Abstract
Prostaglandin E2 (PGE2) is elevated during cardiac injury and we have previously shown that mice lacking the PGE EP4 receptor display dilated cardiomyopathy (DCM) with increased expression of the membrane type matrix metalloproteinase, MMP-14. We thus hypothesized that PGE2 regulates expression of MMP-14 and also affects fibroblast migration. Primary cultures of neonatal rat ventricular fibroblasts (NVFs) were used to test the effects of PGE2. Gene and protein expression was assessed by real time RT-PCR and Western blot, MMP activity was determined by zymography and migration of NVF was assessed by motility in a transwell system. PGE2 reduced expression of MMP-14 and these effects were antagonized by an EP4 antagonist. An EP4 agonist mimicked the effect of PGE2. PGE2 also increased mRNA and protein levels of plasminogen activator inhibitor-1 (PAI-1), an inhibitor of MMP activation. However, PGE2-stimulation of PAI-1 was mediated by the EP1/EP3 receptor and not EP4. Migration of NVF was assessed by motility in a transwell system. Treatment of NVFs with PGE2 reduced the number of cells migrating towards 10% FCS. Treatment with the EP2 agonist also reduced migration but did not affect MMP-14 expression or PAI-1. Our results suggest that PGE2 utilizes different receptors and mechanisms to ultimately decrease MMP expression and NVF migration.
Keywords: Prostaglandin E2, fibroblasts, matrix metalloproteinase, EP receptors, migration, plasminogen activator inhibitor-1
INTRODUCTION
Cardiac remodeling is an essential process for wound healing after myocardial infarction. Too little collagen deposition often results in cardiac rupture, a leading cause of morbidity whereas increased collagen results in fibrosis, increased stiffening and a lack of compliance. Thus, regulation of these processes are critical to optimal cardiac performance. The matrix metalloproteinases (MMPs) are a group of enzymes that are responsible for the breakdown of collagen and other extracellular matrix proteins. Although the contribution of MMP-2 and MMP-9 to cardiac fibrosis is well documented in disease states, the involvement of the membrane-bound MMP-14 (also named MT1-MMP) has also been recently recognized and appears to be more complex. The membrane type I metalloproteinase (MT1-MMP or MMP-14) is a transmembrane metalloproteinase with collagenolytic activity and is known to activate MMP-2 by facilitating its cleavage from the pro form [1]. Rowe et al [2] reported that MMP-14 is essential for pulmonary fibroblast migration though type I collagen and this MMP was described to play a role in the development of aortic stenosis in patients [3].
The plasminogen system is another mechanism responsible for the activation of MMPs. Plasminogen is converted into plasmin by the plasminogen activators uPA and tPA, and subsequently, plasmin converts pro MMP- 2 or 9 into active forms. The action of uPA and tPA are inhibited by the plasminogen activator inhibitor, PAI-1. Thus, activation of MMPs can occur through at least two separate pathways. Recently, we reported that aged male mice lacking the prostaglandin E2 (PGE2) EP4 receptor sub-type in cardiomyocytes (EP4 KO) display a phenotype of dilated cardiomyopathy coupled with increased expression of MMP-14 in the left ventricle [4]. We therefore hypothesized that MMP-14 is negatively regulated by PGE2 in an EP4 dependent manner. We examined the effect of PGE2 on MMP-14 and PAI-1 expression and finally tested the net downstream effects of these pathways by examining whether PGE2 or the 3 various EP agonists alter cardiac fibroblast migration. Our data suggest that PGE2 utilizes its EP/EP3 receptor to increase PAI-1 whereas the decrease in MMP-14 is mediated by EP4 receptor. Thus, PGE2 can elicit downstream effects on MMP activity by two different signaling pathways that are linked to different EP receptors.
MATERIALS AND METHODS
Chemicals
The EP4 agonist, ONO AE1-329, theEP3 antagonist, ONO AE3-240 and the EP4 antagonist, ONO AE3-208 were generously provided by ONO pharmaceuticals, Osaka, Japan. Butaprost free acid (an EP2 agonist), sulprostone (an EP1/EP3 agonist) and PGE2 were from Cayman Chemical (Ann Arbor, MI).
Culture of Neonatal Rat Cardiac Fibroblasts (NVF)
All studies involving the use of animals were approved by the institutional review committee at Henry Ford Hospital, in accordance with federal guidelines. NVF were derived from digestion of neonatal rat hearts as previously described [5] and the pre-plate of attached fibroblasts was used as a source of neonatal fibroblasts. These cells were grown in DMEM supplemented with 10% fetal bovine serum, glutamate and penicillin/streptomycin and were used between passage 2 and 3 for all experiments.
Western Blot for MMP-14
Western blot analysis was performed under reducing conditions using 30 μg of total protein. To ensure that proteins were detected at the correct molecular weight, we also ran an aliquot of the full range molecular weight ladder (GE Amersham, Pittsburgh, PA) on each gel. After electrophoresis, proteins were transferred overnight to a PVDF membrane. Membranes were blocked for 1 hr in 5% milk (v/v in TBS-Tween) and incubated overnight (4°C) with a 1:1000 dilution of MMP-14 antibody (Abcam). After washing with TBS-tween, membranes were incubated with a HRP-conjugated donkey anti-rabbit secondary antibody for 1 hr at room temperature at a dilution of 1:2000. After further washing they were developed using a Super Signal West Pico chemiluminescent substrate (Pierce, Rockford, IL). The membranes were then stripped and re-probed for GAPDH as a loading control.
Fibroblast Migration Assays
Migration of NVF was assessed using a transwell system. Briefly, NVF at passage 2 were grown until approximately 70-80% confluence and were then serum-starved overnight. Cells were then treated for 24 hrs with the appropriate compounds after which, they were trypsinized and washed in serum-minus media. They were then counted and 0.15 × 106 cells were plated on the top of a transwell (8 μm pore size) that had been coated with 0.5% BSA in Hank’s balanced salt solution. The bottom chamber contained 10% serum in media and served as a chemoattractant. After 5 hrs the wells were washed with PBS and the top surface of the transwell was gently wiped with a cotton tip applicator to remove non-migrating cells. Ice-cold methanol was added to the wells for 10 min at room temperature and thereafter, wells were washed five times with tap water. The cells were then stained by the addition of hematoxylin which was allowed to remain in contact for 3 min and finally the wells were rinsed twice in tap water. Photographs of each transwell were taken under the ×20 objective of an Olympus microscope and six randomly selected fields were taken for each well, avoiding the outer rims. All treatments were performed in duplicate wells. Image J was used to quantify the number of cells on each membrane.
Real Time RT-PCR for MMP-14, PAI-1 and Collagen Type I
To detect the expression of PAI-1, MMP-14 and collagen type 1mRNAs in cultured neonatal rat ventricular fibroblasts, we performed real time RT-PCR. Total RNA was extracted from cultured fibroblasts using the Tri-reagent according to the manufacturer’s instructions and was treated with DNase I to remove contaminating DNA. 2 μg of RNA was reverse transcribed using an Omniscript reverse transcriptase kit (Qiagen, Valencia, CA) in a final volume of 20 μl. Two microliters of the reverse transcription reaction were then amplified in a Roche version 2.0 LightCycler PCR instrument (Indianapolis, IN) using SYBR green dye (SA Biosciences) and specific primers. Primers were designed by TIB MolBiol (Adelphia, NJ) and are described as follows: PAI-1 Sense 5′ ccatctccgtgcccatgat 3′ and Antisense 5′ gtcatgttgctcttccattgtct 3′; MMP-14 Sense 5′ tatggaagcaagtcagggtcac 3′ and Antisense 5′ cgctccttgaagacaaacatct 3′; Collagen type I α I: sense 5′ gaccgatggattccmgttcg 3′ and antisense 5′ gtaggctacgctgttcttgca 3′ where m is a Wobbel base. After an initial activation at 94°C for 90 sec, PCR was performed for 35 cycles with the following parameters: denaturation at 94°C for 15 sec, annealing at 58°C for 40 sec, extension at 72 °C for 40 sec. At the end of PCR cycling, melting curve analyses were performed and representative PCR products were run on agarose gels and visualized by ethidium bromide staining. A relative quantitation method [ΔΔCt] [6] was used to evaluate expression of each gene relative to control. RT-PCR of GAPDH was used for normalization of all data.
Zymography
To detect MMP activity, 10 μl media samples were run on Novex ®Tris-glycine zymograms (Invitrogen, Carlsbad, CA) in 1X Novex ®Tris-Glycine SDS running buffer at a constant voltage of 125V. As positive controls, 10 ng of the human recombinant MMP-2 proenzyme (Calbiochem) and the active human recombinant MMP-9 (Calbiochem) were also run on the same zymograms. After electrophoresis, gels were placed into the 1X zymogram renaturing buffer (Invitrogen) for 30 min at room temperature with gentle agitation before the addition of 1X zymogram developing buffer. Gels were incubated in the developing buffer for 30 min at room temperature before fresh developing buffer was added and gels incubated overnight at 37°C. The next day, gels were stained for 30 min at room temperature in 0.5% w/v Coomassie blue R250 stain made up in 50 mM Tris base, 0.2 M NaCl, 5 mM CaCl2. Gels were then destained for the appropriate amount of time in a solution containing 50% methanol, 10% acetic acid and 40% distilled water. Images were taken with the Protein Simple Imaging System (Santa Clara, CA) and analyzed using Image J software with bands for both the pro- and active forms analyzed together
Silencing of MMP-14
For the siRNA experiments, silencing of MMP-14 was achieved using a siRNA against rat MMP-14 (Ambion Silencer Select ID # s135552). NVF at passage 3 and approximately 70% confluent were transfected for 48 hrs with 150 pmol of either a non-targeting control siRNA (Ambion Silencer Select negative control number 1) or the rat MMP-14 siRNA using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions. At the end of the experimental period, media were removed and assayed for MMP2/-9 activity by zymography as decribed above.
PAI-1 ELISA
To determine whether alterations in PAI-1 mRNA correlated with changes in protein, we measured total PAI-1 in the media by ELISA (Molecular Innovations, Novi, MI) following the manufacturer’s instructions.
Statistical Analyses
All statistics were performed by a statistician in the Department of Public Health Sciences of Henry Ford Hospital. All data is described as means ± standard error of the mean (SEM). Groups were compared by one way ANOVA. Significance was determined using Tukey’s’s method for multiple comparisons.
RESULTS
Effect of PGE2 on MMP-14 expression
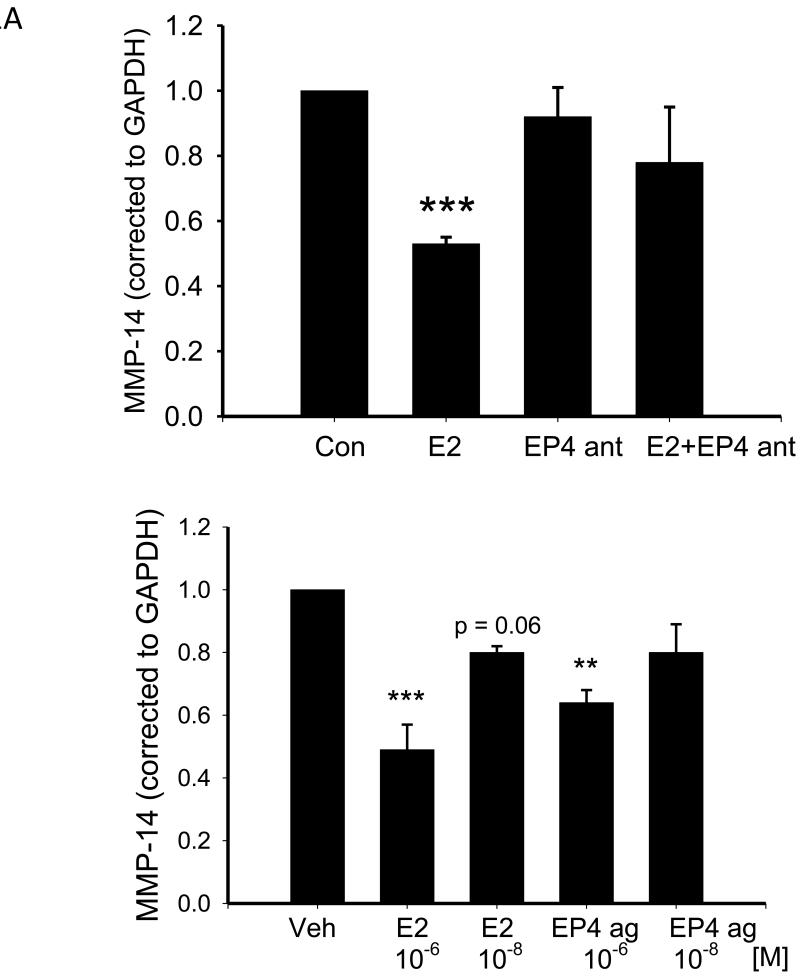
Figure 1A shows that treatment with 10−6M PGE2 for 24 hrs reduced MMP-14 mRNA (normalized to GAPDH) by 47%. The EP4 antagonist alone had no effect on expression of MMP-14 mRNA but prevented the decrease noted with PGE2 and the value noted in the presence of the antagonist was not different from that of vehicle-treated cells. In support of this data, in a separate set of experiments we observed that treatment with 10−6M EP4 agonist also decreased MMP-14 mRNA (Figure 1A lower panel). When the same experiment was performed using cardiac fibroblasts from adult male rats, we noted similar effects with a 32% decrease in MMP-14 mRNA (p = 0.07, n =3).
Figure 1.
A Upper panel A shows the effect of 24 hr treatment with PGE2 and the EP4 antagonist (EP4 ant) on MMP-14 mRNA in NVF as assessed by real time RT-PCR. Statistical significance: *** p ≤ 0.005 versus vehicle, n = 4. Lower panel shows the effect of 24 hr treatment with PGE2 and the EP4 agonist on MMP-14 mRNA in NVF as assessed by real time RT-PCR. Statistical significance: ** p ≤ 0.01 *** p ≤ 0.005 versus vehicle treatment, n = 3.
B shows the effect of 24 hr treatment with PGE2 (E2) and the EP4 agonist (EP4 ag) on MMP-14 protein expression as determined by western blot. Upper panel is a representative blot. Lower panel is mean quantitative data. Statistical analysis: * p ≤ 0.05, ** p < 0.01 versus vehicle, n = 7.
To determine whether the effects of PGE2 on MMP-14 mRNA translated into changes in protein, we performed Western blot for MMP-14. Figure 1B shows that treatment with 10−6M PGE2 for 24 hrs decreased MMP-14 by 54% with a similar effect noted for 10−8M PGE2. Consistent with the EP4-mediated effect observed with the real time RT-PCR data, treatment with 10−6M EP4 agonist for 24 hrs decreased MMP-14 protein levels by 52% (Figure 1B).
Since both EP2 and the EP4 couple to adenylate cyclase to raise cAMP levels, we investigated the effect of the EP2 agonist, butaprost, on MMP-14 levels using Western blot. In contrast to the effect of the EP4 agonist, treatment with butaprost (5×10−6M) did not affect MMP-14 levels (1.0 ± 0.2 vs 1.22 ± 0.13 arbitrary units).
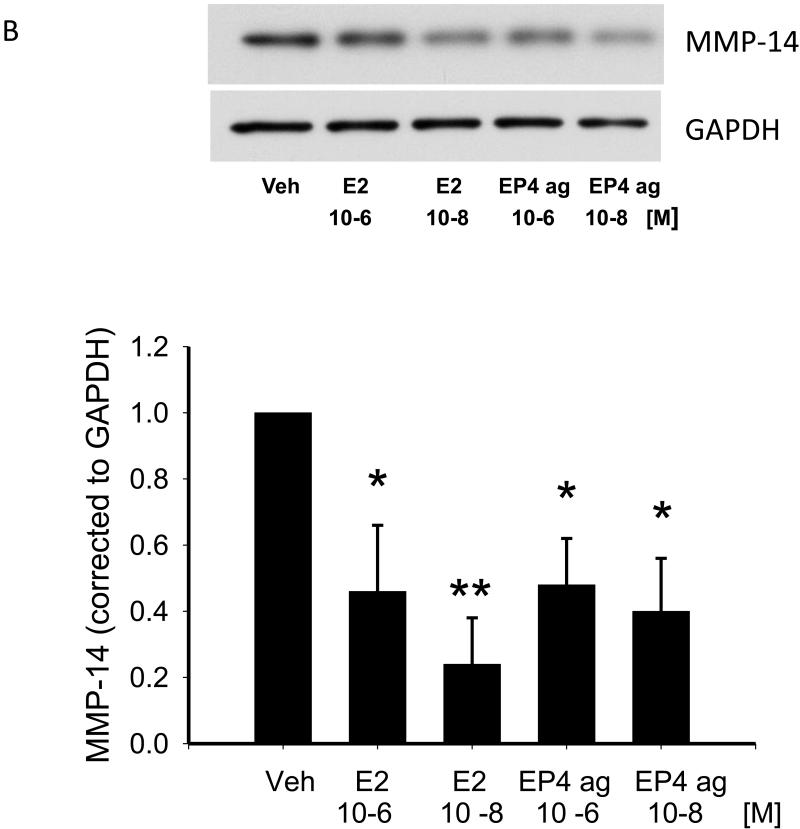
Effect of MMP-14 Silencing on MMP-2 and MMP-9
To determine the relationship between MMP-14 and MMP-2 and -9 in cardiac fibroblasts, we reduced MMP-14 expression using a siRNA approach and examined its effects on MMP-2 activity using zymography on media samples. Silencing of MMP-14 resulted in a 61% decrease in mRNA levels (Figure 2B) and a 63% decrease in MMP-14 protein levels as determined by Western blot (data not shown). Using zymography, bands for MMP-2 were mainly observed and only a very faint band co-migrated with the MMP-9 standard. MMP-2 bands (pro and active) were quantified as a whole and these bands were reduced by approximately 25% with MMP-14 silencing compared to control siRNA (Figure 2C).
Figure 2.
Panel A: Representative zymogram showing effect of 48 hr treatment with a control siRNA (lanes 1-3) or a MMP-14 siRNA (lanes 4-6) on MMP activity as measured by gelatin zymography. Major bands are observed for pro and active MMP-2 with only a faint band visible for MMP-9 as indicated by arrows. Panel B shows the degree of MMP-14 silencing using the MMP-14 siRNA as assessed by real time RT-PCR. Panel C is quantitative analysis of the zymogram presented in panel A.
Effect of PGE2, EP2 and EP agonists, and EP1/EP3 agonist on MMP-2 and MMP-9 Activity
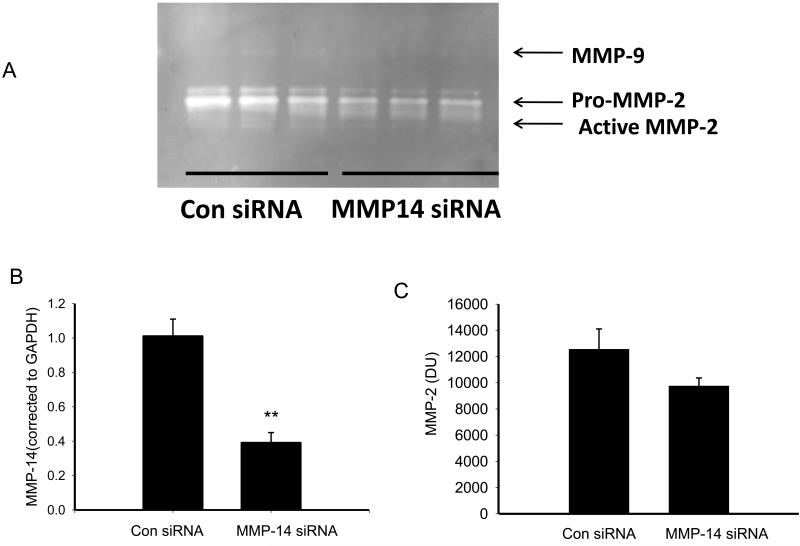
To determine whether changes in MMP-14 resulted in downstream alterations in MMP activity, we performed zymography. Figure 3 shows a representative zymogram and mean data from those experiments. Treatment with either 10−6M PGE2 or the EP4 agonist for 24 hrs reduced active MMP-2 compared to vehicle-treated cells. Surprisingly, treatment with the EP1/EP3 agonist sulprostone also reduced active MMP-2 suggesting the existence of two separate pathways. We also investigated the effect of the EP2 agonist, butaprost, on MMP activity as assessed by zymography. Treatment of NVF with 5×10−6M butaprost reduced MMP activity from a control value of 1.0 to 0.51 ± 0.04, p < 0.05 (n =3).
Figure 3.
Effect of 24 hr treatment with PGE2, the EP4 agonist, and the EP1/EP3 agonist sulprostone on MMP activity (densitometric units, DU) as measured by gelatin zymography. The values on the lower x-axis represent molar concentrations. Statistical significance: * p <7 0.05, ** p < 0.01 and *** p < 0.005 versus vehicle treatment, n = 3-4.
Effect of PGE2 on PAI-1 mRNA and protein
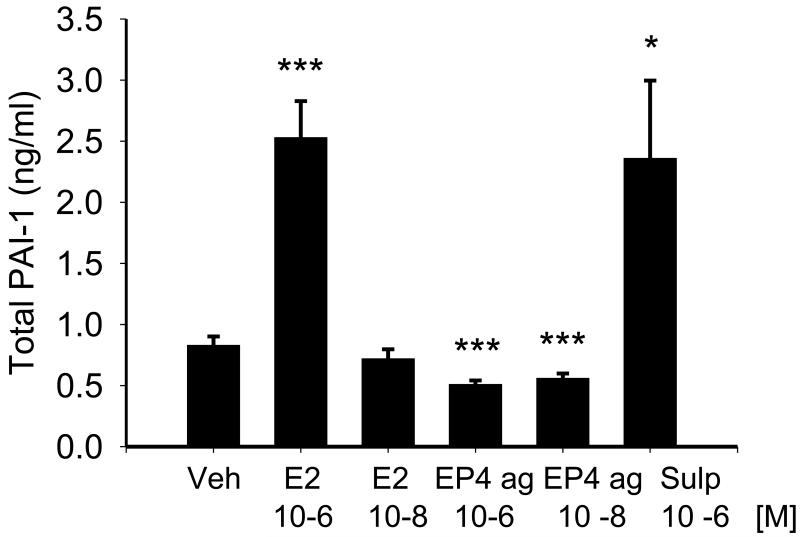
Since the plasminogen system is also known to affect MMP activity and to reconcile the unexpected results described above, we next determined whether PGE2 could affect MMP activity via the plasminogen system. We examined the effect of PGE2, the EP4 agonist and the EP1/EP3 agonist, sulprostone, on expression and activity of PAI-1.Treatment with 10−6M PGE2 for 24 hrs increased PAI-1 mRNA by 2.43 ± 0.62 –fold (p < 0.005). Consistent with the effect of PGE2 on PAI-1 mRNA, both active and total PAI-1 protein were increased after treatment with 10−6M PGE2 for 24 hrs. Active PAI-1 increased from 0.26 ± 0.06 to 0.72 ± 0.19 ng/ml, p < 0.05 and total PAI-1 is shown in Figure 4. The lower concentration of PGE (10−8M) had no effect on PAI-1 protein. The effect of PGE2 was mimicked by the EP1/EP3 agonist sulprostone (1 μM) which also increased total PAI-1 to a value similar to that achieved after PGE2-stimulation (Figure 4). In contrast, both 10−6 M and 10−8 M concentrations of the EP4 agonist reduced total PAI-1. Treatment with the EP2 agonist butaprost (5×10−6M) had no effect on total PAI-1 (0.81 ± 0.04 ng/ml for vehicle, 0.80 ± 0.03 ng/ml for butaprost treatment).
Figure 4.
Effect of 24 hr treatment with PGE2, the EP4 agonist, and the EP1/EP3 agonist sulprostone on total PAI-1 in NVF as measured by ELISA. The values on the lower x-axis represent molar concentrations. Statistical significance: * p < 0.05. *** p < 0.005 versus vehicle treatment, n = 9.
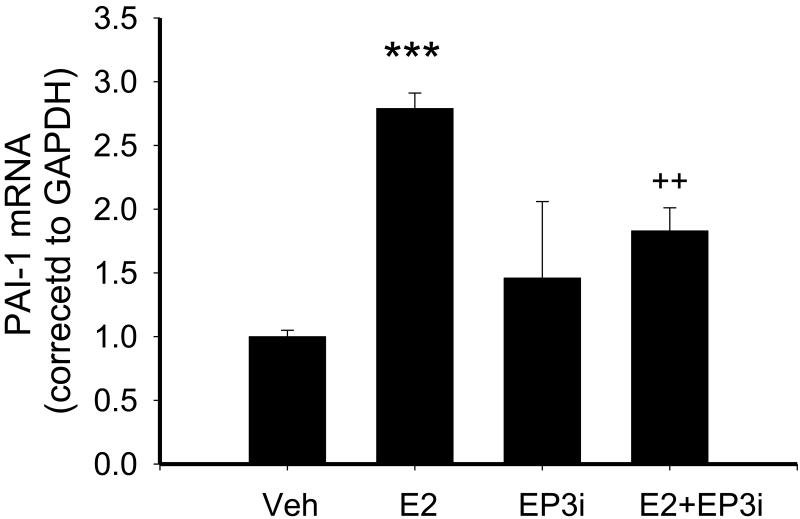
To verify that the effect of PGE2 on PAI-1 was mediated by its EP3 receptor we performed experiments to determine whether the EP3 antagonist, ONO AE3 240 (10 μM), would prevent PGE2-mediated increases in PAI-1 expression as determined by real time RT-PCR. In a separate set of experiments, treatment with PGE2 increased PAI-1 expression 8 and this effect was inhibited by pre-treatment with the EP3 antagonist, although not to control levels (Figure 5).
Figure 5.
Effect of 10−5M EP3 antagonist (EP3i) on PGE2-stimulation of PAI-1 in NVF as measured by real time RT-PCR. Cells were pre-treated with antagonist for 1 hr prior to 24 hr treatment with PGE2. Expression of PAI-1 was corrected to GAPDH as a loading control. Statistical significance: *** p < 0.0001 versus vehicle treatment, ++ p < 0.01 versus PGE2 alone, n = 3-4.
To determine whether the effects of PGE2 were evident at an earlier time point, we treated NVF with either PGE2, sulprostone or the EP4 agonist for 4 hrs and measured PAI-1 mRNA by real time RT-PCR. Similar to the effects noted at 24 hrs of treatment, 10−6M PGE2 and 10−6M sulprostone increased PAI-1 mRNA (corrected to GAPDH) by 3.93 ± 0.82-fold and 3.12 ± 0.84-fold respectively (p < 0.01 and p < 0.05) whereas the EP4 agonist (10−8M) reduced PAI-1 mRNA from a control value of 1.00 to 0.58 ± 0.15 arbitrary units (p < 0.001).
Effect of PGE2 and EP4 agonist on Collagen Type I mRNA
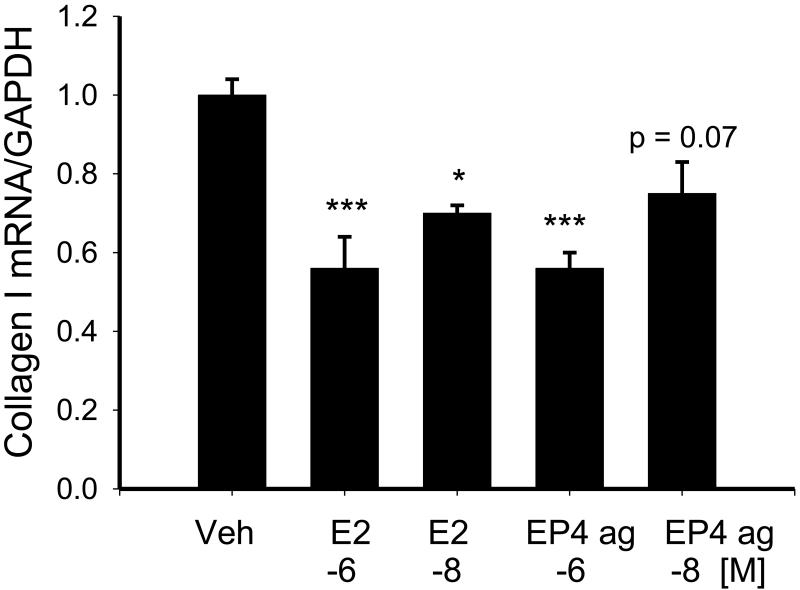
To determine whether treatment with PGE2 or the EP4 agonist affected collagen type I mRNA levels, we treated NVF with 10−6M or 10−8M concentrations of these compounds for 24 hrs under serum-free conditions and examined expression of collagen type I by real time RT-PCR. Figure 6 shows that treatment with either PGE2 or the EP4 agonist reduced collagen type I mRNA by a similar extent.
Figure 6.
Effect of 24 hr treatment with 10−6 M and 10−8M PGE2 and the EP4 agonist (EP4 ag) on collagen type I mRNA as measured by real time RT-PCR. Statistical significance: * p < 0.05. *** p < 0.005 versus vehicle treatment, n = 3.
Effect of PGE2, EP4 agonist and the EP2 agonist on fibroblast migration
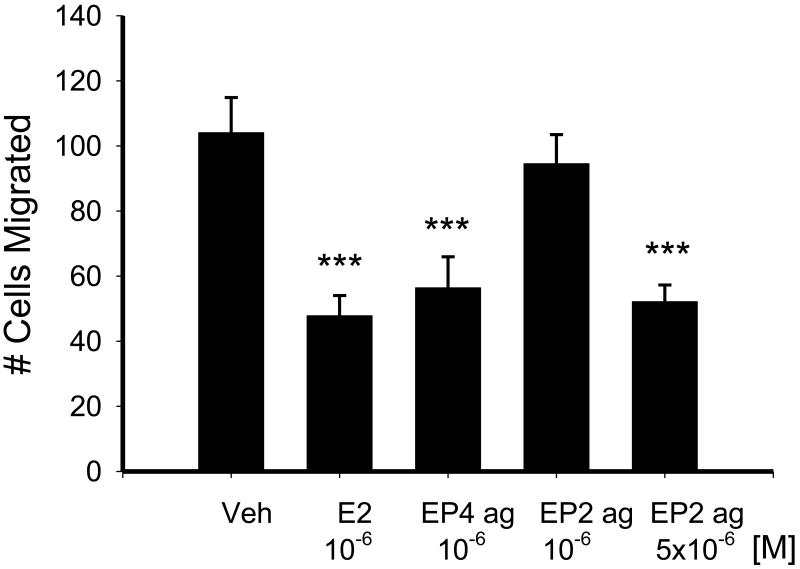
As shown in Figure 7, treatment with either PGE2 or the EP4 agonist for 24 hrs was able to reduce fibroblast migration. A similar effect on migration was observed with the higher concentration of the EP2 agonist, butaprost, although the lower concentration was ineffective.
Figure 7.
Effect of 24 hr treatment with 10−6M PGE2, 10−6M EP4 agonist (EP4 ag) and the EP2 agonist (EP2 ag), butaprost (10−6M and 5 × 10−6M), on subsequent NVF migration stimulated by 10% FCS for 5 hrs. Statistical significance: *** p < 0.005 by one way ANOVA versus vehicle, n = 4-5
DISCUSSION
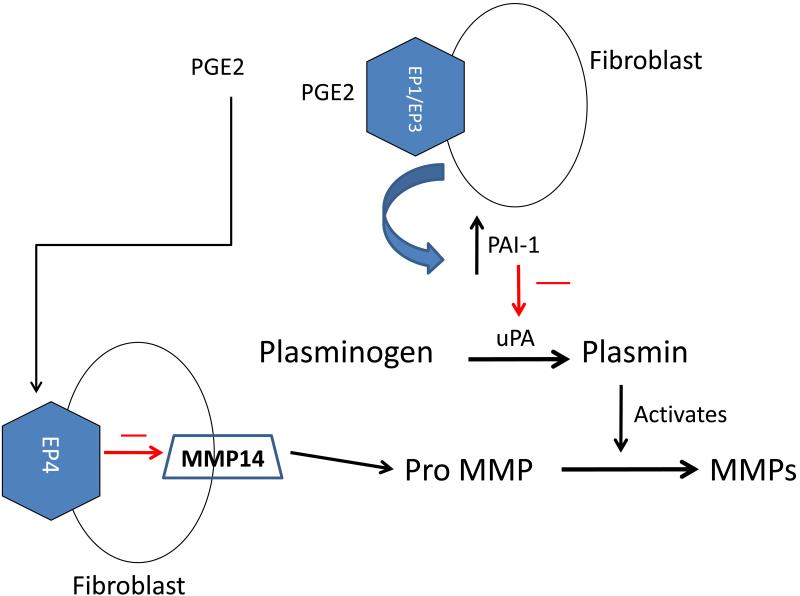
The novel results of this study show that PGE2 can affect MMP expression and activity by different mechanisms that utilize different EP receptors and that this has the ability to impact cardiac fibroblast migration. Overall, we found that PGE2 decreases MMP-14 expression via an EP4-dependent pathway whereas the increase in PAI-1 is independent of EP4 and appears to be mediated via EP1 and/or EP3. The net effect of these processes is decreased MMP2 activity and decreased fibroblast migration as shown in Figure 8.
Figure 8.
Proposed pathway whereby PGE2 reduces MMP-2 activity via EP4-mediated reduction in MMP-14 and EP1/EP3-mediated stimulation of PAI-1. We propose that PGE2, acting on EP1/EP3 receptors increases PAI-1which prevents the conversion of plasminogen to plasmin and thus inhibits activation of MMPs. PGE2 also acts on its EP4 receptor to inhibit MMP-14 and thus, prevents activation of MMPs. These two pathways act in concert to prevent MMP activation.
For the first time, our results show that the membrane bound MMP-14 can be down-regulated by PGE2 in an EP4 dependent manner; a result that is consistent with our previous findings that the cardiac specific EP4 KO mice show increased MMP-14 mRNA in the left ventricle. MMP-14 is a known activator of pro MMP-2 [1,7] and our present data indicating that decreases in MMP-14 after PGE2 treatment correlate with decreased MMP-2 activity support this idea. Spinale et al reported that overexpression of MMP-14 caused adverse cardiac remodeling after myocardial infarction [8]. Although activation of MMPs was believed to decrease the extracellular matrix, the above study challenged that dogma, showing that over-expression of MMP-14 increased total collagen content by a mechanism that included the proteolytic cleavage of TGFβ from its latency associated binding protein (LTBP-1). Thus, overexpression of MMP-14 activated TGFβ1and Smad 2 signaling pathways in addition to activation of other MMPs. In contrast to our present study, Dery et al [9] reported that oxytocin increases the invasiveness of endometrial cancer cells by a mechanism that is dependent on cyclooxygenase (COX) and MMP-14, with the COX inhibitor indomethacin preventing oxytocin-induced MMP-14 expression. However, that study implicated EP1 receptors in cancer cell migration and whether the conflicting results relate to different cell types used is currently unknown. Taken altogether these results indicate that regulation of extracellular matrix production and degradation is complex, with interplay of a variety of factors. Moreover, in diseases such as myocardial infarction, an additional layer of complexity is added with temporal regulation of these factors [10].
In addition to activation of MMP-2 by MMP-14, MMPs are also activated by the plasminogen-plasmin system and this process is inhibited by plasminogen activator inhibitor-1 (PAI-1). In the present study, our surprising results with sulprostone on MMP activity, lead us to investigate the possibility that the EP1/EP3 agonist acts via a different mechanism to decrease MMP activity. Our present study shows that PGE2 increases PAI-1 mRNA and protein levels by mechanism(s) that are independent of EP4 and most likely mediated via EP1/EP3 as they are reproduced by sulprostone treatment. The stimulatory effect of PGE2 on PAI-1 is dose dependent with no effect at the 10−8M concentration. In contrast, treatment with the EP4 agonist elicited a small but significant decrease in PAI-1. Thus, it appears as though pathological but not physiologic amounts of PGE2 increase PAI-1 in cardiac fibroblasts and our previous studies show that the concentration of PGE2 in the heart after myocardial infarction reaches these concentrations. PAI-1 plays a crucial role in the development of fibrosis in many organs. Mice lacking PAI-1 are protected from bleomycin-induced pulmonary fibrosis [11] and are reported to have less cardiac fibrosis after myocardial infarction than wild-type mice [12]. Although there are reports that PGE2 has divergent effects on PAI-1 [13-15], our results in cardiac fibroblasts are similar to those of Markosyan and Duffy [13] who studied the effects of PGE2 on PAI-1 in granulosa cells, reporting that the increase noted was due to activation of EP1/EP3 receptors.
Migration of fibroblasts to the site of injury is a common feature in wound repair such as that which occurs in myocardial infarction where collagen deposition is needed for scar formation to prevent cardiac rupture. Although the effect of PGE2 and related EP agonists has been evaluated in many cell types, few studies have examined their effect in cultured cardiac fibroblasts. Previously, we reported that cultured cardiac fibroblasts express all four EP receptors and that the addition of PGE2 causes cell proliferation via an EP1/EP3 effect that involves stimulation of cyclin D with involvement of both the p42/44 MAP kinase pathway and the PI3 kinase pathway [16]. In vivo studies [17] using the COX-2 inhibitor, rofecoxib, showed inhibition of fibroblast proliferation in a rat model of MI; consistent with our former results discussed above. The results of our present study suggest that PGE2 has inhibitory effects on migration and that this effect is mediated via EP2 and/or EP4 receptors as both the EP2 agonist and the EP4 agonist reduced migration, recapitulating the effect of PGE2. Consistent with the results of our present study, White et al [18] also reported an inhibitory effect of PGE2 on migration of human lung fibroblasts that was mediated by the EP2 receptor and involved increased PTEN (Phosphatase and tensin homolog on chromosome ten) activity. The inhibitory effect of the EP2 agonist, butaprost, was abolished in fibroblasts obtained from EP2 KO mice and furthermore, PTEN null mice showed increased baseline migration that was further increased by fibroblast growth factor but was not antagonized by butaprost. Previously, low concentrations of PGE2 were reported to inhibit interleukin-1β stimulation of MMPs in human synovial fibroblasts via a cAMP dependent pathway [14] but in contrast, Funck et al [19] reported that PGE2 stimulated MMP1 activity in cultured cardiac fibroblasts. However, they did not evaluate other MMPs nor did they examine the effect of the different EP receptors. Biphasic effects of PGE2 have also been noted concerning fibroblast proliferation [20]. Thus, the effects of PGE2 are complex and depend on its concentration, location and which receptor is activated. Our results showing PGE2-mediated reduction of MMP activity by zymography could be due to additive effects including the reduction of MMP-14 and the increase in PAI-1.
In our present study, although butaprost reduced fibroblast migration and MMP-2/9 activity, it did not affect MMP-14 expression nor PAI-1 expression suggesting that its effect on MMP-2 and/or -9 activity is not mediated by decreased MMP-14 leading to reduced activation of MMP-2 or -9. Rather, our results indicate a direct effect of butaprost on those pathways regulating MMP activity. Whether PTEN is involved in this mechanism in cardiac fibroblasts remains open to speculation. Nevertheless, our data support the concept that PGE2 utilizes multiple receptors and pathways to regulate the extracellular matrix. This may have significance during events such as myocardial infarction in which one may speculate that the increased PGE2 plays a role during the early phase of wound repair to prevent cardiac rupture. On the contrary, prolonged and excessive reduction of MMPs might lead to cardiac fibrosis with a negative impact on contractility. Our current study showed that PGE2 reduced collagen type I mRNA expression but we did not measure collagen protein. Nevertheless, one would anticipate that the 50% reduction we observed would also affect protein levels. However, the in vivo effects of PGE2 were not evaluated in the present study and remain to be investigated in future studies. In conclusion, PGE2 utilizes multiple receptors and pathways to affect degradation of the extracellular matrix. This redundancy means that blockade of prostaglandin E synthase, rather than selective EP antagonism, may be more effective in reducing cardiac fibrosis in conditions characterized by elevated PGE2.
Highlights.
PGE2 decreases MMP-14 in NVF via its EP4 receptor but increases PAI-1 through EP1 and/or EP3.
PGE2 utilizes different mechanisms to alter MMP activity.
This study has relevance to cardiac disease characterized by inflammation and high PGE2 levels.
There is redundancy in PGE2 signaling system whereby the same net effect is achieved using different EP receptors.
Blockade of prostaglandin E synthase, rather than a specific receptor sub-type may be effective in preventing cardiac fibrosis.
ACKNOWLEDGEMENTS
The authors wish to thank David Taube and Gulser Gurocak for excellent technical assistance.
SOURCES OF FUNDING
These studies were funded by NIH grant 5P01HL028982 (sub-project 2) to PH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The authors have nothing to disclose.
REFERENCES
- 1.Osenkowski P, Toth M, Fridman R. Processing, shedding, and endocytosis of membrane type 1-matrix metalloproteinase (MT1-MMP) J Cell Physiol. 2004;200:2–10. doi: 10.1002/jcp.20064. [DOI] [PubMed] [Google Scholar]
- 2.Rowe RG, Keena D, Sabeh F, Willis AL, Weiss SJ. Pulmonary fibroblasts mobilize the membrane-tethered matrix metalloprotease, MT1-MMP, to destructively remodel and invade interstitial type I collagen barriers. Am J Physiol Lung Cell Mol Physiol. 2011;301:L683–L692. doi: 10.1152/ajplung.00187.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joghetaei N, Akhyari P, Rauch BH, Cullen P, Lichtenberg A, Rudelius M, Pelisek J, Schmidt R. Extracellular matrix metalloproteinase inducer (CD147) and membrane type 1-matrix metalloproteinase are expressed on tissue macrophages in calcific aortic stenosis and induce transmigration in an artificial valve model. J Thorac Cardiovasc Surg. 2011;142:191–198. doi: 10.1016/j.jtcvs.2010.09.051. [DOI] [PubMed] [Google Scholar]
- 4.Harding P, Yang XP, Yang J, Shesely E, He Q, LaPointe MC. Gene expression profiling of dilated cardiomyopathy in older male EP4 knockout mice. Am J Physiol Heart Circ Physiol. 2010;298:H623–H632. doi: 10.1152/ajpheart.00746.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harding P, Carretero OA, LaPointe MC. Effects of interleukin-1 beta and nitric oxide on cardiac myocytes. Hypertension. 1995;25:421–430. doi: 10.1161/01.hyp.25.3.421. [DOI] [PubMed] [Google Scholar]
- 6.Winer J, Jung CK, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 1999;270:41–49. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]
- 7.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Spinale FG, Mukherjee R, Zavadzkas JA, Koval CN, Bouges S, Stroud RE, Dobrucki LW, Sinusas AJ. Cardiac restricted overexpression of membrane type-1 matrix metalloproteinase causes adverse myocardial remodeling following myocardial infarction. J Biol Chem. 2010;285:30316–30327. doi: 10.1074/jbc.M110.158196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dery MC, Chaudhry P, Leblanc V, Parent S, Fortier AM, Asselin E. Oxytocin increases invasive properties of endometrial cancer cells through phosphatidylinositol 3-kinase/AKT-dependent up-regulation of cyclooxygenase-1, -2, and X-linked inhibitor of apoptosis protein. Biol Reprod. 2011;85:1133–1142. doi: 10.1095/biolreprod.111.093278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindsey ML, Zamilpa R. Temporal and spatial expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases following myocardial infarction. Cardiovasc Ther. 2012;30:31–41. doi: 10.1111/j.1755-5922.2010.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauman KA, Wettlaufer SH, Okunishi K, Vannella KM, Stoolman JS, Huang SK, Courey AJ, White ES, Hogaboam CM, Simon RH, Toews GB, Sisson TH, Moore BB, Peters-Golden M. The antifibrotic effects of plasminogen activation occur via prostaglandin E2 synthesis in humans and mice. J Clin Invest. 2010;120:1950–1960. doi: 10.1172/JCI38369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeshita K, Hayashi M, Iino S, Kondo T, Inden Y, Iwase M, Kojima T, Hirai M, Ito M, Loskutoff DJ, Saito H, Murohara T, Yamamoto K. Increased expression of plasminogen activator inhibitor-1 in cardiomyocytes contributes to cardiac fibrosis after myocardial infarction. Am J Pathol. 2004;164:449–456. doi: 10.1016/S0002-9440(10)63135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markosyan N, Duffy DM. Prostaglandin E2 acts via multiple receptors to regulate plasminogen-dependent proteolysis in the primate periovulatory follicle. Endocrinology. 2009;150:435–444. doi: 10.1210/en.2008-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiBattista JA, Martel-Pelletier J, Morin N, Jolicoeur FC, Pelletier JP. Transcriptional regulation of plasminogen activator inhibitor-1 expression in human synovial fibroblasts by prostaglandin E2: mediation by protein kinase A and role of interleukin-1. Mol Cell Endocrinol. 1994;103:139–148. doi: 10.1016/0303-7207(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 15.Neil JR, Johnson KM, Nemenoff RA, Schiemann WP. Cox-2 inactivates Smad signaling and enhances EMT stimulated by TGF-beta through a PGE2-dependent mechanisms. Carcinogenesis. 2008;29:2227–2235. doi: 10.1093/carcin/bgn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harding P, LaPointe MC. Prostaglandin E2 increases cardiac fibroblast proliferation and increases cyclin D expression via EP1 receptor. Prostaglandins Leukot Essent Fatty Acids. 2011 doi: 10.1016/j.plefa.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheuren N, Jacobs M, Ertl G, Schorb W. Cyclooxygenase-2 in Myocardium Stimulation by Angiotensin-II in Cultured Cardiac Fibroblasts and Role at Acute Myocardial Infarction. J Mol Cell Cardiol. 2002;34:29–37. doi: 10.1006/jmcc.2001.1484. [DOI] [PubMed] [Google Scholar]
- 18.White ES, Atrasz RG, Dickie EG, Aronoff DM, Stambolic V, Mak TW, Moore BB, Peters-Golden M. Prostaglandin E(2) inhibits fibroblast migration by E-prostanoid 2 receptor-mediated increase in PTEN activity. Am J Respir Cell Mol Biol. 2005;32:135–141. doi: 10.1165/rcmb.2004-0126OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Funck RC, Wilke A, Rupp H, Brilla CG. Regulation and role of myocardial collagen matrix remodeling in hypertensive heart disease. Adv Exp Med Biol. 1997;432:35–44. doi: 10.1007/978-1-4615-5385-4_4. [DOI] [PubMed] [Google Scholar]
- 20.White KE, Ding Q, Moore BB, Peters-Golden M, Ware LB, Matthay MA, Olman MA. Prostaglandin E2 mediates IL-1beta-related fibroblast mitogenic effects in acute lung injury through differential utilization of prostanoid receptors. J Immunol. 2008;180:637–646. doi: 10.4049/jimmunol.180.1.637. [DOI] [PMC free article] [PubMed] [Google Scholar]