Abstract
Background
Rett syndrome is a unique neurodevelopmental disorder, affecting approximately 1 in 10,000 live female births, most experiencing reduced growth. We characterized pubertal trajectories in females with Rett syndrome. We hypothesized that pubertal trajectory deviates from the general female population with early pubertal onset and delayed menarche.
Methods
Participants were individuals enrolled in the Rett Syndrome Natural History Study with clinical diagnosis of Rett syndrome or mutations in MECP2. Intervals to thelarche, adrenarche, and menarche were assessed by survival analysis; BMI, mutation type, clinical severity, and pubertal milestone relationships were assessed by log-likelihood test; pathway synchrony (relationship between thelarche, adrenarche, and menarche) was assessed by Chi-squared analysis.
Results
Compared to the general female population, over 25% initiated puberty early, yet entered menarche later (median age 13.0 years). 19% experienced delayed menarche. Median length of puberty, from thelarche to menarche, was 3.9 years. Higher BMI correlated with earlier thelarche and adrenarche but not menarche; milder mutations correlated with earlier menarche; and milder clinical presentation correlated with earlier thelarche and menarche. Fifty-two percent entered puberty in synchrony, but differing from general population, 15% led with thelarche, and 32% with adrenarche.
Conclusions
Pubertal trajectories in Rett syndrome differ from general population, entering puberty early and reaching menarche later. BMI affects pubertal timing, but the relationship between specific mutations, clinical presentation, and underlying neuroendocrine pathology is less clear.
Keywords: Rett syndrome, MECP2 mutations, Neurodevelopmental disorder, Puberty, Menarche, Body mass index
Introduction
Rett syndrome (RTT) is a neurodevelopmental disorder that affects approximately 1 in 10,000 live female births. Mutations in the Methyl-CpG-binding protein 2 gene (MECP2) have been implicated in >95% of classic RTT. The main diagnostic criteria for classic RTT are as follows: loss of hand skills, loss of acquired spoken language, gait abnormalities, and stereotypic hand movements.1 Multiple developmental issues are present in RTT, particularly growth retardation and orthopedic problems including scoliosis, bone under-mineralization, and joint contractures.2-4 Studies of gonadal and adrenal steroids suggest normal sex hormones,5 yet one study showed precocious pubertal onset with normal age of pubertal completion in a population of 494 girls with RTT,6 and another supported these findings with endocrinological data in one case.7 Neither pubertal trajectories nor factors associated with deviation in pubertal onset in RTT have been definitively characterized. Current research has not achieved consensus on the ages of pubertal milestones or the underlying mechanisms driving puberty in RTT individuals, particularly in view of their generally small stature. In conjunction with these milestones, underlying developmental biologic issues are believed to prevent the traditional pubertal growth spurt.4,6 Using Tanner staging criteria, adrenarche is classified as stage 2 in pubic hair development, and thelarche is classified as stage 2 in breast development.8 US population-based data demonstrate that the mean age of stage 2 breast development (B2) is 9.8 years and stage 2 pubic hair development (PH2) is 10.2 years, and that B2 occurs, on average, 5 months prior to PH2.9 In a large longitudinal study, the median age of onset of B2 was 9.7 years for white non-Hispanic girls.10 Other studies focusing specifically on white females have found similar results: B2 at 10.0 years, PH2 at 10.5 years, and menarche at 12.6 years.11,12 In general, menarche should follow thelarche within three years;13 early breast development typically predicts a longer interval to menarche.13,14
Breast or pubic hair development earlier than age 8 is classified as premature.8,15 Menarche is premature before 11.2 years.15 Breast development is defined as delayed after 13.3 years,15 and menarche is delayed after age 16 or if it does not occur within 5 years of the initiation of puberty.16
Many factors could influence pubertal trajectories, including general health, genetics, and environment. Both race and ethnicity can influence the timing of puberty in typical individuals,17 as can growth, especially malnutrition or obesity as defined by BMI. In RTT, bone age and BMI hold particular clinical significance and may be related to pubertal timing. In the general population, pubertal trajectories correlate more strongly with bone age than with chronologic age;18 higher BMI is correlated with earlier maturation in girls.19
Pubertal development research in RTT is limited. One study (n=494) found B2 at an average age of 7.1 +/- 2.5 (SD) years, with an average age of menstruation at 12.7+/- 2.4 years.6 A second study (n=213) found that half of girls achieved adrenarche by 11 years; half reached thelarche by 11.5 years; and half reached menarche by 14 years.20 The present study utilizing the US Natural History Study database reexamines not only the age at thelarche, adrenarche, and menarche but also correlates these with BMI, specific MECP2 mutations, and clinical presentation. We hypothesized that pubertal trajectories in RTT deviate from the general female population, with earlier onset of puberty and later menarche in RTT.
Methods
Participants
Through the multicenter RTT Natural History study (RNHS), individuals with classic RTT were recruited from 2006 to 2013 and evaluated as described previously.21 A RNHS neurologist or geneticist (D.G.G., J.L.N., A.K.P., S.A.S., and W.E.K.) confirmed the diagnosis of classic RTT based upon diagnostic criteria.1,22 Two scales were used to assess overall severity, the clinical severity scale (CSS) and motor behavioral analysis (MBA), as previously reported.4 All participants had MECP2 testing by a qualified laboratory, >95% of classic RTT having a mutation. Pubertal development was assessed by the clinicians at each study visit which was semi-annually until age 6 years and annually thereafter. Only female participants were included. Institutional review board approval was obtained for each participating institution. Participants’ families granted informed assent. The RNHS is registered as clinical trial NCT00296764.
Data Coding
Data were coded in order to provide specific ages for Tanner stages B2, PH2, and menarche. If a participant was staged at B1 at age 10.0 and B2 at age 11.0, she was scored as transitioning to B2 at the midpoint of those ages, that is, at 10.5. If a participant was staged at B1 at age 10.0 and B3 at age 11.0, she was scored as transitioning to B2 at age 10.33 (a third of the time elapsed between the two ages). If a participant was judged to regress in the Tanner staging, the earlier evaluations were disregarded. For example, if a participant was scored at B1 at age 8.0, at B2 at age 9.0, at B1 at age 10.0, and B2 at age 11.0, the first transition was disregarded, and the participant was scored as reaching B2 at age 10.5 (the midpoint of 10.0 and 11.0). A study that used self-reported data from children, parents, or guardians chose to exclude data when a lower stage was reported;23 however, we believe that due to the use of Tanner staging by trained professionals, our approach is warranted. This same paper also chose to use the first age at menarche reported if multiple ages were reported for a given participant. We followed this approach, using the first reported menarchal age. Any different ages reported in subsequent visits were disregarded, assuming the initial parent report was more accurate. BMI z-scores were obtained using CDC growth charts for BMI and the LMS (lambda-mu-sigma) technique.24,25 Although BMI at age 8 years has been used in other studies,26 because of the possibility of an earlier pubertal onset, we chose to use BMI at age 7 +/− 1 year. (6.00 to 8.00 years of age). Classification of precocious or delayed puberty compared to typical individuals was as follows: precocious B2 and PH2 occurred before 8.0 years and menarche before 11.2 years; and delayed B2 occurred after 13.3 years, PH2 after 12.6 years, and menarche after 15.6 years.15
If B2 and PH2 occurred within 5 months of each other, the pathway was classified as synchronous. If B2 and PH2 occurred at greater than 5 months apart, the pathway was classified as asynchronous. If neither milestone was reached, the participant was excluded from synchrony calculation. And if the participant had reached both B2 and PH2 prior to the initial observation, the participant was also excluded from the synchrony calculations.
Statistical Analysis
Descriptive statistics were calculated. Percentiles for age of adrenarche, thelarche, and menarche were generated using the Kaplan-Meier estimator. Because the participants in the RNHS were different ages and were observed for different time periods, not all participants were observed for all three pubertal milestones (Table 1). The Kaplan-Meier estimator allows inclusion of observed milestones and censors or excludes participants that enter the study after achieving these pubertal milestones or leave the study prior to achieving certain pubertal milestones. To examine the effects of BMI, mutation type, race, ethnicity, MBA, and CSS on pubertal trajectories, these variables were categorized based on clinical significance, and survival curves were compared using the Log-rank test. Categorization of BMI was based on published recommendations, specific to RTT, regarding overweight and obesity (85th and 98th percentiles respectively), and under-nutrition (25th percentile).27,28 Categorization of MECP2 mutation based on phenotypic similarities29,30 was as follows: mild mutations included R133C, R294X, R306C, and 3’ truncations; moderate included T158M and other mutations; and severe included R106W, R168X, R255X, R270X, early truncations, and large deletions. Mutation negative participants were excluded from mutation analysis. The association between predictor variables and synchrony was analyzed using the Chi-squared test. All analyses were completed in SPSS version 21,31 and a p-value of <.05 was considered significant.
Table 1.
Demographics of Studied Population.
| Overall | Percent | B2 Observations | PH2 Observations | Menarche Observations | |
|---|---|---|---|---|---|
| American Indian | 6 | 0.7 | 3 | 3 | 5 |
| Asian | 35 | 4.4 | 10 | 10 | 9 |
| Native Hawaiian | 2 | 0.3 | 2 | 2 | 1 |
| Black | 34 | 4.2 | 9 | 7 | 14 |
| White | 697 | 86.9 | 150 | 138 | 282 |
| Mixed Black and White | 13 | 1.6 | 2 | 2 | 4 |
| Missing Demographic Data | 15 | 1.9 | 3 | 4 | 3 |
Demographic data were not reported for 15 of 802 participants
Results
Description of Population
After excluding male participants (n=47) and those who did not meet criteria for classic RTT (n=147), 802 participants were analyzed. Each participant was observed for an average of 4.1 visits with a range of 1 to 8 visits. Each participant was observed for an average of 3.4 years with a range of 0 years (indicating a single baseline visit) to 7 years. Dates of birth ranged from 1943 to 2010. A total of 34 (4.2%) participants died during the study and 188 (23.4%) missed one or more appointments. For the age ranges analyzed, visits were conducted annually. Regarding B2, 314 were too old to be observed at this transition, 309 did not reach B2 during the study and were censored or excluded from further analysis, and 179 reached B2. Regarding PH2, 329 were too old to be observed at this transition, 307 did not reach PH2 during the study and were censored or excluded from further analysis, and 166 reached PH2. Regarding menarche, 483 did not reach menarche and were censored or excluded from further analysis, one participant had a hysterectomy and was not included, and 318 reached menarche. Of these 318 participants, 112 reached menarche during the RNHS. For these 112, 50% reached menarche by 13.0 years (SE = 0.25 years). Of the 318, 206 reached menarche prior to their enrollment in the RNHS. For these 206, 50% reached menarche by 13.0 years (SE = 0.17 years). Of the 802 participants, BMI data at age 7 +/− 1 year were available for 299 participants.
Percentiles for Milestones and Precocious and Delayed Puberty
B2
The proportions of the population who achieved B2 were as follows: 10% by 7.2 years (SE = 0.22 years), 50% by 9.3 years (SE = 0.19 years), and 90% by 11.3 years (SE= 0.37 years). Compared to typical individuals, 25% of females reached thelarche prematurely, whereas 4% experienced delayed thelarche (Figure 1A).
Figure 1.
Proportion of participants experiencing onset of puberty. The Y-axis is expressed as one minus the cumulative survival. Cutoffs for precocious and delayed puberty in the general population are indicated by vertical lines.
PH2
The proportions for those who achieved PH2 were as follows: 10% by 6.8 years (SE = 0.23 years), 50% by 9.0 years (SE = 0.15 years), and 90% by 11.9 years (SE = 0.47 years). Compared to typical individuals, 28% of females reached adrenarche prematurely, whereas 5% experienced delayed adrenarche (Figure 1B).
Menarche
The proportions for those who achieved menarche were as follows: 10% by 10.0 years (SE = 0.11 years), 50% by 13.0 years (SE = 0.09 years), and 90% by 16.0 years (SE = 0.26 years). Compared to typical individuals, 13% of females reached menarche prematurely, whereas 19% experienced delayed menarche (Figure 1C).
Duration of Puberty
The median interval from B2 to menarche was 3.9 years (SE = 0.40 years), and from PH2 to menarche was 4.5 years (SE = 0.27 years).
Predictor Variables
BMI
B2 and PH2 occurred earlier in those categorized as overweight or obese compared to normal or underweight individuals (p<.001, Figures 2A and 2B). Onset of menarche was similar among these three categories (Figure 2C).
Figure 2.
Proportion of participants experiencing onset of puberty based on clinical variables. The Y-axis is expressed as one minus the cumulative survival. Cutoffs for precocious and delayed puberty in the general population are indicated by vertical lines. Significant difference among distributions indicated by *.
Mutation type
Menarche occurred earlier in those with a milder mutation type compared to those with moderate and severe mutations (p=.032, Figure 2F). Onset of B2 or PH2 was similar among mutation types (Figure 2D and Figure 2E). B2 did trend earlier with severe mutations; however, this relationship was not significant (p=.095).
Disease severity
Those with a milder clinical presentation experienced B2 (p=.001, Figure 2G) and menarche (p=.026, Figure 2I) earlier than those with a moderate or severe phenotype. However, age of PH2 was similar among severity categories (Figure 2H).
Synchrony
Compared to expected percentages of 46% synchronous, 42% thelarche pathway, 11% pubic hair pathway in typical individuals,23 a higher proportion of RTT participants led with adrenarche (32%), while only 15% led with thelarche (p<0.001). The majority of RTT participants entered in synchrony (52%).
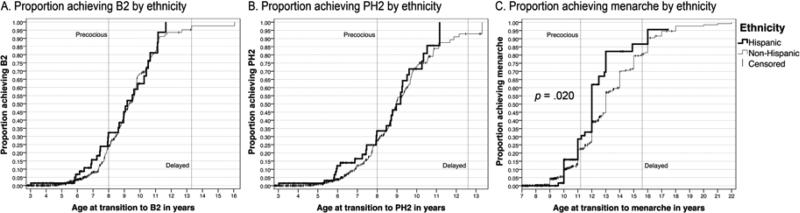
The majority of participants (Table 1) were white, and no differences were found among the three largest groups, whites (88%), Asians (4.5%) and blacks (4.5%) for age at B2, PH2, or menarche. Fifteen percent of all participants were Hispanic. Although, the average age of transition to B2 and PH2 was similar between the two groups, Hispanic participants experienced earlier menarche (median 12.4y, 95% CI 11.8-13.0) compared to non-Hispanic participants (median 13.3y, 95% CI 13.0-13.6; p = .020, Figure 3).
Figure 3.
Proportion of participants experiencing onset of puberty based on ethnicity. The Y-axis is expressed as one minus the cumulative survival. Cutoffs for precocious and delayed puberty in the general population are indicated by vertical lines. Significant difference between distributions indicated by *.
Discussion
Using survival analysis, we calculated median entry times for B2, PH2, and menarche as well as intervals between B2 and menarche. The 50th percentile for B2 in this study, 9.3 years, is considerably earlier than that reported in previous work, 11 years, and the 50th percentile for menarche in RTT in this study differs by a full year from that reported in previous work.20 A significant percentage of participants would be classified as entering puberty prematurely, with 25% and 28% of females reaching B2 and PH2, respectively, before 8 years. These high percentages have not been reported in previous work.20 One possible explanation for these differences in B2 and PH2 is that when participants had experienced B2 or PH2 prior to data collection, one study estimated B2 and PH2 as 1 year prior to return of the participant's questionnaire; in fact, B2 and PH2 may have occurred at any point prior to the study and could have altered these results accordingly.20 Because such a high proportion of participants experienced premature B2 and PH2, and based on the 10th percentiles of B2 and PH2 in RTT, 7.2 years and 6.8 years respectively, population-based norms may not be an accurate clinical measure of precocity in RTT. While a smaller percentage of participants experienced precocious menarche, 19% experienced delayed menarche. Considering that pubertal trajectories seem to be shifted earlier in RTT, the mechanisms driving delayed menarche in almost a fifth of the population remain unclear.
Our findings on the interval from B2 to menarche, typically 3 years for the general population, also differ from the previously reported value for RTT of 2.5 years.13,20 The median length of time from B2 to menarche in this study was 3.9 years. While early onset of puberty tends to lengthen the interval until menarche, the mechanism in RTT is unclear, as menarche is also delayed relative to typical individuals. However, we did not find menarche to be as delayed as reported previously for RTT.20
BMI plays an important role in pubertal trajectory. BMI at age 7 was associated with age of onset of B2 and PH2, but not menarche. The interval from B2 to menarche may buffer the effects of BMI on puberty.
From a genetic perspective, 8 common mutations in the MECP2 gene (R106W, R133C, T158M, R168X, R225X, R270X, R294X, and R306C) account for about 60% of typical RTT and insertions and deletions another 20%. These mutations have been shown to have significantly different levels of clinical severity, particularly for gait, language, and hand use. R133C and R306C are associated with milder phenotypes and R168X and large deletions with worse phenotypes.29,30 R133C, R294X, and C-terminal deletions produced milder phenotypes than R255X and R270X.32,33 The association between mutation and pubertal milestones varies among studies. In a smaller study, MECP2 mutations did not affect age of pubertal onset.4 However, in a larger study, R168X was associated with later adrenarche, thelarche, and menarche, R255X was associated with later menarche, and C-terminal mutations and early truncating mutations were associated with earlier thelarche and menarche.34
Regarding genetic effects, mutation type was significantly associated with menarche, with the most severe mutation types predicting later menarche. This finding agrees with earlier work that found severe mutations (R168X and 255X) predicted later menarche. Contrary to BMI, mutation influenced menarche but had no significant effect on either B2 or PH2; these findings differed from previous research which found that all three pubertal stages were associated with mutation type.20 Again, their results with respect to BMI may have been biased by their method of estimating B2 and PH2.
The underlying mechanism by which a MECP2 mutation could impact pubertal pathways remains unclear. MECP2-null mouse models, modeling RTT, have shown modified gene expression in the hypothalamus.35 In another mouse model with a truncating deletion, MECP2 dysfunction altered estrogen receptor expression.36 These effects could provide a biological link through the hypothalamic-pituitary-gonadal (HPG) axis, which, through its production of estrogen is believed to stimulate breast development.8,18,37 However, B2 was not correlated with mutation type, and research suggests that the mechanisms determining menarche are more complex than estrogen-driven breast budding.37 At this point, we cannot explain the possible genetic mechanisms contributing to the different pubertal milestones in RTT. Notably, greater clinical severity (MBA), like more severe mutation type, predicted later menarche. However, a more severe MBA score also predicted later B2, an effect that was not seen with mutation type.
Previous research has examined the synchrony of B2 and PH2, finding that 46% of females traditionally undergo a synchronous transition, whereas an asynchronous pattern is noted where 42% lead with thelarche, and 11% lead with adrenarche.23 However, our data suggest a reversal of the normal pattern of asynchrony, namely, 32% leading with adrenarche and 11% with thelarche. The mechanism for this possible change is not clear. Interestingly, in addition to possible HPG axis effects, the hypothalamic-pituitary-adrenal (HPA) axis has been shown to be hyperactive in a RTT mouse model.38 An increase in corticotropin-releasing hormone and the increased synthesis of adrenal hormones could possibly promote earlier adrenarche.
One of the weaknesses of our study is the lack of hormonal measurements. Although only a single case documents precocious puberty using hormonal stimulation testing,7 evidence from a mouse model of RTT suggests that regulation of gonadotropin releasing hormone by MECP2 could influence the onset of puberty.6 In addition, while pubertal assessment was made by direct observation, menarche was retrospective as it was based on parent report and could have been subject to error.
These findings raise additional questions regarding pubertal trajectory in RTT. The neuroendocrine control of puberty, particularly through MECP2 regulation, warrants further examination. The effects of BMI and MECP2 mutations on pubertal trajectories have important clinical consequences for RTT beyond the timing of these pubertal milestones – impacting bone development, growth, and the psychosocial issues surrounding puberty. Additional research will help provide a better understanding of the predictors of pubertal milestones in RTT beyond the effects of BMI and MECP2 mutation type.
Acknowledgements
This study was supported by NIH U54 grants RR019478 and HD061222, Office of Rare Disease Research, and IDDRC grant HD38985, funds from the International Rett Syndrome Foundation and the Civitan International Research Center. The Rett Syndrome Natural History Study (U54 HD061222) is a part of the National Institutes of Health (NIH) Rare Disease Clinical Research Network (RDCRN), supported through collaboration between the NIH Office of Rare Diseases Research (ORDR) at the National Center for Advancing Translational Science (NCATS), and the Eunice Kennedy Shriver Child Health and Human Development Institute (NICHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors report no conflicts of interest. The authors acknowledge the gracious participation and provision of information by the families of the reported participants. Dr. Mary Lou Oster-Granite, Health Scientist Administrator at NICHD, provided invaluable guidance, support, and encouragement for this Rare Disease initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trial #: NCT00296764
Author Contributions
JK : Conceptualization, database review and analysis, statistical methodology, writing the final document.
JBL: Conceptualization, data acquisition, database review, revision and review of final document.
AKP: Conceptualization, data acquisition, database review, revision and review of final document.
All other authors: Data acquisition, review of manuscript
Disclosure
The authors report no disclosures.
References
- 1.Neul JL, Kaufmann WE, Glaze DG, et al. Rett syndrome: revised diagnostic criteria and nomenclature. Annals of neurology. 2010 Dec;68(6):944–950. doi: 10.1002/ana.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamilton A, Marshal MP, Sucato GS, Murray PJ. Rett syndrome and menstruation. Journal of pediatric and adolescent gynecology. 2012 Apr;25(2):122–126. doi: 10.1016/j.jpag.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Leonard H, Thomson M, Bower C, Fyfe S, Constantinou J. Skeletal abnormalities in Rett syndrome: increasing evidence for dysmorphogenetic defects. American journal of medical genetics. 1995 Sep 11;58(3):282–285. doi: 10.1002/ajmg.1320580316. [DOI] [PubMed] [Google Scholar]
- 4.Tarquinio DC, Motil KJ, Hou W, et al. Growth failure and outcome in Rett syndrome: specific growth references. Neurology. 2012 Oct 16;79(16):1653–1661. doi: 10.1212/WNL.0b013e31826e9a70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huppke P, Roth C, Christen HJ, Brockmann K, Hanefeld F. Endocrinological study on growth retardation in Rett syndrome. Acta paediatrica. 2001 Nov;90(11):1257–1261. doi: 10.1080/080352501317130281. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Rudaz C, Deng V, Matagne V, et al. FXYD1, a modulator of Na,K-ATPase activity, facilitates female sexual development by maintaining gonadotrophin-releasing hormone neuronal excitability. Journal of neuroendocrinology. 2009 Feb;21(2):108–122. doi: 10.1111/j.1365-2826.2008.01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bas VN, Cetinkaya S, Agladioglu SY, Aksoy A, Gulpinar B, Aycan Z. Report of the first case of precocious puberty in Rett syndrome. J Pediatr Endocrinol Metab. 2013 Apr 2;:1–3. doi: 10.1515/jpem-2012-0418. [DOI] [PubMed] [Google Scholar]
- 8.Bordini B, Rosenfield RL. Normal pubertal development: part II: clinical aspects of puberty. Pediatrics in review / American Academy of Pediatrics. 2011 Jul;32(7):281–292. doi: 10.1542/pir.32-7-281. [DOI] [PubMed] [Google Scholar]
- 9.Susman EJ, Houts RM, Steinberg L, et al. Longitudinal development of secondary sexual characteristics in girls and boys between ages 91/2 and 151/2 years. Archives of pediatrics & adolescent medicine. 2010 Feb;164(2):166–173. doi: 10.1001/archpediatrics.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biro FM, Greenspan LC, Galvez MP, et al. Onset of breast development in a longitudinal cohort. Pediatrics. 2013 Dec;132(6):1019–1027. doi: 10.1542/peds.2012-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chumlea WC, Schubert CM, Roche AF, et al. Age at Menarche and Racial Comparisons in US Girls. Pediatrics. 2003;111(1):110–113. doi: 10.1542/peds.111.1.110. [DOI] [PubMed] [Google Scholar]
- 12.Herman-Giddens ME. Recent data on pubertal milestones in United States children: the secular trend toward earlier development. International journal of andrology. 2006 Feb;29(1):241–246. doi: 10.1111/j.1365-2605.2005.00575.x. discussion 286-290. [DOI] [PubMed] [Google Scholar]
- 13.American Academy of Pediatrics Committee on A, American College of O, Gynecologists Committee on Adolescent Health C. Diaz A, Laufer MR, Breech LL. Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Pediatrics. 2006 Nov;118(5):2245–2250. doi: 10.1542/peds.2006-2481. [DOI] [PubMed] [Google Scholar]
- 14.Marti-Henneberg C, Vizmanos B. The duration of puberty in girls is related to the timing of its onset. The Journal of pediatrics. 1997 Oct;131(4):618–621. doi: 10.1016/s0022-3476(97)70073-8. [DOI] [PubMed] [Google Scholar]
- 15.Bramswig J, Dubbers A. Disorders of pubertal development. Deutsches Arzteblatt international. 2009 Apr;106(17):295–303. doi: 10.3238/arztebl.2009.0295. quiz 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosen DS, Foster C. Delayed Puberty. Pediatrics in Review. 2001;22(9):309–315. doi: 10.1542/pir.22-9-309. [DOI] [PubMed] [Google Scholar]
- 17.Karapanou O, Papadimitriou A. Determinants of menarche. Reproductive biology and endocrinology : RB&E. 2010;8:115. doi: 10.1186/1477-7827-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bordini B, Rosenfield RL. Normal pubertal development: Part I: The endocrine basis of puberty. Pediatrics in review / American Academy of Pediatrics. 2011 Jun;32(6):223–229. doi: 10.1542/pir.32-6-223. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y. Is Obesity Associated With Early Sexual Maturation? A Comparison of the Association in American Boys Versus Girls. Pediatrics. 2002;110(5):903–910. doi: 10.1542/peds.110.5.903. [DOI] [PubMed] [Google Scholar]
- 20.Knight O, Bebbington A, Siafarikas A, Woodhead H, Girdler S, Leonard H. Pubertal trajectory in females with Rett syndrome: a population-based study. Brain & development. 2013 Nov;35(10):912–920. doi: 10.1016/j.braindev.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Glaze DG, Percy AK, Skinner S, et al. Epilepsy and the natural history of Rett syndrome. Neurology. 2010 Mar 16;74(11):909–912. doi: 10.1212/WNL.0b013e3181d6b852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagberg B, Hanefeld F, Percy A, Skjeldal O. An update on clinically applicable diagnostic criteria in Rett syndrome. Comments to Rett Syndrome Clinical Criteria Consensus Panel Satellite to European Paediatric Neurology Society Meeting, Baden Baden, Germany, 11 September 2001. Eur J Paediatr Neurol. 2002;6(5):293–297. doi: 10.1053/ejpn.2002.0612. [DOI] [PubMed] [Google Scholar]
- 23.Christensen KY, Maisonet M, Rubin C, et al. Pubertal pathways in girls enrolled in a contemporary british cohort. International journal of pediatrics. 2010;2010:329261. doi: 10.1155/2010/329261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. 1992 Jul;11(10):1305–1319. doi: 10.1002/sim.4780111005. [DOI] [PubMed] [Google Scholar]
- 25.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000 Jun 8;(314):1–27. [PubMed] [Google Scholar]
- 26.Nathan BM, Palmert MR. Regulation and disorders of pubertal timing. Endocrinology and metabolism clinics of North America. 2005 Sep;34(3):617–641, ix. doi: 10.1016/j.ecl.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 27.Barlow SE, Expert C. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007 Dec;120(Suppl 4):S164–192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 28.Leonard H, Ravikumara M, Baikie G, et al. Assessment and Management of Nutrition and Growth in Rett Syndrome. Journal of pediatric gastroenterology and nutrition. 2013 Oct;57(4):451–460. doi: 10.1097/MPG.0b013e31829e0b65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neul JL, Fang P, Barrish J, et al. Specific mutations in methyl-CpG-binding protein 2 confer different severity in Rett syndrome. Neurology. 2008 Apr 15;70(16):1313–1321. doi: 10.1212/01.wnl.0000291011.54508.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cuddapah VA, Pillai RB, Shekar KV, et al. Methyl-CpG-binding protein 2 (MECP2) mutation type is associated with disease severity in Rett syndrome. J Med Genet. 2014 Mar;51(3):152–158. doi: 10.1136/jmedgenet-2013-102113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.IBM SPSS Statistics for Windows [computer program]. Version 21.0. IBM Corp; Armonk, NY: 2012. Released. [Google Scholar]
- 32.Bebbington A, Anderson A, Ravine D, et al. Investigating genotype-phenotype relationships in Rett syndrome using an international data set. Neurology. 2008 Mar 11;70(11):868–875. doi: 10.1212/01.wnl.0000304752.50773.ec. [DOI] [PubMed] [Google Scholar]
- 33.Bebbington A, Percy A, Christodoulou J, et al. Updating the profile of C-terminal MECP2 deletions in Rett syndrome. Journal of medical genetics. 2010 Apr;47(4):242–248. doi: 10.1136/jmg.2009.072553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knight O, Bebbington A, Siafarikas A, Woodhead H, Girdler S, Leonard H. Pubertal trajectory in females with Rett syndrome: A population-based study. Brain & development. 2012 Dec 24; doi: 10.1016/j.braindev.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Ben-Shachar S, Chahrour M, Thaller C, Shaw CA, Zoghbi HY. Mouse models of MeCP2 disorders share gene expression changes in the cerebellum and hypothalamus. Human molecular genetics. 2009 Jul 1;18(13):2431–2442. doi: 10.1093/hmg/ddp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Westberry JM, Trout AL, Wilson ME. Epigenetic regulation of estrogen receptor alpha gene expression in the mouse cortex during early postnatal development. Endocrinology. 2010 Feb;151(2):731–740. doi: 10.1210/en.2009-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocrine reviews. 2003 Oct;24(5):668–693. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- 38.McGill BE, Bundle SF, Yaylaoglu MB, Carson JP, Thaller C, Zoghbi HY. Enhanced anxiety and stress-induced corticosterone release are associated with increased Crh expression in a mouse model of Rett syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2006 Nov 28;103(48):18267–18272. doi: 10.1073/pnas.0608702103. [DOI] [PMC free article] [PubMed] [Google Scholar]