Abstract
Hepatocytes, the main epithelial cell type of the liver, function like all epithelial cells to mediate the vectorial flow of macromolecules into and out of the organ they encompass. They do so by establishing polarized surface domains and by restricting paracellular flow via their tight junctions and cell-cell adhesion. Yet, the cell and tissue organization of hepatocytes differs profoundly from that of most other epithelia, including those of the digestive and urinary tracts, the lung or the breast. The latter form monolayered tissues in which the apical domains of individual cells align around a central continuous luminal cavity that constitutes the tubules and acini characteristic of these organs. Hepatocytes, by contrast, form capillary-sized lumina with multiple neighbors resulting in a branched, tree-like bile canaliculi network that spreads across the liver parenchyme. I will discuss some of the key molecular features that distinguish the hepatocyte polarity phenotype from that of monopolar, columnar epithelia.
Keywords: hepatocyte polarity, ECM-signaling, tissue architecture, polarized protein trafficking
The hepatocytic building plan
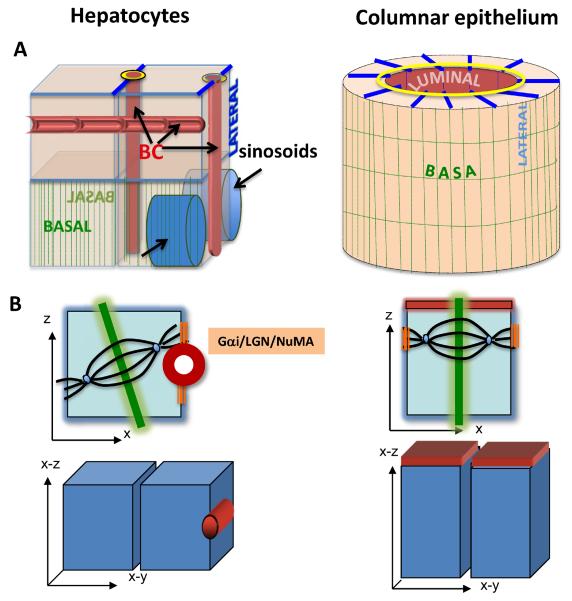
Hepatocytes constitute the parenchyme of the liver, our largest metabolic organ. They are engaged in two counter-current flow systems – the synthesis and secretion of bile, and the uptake, processing, and secretion of sinusoidal blood components, including components of the bile itself that return to hepatocytes via the portal venous blood. To accommodate such intense macromolecular exchange hepatocytes exhibit a polarity phenotype that is unique among vertebrate epithelia. The familiar monolayered epithelia that line the majority of our organs feature columnar cells with a single luminal domain opposite a single basal surface and flanked by the lateral domain at cell-cell contact sites. Hepatocytes, by contrast, organize in one- or two- cell thick plates with multiple luminal and basal domains. Hepatocyte luminal surfaces are not set perpendicular to their lateral surface as in monopolar epithelia, but their narrow luminal surfaces interrupt the lateral domain between neighboring cells (see Figure 1A). The capillary-sized hepatocyte lumina form an interconnected network, the bile canalicular network that empties into the common hepatic duct and transports bile acids to be used as digestive aid in the duodenum. When in a two-cell layer, a hepatocyte may form bile canaliculi with three of its neighbors as seen in Figure 1A (BC arrows); when part of a one-cell thick plate, a hepatocytic cell has at least two basal surfaces (green striped surfaces in Figure 1A), called sinusoidal membranes because they are in intimate contact with sinusoidal blood vessels that have a fenestrated, discontinuous endothelium. Of further importance, while most epithelia deposit a basal lamina, hepatocytes don't assemble extra cellular matrix (ECM)-molecules into a proper dense matrix, because laminin, an obligate basement membrane component and nidogen, a matrix crosslinker, are absent from the ECM surrounding mature hepatocytes (1). The presence of multiple sinusoidal surfaces on a single cell and the lack of a dense basement membrane might have evolved to maximize the bidirectional exchange of macromolecules between the hepatocytes and the blood. In fact, basement membrane deposition in the hepatic sinusoids, as it is observed in liver cirrhosis, disrupts tissue organization and results in impaired hepatocyte function. As we will see below, emerging in vitro evidence suggests that the unique hepatocyte polarity phenotype might even be contingent on the lack of a bona fide basement membrane.
Figure 1. The hepatocytic and columnar epithelial phenotypes.
A) Hepatocytes form branched bile canaliculi (BC arrows, red domains) that interrupt their cell-cell contacting domains (LATERAL, blue domains) and are surrounded by a tight junction belt (yellow). A single hepatocyte can form lumina with three neighbors (BC arrows). Hepatocytes can also have two basal domains (BASAL, green stripped domains) that face the adjacent sinusoids (blue capillaries with black arrows). Columnar epithelia feature a central lumen (LUMINAL, red) formed by the apical domains of individual cells, which are perpendicular to their cell-cell contacting domains (LATERAL, blue lines) and separated from the latter by tight junctions (yellow). The basal domains (BASAL, green stripped domains) are in contact with a basal lamina. B) In dividing rat hepatoma cells (HepG2) one set of astral spindle microtubules anchors via the attachment complex of G i/LGN/NuMA (orange) adjacent to the luminal domain (red), while the other astral microtubule fan faces the opposite surface domain. The resulting cleavage furrow (green) will execute an asymmetric division. In dividing kidney-derived MDCK cells the sub-apical G i/LGN/NuMA patches align the spindle parallel to the substratum, resulting in symmetric divisions.
Although few systematic morphological studies on hepatocyte polarization have been conducted, it has been reported that during rat embryogenesis hepatocytes initially cluster to form central lumen-sharing acini akin to the acini formed by monopolar epithelia, before they acquire their characteristic polarity phenotype, which is fully established only after birth (2). A re-organization of hepatocytes from acini into plates is also observed during liver regeneration after partial hepatectomy. Conversely, it has been suggested that formation of hepatocytic acini is an early sign of transformation during progression to hepatocellular carcinoma (3). Thus, re-polarization from columnar or cuboidal to hepatocytic polarity might constitute an aspect of the hepatocyte differentiation program that can be recalled in the adult liver after injury and can be reversed in cancer. The capability of liver cells to switch between monopolar and hepatocytic polarity phenotypes is further suggested by liver regeneration studies that have shown that hepatocytes can give rise to biliary cells, which form the bile duct and are of columnar polarity (4) and vice versa (5, 6).
WIFB cells, a hybrid cell line obtained by fusion of non-polarized rat hepatic Fao cells with human fibroblasts and one of the few hepatocytic cell lines that develop polarized surface domains, mimic the two-step process proposed for the developing liver: Upon plating at low confluency, they initially adopt simple columnar polarity. Then, over a two-week period, columnar WIFB cells first lose their luminal domains to become non-polarized and proliferate before they subsequently re-polarize with hepatocytic polarity (7).
Tissue organization is critically dependent on the mechanism of cell division. Columnar epithelia align their mitotic spindle parallel to their apical and basal domains so that the cleavage furrow, which forms perpendicular to the spindle axis, bisects the luminal domain, resulting in symmetric cell divisions in which both daughters remain in the plane of the monlayer (Figure 1B). In hepatocytes, such mode of division would cause their organization in acini and abrogate the canalicular network. Mature hepatocytes, although largely non-dividing, re-enter the cell cycle and proliferate after injury, such as partial hepatectomy. Observations of the abundant mitotic profiles that can be found in sections of such regenerating livers indicated that the hepatocyte cleavage furrow rarely bifurcated their bile canalicular domains, instead distributing individual bile canalicular domains between the daughters (8). WIFB cells and the polarized rat hepatoma line HepG2 mimic hepatocytes in this respect (9, 10). These cultures mostly feature only a single luminal domain per cell, which is distributed asymmetrically to only one of the daughters during cell divisions. As in columnar epithelia, mitotic spindle alignment is driven by the capture of astral microtubules by cortical dynein that in metaphase is anchored via an evolutionary conserved complex of G i/LGN/NuMA to the lateral membrane, coinciding with the location of adherens junctions. An x-z view of columnar metaphase cells shows the astral microtubule anchoring sites at equi-distance from the basement membrane and on opposite lateral domains (Figure 1B). By contrast, the lumen architecture of WIFB and HepG2 cells likely precludes the spindle from “curling around” the lumen to attach to both its adjacent anchoring domains. Instead, the subluminal LGN/NuMA patch anchors only one of the two astral microtubule fans, with the other facing the opposite basolateral surface. This spindle orientation, in which one spindle pole always faces the luminal domain and the other away from it, results in the observed asymmetric divisions. Hence, despite employing the same machinery, the different lumen architecture and positioning in columnar and hepatocytic cells results in different outcomes of their cell divisions. It should be added that bifurcation of the luminal domain during division has recently been reported for a hepatocytic cell line in which neighboring cells aligned their luminal surfaces to form tubular structures that resemble the bile canalicular tree seen in hepatocyte tissue (11). Such symmetric divisions could serve in vivo to initiate the formation of a bile canalicular branch.
Signaling mechanisms for hepatocytic polarity
Gene targeting approaches and signaling studies on primary rat hepatocytes have revealed sequentially operating signaling pathways that are associated with the acquisition of epithelial polarity. The underlying mechanisms for the distinctive hepatocytic polarity phenotype, however, have not yet been addressed in these systems. Instead, clues have emerged from non-hepatocytic experimental systems. Utilizing the kidney-derived epithelial line MDCK as a model my group discovered with Par1b the first candidate protein to regulate the branching of the monopolar and hepatocytic epithelial differentiation programs. Par1b/MARK2/EMK1 is a ubiquitously expressed mammalian paralogue of Par1, a serine/threonine kinases originally identified as polarity determinant in the one-cell embryo of C. elegans (12, 13). When overexpressed in MDCK cells Par1b caused these columnar epithelial cells to exhibit several hallmarks of the hepatocytic polarity phenotype, namely (i) the formation of bile canaliculi-like lateral rather than apical lumina (13), (ii) the hepatocytic-specific mechanism of cell divisions (9, 10), and (iii) hepatocytic specific trafficking itineraries for luminal proteins that we will discuss later (14). Our recent evidence suggests that the converse also applies, namely that hepatocytic WIFB cells adopt features of the monopolar phenotype when Par1b levels are reduced. Abrogation of Par1 activity in columnar epithelia in vivo and in vitro leads to a disorganized monolayer. Reduced Par1b activity in WIFB cells, however, resulted in areas of the monolayer that exhibited a chickenwire arrangement of tight junctional and apical junctional markers, and in luminal surfaces at the cell apex, although the phenotype reversal was not perfect and many cells simply lost polarity (10). Nevertheless, the combined gain and loss of function data suggest that Par1b-regulated signaling pathways are central to the development of the distinct epithelial polarity phenotypes.
The best-established signaling pathways linked to hepatocyte polarization are centered around the ubiquitously expressed serine/threonine kinase and classified tumor suppressor LKB1/Par4. LKB1 activates AMPK and 11 AMPK-related kinases that include all Par1 paralogues. Liver-specific LKB1-deletion caused defective canaliculi in hepatocytes and a lack of open tubular bile ducts in the developing liver, which prevented the formation of a normal biliary tree and caused impaired bile acid clearance and accumulation of bile acids in serum and liver (15). The reason for the hepatocyte phenotype appeared to be the retention of the canalicular bile salt export pump BSEP in intracellular pools. In addition, the amount of the bile acid influx transporters Oatp1 and Ntcp at the sinusoidal membrane was reduced. Thus, it is plausible that defects in protein trafficking to or their retention at the luminal and sinusoidal poles could be the primary defect in LKB1-deficient hepatocytes. The trafficking defects at the luminal domain in turn could be caused by defects in the organization of the sub-luminal actin cytoskeleton, which is crucial for the retention of membrane proteins at this pole (16). Radixin, a linker protein that connects the actin cytoskeleton with the luminal membrane was reduced at the canalicular domain in LKB1-depleted hepatocytes. The hepatic polarity phenotype of LKB1-KO mice is consistent with the effect Arias and colleagues observed when they prepared primary hepatocytic sandwich cultures from the liver-specific LKB1-knock-out mice (17) or expressed a dominant negative (DN) form of LKB1 in rat hepatocyte cultures (18). Ablation of LKB1 function inhibited the formation of an extensive branched bile canalicular-network that developed over 6 days in the cultures while LKB1 activators accelerated bile canalicular-network formation. The authors identified AMPK as the LKB1 substrate responsible for polarization. One likely mechanism for AMPK function is to promote tight junction-assembly (19, 20), possibly through the phosphorylation of myosin regulatory light chain, which occurs downstream of AMPK activation in the kidney derived epithelial culture model MDCK (21). Indeed, activators of LKB1 and AMPK all prevented the disruption of tight junctions that were induced by Ca2+-withdrawal in the hepatocyte sandwich model. Intriguingly, AMPK also contributed to polarization via its classic metabolic function as an energy-sensor that boosts mitochondrial catabolic activities and activates macroautophagy. AMPK-activation was required for the switch from glycolysis to the more efficient ATP-generation via respiratory oxidative phosphorylation at the onset of hepatocyte differentiation in their primary culture model (22, 23). Additional work form Arias' group suggested that the major primary bile acid taurocholate might represent the physiological stimulus for the elevated LKB1/AMPK activity that triggers hepatocyte polarization (24). It was already known that bile acids induce polarization in a nonpolarized rat hepatoma cell line (25). When Fu et al. studied this phenomenon in their primary cell culture system, they delineated a taurocholate-induced G-protein coupled signaling cascade that led to a MEK dependent activation of LKB1/AMPK. Indeed, bile acid synthesis, turnover, and secretion are sparse in the fetal liver, but rapidly increase, concomitant with hepatocyte polarization and development of a branched canalicular network that occurs postnatally (26).
An independent line of research related to hepatocyte polarization was driven by longstanding efforts to develop a bioartificial liver, which spurred constant improvements in culture conditions to maintain highly differentiated and polarized hepatocytes in either monolayers or spheroids for weeks. These studies taught us about the importance of cell-extracellular matrix (ECM) signaling processes for the development of polarity in cultured hepatocytes. When plated on a matrix of either collagen or matrigel, hepatocytes rapidly de-differentiate. The degree of polarity loss inversely correlates with the density of the matrix and the extent of cell spreading. Even de-differentiated monolayers can be “rescued”, however, when overlaid with a gelling matrix at the free cell surface (27). It is less the nature of the ECM gel that appears to matter since even agarose is effective (27), than the presence of a suitable scaffold that favors accumulation of ECM molecules secreted by the hepatocytes themselves. Such a scaffold may sequester other factors such as cytokines and growth factors. Indeed, cultured hepatocytes in collagen sandwiches secrete their own matrix proteins including laminin, collagen and fibronectin (28). Thus, being surrounded by hepatocyte-derived ECM-molecules on both non-contacting surfaces appears to be crucial for polarization in vitro. Does it also hold the key for the hepatocyte-specific lumen polarity? We know from collagen-sandwich cultures of monopolar epithelia, including MDCK cells, that luminal surfaces cannot be maintained when in contact with ECM. MDCK cells grown on collagen matrices rapidly remove their apical surfaces when overlaid with additional collagen on their apex. The sandwich cultures initially re-establish their luminal domains between the lateral surfaces of neighboring cells, just like hepatocytic cells, but eventually organize in two cell layers to form a central lumen between them, away from the surrounding ECM (29). It is likely that the ECM-filled space of Disse that separates hepatocytes from the endothelium similarly prevents the establishment of an apical pole at either of the sinusoids, leaving it for the cells to carve out luminal surfaces between neighboring cells. In agreement with this model, Par1b-overexpressing MDCK cells, which organize with hepatocytic polarity, show interruptions in collagen IV and laminin staining at the basal surface compared to the corresponding control monolayers and at the same time feature laminin and collagen IV at their apex, opposite the substrate-contacting domain (10). When plated on a collagen IV matrix, MDCK-Par1b cells reverted back to a columnar polarity phenotype. These findings indicate that strengthening the basal lamina at the substrate-contacting domain and thereby increasing the ECM asymmetry between the contacting surface and its opposite domain, favors columnar over hepatocytic lumen organization in this experimental model. RhoA activity appears to be a crucial target of ECM signaling for the phenotype conversion: Not only did ECM-reconstitution antagonize the RhoA inhibition caused Par1b overexpression in MDCK cells, RhoA depletion also phenocopied the Par1b-polarity phenotype. RhoA has also been identified as effector of ECM signaling that modulates the lumen organization of HepG2 cells (30). The GTPase has emerged as an important signaling node that regulates cytoskeletal changes induced by force (31). The force sensors and transducers that connect to RhoA GEFs and GAPs are integrins but they also include cell-cell adhesion molecules such as Cadherins and proteins of the Ig-superfamily such as the junctional adhesion molecule-A, JAM-A. Both E-cadherin and JAM-A have been implicated in regulating lumen polarity in cultured hepatocyte cell lines and in MDCK cells, respectively, although a connection to RhoA signaling was not established in these cases. Thus, JAM-A depletion in two different hepatocyte cell lines resulted in the loss of bile canalicular-like luminal domains and in the organization of the tight junctions in a chickenwire arrangement, consistent with columnar polarity (32, 33). Substitution of endogenous E-cadherin in MDCK cells for an adhesion-defective mutant that lacked the extracellular cell-cell contact-forming domain and thus was defective in outside-in signaling, but still capable of protein interactions via their cytoplasmic domain, promoted a transient polarization of MDCK cells with hepatocytic polarity (34). Collectively, these data suggest a model in which the nature or extent of cell-cell adhesion signaling also contributes to the decision to polarize with hepatocytic or columnar polarity. Generally, cell adhesion to the environment represents a means to transfer force to and from the cell and is the main vehicle for mechanotransduction, the conversion of mechanical energy into biochemical signals. It is probably this context that explains the remarkable finding by D. Cassio and colleagues who induced bile canaliculi-like luminal surfaces and expression of hepatocytic membrane proteins in the nonpolarized rat hepatoma cell line Fao simply by culturing the cells in spheroids, a three-dimensional system that strengthens cell-cell contacts (35). Precisely how engagement of integrins with the ECM and of cell-cell adhesion molecules between cells regulate the cytoskeletal systems to dictate cell surface architecture in epithelial cells is an important question that poses a formidable challenge to cell biologists.
Protein trafficking in hepatocytes
Hepatocytic cells also differ from all other epithelia studied to date in their strategy to target luminal proteins in the biosynthetic pathway (see Figure 2 for an overview of pathways). Hepatocytes only transport polytopic membrane proteins directly from the Golgi to the bile canalicular domain but lack polarized protein secretion into the bile and target single-spanning and GPI-anchored bile canalicular membrane proteins via transcytosis from the basolateral domain. Although the existence of a transcytotic targeting route for luminal membrane proteins is not unique to hepatocytes, they are the epithelial cell type that relies on it to the largest extent. The tremendous amount of proteins that hepatocytes secrete into the sinusoidal space might have made a protein targeting system that emphasizes basolaterally directed traffic from the TGN an evolutionary favorable solution. A basolateral detour for apical membrane proteins might be detrimental mainly for the vectorial transport of bile acids in the enterohepatic circuit and perhaps that's why ABC transporters evolved a direct route from the TGN to the apical pole.
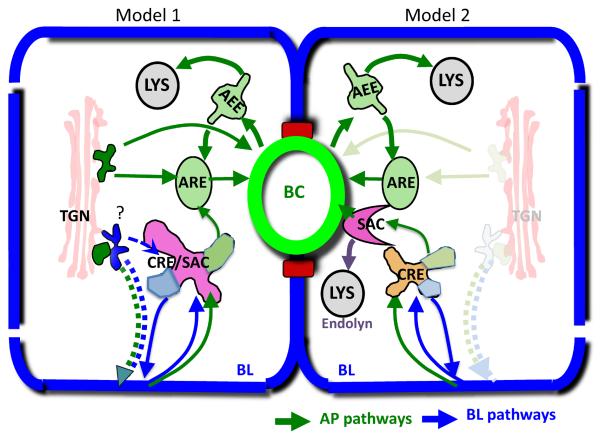
Figure 2. The main known and hypothetical trafficking pathways in hepatocytic cells.
Left cell, Model 1: During biosynthetic targeting, monotopic apical (AP) membrane proteins travel from the TGN to the basolateral (BL) surface. It is unknown whether AP and BL proteins are segregated in this transport leg. Whether BL cargo passes through the Common Recycling Endosome (CRE) from the TGN en route to the BL surface is also unknown. AP polytopic membrane proteins are targeted from the TGN directly to the bile canalicular (BC) membrane or via the Apical Recycling Endosome (ARE). Upon endocytosis from the BL domain, AP cargo is segregated from BL recycling proteins in the CRE that has also been called SubApical Compartment (SAC). From he SAC/CRE AP cargo reaches the BC membrane via the ARE. AREs also mediate the recycling of endocytosed BC proteins after they become diverted in an Apical Early Endosome (AEE)) from the pathway that leads to lysosomal (LYS) degradation. Right cell, Model 2: An alternative model suggests that the SAC is distinct from the CRE and mediates BL-to-AP transcytosis bypassing the ARE. According to this model, the ARE is only utilized for AP protein recycling. The SAC has also been implicated in the lysosomal targeting of the protein Endolyn.
It was Ann Hubbard's group who pioneered in hepatocytes the first and still only in vivo approach to protein trafficking. Her group exploited the fact that 35S-methionine, when injected into the tail vein of rodents, first reaches the liver and is mostly incorporated into newly synthesized hepatocyte proteins. By combining an in vivo pulse-chase protocol with cell fractionation the authors established that the single membrane-spanning bile canalicular proteins they analyzed in rat hepatocytes appeared at the basolateral membrane prior to their arrival at the luminal domain (36). The authors also showed the predominantly basolateral secretion of albumin and combined their in vivo pulse-chase approach with an immune-depletion/adsorption protocol to demonstrate that the bile canalicular membrane protein DPPIV travels with pIgR, a receptor that transports IgA from the blood into the bile, in the same transcytotic vesicles (37, 38). Kipp and Arias later utilized this in vivo approach to document that polytopic membrane proteins such as the ABC transporters MDR1, MPR2 and BSEP/SPGS are targeted to the bile canalicular membrane without first appearing at the basolateral surface (39). The hepatocytic model cell lines HepG2 and WIFB mimic all established hepatocyte targeting pattern (40, 41). Ironically, however, despite solid knowledge of in vivo trafficking itineraries, our understanding of the molecular machinery for protein sorting and trafficking in hepatocytic cells lags behind that from experimental models for monopolar epithelial cells, such as the kidney-derived MDCK cells. The latter segregate apical and basolateral proteins in the trans Golgi network (TGN) into multiple classes of apical and basolateral transport carriers that differ in their trafficking routes to the two surface domains, some involving passage through endosomal intermediates, perhaps for iterative sorting. Basolateral proteins are sorted by recruitment into clathrin-coated vesicles via AP1 adaptors (42). By contrast it is not known where and how monotopic apical and basolateral membrane proteins become segregated along their biosynthetic itineraries in hepatocytes. Do they leave the TGN for the basolateral surface in the same or in distinct transport carriers? Are these vesicles different from those carrying soluble cargo? Early work by Saucan and Palade who showed that basolaterally secreted proteins and membrane proteins are delivered to the basolateral surface in different vesicles in vivo (43) indicates that hepatocytes posses indeed multiple trafficking routes between the TGN and the basolateral domain. Likewise, polytopic membrane proteins appear to take multiple routes from the TGN to the bile canalicular domain because the kinetics of TGN-to-surface transport between BSEP and MDR1 in vivo differ vastly, and BSEP but not MDR1 has been observed in an Apical Recycling Endosome (ARE) (39). In HepG2 cells, MDR1, but not MPR2 targeting was dependent on a Golgi PKA RIIa anchoring protein and on ceramide (44), further supporting the notion of multiple TGN-to-bile canalicular pathways. Thus, hepatocytes like MDCK cells have evolved multiple proteins targeting strategies, which can increase the cells' ability to regulate the exocytosis of different classes of proteins independently from each other. Deciphering protein sorting into these distinct pathways remains a challenge but some inroads have been made. Studies on the lipid raft-associated transmembrane proteins MAL-1 and MAL-2 by Tuma and colleagues have provided the first clues on apical and basolateral protein sorting in the TGN of WIFB cells. In MDCK cells MAL-1 has been attributed a critical role in the clustering of raft-associated proteins into lipid micro-domains that facilitates their sorting into apical-destined transport vesicles. Thus, MAL-1 depletion in MDCK cells impaired the polarized targeting of raft-associated influenza HA (45, 46). Interestingly, MAL-1 is absent in WIFB cells, and upon its exogenous expression a GPI-anchored reporter and several apical proteins with single transmembrane domain were targeted directly from the TGN to the apical pole in these cells (47) supporting the idea that lack of (a) sorting receptor(s) causes apical proteins to be included in basolateral transport carriers that form at the TGN in hepatocytic cells. On the other hand, depletion of MAL-2 (previously only implicated in the transcytotic pathway) had distinct effects on the basolateral surface delivery of pIgR (a basolateral protein) and DPPIV (an apical protein) from the TGN in WIFB cells, suggesting that both proteins utilize different machineries for their TGN-to-surface transport despite being delivered to the same surface (48).
The fate of monotopic apical proteins and of recycling basolateral proteins after their arrival at the basolateral domain is better understood, not least because the accessibility of the sinusoidal membrane to antibodies and labeling reagents makes post-endocytic processes easier to study. Regardless of whether they are internalized via clathrin-dependent or -independent mechanisms from the basolateral domain, bile canaliculi proteins reach the bile canalicular-membrane in common transcytotic transport carriers after being segregated from basolateral proteins in sorting endosomes (49) (50) The nature of the hepatocytic sorting endosomes however, is still controversial. Hoekstra and colleagues as well as Landmann's group refer to an apically localized, tubular endosome that contains apical and basolateral cargo and thus would be the hepatocytic equivalent of the common recycling endosome in MDCK cells, as Sub Apical Compartment (SAC) (51, 52). Their model describes a sub-domain of the SAC that contains sorted apically-destined proteins and is characterized by the presence of Rab11, Rab25 and Rab3 (52, 53). The latter would be the equivalent of the Apical Recycling Endosome (ARE) in MDCK cells. Work from the Hubbard group on the other hand, had defined the SAC as a crescent-shaped organelle adjacent to the apical domain that is distinct from both the CRE and ARE and contains only apical proteins as well as endolyn-78, a lysosomal protein that appears to reach its destination via the SAC (37, 54). In this model the pathways of apically endocytosed proteins and of proteins targeted from the TGN to the apical domain via apical endosomes does not intersect with that of proteins that reach the apical domain via transcytosis from the basolateral domain (see Figure 2). Regardless of the precise role of the ARE in biosynthetic protein targeting, this compartment occupies a central role in luminal surface identity because apical recycling pathways also depend on this transport leg. The ARE in hepatocytes is an important holding cell for bile canalicular proteins that are internalized and re-inserted into the luminal membrane in a signal-dependent and dynamic manner (55). The intrahepatic reservoir for ABC transporters is at least 6-fold greater than the content of those proteins at the canalicular membrane. It is mobilized by the bile acids that circulate in the enterohepatic circuit and by postprandially secreted peptide hormones that increase cAMP production in hepatocytes to cope with increased demand on bile acid secretion (55). Transport between the ARE and the bile canalicular surface is critically dependent on its signature Rab-GTPase Rab11 and the Rab11-effector Myosin Vb. Inhibition of either protein prevented the establishment of a canalicular pole in WIFB-9 cells (56). This is mirrored by findings in the MDCK cell model where Rab11 function is key for lumen development in 3D cultures (57). Interestingly, a Rab11 effector-scaffolding protein, Rab11-FIP1, has been identified as AMPK substrate (58) and might represent a candidate effector of LKB1 signaling in bile canalicular formation.
Clearly, much needs to be learned about epithelial protein trafficking. But based on what we know so far, it might well turn out that the radically different strategies for luminal protein targeting between kidney and hepatocytic epithelial cells are due to only minor but consequential differences in their sorting and trafficking machineries.
To conclude, I have emphasized in this review three molecular hallmarks of the hepatocytic polarity phenotype that relate to lumen polarity, cell division and protein trafficking. They appear to be optimized for the enormous counter-current macromolecular flux hepatocytes manage, making them a true marvel of engineering form for function. For a more detailed discussion of the topics raised here, the interested reader is referred to references (59, 60).
Highlights.
-
>
Hepatocytes form capillary lumina with multiple neighbors and can have 2 basal domains.
-
>
The kinase LKB1 and its substrate AMPK are crucial for hepatocyte polarization.
-
>
Hepatocytes avoid bisecting their luminal domain during cell divisions.
-
>
Hepatocytes lack polarized protein secretion into the bile.
-
>
They target monotopic and GPI-anchored apical membrane proteins via transcytosis.
Acknowledgments
I thank the anonymous reviewers for their comments on the manuscript. Work in my lab is funded by RO1 NIDDK R01KD064842 and RO1 CA160790.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baloch Z, Klapper J, Buchanan L, Schwartz M, Amenta PS. Ontogenesis of the murine hepatic extracellular matrix: an immunohistochemical study. Differentiation. 1992;51(3):209–218. doi: 10.1111/j.1432-0436.1992.tb00698.x. [DOI] [PubMed] [Google Scholar]
- 2.Stamatoglou SC, Enrich C, Manson MM, Hughes RC. Temporal changes in the expression and distribution of adhesion molecules during liver development and regeneration. The Journal of cell biology. 1992;116(6):1507–1515. doi: 10.1083/jcb.116.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogawa K, Medline A, Farber E. Sequential analysis of hepatic carcinogenesis: the comparative architecture of preneoplastic, malignant, prenatal, postnatal and regenerating liver. British journal of cancer. 1979;40(5):782–790. doi: 10.1038/bjc.1979.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michalopoulos GK, Barua L, Bowen WC. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology. 2005;41(3):535–544. doi: 10.1002/hep.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen BE, Zajac VF, Michalopoulos GK. Bile ductular damage induced by methylene dianiline inhibits oval cell activation. Am J Pathol. 1997;151(4):905–909. [PMC free article] [PubMed] [Google Scholar]
- 6.Yin L, Lynch D, Ilic Z, Sell S. Proliferation and differentiation of ductular progenitor cells and littoral cells during the regeneration of the rat liver to CCl4/2-AAF injury. Histol Histopathol. 2002;17(1):65–81. doi: 10.14670/HH-17.65. [DOI] [PubMed] [Google Scholar]
- 7.Decaens C, Rodriguez P, Bouchaud C, Cassio D. Establishment of hepatic cell polarity in the rat hepatoma-human fibroblast hybrid WIF-B9. A biphasic phenomenon going from a simple epithelial polarized phenotype to an hepatic polarized one. Journal of cell science. 1996;109(Pt 6):1623–1635. doi: 10.1242/jcs.109.6.1623. [DOI] [PubMed] [Google Scholar]
- 8.Bartles JR, Hubbard AL. Preservation of hepatocyte plasma membrane domains during cell division in situ in regenerating rat liver. Dev Biol. 1986;118(1):286–295. doi: 10.1016/0012-1606(86)90095-3. [DOI] [PubMed] [Google Scholar]
- 9.Slim CL, et al. Par1b induces asymmetric inheritance of plasma membrane domains via LGN-dependent mitotic spindle orientation in proliferating hepatocytes. PLoS biology. 2013;11(12):e1001739. doi: 10.1371/journal.pbio.1001739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazaro-Dieguez F, et al. Par1b links lumen polarity with LGN-NuMA positioning for distinct epithelial cell division phenotypes. The Journal of cell biology. 2013;203(2):251–264. doi: 10.1083/jcb.201303013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang T, Yanger K, Stanger BZ, Cassio D, Bi E. Cytokinesis defines a spatial landmark for hepatocyte polarization and apical lumen formation. Journal of cell science. 2014;127(Pt 11):2483–2492. doi: 10.1242/jcs.139923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kemphues KJ, Priess JR, Morton DG, Cheng NS. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell. 1988;52(3):311–320. doi: 10.1016/s0092-8674(88)80024-2. [DOI] [PubMed] [Google Scholar]
- 13.Cohen D, Brennwald PJ, Rodriguez-Boulan E, Musch A. Mammalian PAR-1 determines epithelial lumen polarity by organizing the microtubule cytoskeleton. The Journal of cell biology. 2004;164(5):717–727. doi: 10.1083/jcb.200308104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen D, Rodriguez-Boulan E, Musch A. Par-1 promotes a hepatic mode of apical protein trafficking in MDCK cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(38):13792–13797. doi: 10.1073/pnas.0403684101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woods A, et al. LKB1 is required for hepatic bile acid transport and canalicular membrane integrity in mice. Biochem J. 2011;434(1):49–60. doi: 10.1042/BJ20101721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuma PL, Nyasae LK, Hubbard AL. Nonpolarized cells selectively sort apical proteins from cell surface to a novel compartment, but lack apical retention mechanisms. Mol Biol Cell. 2002;13(10):3400–3415. doi: 10.1091/mbc.02-04-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Homolya L, et al. LKB1/AMPK and PKA control ABCB11 trafficking and polarization in hepatocytes. PloS one. 2014;9(3):e91921. doi: 10.1371/journal.pone.0091921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu D, Wakabayashi Y, Ido Y, Lippincott-Schwartz J, Arias IM. Regulation of bile canalicular network formation and maintenance by AMP-activated protein kinase and LKB1. Journal of cell science. 2010;123(Pt 19):3294–3302. doi: 10.1242/jcs.068098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Li J, Young LH, Caplan MJ. AMP-activated protein kinase regulates the assembly of epithelial tight junctions. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(46):17272–17277. doi: 10.1073/pnas.0608531103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng B, Cantley LC. Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(3):819–822. doi: 10.1073/pnas.0610157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bultot L, et al. Myosin light chains are not a physiological substrate of AMPK in the control of cell structure changes. FEBS letters. 2009;583(1):25–28. doi: 10.1016/j.febslet.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 22.Fu D, et al. Coordinated elevation of mitochondrial oxidative phosphorylation and autophagy help drive hepatocyte polarization. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(18):7288–7293. doi: 10.1073/pnas.1304285110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu D, Lippincott-Schwartz J, Arias IM. Increased mitochondrial fusion and autophagy help isolated hepatocytes repolarize in collagen sandwich cultures. Autophagy. 2013;9(12):2154–2155. doi: 10.4161/auto.26167. [DOI] [PubMed] [Google Scholar]
- 24.Fu D, Wakabayashi Y, Lippincott-Schwartz J, Arias IM. Bile acid stimulates hepatocyte polarization through a cAMP-Epac-MEK-LKB1-AMPK pathway. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(4):1403–1408. doi: 10.1073/pnas.1018376108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng KH, Le Goascogne C, Amborade E, Stieger B, Deschatrette J. Reversible induction of rat hepatoma cell polarity with bile acids. Journal of cell science. 2000;113(Pt 23):4241–4251. doi: 10.1242/jcs.113.23.4241. [DOI] [PubMed] [Google Scholar]
- 26.Little JM, Richey JE, Van Thiel DH, Lester R. Taurocholate pool size and distribution in the fetal rat. J Clin Invest. 1979;63(5):1042–1049. doi: 10.1172/JCI109373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunn JC, Tompkins RG, Yarmush ML. Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Prog. 1991;7(3):237–245. doi: 10.1021/bp00009a007. [DOI] [PubMed] [Google Scholar]
- 28.Ezzell RM, et al. Effect of collagen gel configuration on the cytoskeleton in cultured rat hepatocytes. Exp Cell Res. 1993;208(2):442–452. doi: 10.1006/excr.1993.1266. [DOI] [PubMed] [Google Scholar]
- 29.Ojakian GK, Nelson WJ, Beck KA. Mechanisms for de novo biogenesis of an apical membrane compartment in groups of simple epithelial cells surrounded by extracellular matrix. Journal of cell science. 1997;110(Pt 22):2781–2794. doi: 10.1242/jcs.110.22.2781. [DOI] [PubMed] [Google Scholar]
- 30.Herrema H, et al. Rho kinase, myosin-II, and p42/44 MAPK control extracellular matrix-mediated apical bile canalicular lumen morphogenesis in HepG2 cells. Mol Biol Cell. 2006;17(7):3291–3303. doi: 10.1091/mbc.E06-01-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marjoram RJ, Lessey EC, Burridge K. Regulation of RhoA activity by adhesion molecules and mechanotransduction. Current molecular medicine. 2014;14(2):199–208. doi: 10.2174/1566524014666140128104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konopka G, Tekiela J, Iverson M, Wells C, Duncan SA. Junctional adhesion molecule-A is critical for the formation of pseudocanaliculi and modulates E-cadherin expression in hepatic cells. The Journal of biological chemistry. 2007;282(38):28137–28148. doi: 10.1074/jbc.M703592200. [DOI] [PubMed] [Google Scholar]
- 33.Braiterman LT, et al. JAM-A is both essential and inhibitory to development of hepatic polarity in WIF-B cells. Am J Physiol Gastrointest Liver Physiol. 2008;294(2):G576–588. doi: 10.1152/ajpgi.00159.2007. [DOI] [PubMed] [Google Scholar]
- 34.Cohen D, Tian Y, Musch A. Par1b promotes hepatic-type lumen polarity in Madin Darby canine kidney cells via myosin II- and E-cadherin-dependent signaling. Mol Biol Cell. 2007;18(6):2203–2215. doi: 10.1091/mbc.E07-02-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng X, et al. How to induce non-polarized cells of hepatic origin to express typical hepatocyte polarity: generation of new highly polarized cell models with developed and functional bile canaliculi. Cell Tissue Res. 2006;323(2):233–243. doi: 10.1007/s00441-005-0067-2. [DOI] [PubMed] [Google Scholar]
- 36.Bartles JR, Feracci HM, Stieger B, Hubbard AL. Biogenesis of the rat hepatocyte plasma membrane in vivo: comparison of the pathways taken by apical and basolateral proteins using subcellular fractionation. The Journal of cell biology. 1987;105(3):1241–1251. doi: 10.1083/jcb.105.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barr VA, Scott LJ, Hubbard AL. Immunoadsorption of hepatic vesicles carrying newly synthesized dipeptidyl peptidase IV and polymeric IgA receptor. The Journal of biological chemistry. 1995;270(46):27834–27844. doi: 10.1074/jbc.270.46.27834. [DOI] [PubMed] [Google Scholar]
- 38.Larkin JM, et al. Intracellular accumulation of pIgA-R and regulators of transcytotic trafficking in cholestatic rat hepatocytes. Hepatology. 2003;38(5):1199–1209. doi: 10.1053/jhep.2003.50419. [DOI] [PubMed] [Google Scholar]
- 39.Kipp H, Arias IM. Newly synthesized canalicular ABC transporters are directly targeted from the Golgi to the hepatocyte apical domain in rat liver. The Journal of biological chemistry. 2000;275(21):15917–15925. doi: 10.1074/jbc.M909875199. [DOI] [PubMed] [Google Scholar]
- 40.Bastaki M, Braiterman LT, Johns DC, Chen YH, Hubbard AL. Absence of direct delivery for single transmembrane apical proteins or their “Secretory” forms in polarized hepatic cells. Mol Biol Cell. 2002;13(1):225–237. doi: 10.1091/mbc.01-07-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slimane TA, Trugnan G, Van ISC, Hoekstra D. Raft-mediated trafficking of apical resident proteins occurs in both direct and transcytotic pathways in polarized hepatic cells: role of distinct lipid microdomains. Mol Biol Cell. 2003;14(2):611–624. doi: 10.1091/mbc.E02-08-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodriguez-Boulan E, Macara IG. Organization and execution of the epithelial polarity programme. Nature reviews. Molecular cell biology. 2014;15(4):225–242. doi: 10.1038/nrm3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saucan L, Palade GE. Differential colchicine effects on the transport of membrane and secretory proteins in rat hepatocytes in vivo: bipolar secretion of albumin. Hepatology. 1992;15(4):714–721. doi: 10.1002/hep.1840150427. [DOI] [PubMed] [Google Scholar]
- 44.Wojtal KA, de Vries E, Hoekstra D, van Ijzendoorn SC. Efficient trafficking of MDR1/P-glycoprotein to apical canalicular plasma membranes in HepG2 cells requires PKA-RIIalpha anchoring and glucosylceramide. Mol Biol Cell. 2006;17(8):3638–3650. doi: 10.1091/mbc.E06-03-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puertollano R, et al. The MAL proteolipid is necessary for normal apical transport and accurate sorting of the influenza virus hemagglutinin in Madin-Darby canine kidney cells. The Journal of cell biology. 1999;145(1):141–151. doi: 10.1083/jcb.145.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheong KH, Zacchetti D, Schneeberger EE, Simons K. VIP17/MAL, a lipid raft-associated protein, is involved in apical transport in MDCK cells. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(11):6241–6248. doi: 10.1073/pnas.96.11.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramnarayanan SP, Cheng CA, Bastaki M, Tuma PL. Exogenous MAL reroutes selected hepatic apical proteins into the direct pathway in WIF-B cells. Mol Biol Cell. 2007;18(7):2707–2715. doi: 10.1091/mbc.E07-02-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.In JG, Tuma PL. MAL2 selectively regulates polymeric IgA receptor delivery from the Golgi to the plasma membrane in WIF-B cells. Traffic. 2010;11(8):1056–1066. doi: 10.1111/j.1600-0854.2010.01074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tuma P, Hubbard AL. Transcytosis: crossing cellular barriers. Physiological reviews. 2003;83(3):871–932. doi: 10.1152/physrev.00001.2003. [DOI] [PubMed] [Google Scholar]
- 50.Ait-Slimane T, Galmes R, Trugnan G, Maurice M. Basolateral internalization of GPI-anchored proteins occurs via a clathrin-independent flotillin-dependent pathway in polarized hepatic cells. Mol Biol Cell. 2009;20(17):3792–3800. doi: 10.1091/mbc.E09-04-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rahner C, Stieger B, Landmann L. Apical endocytosis in rat hepatocytes In situ involves clathrin, traverses a subapical compartment, and leads to lysosomes. Gastroenterology. 2000;119(6):1692–1707. doi: 10.1053/gast.2000.20233. [DOI] [PubMed] [Google Scholar]
- 52.Hoekstra D, Tyteca D, van ISC. The subapical compartment: a traffic center in membrane polarity development. Journal of cell science. 2004;117(Pt 11):2183–2192. doi: 10.1242/jcs.01217. [DOI] [PubMed] [Google Scholar]
- 53.Hoekstra D, Zegers MM, van Ijzendoorn SC. Membrane flow, lipid sorting and cell polarity in HepG2 cells: role of a subapical compartment. Biochemical Society transactions. 1999;27(4):422–428. doi: 10.1042/bst0270422. [DOI] [PubMed] [Google Scholar]
- 54.Ihrke G, et al. Apical plasma membrane proteins and endolyn-78 travel through a subapical compartment in polarized WIF-B hepatocytes. The Journal of cell biology. 1998;141(1):115–133. doi: 10.1083/jcb.141.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wakabayashi Y, Kipp H, Arias IM. Transporters on demand: intracellular reservoirs and cycling of bile canalicular ABC transporters. The Journal of biological chemistry. 2006;281(38):27669–27673. doi: 10.1074/jbc.R600013200. [DOI] [PubMed] [Google Scholar]
- 56.Wakabayashi Y, Dutt P, Lippincott-Schwartz J, Arias IM. Rab11a and myosin Vb are required for bile canalicular formation in WIF-B9 cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(42):15087–15092. doi: 10.1073/pnas.0503702102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Desclozeaux M, et al. Active Rab11 and functional recycling endosome are required for E-cadherin trafficking and lumen formation during epithelial morphogenesis. American journal of physiology. Cell physiology. 2008;295(2):C545–556. doi: 10.1152/ajpcell.00097.2008. [DOI] [PubMed] [Google Scholar]
- 58.Banko MR, et al. Chemical Genetic Screen for AMPKalpha2 Substrates Uncovers a Network of Proteins Involved in Mitosis. Mol Cell. 2011;44(6):878–892. doi: 10.1016/j.molcel.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hubbard A, Barr V, Scott L. W-5 Hepatocyte Surface Polarity. In: Arias JB I, Chisari F, Fausto N, Schachter D, Schafritz D, editors. The Liver Biology and Pathobiology. Lippincott Williams & Wilkins; Philadelphia: 2001. http://liver.med.tufts.ed. [Google Scholar]
- 60.Treyer A, Musch A. Hepatocyte polarity. Comprehensive Physiology. 2013;3(1):243–287. doi: 10.1002/cphy.c120009. [DOI] [PMC free article] [PubMed] [Google Scholar]