Abstract
Alanine-glyoxylate aminotransferase 2 (AGXT2) is a multifunctional mitochondrial aminotransferase that was first identified in 1978. The physiological importance of AGXT2 was largely overlooked for three decades because AGXT2 is less active in glyoxylate metabolism than AGXT1, the enzyme that is deficient in primary hyperoxaluria type I. Recently, several novel functions of AGXT2 have been “rediscovered” in the setting of modern genomic and metabolomic studies. It is now apparent that AGXT2 has multiple substrates and products and that altered AGXT2 activity may contribute to the pathogenesis of cardiovascular, renal, neurological and hematological diseases. This article reviews the biochemical properties and physiological functions of AGXT2, its unique role at the intersection of key mitochondrial pathways, and its potential as a drug target.
Keywords: alanine, amino acid metabolism, aminotransferase, glyoxylate, methylarginine
AGXT isoforms in glyoxylate metabolism and beyond
There are two known alanine-glyoxylate aminotransferase (AGXT) (see Glossary) isoenzymes in mammals: AGXT1 and AGXT2 [1], both of which catalyze the transfer of an amino group from alanine to glyoxylate (Figure 1A). AGXT1 has been extensively characterized due to its role in the detoxification of glyoxylate and the pathogenesis of primary hyperoxaluria type I [2]. In addition to its alanine-glyoxylate aminotransferase activity, AGXT2 possesses several further enzymatic activities that are not shared by AGXT1. Because AGXT2 catalyzes multiple aminotransferase reactions, several alternative names for this enzyme have been proposed. AGXT2 has been referred to as D-3-aminoisobutyrate-pyruvate aminotransferase [3], (R)-3-amino-2-methylpropionate-pyruvate aminotransferase [4] and BAIB-pyruvate aminotransferase [5] due to its ability to utilize D-β-aminoisobutyric acid (BAIB) as an amino group donor (Figure 1B). It has been called dimethylarginine-pyruvate aminotransferase due to its ability to utilize methylarginines such as asymmetric dimethylarginine (ADMA) as amino group donors [6] (Figure 1C), and alanine-γ, δ-dioxovaleric acid aminotransferase because it can catalyze the transfer of an amino group from alanine to γ,δ-dioxovaleric acid (DOVA) [7] (Figure 1D). AGXT2 also has been shown to exhibit β-lyase activity towards certain exogenous halogenated alkenes such as S-tetrafluoroethyl-L-cysteine [8] (Figure 2).
Figure 1.
Some of the aminotransferase activities of AGXT2. In addition to its alanine-glyoxylate aminotransferase activity (A), AGXT2 catalyzes several additional aminotransferase reactions, including BAIB-pyruvate aminotransferase activity (B), ADMA-glyoxylate aminotransferase activity (C), and alanine-DOVA aminotransferase (DOVA transaminase) activity.
Figure 2. β-lyase activity of AGXT2.

AGXT2 was first identified in 1978 [1], and much of what is known about its biochemical properties stems from experiments performed with the purified enzyme over two decades ago. It has been only recently, however, that the multiple enzymatic activities and potential pathophysiological roles of AGXT2 have been “rediscovered” in the setting of modern genomic and metabolomics studies. It is now becoming apparent that some of the multiple substrates and products of AGXT2 may contribute to the pathogenesis of cardiovascular, renal, neurological and hematological diseases. This article reviews current knowledge about the biochemical properties and multiple physiological functions of AGXT2, its unique role at the intersection of several key mitochondrial catabolic and anabolic pathways, and its potential as a therapeutic drug target.
Discovery of AGXT2 and characterization of its alanine-glyoxylate aminotransferase activity
AGXT activity was first detected in rat liver slices in 1955 [9]. In 1978, Noguchi and colleagues identified two distinct AGXT isoenzymes (now known as AGXT1 and AGXT2) that differed in intracellular localization and biochemical activity [1]. The same group confirmed the presence of these two AGXT isoenzymes in several other mammalian species, including humans [10, 11]. AGXT1 is a pyridoxal-phosphate-dependent enzyme that functions as a homodimer with a subunit molecular weight of 40 kDa [10–12]. In mammals, the expression of AGXT1 is restricted to the liver [13, 14]. AGXT1 plays a major role in the clearance of glyoxylate, preventing it from being oxidized to oxalate, a poorly soluble metabolite. Interestingly, the intracellular localization of AGXT1 varies in different mammalian species depending on the major site of glyoxylate production. The principle dietary precursor of glyoxalate in herbivores is glycolate, which is metabolized to glyoxalate in peroxisomes; accordingly, in most herbivores AGXT1 is localized mainly in peroxisomes [15]. By contrast, the major dietary precursor of glyoxalate in carnivores is hydroxyproline, which is converted to glyoxalate in mitochondria, and in most carnivores AGXT1 is localized in mitochondria [15]. In omnivores, the enzyme is often present in both peroxisomes and mitochondria, although in humans AGXT1 is primarily localized in peroxisomes [15]. This variable subcellular localization pattern is determined by species-specific N-terminal mitochondrial and C-terminal peroxisomal targeting sequences.
AGXT2 is a pyridoxal-phosphate-dependent enzyme that functions as a tetramer with a subunit molecular weight of 50–56 kDa [3, 6, 11, 16]. Unlike AGXT1, AGXT2 is localized only in mitochondria in all examined mammalian species [11]. Human AGXT2 is encoded by a nuclear gene located on chromosome 5. After synthesis in the cytoplasm, AGXT2 is transported to mitochondria, where its 41 amino acid N-terminal mitochondrial targeting sequence is cleaved, which leads to formation of the mature form of the enzyme [17]. AGXT2 is primarily expressed in the kidney and liver [18].
The only “head-to-head” comparison of the relative aminotransferase activity of AGXT1 and AGXT2, using alanine as the amino donor and glyoxylate as the amino acceptor, was performed by Noguchi and colleagues [10]. These authors reported that the Km value for glyoxylate was 14–fold higher with AGXT2 than with AGXT1 in rats (1.0 vs. 0.07 mM) and 4-fold higher with AGXT2 than with AGXT1 in mice (0.72 vs. 0.2 mM). These findings suggest that, at least in rodents, AGXT1 is more efficient than AGXT2 in catalyzing the alanine-glyoxylate aminotransferase reaction. This observation also is consistent with the known importance of AGXT1 in glyoxylate detoxification. Santana and colleagues estimated the Km of recombinant human AGXT1 for glyoxylate as 0.36 mM [19], while the Km of human AGXT2 towards glyoxylate still needs to be determined.
The strongest evidence that AGXT1 rather than AGXT2 is responsible for the bulk of glyoxalate metabolism in humans comes from the observation that patients with inborn AGXT1 deficiency have impaired detoxification of glyoxalate in the liver, which leads to increased oxidation of glyoxylate to oxalate, deposition of insoluble calcium oxalate crystals in the kidney and urinary tract, and subsequent renal failure [20]. Consistent with the human data, Salido and colleagues demonstrated increased urine oxalate levels and predisposition to urolithiasis in AGXT1-deficient mice [21]. Impairment of glyoxalate metabolism in the setting of AGXT1 deficiency occurs despite presumably intact AGXT2 expression and activity, which suggests that the metabolism of glyoxylate by AGXT2 cannot compensate for the absence of AGXT1. The remainder of this review will therefore focus on the alternative catalytic activities of AGXT2.
Metabolism of D-β-aminoisobutyric acid (BAIB)
In 1951, Crumpler and colleagues demonstrated that human urine contains D-β-aminoisobutyric acid (BAIB) [22], a metabolite that was subsequently shown to be a product of thymine catabolism (Figure 3) [23]. In 1953, an autosomal recessive metabolic trait leading to increased BAIB levels in urine (hyper-β-aminoisobutyric aciduria) was described [24]. The prevalence of this trait was shown to be less than 10% in the European population [24] but higher than 30% in Asian populations [25]. Kakimoto and colleagues reported in 1969 that patients with hyper-β-aminoisobutyric aciduria had impaired activity of an enzyme responsible for BAIB metabolism, which they called D-β-aminoisobutyrate-pyruvate aminotransferase [5]. Twenty-four years later, Kontani and coauthors demonstrated the identity of this enzyme with AGXT2 [3]. Deamination of BAIB by AGXT2 leads to formation of methylmalonate semialdehyde (Figure 1B), which is subsequently converted to proprionyl CoA (Figure 3). Both glyoxylate and pyruvate can serve as amino acceptors in the BAIB deamination reaction [6]. Using glyoxylate as the amino acceptor at pH 7.3, the apparent Km of rat AGXT2 towards BAIB is 18 times lower than the Km for alanine (0.12 mM vs. 2.2 mM), which suggests that BAIB might be a preferred substrate for AGXT2 under physiological conditions [3]. On the other hand, Ogawa and colleagues showed that, at pH 10.0 and with glyoxylate as the amino acceptor, the relative activity of rat AGXT2 was 60-fold higher with alanine than with BAIB [6], raising the intriguing possibility that substrate specificity of AGXT2 may be strongly pH-dependent. Recent data suggest that the pH inside mitochondria varies from 7.5 to 8.2 [26]. There are some conditions that influence the mitochondrial pH, such as glucose stimulation [27] and uptake of cations [28]. Mitochondrial pH also plays a role in the generation of reactive oxygen species [29]. However, it remains unanswered whether physiological changes in pH in the matrix of mitochondria are sufficient to influence the substrate specificity of AGXT2.
Figure 3.
Role of AGXT2 in thymine metabolism. D-β-aminoisobutyric acid (BAIB) is a product of thymine catabolism, which is subsequently converted to methylmalonate semialdehyde by AGXT2 and then to proprionyl-CoA by methylmalonate semialdehyde dehydrogenase.
Recent genomic studies have generated a resurgence of interest in the pathophysiology of BAIB and its metabolism by AGXT2. Suhre and colleagues showed in 2011 that hyper-β-aminoisobutyric aciduria is associated with a single nucleotide polymorphism (rs37369) that causes a valine to isoleucine substitution (Val140Ile) in human AGXT2 [30]. Nicholson and colleagues independently reported that hyper-β-aminoisobutyric aciduria is associated with two polymorphisms in AGXT2, rs37369 and rs37370, and suggested that the AGXT2 locus may have undergone recent positive selection in the European population [31]. In agreement with these findings, an association between the AGXT2 rs37370 polymorphism and plasma BAIB levels was detected in a genome-wide association study performed with samples from the Framingham Heart Study [32]. In another recent study, the AGXT2 polymorphisms rs37369 and rs16899974 (Val496Leu) were found to be associated with increased BAIB levels in plasma and urine in healthy Caucasian volunteers [33]. Despite the fact that plasma levels of BAIB in healthy individuals (2.3 ± 1.9 μM) [34] are considerably lower than the estimated Km for BAIB of the rat AGXT2 enzyme, the clear association between AGXT2 polymorphisms and BAIB levels strongly suggests that AGXT2 plays a physiological role in regulating BAIB levels in humans in vivo.
The physiological and pathophysiological roles of BAIB and the potential consequences of its accumulation are still poorly understood. In a cross-sectional analysis of Framingham Heart Study data, elevated plasma levels of BAIB were associated with decreased levels of serum triglycerides [32]. Moreover, in a zebrafish model, knockdown of agxt2 gene expression resulted in alterations in tissue levels of triglycerides and cholesterol esters [32]. The role of BAIB in producing this phenotype is uncertain, however, since levels of BAIB were unexpectedly decreased, rather than increased, in the agxt2 knockdown morphants [32]. Interestingly, Spitsyn and Afanas’eva reported an increased frequency of hyper-β-aminoisobutyric aciduria in patients with coronary atherosclerosis in two selected populations [35]. Clearly, more work is needed to determine whether BAIB directly affects lipid homeostasis or serves as a biometabolomic marker of AGXT2 deficiency and increased cardiovascular risk. Interestingly, a recent metabolomic study identified BAIB as small molecule “myokine” that is secreted from myocytes and induces a brown adipose-like phenotype in both adipocytes and human pluripotent stem cells and improves glucose homeostasis in mice [36]. The authors suggest that BAIB may contribute to exercise-induced protection from metabolic diseases [36].
Metabolism of methylarginines
The endogenous guanidine-methylated analogues of L-arginine, NG–monomethyl–L–arginine (NMMA), asymmetric NG, NG–dimethyl–L–arginine (ADMA) and symmetric NG, N′G–dimethyl–L–arginine (SDMA), have received considerable attention as novel cardiovascular risk factors [37–39]. Both NMMA and ADMA have been postulated to produce adverse cardiovascular effects via inhibition of nitric oxide synthase (NOS). ADMA is considered to be a more physiologically relevant NOS inhibitor than NMMA due to its higher concentration in plasma. Elevated blood levels of ADMA are associated with increased cardiovascular morbidity and mortality, and ADMA has been proposed to be an independent cardiovascular risk factor [39–41]. Elevation of ADMA levels in animal models or human subjects leads to endothelial dysfunction, decreased renal blood flow, increased renovascular resistance, renal sodium retention, and elevated systemic blood pressure [42].
Elevation of SDMA also has been found to be associated with some cardiovascular and renal pathologies [42]. Unlike NMMA and ADMA, however, SDMA does not directly inhibit NOS, and the exact mechanisms of the potential adverse biological effects of SDMA are not entirely understood. SDMA has been proposed to compete with L-arginine for the common transporter and thus indirectly decrease intracellular L-arginine concentration and NO production [43]. A recent report has suggested that SDMA can modify high-density lipoprotein (HDL) particles to become activators of toll-like receptor-2, triggering an innate immune pathway leading to vascular oxidative stress and endothelial dysfunction [44]. SDMA has also been proposed to stimulate production of reactive oxygen species in monocytes by augmenting calcium entry inside the cells via store-operated Ca2+-channels [45]. Further work is needed to confirm this finding and clarify the relative importance of SDMA vs. NMMA and ADMA in cardiovascular pathophysiology.
The primary pathway of methylarginine catabolism is thought to occur via a hydrolysis reaction catalyzed by dimethylarginine dimethylaminohydrolase (DDAH), which can hydrolyze both ADMA and NMMA, but not SDMA. It has been recognized since at least 1990, however, that methylarginines also can undergo transamination reactions catalyzed by AGXT2 [6]. AGXT2 has broad substrate specificity for methylarginines; it can utilize not only ADMA or NMMA, but also SDMA, as a substrate [6, 46, 47]. The product of ADMA deamination catalyzed by AGXT2 is α-keto-δ-(NG, NG-dimethylguanidino)valeric acid (DMGV) (Figures 1C and 4). The AGXT2-mediated pathway of ADMA metabolism received relatively little attention until our group cloned the human AGXT2 gene in 2010 [17]. We found that overexpression of human AGXT2 in mice led to decreases in plasma and tissue levels of ADMA [17]. Recent experiments with stable isotope-labeled ADMA have demonstrated endogenous AGXT2 activity towards ADMA, detected by conversion of ADMA to DMGV in the liver and kidney of mice [48]. In agreement with these findings, Caplin and colleagues showed that AGXT2-deficient mice have increased plasma concentrations of both ADMA and SDMA, suggesting that endogenous AGXT2 contributes to the regulation of plasma levels of methylarginines [46]. Elevation of plasma ADMA and SDMA also occurs after systemic infusion of BAIB in mice, presumably due to competitive inhibition of AGXT2’s activity toward methylarginines [47]. Finally, ADMA and SDMA levels were found to inversely correlate with AGXT2 expression in allografts of renal transplant recipients [46], which suggests that AGXT2 also regulates methylarginine metabolism in humans. Caplin and coworkers also provided experimental evidence that AGXT2 isolated from murine kidney mitochondria can metabolize ADMA at physiological concentrations [46]. Another recent study found the AGXT2 polymorphism rs37369 to be associated with increased SDMA levels in the plasma and urine of healthy human volunteers [33].
Figure 4. Role of AGXT2 in ADMA metabolism.
Asymmetric NG, NG–dimethyl–L–arginine (ADMA), derived from hydrolysis of proteins containing methylarginine, can be converted either to citrulline by dimethylarginine dimethylaminohydrolase (DDAH) in the cytoplasm or to α-keto-δ-(NG, NG-dimethylguanidino) valeric acid (DMGV) by AGXT2 in the mitochondrion.
Thus, the current experimental evidence strongly suggests that endogenous AGXT2 regulates systemic levels of ADMA and other methylarginines in vivo. These results, however, stand in puzzling contrast with the observation that the estimated Km of purified rat AGXT2 towards ADMA is in the range of 10 mM [6], which is about four orders of magnitude higher than plasma ADMA concentrations in rodents or humans [49–51]. One potential explanation for this paradox is that intracellular ADMA concentrations might be higher than plasma levels [52], although it is likely that the intracellular concentrations are still considerably lower than the estimated Km. Another potential explanation might be that the kinetic properties of ADMA transamination by AGXT2 in situ within the mitochondrial milieu might differ from those of the purified enzyme, for example due to the presence of as yet unidentified cofactors.
The kidney appears to be the major site of metabolism of ADMA by AXGT2. In a landmark experiment, Ogawa et al. injected radiolabeled ADMA into rats, and found that most of the radioactivity recovered in the urine within 12 hours was in the form of either ADMA, DMGV, or the DMGV-metabolite α-keto-δ-(NG, NG-dimethylguanidyno)butyric acid (DMGB) [53]. This observation suggests that there may be two mechanisms for renal clearance of ADMA: a direct mechanism wherein ADMA is excreted into the urine unchanged, and an indirect mechanism in which ADMA is first metabolized by AGXT2 and then excreted into the urine as DMGV or DMGB. Interestingly, kidney extracts had 8-fold higher levels of radiolabeled ADMA than DMGV and DMGB, whereas the ratio of radioactive ADMA to DMGV and DMGB in urine was approximately 1:1 [53], which suggests that clearance of ADMA through the AGXT2-mediated mechanism is much more efficient than the direct excretion of ADMA. A possible role of AGXT2 in renal ADMA metabolism and clearance is further supported by the observation that AGXT2 is specifically localized to the epithelium of Henle’s loop [18].
The discovery that endogenous AGXT2 can regulate systemic levels of methylarginines suggests a mechanism by which AGXT2 may protect from cardiovascular diseases. This hypothesis is consistent with the finding that upregulation of AGXT2 can protect from ADMA-mediated impairment of NO production in cultured endothelial cells [17], and with the observed phenotype of endothelial dysfunction and hypertension in AGXT2 knockout mice [46]. It is also consistent with the results of a study demonstrating an association between the AGXT2 gene locus with plasma levels of SDMA and heart rate variability in young adults [54].
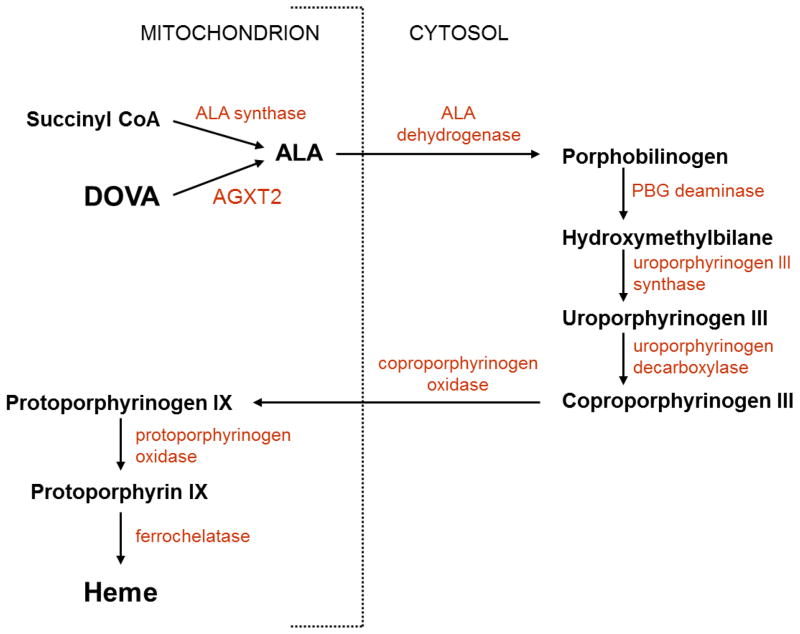
Metabolism of γ, δ-dioxovalerate (DOVA)
The formation of δ-aminolevulinic acid (ALA) is the first committed step in the porphyrin biosynthetic pathway leading to the synthesis of heme. ALA is synthesized from glycine and succinyl Co-A by the mitochondrial enzyme ALA synthase [55, 56] (Figure 5). An alternative pathway for the synthesis of ALA was proposed in the early 1980s to be catalyzed by a transamination reaction between alanine and γ, δ-dioxovalerate (DOVA), yielding pyruvate and δ-aminolevulinic acid (Figures 1D and 5). This alternative reaction was first described by the group of Varticovski, who isolated a pyridoxal-phosphate-dependent DOVA transaminase from bovine liver mitochondria [7]. Using radiolabelled DOVA, Morton and colleagues showed that DOVA transaminase-mediated formation of ALA can occur in liver cells [57] and whole animals [58]. Okuno and colleagues demonstrated the biosynthesis of ALA by DOVA transamination in the liver of rats, rabbits and humans, which further supported the potential in vivo importance of this alternative pathway for ALA synthesis [59]. DOVA transaminase was reported to be identical to AGXT2 by Noguchi and colleagues [60], although this conclusion was questioned by another group [61]. Thus, there is still considerable uncertainty about the role of AGXT2 in catalyzing DOVA transamination. Perhaps the time is now right to re-explore this neglected metabolic activity of AGXT2 and determine the potential impact of AGXT2 on porphyrin and heme biosynthesis.
Figure 5.
Potential role of AGXT2 in heme synthesis. The heme precursor molecule δ-aminolevulinic acid (ALA) is produced from succinyl-CoA in the mitochondrion by the enzyme ALA synthase. An alternative source of ALA in the mitochondrion has been proposed to be mediated by the DOVA transaminase activity of AGXT2.
Other aminotransferase activities of AGXT2
AGXT2 clearly has broad aminotransferase substrate specificity, allowing it to utilize many different amino donors and acceptors in different combinations. In addition to the substrates described above, AGXT2 also can utilize amino donors such as β-alanine (a product of uracil catabolism), L-ornithine (a metabolite of the urea cycle), α-aminobutyric acid (a precursor of ophthalmic acid) and several other endogenous amino compounds. Furthermore, ex vivo experiments have suggested that AGXT2 may exhibit aminotransferase activity towards exogenous substrates such as α-fluoro-β-alanine (a metabolite of 5-fluorouracil) and β-chloro-L-alanine [62]. Pyruvate and glyoxylate are the most common AGXT2 amino acceptors in these reactions, but several other ketone compounds, including DOVA and oxaloacetate, also can serve as amino acceptors. The potential pathophysiological significance of these reactions remains to be defined.
β-lyase activity
Halogenated alkenes (e.g. trichloroethylene, tetrachloroethylene and tetrafluoroethylene) are common in both industrial and commercial use. They have nephrotoxic effects in rodents and are associated with kidney and liver tumors in experimental animals and humans [63]. One mechanism of the toxicity of halogenated alkenes is the formation of corresponding S-conjugates, which are subsequently metabolized by cysteine S-conjugate β-lyases into pyruvate, ammonium, and reactive sulfur-containing fragments (Figure 2) [64]. Some of the resulting sulfur containing fragments are reactive molecular species that can thioacylate macromolecules, especially at ε-amino groups of protein lysine residues [65]. Several S-conjugate β-lyases have been identified in mitochondria. Their presence makes mitochondrial proteins common targets of halogenated alkenes [66]. Recently Cooper and colleagues demonstrated that AGXT2 isolated from rat kidney mitochondria possess β-lyase activity towards two halogenated alkene S-conjugates (S-(1,1,2,2-tetrafluoroethyl)-L-cysteine and S-(benzothiazolyl)-L-cysteine) [8]. This observation suggests that AGXT2 may promote the toxicity of halogenated alkenes and that AGXT2 polymorphisms associated with decreased β-lyase activity may be protective. Further studies are necessary to further define the exogenous and endogenous substrates for the β-lyase activity of AGXT2.
Concluding remarks
It has been recognized for three decades that AGXT2 is a promiscuous aminotransferase capable of utilizing a wide array of endogenous and exogenous substrates. In recent years, there has been a resurgence of interest in the multiple roles of AGXT2 in amino group metabolism due to findings from molecular, genomic, and metabolomic studies. The picture that is emerging is one in which AGXT2 plays a complex role in mitochondrial function that may extend well beyond amino acid metabolism.
Several experimental approaches are now available to investigate the physiological and pathophysiological roles of AGXT2. AGXT2 gene polymorphisms have been found in relatively high allele frequency in many populations, providing the ability to assess pathophysiological and metabolomics phenotypes via genome-wide association studies [30–32, 54, 67, 68]. More work is needed to better define the functional consequences of these polymorphisms on the enzymatic activities of AGXT2 and their associated clinical phenotypes. A mouse model of AGXT2 deficiency has been developed [46]. AGXT2-deficient mice develop vascular dysfunction and hypertension, which has been hypothesized to be caused by elevated levels of endogenous methylargininines such as ADMA [46]. It is possible, however, that the accumulation of other AGXT2 substrates such as BAIB also contributes to the vascular phenotype of these mice. It remains to be determined whether the observed association between hyper-β-aminoisobutyric aciduria and coronary heart disease prevalence [35] is related to elevated concentrations of BAIB, other AGXT2 substrates such as methylarginines, or another mechanism. There is also a need to better define the potential pathophysiological effects of AGXT2’s activity toward its other substrates, including SDMA, DOVA, β-alanine, L-ornithine, and α-aminobutyric acid. Though AGXT2 does not appear to play a substantive role in the detoxification of glyoxylate, it remains possible that its alanine-glyoxylate aminotransferase activity may serve another function within mitochondria.
Because it is a multifunctional enzyme, AGXT2 may be an attractive potential therapeutic drug target because it offers the potential for the development of compounds that selectively inhibit a subset of its activities. Several small molecule inhibitors of AGXT2 have already been described [3, 4, 7, 10, 69, 70], some of which are endogenous molecules and others are analogs of drugs or toxins. Further studies are needed to better characterize the known inhibitors and develop new lead compounds that selectively inhibit a subset of the specific metabolic pathways regulated by AGXT2 without inhibiting its other enzymatic activities. Selective AGXT2 inhibitors would also be very useful research tools. Likewise, the development of approaches to increase or upregulate AGXT2 activity would not only facilitate research but also could potentially lead to new therapies for the prevention and treatment of cardiovascular and metabolic diseases.
Highlights.
AGXT2 is a mitochondrial aminotransferase that has diverse functions in cellular physiology
Several substrates and products of AGXT2 are biomarkers of cardiovascular and metabolic diseases
AGXT2 may be an attractive therapeutic drug target for cardiovascular disease
Glossary
- ADMA (asymmetric dimethylarginine)
NG, NG dimethyl L arginine, a product of catabolism of proteins methylated on arginine residues; an endogenous inhibitor of nitric oxide synthesis;a biomarker of cardiovascular, neural and renal diseases
- ALA (δ-aminolevulinic acid)
a precursor in the porphyrin biosynthetic pathway leading to the synthesis of heme
- Alanine-glyoxylate aminotransferase 1 (AGXT1)
an aminotransferase that transfers an amino group from alanine to glyoxylate; deficiency of AGXT1 causes primary hyperoxaluria type I
- Alanine-glyoxylate aminotransferase 2 (AGXT2)
a multifunctional aminotransferase that is localized in mitochondria
- Aminotransferase (or transaminase)
an enzyme that catalyzes the transfer of an amino group between an amino acid and an α-keto acid
- BAIB (D-β-aminoisobutyric acid)
a product of thymine catabolism and one of multiple substrates for AGXT2l
- Dimethylarginine dimethylaminohydrolase (DDAH)
An enzyme that hydrolyzes methylarginines such as ADMA and NMMA
- DMGB (α-keto-δ-(NG,NG-dimethylguanidyno)butyric acid)
a metabolite of DMGV
- DMGV (α-keto-δ-(NG,NG-dimethylguanidino) valeric acid)
a product of ADMA metabolism by AGXT2
- DOVA (γ, δ-dioxovaleric acid)
an alternative substrate for the synthesis of ALA via DOVA transamination
- High density lipoprotein (HDL)
an atheroprotective lipoprotein
- Hyper-β-aminoisobutyric aciduria
an autosomal recessive metabolic trait that causes increased BAIB levels in urine
- NMMA (NG monomethyl L arginine)
an endogenous guanidine-methylated analogue of L-arginine that can inhibit nitric oxide synthase
- Nitric oxide synthase (NOS)
an enzyme that catalyzes the production of nitric oxide from L-arginine; can be inhibited by ADMA and NMMA
- Primary hyperoxaluria type I
an autosomal recessive disorder caused by deficiency of AGXT1. Absence of AGXT1 leads to excessive conversion of glyoxylate to oxalate, a poorly soluble metabolite that accumulates in the kidney and other organs
- SDMA (symmetric dimethylarginine)
NG, N′G dimethyl L arginine, an endogenous guanidine-methylated analogue of L-arginine; a cardiovascular risk factor but, unlike ADMA and NMMA, does not inhibit nitric oxide synthase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Noguchi T, et al. Subcellular distribution of pyruvate (glyoxylate) aminotransferases in rat liver. Biochem J. 1978;170:173–175. doi: 10.1042/bj1700173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danpure CJ, et al. An enzyme trafficking defect in two patients with primary hyperoxaluria type 1: peroxisomal alanine/glyoxylate aminotransferase rerouted to mitochondria. J Cell Biol. 1989;108:1345–1352. doi: 10.1083/jcb.108.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kontani Y, et al. Identity of D-3-aminoisobutyrate-pyruvate aminotransferase with alanine-glyoxylate aminotransferase 2. Biochim Biophys Acta. 1993;1156:161–166. doi: 10.1016/0304-4165(93)90131-q. [DOI] [PubMed] [Google Scholar]
- 4.Tamaki N, et al. Purification, characterization and inhibition of D-3-aminoisobutyrate aminotransferase from the rat liver. Eur J Biochem. 1990;189:39–45. doi: 10.1111/j.1432-1033.1990.tb15457.x. [DOI] [PubMed] [Google Scholar]
- 5.Kakimoto Y, et al. D-beta-aminoisobutyrate:pyruvate aminotransferase in mammalian liver and excretion of beta-aminoisobutyrate by man. J Biol Chem. 1969;244:335–340. [PubMed] [Google Scholar]
- 6.Ogawa T, et al. Dimethylarginine:pyruvate aminotransferase in rats. Purification, properties, and identity with alanine:glyoxylate aminotransferase 2. J Biol Chem. 1990;265:20938–20945. [PubMed] [Google Scholar]
- 7.Varticovski L, et al. Biosynthesis of porphyrin precursors. Purification and characterization of mammalian L-alanine:gamma,delta-dioxovaleric acid aminotransferase. J Biol Chem. 1980;255:3742–3747. [PubMed] [Google Scholar]
- 8.Cooper AJ, et al. L-alanine-glyoxylate aminotransferase II of rat kidney and liver mitochondria possesses cysteine S-conjugate beta-lyase activity: a contributing factor to the nephrotoxicity/hepatotoxicity of halogenated alkenes? Biochem J. 2003;376:169–178. doi: 10.1042/BJ20030988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pavlova NA. Biosynthesis of glycocoll from glyoxylic and amino acids. Doklady Akademii nauk SSSR. 1955;100:947–949. [PubMed] [Google Scholar]
- 10.Noguchi T, et al. Characteristics of hepatic alanine-glyoxylate aminotransferase in different mammalian species. Biochem J. 1978;169:113–122. doi: 10.1042/bj1690113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takada Y, Noguchi T. Subcellular distribution, and physical and immunological properties of hepatic alanine: glyoxylate aminotransferase isoenzymes in different mammalian species. Comp Biochem Physiol B. 1982;72:597–604. doi: 10.1016/0305-0491(82)90512-0. [DOI] [PubMed] [Google Scholar]
- 12.Noguchi T, Takada Y. Purification and properties of peroxisomal pyruvate (glyoxylate) aminotransferase from rat liver. Biochem J. 1978;175:765–768. doi: 10.1042/bj1750765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi S, et al. Identity of alanine:glyoxylate aminotransferase with alanine:2-oxoglutarate aminotransferase in rat liver cytosol. Biochimie. 1989;71:471–475. doi: 10.1016/0300-9084(89)90177-6. [DOI] [PubMed] [Google Scholar]
- 14.Noguchi T, et al. Intraperoxisomal and intramitochondrial localization, and assay of pyruvate (glyoxylate) aminotransferase from rat liver. Hoppe-Seyler’s Zeitschrift fur physiologische Chemie. 1979;360:919–927. doi: 10.1515/bchm2.1979.360.2.919. [DOI] [PubMed] [Google Scholar]
- 15.Birdsey GM, et al. Differential enzyme targeting as an evolutionary adaptation to herbivory in carnivora. Molecular biology and evolution. 2004;21:632–646. doi: 10.1093/molbev/msh054. [DOI] [PubMed] [Google Scholar]
- 16.Okuno E, et al. Co-purification of alanine-glyoxylate aminotransferase with 2-aminobutyrate aminotransferase in rat kidney. Biochimica et biophysica acta. 1982;715:97–104. doi: 10.1016/0304-4165(82)90054-x. [DOI] [PubMed] [Google Scholar]
- 17.Rodionov RN, et al. Human alanine-glyoxylate aminotransferase 2 lowers asymmetric dimethylarginine and protects from inhibition of nitric oxide production. J Biol Chem. 2010;285:5385–5391. doi: 10.1074/jbc.M109.091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee IS, et al. Specific expression of alanine-glyoxylate aminotransferase 2 in the epithelial cells of Henle’s loop. Nephron. 1999;83:184–185. doi: 10.1159/000045507. [DOI] [PubMed] [Google Scholar]
- 19.Santana A, et al. Primary hyperoxaluria type 1 in the Canary Islands: a conformational disease due to I244T mutation in the P11L-containing alanine:glyoxylate aminotransferase. Proc Natl Acad Sci U S A. 2003;100:7277–7282. doi: 10.1073/pnas.1131968100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cellini B, et al. Human liver peroxisomal alanine:glyoxylate aminotransferase: Different stability under chemical stress of the major allele, the minor allele, and its pathogenic G170R variant. Biochimie. 2010;92:1801–1811. doi: 10.1016/j.biochi.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Salido EC, et al. Alanine-glyoxylate aminotransferase-deficient mice, a model for primary hyperoxaluria that responds to adenoviral gene transfer. Proc Natl Acad Sci U S A. 2006;103:18249–18254. doi: 10.1073/pnas.0607218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crumpler HR, et al. beta-Aminoisobutyric acid (alpha-methyl-beta-alanine); a new amino-acid obtained from human urine. Nature. 1951;167:307–308. doi: 10.1038/167307a0. [DOI] [PubMed] [Google Scholar]
- 23.Fink K, et al. Metabolism of thymine (methyl-C14 or -2-C14) by rat liver in vitro. J Biol Chem. 1956;221:425–433. [PubMed] [Google Scholar]
- 24.Harris H. Family studies on the urinary excretion of beta-aminoisobutyric acid. Annals of eugenics. 1953;18:43–49. doi: 10.1111/j.1469-1809.1952.tb02496.x. [DOI] [PubMed] [Google Scholar]
- 25.Yanai J, et al. Genetic study of beta-aminoisobutyric acid excretion by Japanese. Am J Hum Genet. 1969;21:115–132. [PMC free article] [PubMed] [Google Scholar]
- 26.Santo-Domingo J, Demaurex N. Perspectives on: SGP symposium on mitochondrial physiology and medicine: the renaissance of mitochondrial pH. J Gen Physiol. 2012;139:415–423. doi: 10.1085/jgp.201110767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiederkehr A. Matrix alkalinisation unleashes beta-cell mitochondria. Islets. 2009;1:154–156. doi: 10.4161/isl.1.2.9058. [DOI] [PubMed] [Google Scholar]
- 28.Abad MF, et al. Mitochondrial pH monitored by a new engineered green fluorescent protein mutant. J Biol Chem. 2004;279:11521–11529. doi: 10.1074/jbc.M306766200. [DOI] [PubMed] [Google Scholar]
- 29.Selivanov VA, et al. The role of external and matrix pH in mitochondrial reactive oxygen species generation. J Biol Chem. 2008;283:29292–29300. doi: 10.1074/jbc.M801019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suhre K, et al. A genome-wide association study of metabolic traits in human urine. Nature genetics. 2011;43:565–569. doi: 10.1038/ng.837. [DOI] [PubMed] [Google Scholar]
- 31.Nicholson G, et al. A genome-wide metabolic QTL analysis in Europeans implicates two loci shaped by recent positive selection. PLoS genetics. 2011;7:e1002270. doi: 10.1371/journal.pgen.1002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhee EP, et al. A Genome-wide Association Study of the Human Metabolome in a Community-Based Cohort. Cell Metab. 2013;18:130–143. doi: 10.1016/j.cmet.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kittel A, et al. Alanine-glyoxylate aminotransferase 2 (AGXT2) polymorphisms have considerable impact on methylarginine and beta-aminoisobutyrate metabolism in healthy volunteers. PLoS One. 2014;9:e88544. doi: 10.1371/journal.pone.0088544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Kuilenburg AB, et al. Beta-alanine and beta-aminoisobutyric acid levels in two siblings with dihydropyrimidinase deficiency. Nucleosides, nucleotides & nucleic acids. 2008;27:825–829. doi: 10.1080/15257770802146445. [DOI] [PubMed] [Google Scholar]
- 35.Spitsyn VA, Afanas’eva IS. Differences in allele frequency at the BAIB locus, determining the level of expression of beta-aminoisobutyric acid, in healthy donors and coronary artery atherosclerosis patients from Buryat and Lithuanian populations. Genetika. 2001;37:1713–1716. [PubMed] [Google Scholar]
- 36.Roberts LD, et al. beta-Aminoisobutyric acid induces browning of white fat and hepatic beta-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 2014;19:96–108. doi: 10.1016/j.cmet.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boger RH, et al. ADMA: an emerging cardiovascular risk factor. Vasc Med. 2005;10(Suppl 1):S1–2. doi: 10.1177/1358836X0501000101. [DOI] [PubMed] [Google Scholar]
- 38.Cooke JP. Asymmetrical dimethylarginine: the Uber marker? Circulation. 2004;109:1813–1818. doi: 10.1161/01.CIR.0000126823.07732.D5. [DOI] [PubMed] [Google Scholar]
- 39.Anthony S, et al. Endogenous production of nitric oxide synthase inhibitors. Vasc Med. 2005;10(Suppl 1):S3–9. doi: 10.1191/1358863x05vm595oa. [DOI] [PubMed] [Google Scholar]
- 40.Cooke JP. ADMA: its role in vascular disease. Vasc Med. 2005;10(Suppl 1):S11–17. doi: 10.1177/1358836X0501000103. [DOI] [PubMed] [Google Scholar]
- 41.Boger RH. Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the “L-arginine paradox” and acts as a novel cardiovascular risk factor. J Nutr. 2004;134:2842S–2847S. doi: 10.1093/jn/134.10.2842S. discussion 2853S. [DOI] [PubMed] [Google Scholar]
- 42.Schwedhelm E, Boger RH. The role of asymmetric and symmetric dimethylarginines in renal disease. Nature reviews. Nephrology. 2011;7:275–285. doi: 10.1038/nrneph.2011.31. [DOI] [PubMed] [Google Scholar]
- 43.Bode-Boger SM, et al. Symmetrical dimethylarginine: a new combined parameter for renal function and extent of coronary artery disease. J Am Soc Nephrol. 2006;17:1128–1134. doi: 10.1681/ASN.2005101119. [DOI] [PubMed] [Google Scholar]
- 44.Speer T, et al. Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity. 2013;38:754–768. doi: 10.1016/j.immuni.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 45.Schepers E, et al. Role of symmetric dimethylarginine in vascular damage by increasing ROS via store-operated calcium influx in monocytes. Nephrol Dial Transplant. 2009;24:1429–1435. doi: 10.1093/ndt/gfn670. [DOI] [PubMed] [Google Scholar]
- 46.Caplin B, et al. Alanine-Glyoxylate Aminotransferase-2 Metabolizes Endogenous Methylarginines, Regulates NO, and Controls Blood Pressure. Arterioscler Thromb Vasc Biol. 2012 doi: 10.1161/ATVBAHA.112.254078. [DOI] [PubMed] [Google Scholar]
- 47.Kittel A, et al. In vivo evidence that Agxt2 can regulate plasma levels of dimethylarginines in mice. Biochem Biophys Res Commun. 2012 doi: 10.1016/j.bbrc.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 48.Martens-Lobenhoffer J, et al. Probing AGXT2 enzyme activity in mouse tissue by applying stable isotope-labeled asymmetric dimethyl arginine as substrate. Journal of mass spectrometry: JMS. 2012;47:1594–1600. doi: 10.1002/jms.3125. [DOI] [PubMed] [Google Scholar]
- 49.Wang D, et al. Isoform-Specific Regulation by NG-NG-Dimethylarginine Dimethylaminohydrolase of Rat Serum Asymmetric Dimethylarginine and Vascular Endothelium-Derived Relaxing Factor/NO. Circ Res. 2007 doi: 10.1161/CIRCRESAHA.107.158915. [DOI] [PubMed] [Google Scholar]
- 50.Rodionov RN, et al. Overexpression of dimethylarginine dimethylaminohydrolase protects against cerebral vascular effects of hyperhomocysteinemia. Circ Res. 2010;106:551–558. doi: 10.1161/CIRCRESAHA.109.200360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caplin B, Leiper J. Endogenous nitric oxide synthase inhibitors in the biology of disease: markers, mediators, and regulators? Arterioscler Thromb Vasc Biol. 2012;32:1343–1353. doi: 10.1161/ATVBAHA.112.247726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Masuda H, et al. Accelerated intimal hyperplasia and increased endogenous inhibitors for NO synthesis in rabbits with alloxan-induced hyperglycaemia. Br J Pharmacol. 1999;126:211–218. doi: 10.1038/sj.bjp.0702298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogawa T, et al. Metabolism of NG,NG-and NG,N′G-dimethylarginine in rats. Arch Biochem Biophys. 1987;252:526–537. doi: 10.1016/0003-9861(87)90060-9. [DOI] [PubMed] [Google Scholar]
- 54.Seppala I, et al. Genome-wide association study on dimethylarginines reveals novel AGXT2 variants associated with heart rate variability but not with overall mortality. Eur Heart J. 2013 doi: 10.1093/eurheartj/eht447. [DOI] [PubMed] [Google Scholar]
- 55.Bonkovsky HL, et al. Porphyrin and heme metabolism and the porphyrias. Compr Physiol. 2013;3:365–401. doi: 10.1002/cphy.c120006. [DOI] [PubMed] [Google Scholar]
- 56.Dailey HA, Meissner PN. Erythroid heme biosynthesis and its disorders. Cold Spring Harb Perspect Med. 2013;3:a011676. doi: 10.1101/cshperspect.a011676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morton KA, et al. Biosynthesis of porphyrins and heme from gamma, delta-dioxovalerate by intact hepatocytes. Proc Natl Acad Sci U S A. 1981;78:5325–5328. doi: 10.1073/pnas.78.9.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morton KA, et al. Biosynthesis of 5-aminolevulinic acid and heme from 4,5-dioxovalerate in the rat. J Clin Invest. 1983;71:1744–1749. doi: 10.1172/JCI110929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okuno E, et al. A new biosynthetic route of porphyrin precursors in common between animals and plants. Biochem Biophys Res Commun. 1983;112:986–990. doi: 10.1016/0006-291x(83)91715-1. [DOI] [PubMed] [Google Scholar]
- 60.Noguchi T, Mori R. Biosynthesis of porphyrin precursors in mammals. Identity of alanine: gamma, delta-dioxovalerate aminotransferase with alanine:glyoxylate aminotransferase. J Biol Chem. 1981;256:10335–10339. [PubMed] [Google Scholar]
- 61.Tyagi RK, Datta K. In vitro translocation of L-alanine:4,5-dioxovalerate transaminase into rat kidney mitochondria. J Biochem (Tokyo) 1993;113:557–562. doi: 10.1093/oxfordjournals.jbchem.a124082. [DOI] [PubMed] [Google Scholar]
- 62.Porter DJ, et al. Enzymatic elimination of fluoride from alpha-fluoro-beta-alanine. Biochemical pharmacology. 1995;50:1475–1484. doi: 10.1016/0006-2952(95)02053-5. [DOI] [PubMed] [Google Scholar]
- 63.Lash LH, et al. Modes of action of trichloroethylene for kidney tumorigenesis. Environmental health perspectives. 2000;108(Suppl 2):225–240. doi: 10.1289/ehp.00108s2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Green T, Odum J. Structure/activity studies of the nephrotoxic and mutagenic action of cysteine conjugates of chloro- and fluoroalkenes. Chemico-biological interactions. 1985;54:15–31. doi: 10.1016/s0009-2797(85)80149-6. [DOI] [PubMed] [Google Scholar]
- 65.Cooper AJ, et al. Toxic, halogenated cysteine S-conjugates and targeting of mitochondrial enzymes of energy metabolism. Biochemical pharmacology. 2002;64:553–564. doi: 10.1016/s0006-2952(02)01076-6. [DOI] [PubMed] [Google Scholar]
- 66.Cooper AJ, et al. Mitochondrial aspartate aminotransferase catalyses cysteine S-conjugate beta-lyase reactions. Biochem J. 2002;368:253–261. doi: 10.1042/BJ20020531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anderssohn M, et al. Genetic and environmental determinants of dimethylarginines and association with cardiovascular disease in patients with type 2 diabetes. Diabetes care. 2013 doi: 10.2337/dc13-0546. [DOI] [PubMed] [Google Scholar]
- 68.Altshuler DM, et al. Integrating common and rare genetic variation in diverse human populations. Nature. 2010;467:52–58. doi: 10.1038/nature09298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaneko M, et al. Inhibition of D-3-aminoisobutyrate-pyruvate aminotransferase by 5-fluorouracil and alpha-fluoro-beta-alanine. Biochimica et biophysica acta. 1992;1122:45–49. doi: 10.1016/0167-4838(92)90125-w. [DOI] [PubMed] [Google Scholar]
- 70.Kaneko M, et al. Irreversible inhibition of D-3-aminoisobutyrate-pyruvate aminotransferase by gabaculine. FEBS Lett. 1990;276:115–118. doi: 10.1016/0014-5793(90)80521-j. [DOI] [PubMed] [Google Scholar]