Abstract
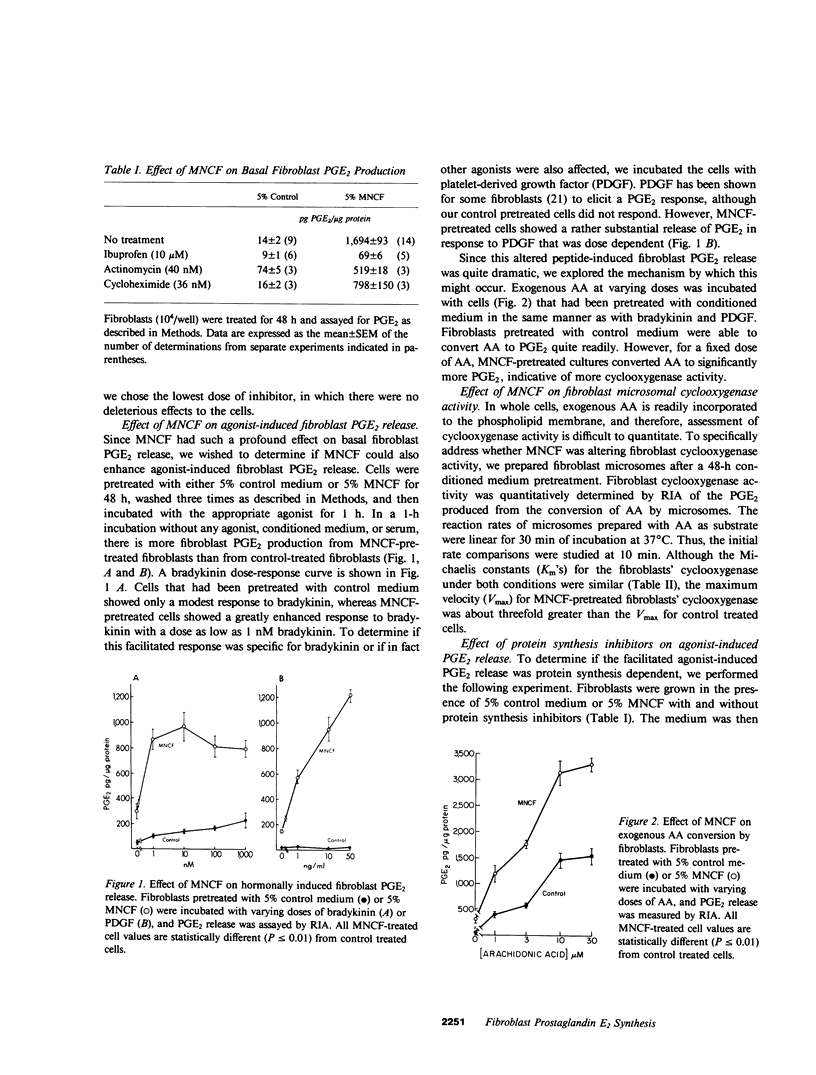
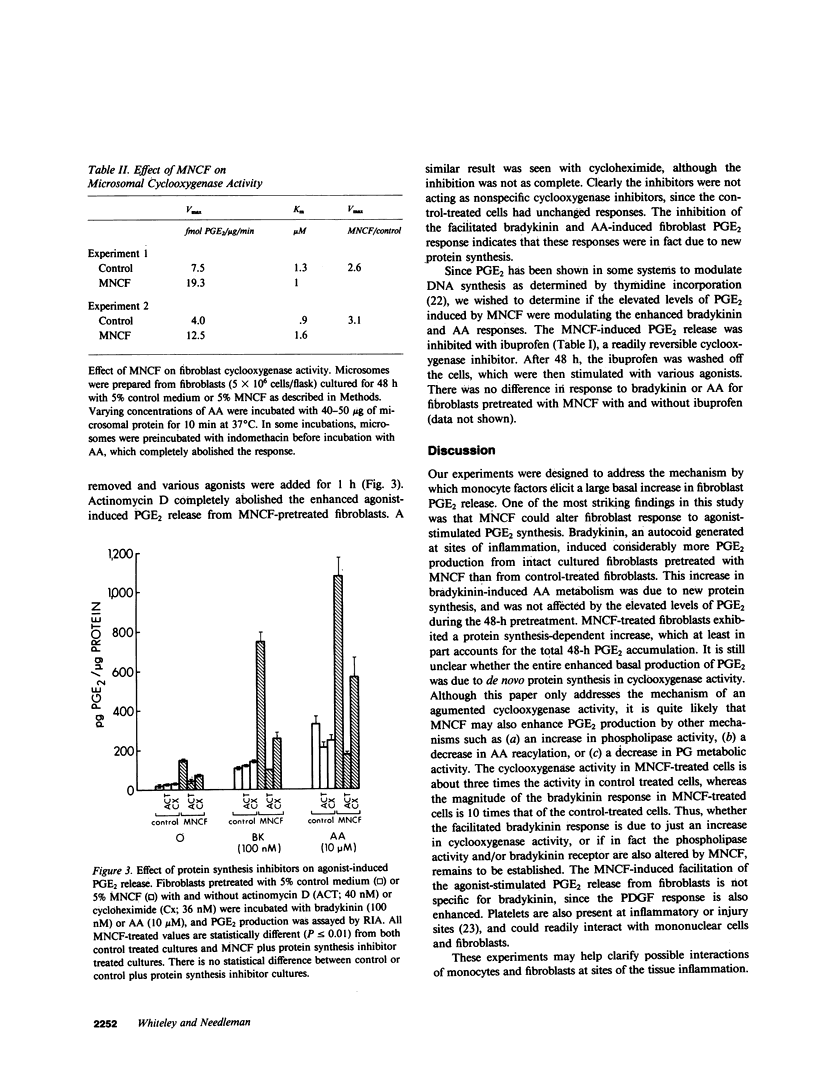
Chronic inflammation is associated with an infiltration of mononuclear cells, fibroblast proliferation, and elevated levels of prostaglandin (PG) E2. Mononuclear cell conditioned factor (MNCF) medium (5%) stimulated a 100-fold increase in basal human dermal fibroblast PGE2 release over 48 h as compared with fibroblasts that were incubated with control medium (conditioned medium prepared without cells). The MNCF-induced PGE2 production was suppressed by protein synthesis inhibitors. Fibroblasts pretreated with control medium released PGE2 only modestly in response to 1 nM bradykinin for 1 h (basal, 50 +/- 7 pg PGE2/micrograms protein; stimulated, 104 +/- 12 pg PGE2/micrograms protein), whereas cells that had been pretreated with MNCF showed a greatly facilitated bradykinin-induced release of PGE2. (basal, 297 +/- 59 pg PGE2/micrograms protein; stimulated, 866 +/- 85 pg PGE2/micrograms protein). The exaggerated agonist response is not specific for bradykinin because platelet-derived growth factor elicits a similar response. Exogenous arachidonic acid conversion to PGE2 was also facilitated (two- to threefold) by MNCF pretreatment as compared with control. Both the enhanced agonist-stimulated and exogenous arachidonic acid-induced PGE2 release from the MNCF pretreated cells were inhibited by actinomyin D or cycloheximide. A kinetic study of microsomal cyclooxygenase prepared from fibroblasts pretreated with MNCF showed a threefold increase in the maximum velocity (Vmax) but the same Michaelis constant (Km) as control-treated cells. This augmented arachidonic acid metabolism and subsequent enhanced PGE2 production may play an important role in macrophage-fibroblast interactions at sites of inflammation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barabé J., Marceau F., Thériault B., Drouin J. N., Regoli D. Cardiovascular actions of kinins in the rabbit. Can J Physiol Pharmacol. 1979 Jan;57(1):78–91. doi: 10.1139/y79-012. [DOI] [PubMed] [Google Scholar]
- Becherer P. R., Mertz L. F., Baenziger N. L. Regulation of prostaglandin synthesis mediated by thrombin and B2 bradykinin receptors in a fibrosarcoma cell line. Cell. 1982 Aug;30(1):243–251. doi: 10.1016/0092-8674(82)90030-7. [DOI] [PubMed] [Google Scholar]
- Clark J. G., Kostal K. M., Marino B. A. Bleomycin-induced pulmonary fibrosis in hamsters. An alveolar macrophage product increases fibroblast prostaglandin E2 and cyclic adenosine monophosphate and suppresses fibroblast proliferation and collagen production. J Clin Invest. 1983 Dec;72(6):2082–2091. doi: 10.1172/JCI111173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza S. M., Englis D. J., Clark A., Russell R. G. Stimulation of production of prostaglandin E in gingival cells exposed to products of human blood mononuclear cells. Biochem J. 1981 Aug 15;198(2):391–396. doi: 10.1042/bj1980391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Robinson D. R., Krane S. M. Prostaglandin production by rheumatoid synovial cells: stimulation by a factor from human mononuclear Cells. J Exp Med. 1977 May 1;145(5):1399–1404. doi: 10.1084/jem.145.5.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick W. C., Grennan D. M., Zeitlin I. J. Studies on the relative effects of prostaglandins, bradykinin, 5-hydroxytryptamine and histamine on the synovial microcirculation in dogs. Br J Pharmacol. 1976 Mar;56(3):313–316. doi: 10.1111/j.1476-5381.1976.tb07644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein L. P., Schneeberger E. E., Colten H. R. Synthesis of the second component of complement by long-term primary cultures of human monocytes. J Exp Med. 1976 Jan 1;143(1):114–126. doi: 10.1084/jem.143.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gery I., Waksman B. H. Potentiation of the T-lymphocyte response to mitogens. II. The cellular source of potentiating mediator(s). J Exp Med. 1972 Jul 1;136(1):143–155. doi: 10.1084/jem.136.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. S., Ceuppens J. Regulation of the immune response by prostaglandins. J Clin Immunol. 1983 Oct;3(4):295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- Grennan D. M., Mitchel W., Miller W., Zeitlin I. J. The effects of prostaglandin E1, bradykinin and histamine on canine synovial vascular permeability. Br J Pharmacol. 1977 Jun;60(2):251–254. doi: 10.1111/j.1476-5381.1977.tb07747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S. L., Levine L. Stimulation of prostaglandin synthesis by bradykinin and thrombin and their mechanisms of action on MC5-5 fibroblasts. J Biol Chem. 1976 Sep 25;251(18):5814–5816. [PubMed] [Google Scholar]
- Humes J. L., Bonney R. J., Pelus L., Dahlgren M. E., Sadowski S. J., Kuehl F. A., Jr, Davies P. Macrophages synthesis and release prostaglandins in response to inflammatory stimuli. Nature. 1977 Sep 8;269(5624):149–151. doi: 10.1038/269149a0. [DOI] [PubMed] [Google Scholar]
- Jonas P. E., Leahy K. M., DeSchryver-Kecskemeti K., Needleman P. Cellular interactions and exaggerated arachidonic acid metabolism in rabbit renal injury. J Leukoc Biol. 1984 Jan;35(1):55–64. doi: 10.1002/jlb.35.1.55. [DOI] [PubMed] [Google Scholar]
- Korn J. H., Halushka P. V., LeRoy E. C. Mononuclear cell modulation of connective tissue function: suppression of fibroblast growth by stimulation of endogenous prostaglandin production. J Clin Invest. 1980 Feb;65(2):543–554. doi: 10.1172/JCI109698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier H. L., Pierce J. V., Colman R. W., Kaplan A. P. Activation and function of human Hageman factor. The role of high molecular weight kininogen and prekallikrein. J Clin Invest. 1977 Jul;60(1):18–31. doi: 10.1172/JCI108754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Needleman P., Key S. L., Denny S. E., Isakson P. C., Marshall G. R. Mechanism and modification of bradykinin-induced coronary vasodilation. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2060–2063. doi: 10.1073/pnas.72.6.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okegawa T., Jonas P. E., DeSchryver K., Kawasaki A., Needleman P. Metabolic and cellular alterations underlying the exaggerated renal prostaglandin and thromboxane synthesis in ureter obstruction in rabbits. Inflammatory response involving fibroblasts and mononuclear cells. J Clin Invest. 1983 Jan;71(1):81–90. doi: 10.1172/JCI110754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto A. M., Nilsen-Hamilton M., Boss B. D., Ulrich M. O., Jimenez De Asua L. Prostaglandins E1 and E2 interact with prostaglandin F2alpha to regulate initiation of DNA replication and cell division in swiss 3T3 cells. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4992–4996. doi: 10.1073/pnas.79.16.4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reingold D. F., Watters K., Holmberg S., Needleman P. Differential biosynthesis of prostaglandins by hydronephrotic rabbit and cat kidneys. J Pharmacol Exp Ther. 1981 Mar;216(3):510–515. [PubMed] [Google Scholar]
- Rozengurt E., Stroobant P., Waterfield M. D., Deuel T. F., Keehan M. Platelet-derived growth factor elicits cyclic AMP accumulation in Swiss 3T3 cells: role of prostaglandin production. Cell. 1983 Aug;34(1):265–272. doi: 10.1016/0092-8674(83)90157-5. [DOI] [PubMed] [Google Scholar]
- Sheng W. Y., Lysz T. A., Wyche A., Needleman P. Kinetic comparison and regulation of the cascade of microsomal enzymes involved in renal arachidonate and endoperoxide metabolism. J Biol Chem. 1983 Feb 25;258(4):2188–2192. [PubMed] [Google Scholar]


