Abstract
Background and Purpose
Stereotactic body radiotherapy (SBRT) to central lung tumors can cause esophageal toxicity, but little is known about the incidence or risk factors. We reviewed central lung SBRT patients to identify dosimetric factors predictive of esophageal toxicity.
Material and Methods
We assessed esophageal toxicity in 125 SBRT patients. Using biological equivalent doses with α/β=10 Gy (BED10), dose-volume histogram variables for the esophagus (Dv and Vd) were assessed for correlation with grade ≥2 acute toxicity.
Results
Incidence of grade ≥2 acute toxicity was 12% (n=15). Highly significant logistic models were generated for D5cc and Dmax (p<0.001). To keep the complication rate < 20%, the model requires that D5cc ≤ 26.3 BED10. At 2 years, the probability of complication with BED10 D5cc > 14.4 Gy was 24%, compared to 1.6% if ≤14.4 Gy.
Conclusions
This novel analysis provides guidelines to predict acute esophageal toxicity in lung SBRT. Dose to the hottest 5cc and Dmax of the esophagus were the best predictors of toxicity. Converting the BED10 limits to physical doses, D5cc to the esophagus should be kept less than 16.8, 18.1 and 19.0 Gy for 3, 4, and 5 fractions, respectively, to keep the acute toxicity rate < 20%.
Keywords: Hypofractionated, esophagitis, esophagus, SBRT, lung, central
INTRODUCTION
Stereotactic body radiotherapy (SBRT) has revolutionized the non-operative management of early-stage non-small cell lung cancer (NSCLC) due to its excellent local control, particularly compared to conventionally fractionated radiation therapy.[1] Lung SBRT has been associated with relatively modest rates of significant toxicity.[1] However, seminal work by Timmerman et al. revealed disproportionately and unacceptably high rates of severe pulmonary toxicity when delivering high-dose-per-fraction SBRT to tumors near the proximal bronchial tree.[2] As a result, subsequent trials of lung SBRT have generally excluded tumors in this location.
A multicenter, phase I/II dose-escalation trial of SBRT for central lung tumors has recently completed accrual, but results are not yet available.[3] Until then, many centers including ours have opted to treat carefully selected patients with central lung tumors using more conservative fractionation schemes, with fraction sizes on the order of 6–12Gy instead of 18–20Gy. Retrospective reports have indicated acceptably low rates of severe pulmonary toxicity with such risk-adapted schemes.[4–8] However, SBRT in this anatomic region often also results in high dose to other critical structures besides the lungs, notably the heart and the esophagus.
Esophageal toxicity, including esophagitis, stricture or perforation, is a well-known complication of radiotherapy involving the mediastinum, such as for NSCLC or esophageal cancer. Dose guidelines to predict and minimize the risk of esophageal toxicity are available for conventional RT.[9] However, these guidelines cannot be readily extrapolated to SBRT, because the relationship between fraction size and esophageal toxicity is largely unknown. Furthermore, whereas mean dose to the whole esophagus is commonly used to evaluate risk of toxicity in conventional RT, SBRT is associated with much smaller target and esophageal volumes and therefore it is less likely that a mean dose constraint would be clinically robust. Although ongoing SBRT trials stipulate dosimetric guidelines for esophageal dose[3], firm data to justify these guidelines do not yet exist.
Our institution has extensive experience treating lung tumors in the central lung zone with SBRT. We therefore reviewed our experience with the aim of characterizing the nature and incidence of esophageal toxicity. In addition, we undertook a quantitative dosimetric analysis with the specific aim of identifying dosimetric parameters that may predict esophageal toxicity.
MATERIALS AND METHODS
Patient Selection
Institutional review and privacy boards approved this study, and patient confidentiality was maintained as required by the Health Insurance Portability and Accountability Act. Institutional databases were queried to identify all patients receiving SBRT to tumors within the lung, including metastases as well as primary NSCLC. SBRT was defined as fraction size of 600cGy or greater and delivered in five fractions or fewer, using linear accelerators with on-board CT guidance. Patients who had received prior radiotherapy to the thorax were excluded, as were patients receiving synchronous RT to two or more lesions within the lung. Radiation treatment plans were reviewed to identify patients with central lung tumors, as defined by one of the following two criteria: 1. Tumor within 2cm of the proximal bronchial tree (the definition utilized in the RTOG 0236 trial, also known as the “no-fly-zone”), or 2. Planning target volume (PTV) intersecting mediastinal structures (the definition used in the RTOG 0813 trial).
Radiation Technique
Our SBRT technique has been previously described.[10] Typically, patients underwent simulation with custom immobilization using an Alpha Cradle (Smithers Medical Products, North Canton, OH). A 2mm reconstructed CT slice thickness was used, as well as a four-dimensional CT (4DCT) scan to characterize the degree of respiratory motion. The tumor was contoured on all respiratory phases to generate an internal target volume (ITV). This was then expanded by 2–3mm to account for subclinical spread and generate a clinical target volume (CTV). The CTV was uniformly expanded by 5mm in all directions to generate a PTV. An IMRT plan was generated using custom in-house treatment planning software, and dose was prescribed to the 100% isodose line (IDL). PTV coverage was kept as homogeneous as possible, with tolerance of a hotspot up to 110% of the prescription dose. Per our institutional guidelines, the maximum point dose to the esophagus was to be kept ≤30Gy, unless the PTV overlapped with esophagus, in which case up to 45Gy in 5 fractions was allowed. Four to 7 co-planar 6MV beams were typically used to deliver an IMRT plan prescribed to the 100% IDL covering the PTV. Cone-beam CT guidance was used at each fraction to ensure accurate patient setup. Patients were treated every other weekday. Patients were followed up one month after completion of SBRT, then every three months for the first two years and every 6 to 12 months thereafter.
A wide variety of fractionation schemes were prescribed, at the discretion of the treating physician (see Table 1). Most commonly, patients who had been identified by the treating physician as having high-risk tumors due to central location were treated in five fractions of 9 or 10Gy each, which is our current institutional practice. In other cases, higher doses per fraction and 3 or 4-fraction schemes were utilized, typically because the tumor was not considered “central” by the treating physician. Less aggressive fractionation schemes (e.g. 3000cGy in 5 fractions) were also sometimes employed based on the clinical scenario, or in some cases because treatment was delivered at a time when institutional guidelines for SBRT dose had not yet been implemented.
Table 1.
Patient and Treatment Characteristics (N=125)
| Characteristic | No. of Patients (%) |
|---|---|
| Disease | |
| Primary NSCLC | 91 |
| Recurrent NSCLC | 12 |
| Lung Metastasis | 22 |
| Median age at diagnosis, years (range) | 76 (32–95) |
| Sex | |
| Male | 62 |
| Female | 63 |
| Dose | |
| 60 Gy in 3 fx (BED10= 180) | 4 |
| 54 Gy in 3 fx (BED10= 151.2) | 9 |
| 48 Gy in 4 fx (BED10= 105.6) | 21 |
| 36 Gy in 2 fx (BED10= 100.8) | 1 |
| 50 Gy in 5 fx (BED10= 100) | 14 |
| 44 Gy in 4 fx (BED10= 92.4) | 1 |
| 45 Gy in 5 fx (BED10= 85.5) | 56 |
| 40 Gy in 4 fx (BED10= 80) | 2 |
| 36 Gy in 3 fx (BED10= 79.2) | 1 |
| 40 Gy in 5 fx (BED10= 72) | 6 |
| 30 Gy in 5 fx (BED10= 48) | 7 |
| Other | 3 |
| Median PTV size, cm3 (range) | 63.0 (17.3–401.7) |
| Median GTV size, cm3 (range) | 13.1 (0.6–195.4) |
Abbreviations: BED10, Biologically equivalent dose for α/β = 10; PTV, planning treatment volume; GTV, gross tumor volume; Gy, gray; fx, fraction.
Dosimetric Analysis
The primary endpoint was grade 2 or greater esophageal toxicity (E2), as defined by the Common Terminology Criteria for Adverse Events, version 4.0. We included all events occurring during RT or within 120 days of its completion. Only one instance of E2 occurred outside of this timeframe and was significantly later (371 days after RT), therefore we limited our dosimetric analysis to acute and subacute events only (E2a). Esophageal contours were reviewed in all patients and where necessary, revised to ensure that the outer wall of the entire organ was contoured, starting from the cricoid cartilage and extending to the gastroesophageal junction. The proximal bronchial tree and no-fly-zone (NFZ) were also contoured according to RTOG 0236 guidelines in all patients, and treatment plans reviewed to identify all patients with lung tumors inside the NFZ. As noted above, tumors outside the NFZ, but with the PTV abutting mediastinal structures, were included in the analysis.
Due to the wide range of fractionation schemes used, doses were converted into biological equivalent doses , using α/β=10 Gy (BED10) since the analyzed esophageal events were acute. However, to validate this choice of α/β and to check the dependence of α/β in our results, the analysis was repeated for α/β values between 0.1 and 30 Gy, in steps of 0.1 Gy.
Two primary dose-volume variables were assessed for their correlation to the primary endpoint: Dv, in which D is the minimum dose to the hottest absolute esophageal volume v; and Vd, in which V is the absolute esophageal volume exposed to at least the dose d. These variables were calculated from each patient DVH, and correlation with toxicity was assessed using logistic regression and Cox proportional hazards modeling. Models were constructed for Dv with 0<v<180cc in steps of 1cc, and for Vd with 0<d<75 BED10. Based on the variables that were determined to be significant, log-rank tests were then performed using the median splits for each variable.
In view of the controversy in applicability of the linear-quadratic (LQ) model to treatment regimens using doses per fraction > 10Gy, we also examined Cox models based on Dv using physical dose, and multivariate Cox models based on Dv (physical dose) and fraction number. These were compared with the models based on Dv (BED10) using the Akaike Information Criterion (AIC).
For the purposes of future data synthesis[11, 12], dose-volume atlases of the incidence of E2a [13, 14], based on physical dose to the esophagus, are provided in a Microsoft Excel file in electronic Appendix A1 for each number of fractions separately. The format of this file is described in electronic Appendix A2.
RESULTS
The median follow-up for the entire cohort of 125 patients was 14.3 months from the completion of SBRT. The median prescription dose was 45Gy in 5 fractions, corresponding to a BED10 of 85.5 Gy. The overall rate of E2a was 12% (n=15). No patients had baseline esophageal toxicity. There were two grade 3 events; one was an upper gastrointestinal bleed attributed to SBRT, and the second was an esophageal fistula requiring hospitalization and stent placement. No grade 4 or 5 events occurred.
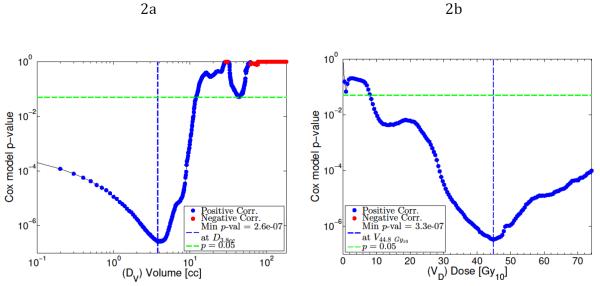
Cox proportional hazards modeling for Dv showed significant correlation with E2a for values of v below 12cc, with the highest statistical significance at v=3.8cc (p<0.0001, see Fig. 1a). Cox models of using Vd showed significant correlation with E2a for values of d above 8.1 BED10, with the greatest statistical significance at d=44.8 BED10 (See Fig. 1b). A combined Cox model using D3.8cc and V44.8 bed10 was generated but not found to be statistically significant, due to the high degree of correlation between the two variables. To facilitate clinical applicability, the variables Dmax and D5cc were selected for logistic modeling and logrank tests.
Figure 1.
P-values from Cox proportional hazards model of grade ≥ 2 acute esophageal toxicity fit as a function of Dv (a) and Vd (b), shown by blue or red dots (denoting correlation or anti-correlation respectively). Minimum p-values are marked by vertical blue dashed lines, p = 0.05 is marked by the horizontal green lines. Most significant model using Dv occurs when v = 3.8cc. Most significant model using Vd occurs when d = 44.8 Gy10.
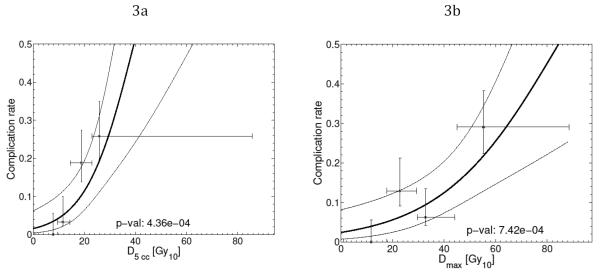
The fitted logistic regression response curves, shown in Figure 2, suggest that values of Dmax ≤52.9 BED10, and D5cc ≤ 26.3 BED10, result in predicted probabilities of complication less than 20%. Converting the BED10 limits to physical doses using common lung SBRT fractionation schemes results in D5cc limits of 16.8Gy, 18.1Gy, and 19.0Gy for treatment regimens of 3, 4 and 5 fractions respectively. For Dmax, this corresponds to respective physical dose limits of 27.6Gy, 30.2Gy, and 32.2Gy.
Figure 2.
Fitted logistic regression response functions for grade ≥ 2 acute esophageal toxicity models (bold line), based on D5cc (a) and Dmax (b). Dose variables are BED10 values. 95% confidence intervals on the response rates are shown by the thin lines. For comparison, observed complication rates for quartiles in the respective dose variables are plotted as points at the quartile median dose value with associated ranges (horizontal bars) together with their 68% confidence intervals (vertical bars).
Best fit model parameters and their uncertainties for the D5cc and Dmax (BED10) Cox and logistic regression models are given in Table 2.
Table 2.
Best fit model parameters, their uncertainties, standard errors (SE) and p-values for the D5cc and Dmax (BED10) Cox and logistic regression models of ≥ grade 2 esophageal complications.
| Model (BED10) | Coefficient [68% CI] | SE | p-value |
|---|---|---|---|
| Cox PH Models: | |||
| D5.0cc | 0.082 [0.065–0.098] | 0.016 | 4.7 × 10−7 |
| Dmax | 0.036 [0.023–0.046] | 0.010 | 5.5×10−4x |
| Logistic Regression Models: | |||
| D5.0cc | 0.106 [0.076–0.135] | 0.030 | 4.3 × 10−4 |
| Dmax | 0.044 [0.031−0.057] | 0.013 | 7.4×10−4 |
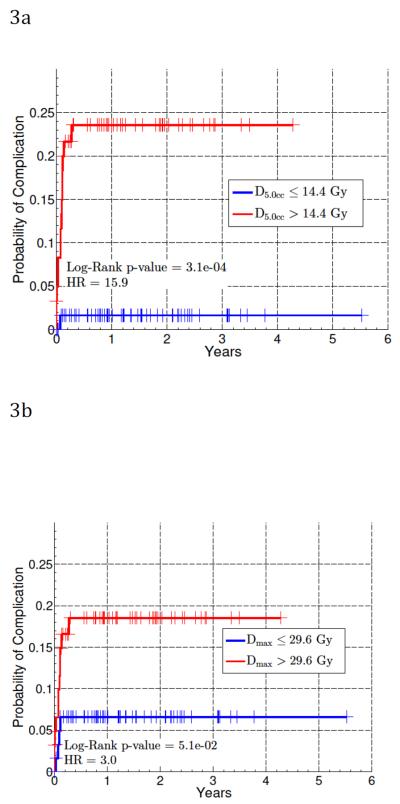
Logrank tests were then performed using the median splits for D5cc and Dmax, shown in Figure 3. These were significant for all three Dv values. At two years, the probability of complication for those with D5cc > 14.4 BED10 was 24% (p<0.001), and for those with Dmax > 29.6 BED10 was 21% (p=0.051). The probability of complication for those with a D5cc, and Dmax (BED10) less than or equal to the above limits were 2% and 7%, respectively.
Figure 3.
Kaplan-Meier cumulative incidence plots for grade ≥ 2 acute esophageal toxicity with cohort split at median of D5cc (a) and Dmax (b). Doses are BED10 values. The number of patients at risk after 1, 2 and 3 years are 54, 28, and 11, respectively. Log-rank p-value and Cox model hazard ratios are also shown.
Based on maximum log-likelihood fit, a Cox model based on Dv with v=5 cc was the best Dv model (using either physical dose or BED10). The D5cc model based on BED10 was a better model than that based on the physical D5cc. Based on AIC, it was also superior to the multivariate model based on physical D5cc and fraction number.
Due to the uncertainty of the appropriate α/β value for the esophagus, a range of α/β values (0.1≤ α/β ≤30 in α/β increments of 0.1) were used to calculate D5cc and Dmax, and to test logistic regression models. The model fits for these three variables remained significant across the range of α/β tested, ensuring the analysis is insensitive to the choice of α/β.
DISCUSSION
Esophageal toxicity is a significant complication of conventionally fractionated radiotherapy to the thorax. Various metrics to predict and prevent esophagitis in this context have been reported, such as the mean esophageal dose. More recently, a number of detailed analyses suggest that the volume of esophagus exposed to higher doses of radiation is the most meaningful metric, in particular, values of Vd ranging from V40Gy to V60Gy.[9, 15] Previous analysis from our institution identified predictors of late esophageal toxicity in the setting of single-fraction SBRT to paraspinal targets.[14] However, there is little data to derive constraints for esophageal dose in the setting of multi-fraction lung SBRT. Severe esophageal toxicities after lung SBRT have been described, including one fatal complication, but due to the rarity of these cases they provide little concrete guidance to the clinician considering SBRT to lung tumors near the esophagus.[16, 17]
Because of the uncertainty regarding the effect of fractionation on esophageal tolerance, and the smaller treatment volumes involved in hypofractionated therapy, existing dosimetric constraints cannot be readily extrapolated to the hypofractionated setting. However, with increasing application of SBRT in the central lung zone, it will become increasingly important to define and prevent esophageal complications from SBRT.
The application of the LQ model to SBRT with fraction sizes of 9–20 Gy is controversial.[18–21] Should some other model be shown to have superior validity, a similar analysis of this and future data would have to be done. For this purpose, the data in the atlas provided in the appendix is separated by fraction number. However, we note that threshold doses for models derived here are at physical doses of 17–19 Gy with doses per fraction of approximately 4–6 Gy, where the LQ model is routinely used. We also note that our D5cc guidelines essentially confirm the constraints that were previously suggested by the American Association of Physicists in Medicine (AAPM) task group.[22]
To our knowledge, there is only one published series focusing on esophageal toxicity after lung SBRT.[23] This was a smaller series and included patients receiving SBRT as a boost to conventionally fractionated therapy, and the authors only analyzed a limited number of predetermined Dv endpoints. Another series of central lung SBRT included a subset of 15 patients with tumors near the esophagus treated with 6 fractions of 8Gy and observed an overall 11% rate of acute Grade 1–2 esophagitis.[8] The current analysis represents the first systematic, quantitative attempt to identify dosimetric predictors of clinically significant esophageal toxicity after hypofractionated SBRT for lung tumors. We found that doses to small volumes of esophagus were the most predictive of acute esophageal toxicity, with the best-fitting model using Dv with a v value of approximately 5cc, but strong correlation was also seen for a range of low volumes below 12cc. Logrank tests indicated that these metrics had significant ability to discriminate patients at high vs. low risk for acute esophageal complication, with Dv values above the median splits associated with complication rates in excess of 20%, while Dv values below the median splits were associated with complication rates well under 10%. In the case of D5cc, esophageal doses above the median split resulted in complication probability nearly 16 times that of doses below the median split.
Because of the wide range of fraction numbers and sizes used in lung SBRT, our analysis was performed converting all doses to BEDs. Given that analyzed events were acute or subacute, we reported our results using an assumption of α/β=10Gy, but the selected metrics retained validity regardless of the α/β value over the range tested. Converting the BED values to physical doses for each fractionation scheme resulted in suggested Dv limits that may be useful in guiding treatment planning and counseling patients regarding their risk of acute esophageal complications.
It should be noted that this study, while novel, has significant limitations. We included any toxicity of Grade 2 or greater in our analysis, but Grade 2 events do not result in prolonged serious injury or hospitalization for the patient. Dosimetric predictors of grade ≥3 toxicity would have great clinical value, but this was not feasible in this dataset given the paucity of grade 3 events. Given that lung SBRT is typically indicated for early-stage NSCLC where there is significant curative potential, it may not be clinically appropriate to significantly compromise SBRT dose or plan quality in order to avert a likely Grade 2, self-resolving esophagitis. In the context of SBRT regimens prescribing a physical dose on the order of 50Gy to the PTV, these Dv limits of approximately 20Gy would require that the 40% isodose line be kept outside of the esophagus entirely, which would not be possible if the PTV abuts or overlaps the esophagus itself.
However, in situations where the PTV is near but not abutting the esophagus, careful plan optimization may allow for PTV coverage to be maintained while still meeting, or approaching, these constraints. In cases where anatomic proximity of the PTV to the esophagus does not allow these constraints to be met, these guidelines will still be useful to counsel patients regarding their elevated risk of esophageal toxicity, and to alert the clinical team to the likely development of such complications after treatment.
Based on a large cohort of patients receiving SBRT to central lung tumors, this novel analysis identified dosimetric factors predictive of clinically significant acute esophageal toxicity. Dose to small volumes of esophagus, particularly the D5cc, correlated best with the development of acute esophageal complications. To keep the acute esophageal complication rate < 20%, D5cc should be < 16.8, 18.1 and 19.0 Gy for treatments delivered in 3, 4, and 5 fractions respectively. This and other quantitative guidelines derived from this data can aid in treatment planning and counseling patients regarding their risk of this important toxicity.
Supplementary Material
Acknowledgements
Andrew Jackson and Eric Williams are supported by NIH grant R01 CA129182. The NIH had no role in the study design; data collection, analysis, or interpretation; nor the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: Andreas Rimner has served as a consultant for Varian Medical Systems and General Electric.
REFERENCES
- [1].Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–6. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Timmerman R, McGarry R, Yiannoutsos C, Papiez L, Tudor K, DeLuca J, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:4833–9. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- [3].Group RTO . Seamless phase I/II study of stereotactic lung radiotherapy for early stage, centrally located non-small cell lung cancer in medically inoperable patients. [Google Scholar]
- [4].Chang JY, Balter PA, Dong L, Yang Q, Liao Z, Jeter M, et al. Stereotactic body radiation therapy in centrally and superiorly located stage I or isolated recurrent non-small-cell lung cancer. International journal of radiation oncology, biology, physics. 2008;72:967–71. doi: 10.1016/j.ijrobp.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rowe BP, Boffa DJ, Wilson LD, Kim AW, Detterbeck FC, Decker RH. Stereotactic body radiotherapy for central lung tumors. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2012;7:1394–9. doi: 10.1097/JTO.0b013e3182614bf3. [DOI] [PubMed] [Google Scholar]
- [6].Senthi S, Haasbeek CJ, Slotman BJ, Senan S. Outcomes of stereotactic ablative radiotherapy for central lung tumours: a systematic review. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2013;106:276–82. doi: 10.1016/j.radonc.2013.01.004. [DOI] [PubMed] [Google Scholar]
- [7].Haasbeek CJ, Lagerwaard FJ, Slotman BJ, Senan S. Outcomes of stereotactic ablative radiotherapy for centrally located early-stage lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2011;6:2036–43. doi: 10.1097/JTO.0b013e31822e71d8. [DOI] [PubMed] [Google Scholar]
- [8].Nuyttens JJ, van der Voort van Zyp NC, Praag J, Aluwini S, van Klaveren RJ, Verhoef C, et al. Outcome of four-dimensional stereotactic radiotherapy for centrally located lung tumors. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2012;102:383–7. doi: 10.1016/j.radonc.2011.12.023. [DOI] [PubMed] [Google Scholar]
- [9].Werner-Wasik M, Yorke E, Deasy J, Nam J, Marks LB. Radiation dose-volume effects in the esophagus. International journal of radiation oncology, biology, physics. 2010;76:S86–93. doi: 10.1016/j.ijrobp.2009.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cuaron JJ, Yorke ED, Foster A, Hsu M, Zhang Z, Liu F, et al. Stereotactic body radiation therapy for primary lung cancers >3 centimeters. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2013;8:1396–401. doi: 10.1097/JTO.0b013e3182a47181. [DOI] [PubMed] [Google Scholar]
- [11].Deasy JO, Bentzen SM, Jackson A, Ten Haken RK, Yorke ED, Constine LS, et al. Improving normal tissue complication probability models: the need to adopt a “data-pooling” culture. International journal of radiation oncology, biology, physics. 2010;76:S151–4. doi: 10.1016/j.ijrobp.2009.06.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jackson A, Marks LB, Bentzen SM, Eisbruch A, Yorke ED, Ten Haken RK, et al. The lessons of QUANTEC: recommendations for reporting and gathering data on dose-volume dependencies of treatment outcome. International journal of radiation oncology, biology, physics. 2010;76:S155–60. doi: 10.1016/j.ijrobp.2009.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jackson A, Yorke ED, Rosenzweig KE. The atlas of complication incidence: a proposal for a new standard for reporting the results of radiotherapy protocols. Semin Radiat Oncol. 2006;16:260–8. doi: 10.1016/j.semradonc.2006.04.009. [DOI] [PubMed] [Google Scholar]
- [14].Cox BW, Jackson A, Hunt M, Bilsky M, Yamada Y. Esophageal toxicity from high-dose, single-fraction paraspinal stereotactic radiosurgery. International journal of radiation oncology, biology, physics. 2012;83:e661–7. doi: 10.1016/j.ijrobp.2012.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Palma DA, Senan S, Oberije C, Belderbos J, de Dios NR, Bradley JD, et al. Predicting esophagitis after chemoradiation therapy for non-small cell lung cancer: an individual patient data meta-analysis. International journal of radiation oncology, biology, physics. 2013;87:690–6. doi: 10.1016/j.ijrobp.2013.07.029. [DOI] [PubMed] [Google Scholar]
- [16].Le QT, Loo BW, Ho A, Cotrutz C, Koong AC, Wakelee H, et al. Results of a phase I dose-escalation study using single-fraction stereotactic radiotherapy for lung tumors. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2006;1:802–9. [PubMed] [Google Scholar]
- [17].Onimaru R, Shirato H, Shimizu S, Kitamura K, Xu B, Fukumoto S, et al. Tolerance of organs at risk in small-volume, hypofractionated, image-guided radiotherapy for primary and metastatic lung cancers. International journal of radiation oncology, biology, physics. 2003;56:126–35. doi: 10.1016/s0360-3016(03)00095-6. [DOI] [PubMed] [Google Scholar]
- [18].Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: are more than the 5 Rs involved? International journal of radiation oncology, biology, physics. 2014;88:254–62. doi: 10.1016/j.ijrobp.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Park C, Papiez L, Zhang S, Story M, Timmerman RD. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. International journal of radiation oncology, biology, physics. 2008;70:847–52. doi: 10.1016/j.ijrobp.2007.10.059. [DOI] [PubMed] [Google Scholar]
- [20].Guckenberger M, Klement RJ, Allgauer M, Appold S, Dieckmann K, Ernst I, et al. Applicability of the linear-quadratic formalism for modeling local tumor control probability in high dose per fraction stereotactic body radiotherapy for early stage non-small cell lung cancer. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2013;109:13–20. doi: 10.1016/j.radonc.2013.09.005. [DOI] [PubMed] [Google Scholar]
- [21].Scheenstra AE, Rossi MM, Belderbos JS, Damen EM, Lebesque JV, Sonke JJ. Alpha/beta ratio for normal lung tissue as estimated from lung cancer patients treated with stereotactic body and conventionally fractionated radiation therapy. International journal of radiation oncology, biology, physics. 2014;88:224–8. doi: 10.1016/j.ijrobp.2013.10.015. [DOI] [PubMed] [Google Scholar]
- [22].Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, et al. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Medical Physics. 2010;37:4078. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- [23].Abelson JA, Murphy JD, Loo BW, Jr., Chang DT, Daly ME, Wiegner EA, et al. Esophageal tolerance to high-dose stereotactic ablative radiotherapy. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus / ISDE. 2012;25:623–9. doi: 10.1111/j.1442-2050.2011.01295.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.