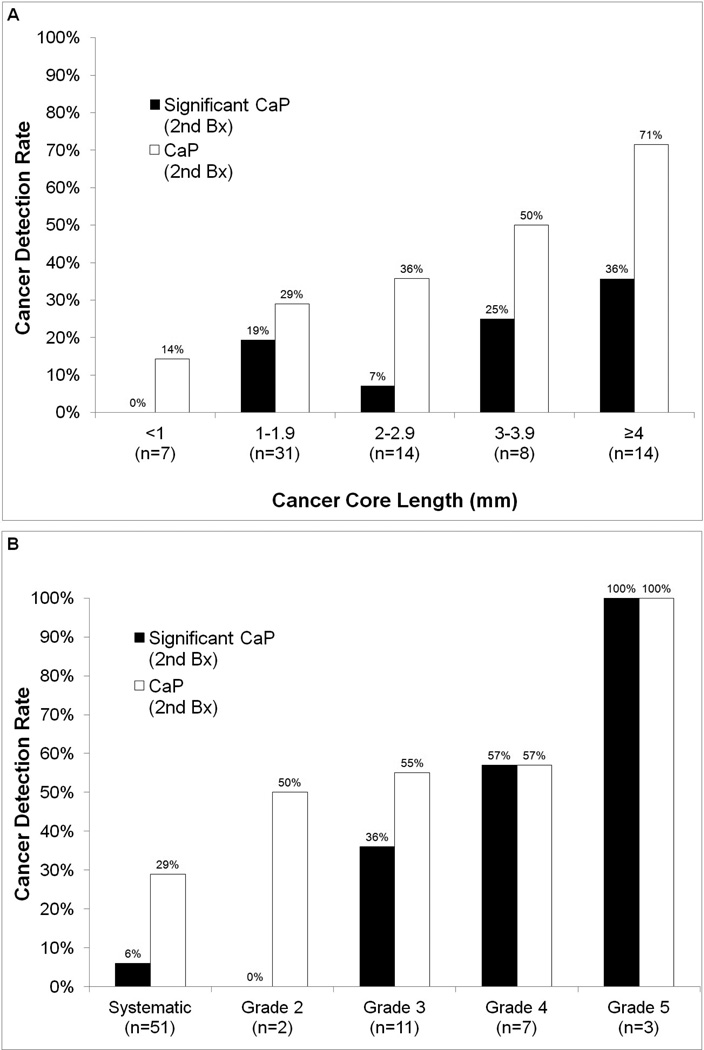
The cancer yield on repeat biopsy varied directly with initial CCL and inversely with prostate volume. Figure 2A demonstrates the effect of maximum CCL on biopsy 2. When the CCL was <1mm on biopsy 1, only 1 of 7 sites (14%) had cancer on biopsy 2. When that length was ≥4mm, 10 of 14 sites (71%) contained cancer on biopsy 2. Of sites with a CCL ≥2mm on biopsy 1, 19 of 36 (53%) sites were found on biopsy 2 to contain cancer; only 10 of 38 (26%) sites containing a CCL <2mm were found to contain cancer on repeat biopsy (p<0.02). For positive sites from prostates less than 40cc in volume (n=27), re-sampling showed cancer in 59% of sites; however, for sites in prostates over 40cc (n=47), re-sampling showed cancer in 28% of sites (p=0.01).
Figure 2. Graphical representation of the factors associated with cancer yield on re-sampling biopsy.
Panel A: Probability of cancer detection on re-sampling biopsy according to cancer core length on initial biopsy. White bars represent all cancers and black bars represent clinically signifiicant cancers.[10] Cancer detection varies from 14% for CCL <1mm to 71% for CCL ≥4mm. Panel B: Results of repeat biopsy comparing the detection of all cancers (white bars) and clinically significant cancers (black bars) stratified by image grade of the target. For example, the cancer containing sites include 7 from image grade 4 targets, of which 57% contained cancer and 57% contained significant cancer upon re-biopsy.
Cancer yield was also related to the location of the initial positive core (i.e., within an MRI region of interest vs. systematic site). Specifically, 14 of 23 (61%) re-biopsies of MRI targeted cores showed cancer. In comparison, 15 of 51 (29%) re-biopsies of systematic sites showed cancer (p=0.005). Figure 2B displays the relationship of MRI grade to cancer yield at biopsy 2; tumors were more likely to be clinically significant with increased grading on MRI. MRI targets containing cancer were found on repeat biopsy to contain longer CCLs than systematic sites (2.8 mm vs. 2.0 mm, p< 0.005). When cancerous sites were within an MRI target and the initial CCL >4mm, 5 of 6 (83%) showed cancer upon repeat biopsy. Of all the variables examined in a multivariate logistic regression analysis, the most important in predicting cancer on 2nd biopsy was MRI grade of target (odds ratio 1.48, 95% confidence interval 1.09 – 2.01). When no MRI target was present, CCL was the most important predictor (odds ratio 1.40, 95% confidence interval 1.02 – 1.92).
In the spatial accuracy analysis, actual biopsy location was judged to have been outside the region of interest (but always within the sextant) in 15 of 74 sites. Such targeting inaccuracies, even though most were ‘near misses,’ were strongly associated with biopsy outcome. In cases where the actual biopsy location matched the planned location, cancer was found in 28 of 59 (47%) sites. In contrast, cancer was found in only 1 of 15 (7%) sites where the biopsy missed the planned site.
COMMENT
Prostate biopsy is the cardinal element of active surveillance for men with apparent low-risk tumors. Varying regimens of biopsy follow-up have been employed, [1–5] often beginning with a confirmatory biopsy within the first year of the diagnostic biopsy. The confirmatory and subsequent follow-up biopsies are aimed at verifying the low-risk nature of the condition and are integral parts of existing protocols. However, the biopsy method described in most active surveillance reports is an ultrasound-guided, random, systematic sampling of the prostate. Subsequent re-sampling of specific cancers in the same patient has not been studied, perhaps because technology to allow it has not been available until recently.
Using an MR-US fusion device (Artemis, Eigen, Grass Valley, CA), we reported in 2011 the ability to accurately re-sample a prior biopsy site under ideal circumstances.[8] Men in that study were re-biopsied in a single session without a position change; image-capture software was used to determine the original and repeat biopsy locations in three dimensions. Under the conditions of that study, the center of the repeat biopsy could be placed within two mm of the original biopsy center. Accuracy was independent of prostate volume or biopsy site. These data provided validation of preliminary work using phantoms[13] and impelled the present clinical investigation.
In the present study, the possibility of using a fusion device to re-sample specific tumor-bearing sites under typical clinical conditions is given an initial exploration. Overall, tumor was found in 39% of 74 cancerous sites subjected to follow-up biopsy, a figure somewhat lower than the overall cancer yield in other active surveillance studies which utilize conventional systematic biopsy and a per-patient analysis.[14] However, in the present study, only the prior positive sites were re-sampled and the data were analyzed on a per-site basis. Importantly, in subset analysis, cancerous sites located within a target seen on MRI had a 61% rate of tumor detection on repeat biopsy. Furthermore, our finding—that every repeat biopsy core from the most suspicious MRI targets showed clinically significant cancer—confirms the importance of sampling targets identified by MRI (as shown previously in men undergoing targeted biopsy to diagnose cancers missed on conventional biopsy). [15–17]
In addition, we showed that at second biopsy, the histology revealed an upgrade to primary Gleason pattern ≥4 only 3 times. The rare instance of increased primary Gleason score on follow-up targeted biopsy implies that, within the confines of our 11-month median follow-up, sampled tumors rarely undergo grade progression. Thus, Gleason upgrades, reported by others on subsequent conventional TRUS guided biopsy [1] may result from sampling tumors that were not sampled initially. Recent epidemiological studies have suggested that Gleason grade progression is uncommon. [18] Further research (e.g., genotype analyses) could be used to conclusively determine if tumors found on initial and repeat biopsies were identical.
We also found that successful garnering of site-specific tumor was directly related to volume of tumor found at first biopsy. Tumor volume was quantified in terms of maximum length of tumor (CCL) in any core. The chance of finding tumor on repeat biopsy was directly related to maximal CCL at initial biopsy, ranging from 14% when the initial tumor was <1mm in length, up to 71% when the initial tumor length was ≥4mm. The vast majority of Gleason upgrades or significant volume increases occurred in either a high-grade MRI target or a high-volume initial tumor site. This observation, if confirmed, could lead to a change in follow-up biopsy regimens, decreasing the necessity for repeat sampling of small foci outside of MRI targets.
In a post-hoc analysis, we found that targeting accuracy was another important factor in finding tumor on follow-up biopsy. In the present study, each Artemis recording was manually reviewed after the study conclusion to determine if the follow-up biopsies were truly obtained from the targeted site. The actual biopsy location was judged to have missed the planned location in 15 of 74 sites. Even though these repeat biopsies were only “near misses,” they were less likely to identify the presence of tumor. Overall, in cases where the actual biopsy location matched the planned location, cancer was found in 28 of 59 (47%) sites. In contrast, only 1 of 15 (7%) sites where the actual biopsy missed the planned site showed cancer. In light of this finding, we are developing software to evaluate targeting accuracy during each biopsy procedure, allowing corrections to be made in real-time.
Additional limitations of the present study include lack of a demonstrated correlation with either trans-perineal template biopsies or radical prostatectomy specimens to know the actual size and Gleason score of the sampled tumors. Furthermore, our definition of cancer core length for biopsy cores with discontinuous cancer foci is somewhat controversial. Some studies have suggested that the total length between foci (including the bridge of benign tissue) correlates better with disease burden at prostatectomy. [19] Ultimately, how our definition impacts outcomes related to active surveillance remains unanswered, and correlating prostatectomy specimens with biopsy results will help clarify how best to assign cancer core length in such cases. Also, because our biopsy protocol did not include repeat systematic sampling each time, we are unable to compare the yield of site-specific re-sampling to repeat systematic biopsy. Along those lines, we did not have a control group to allow a comparison of outcomes to this cohort of men in active surveillance. Future studies evaluating long-term outcomes (e.g., survival) will have to incorporate a comparison group to better understand the potential for this system to accurately track prostate tumors over time. Further follow-up will be required before the long-term data is available to contrast the outcomes of men who have tumor on re-sampling biopsy to those who do not. However, if the concept of an index tumor prevails for men in active surveillance, biopsy-site tracking could become an important benefit of the new technology. Important areas for future research also include the impact on other functional outcomes and costs associated with this approach. For instance, although cost-effectiveness of targeted biopsies using MR-US fusion has been confirmed in general, [20] it remains unclear whether this approach is cost-effective for men undergoing active surveillance.
CONCLUSIONS
Electronically tracked biopsy has the potential to allow repeat sampling of specific tumor foci in some men on active surveillance. In this preliminary study, cancer detection rate upon repeat biopsy was directly related to (1) tumor volume (maximal cancer core length in mm) and (2) presence of tumor within an MRI region of interest at initial biopsy. Further research is required to improve the accuracy of this biopsy system and more clearly understand long-term outcomes associated with this surveillance approach.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28:126–131. doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 2.Eggener SE, Mueller A, Berglund RK, Ayyathurai R, Soloway C. A multi-institutional evaluation of active surveillance for low risk prostate cancer. J Urol. 2013;189:S19–S25. doi: 10.1016/j.juro.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 3.Tosoian JJ, Trock BJ, Landis P, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol. 2011;29:2185–2190. doi: 10.1200/JCO.2010.32.8112. [DOI] [PubMed] [Google Scholar]
- 4.Dall'Era MA, Konety BR, Cowan JE, et al. Active surveillance for the management of prostate cancer in a contemporary cohort. Cancer. 2008;112:2664–2670. doi: 10.1002/cncr.23502. [DOI] [PubMed] [Google Scholar]
- 5.Soloway MS, Soloway CT, Williams S, Ayyathurai R, Kava B, Manohara M. Active surveillance; a reasonable management alternative for patients with prostate cancer: the Miami experience. BJU Int. 2008;101:165–169. doi: 10.1111/j.1464-410X.2007.07190.x. [DOI] [PubMed] [Google Scholar]
- 6.Porten SP, Whitson JM, Cowan JE, Perez N, Shinohara K, Carroll PR. Changes in cancer volume in serial biopsies of men on active surveillance for early stage prostate cancer. J Urol. 2011;186:1825–1829. doi: 10.1016/j.juro.2011.06.055. [DOI] [PubMed] [Google Scholar]
- 7.Porten SP, Whitson JM, Cowan JE, et al. Changes in prostate cancer grade on serial biopsy in men undergoing active surveillance. J Clin Oncol. 2011;29:2795–2800. doi: 10.1200/JCO.2010.33.0134. [DOI] [PubMed] [Google Scholar]
- 8.Natarajan S, Marks LS, Margolis DJ, et al. Clinical application of a 3D ultrasound-guided prostate biopsy system. Urol Oncol. 2011;29:334–342. doi: 10.1016/j.urolonc.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 10.Epstein JI, Allsbrook WC, Amin MB, et al. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Path. 2005;29:1228–1242. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 11.Brimo F, Vollmer RT, Corcos J, et al. Prognostic value of various morphometric measurements of tumour extent in prostate needle core tissue. Histopathology. 2008;53:177–183. doi: 10.1111/j.1365-2559.2008.03087.x. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed HU, Hu Y, Carter T, et al. Characterizing clinically significant prostate cancer using template prostate mapping biopsy. J Urol. 2011;186:458–464. doi: 10.1016/j.juro.2011.03.147. [DOI] [PubMed] [Google Scholar]
- 13.Bax J, Cool D, Gardi L, et al. Mechanically assisted 3D ultrasound guided prostate biopsy system. Med Phys. 2008;35:5397–5410. doi: 10.1118/1.3002415. [DOI] [PubMed] [Google Scholar]
- 14.Dall'Era MA, Albertsen PC, Bangma C, et al. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol. 2012;62:976–983. doi: 10.1016/j.eururo.2012.05.072. [DOI] [PubMed] [Google Scholar]
- 15.Sonn GA, Chang E, Natarajan S, et al. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol. 2013 doi: 10.1016/j.eururo.2013.03.025. e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vourganti S, Rastinehad A, Yerram NK, et al. Multiparametric magnetic resonance imaging and ultrasound fusion biopsy detect prostate cancer in patients with prior negative transrectal ultrasound biopsies. J Urol. 2012;188:2152–2157. doi: 10.1016/j.juro.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hambrock T, Somford DM, Hoeks C, et al. Magnetic resonance imaging guided prostate biopsy in men with repeat negative biopsies and increased prostate specific antigen. J Urol. 2010;183:520–527. doi: 10.1016/j.juro.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 18.Penney KL, Stampfer MJ, Jahn JL, et al. Gleason grade progression is uncommon. Cancer Res. 2013;73:5163–5168. doi: 10.1158/0008-5472.CAN-13-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karram S, Trock BJ, Netto GJ, et al. Should intervening benign tissue be included in the measurement of discontinuous foci of cancer on prostate needle biopsy? Correlation with radical prostatectomy findings. Am J Surg Pathol. 2011;35:1351–1355. doi: 10.1097/PAS.0b013e3182217b79. [DOI] [PubMed] [Google Scholar]
- 20.de Rooij M, Crienen S, Witjes JA, et al. Cost-effectiveness of Magnetic Resonance (MR) Imaging and MR-guided Targeted Biopsy Versus Systematic Transrectal Ultrasound–Guided Biopsy in Diagnosing Prostate Cancer: A Modelling Study from a Health Care Perspective. Eur Urol. 2014 doi: 10.1016/j.eururo.2013.12.012. in press. [DOI] [PubMed] [Google Scholar]