Abstract
Decreased NK cell numbers and impairment of NK cell function are reported in patients with multiple sclerosis (MS). Interleukin-7 (IL-7) is a member of the common gamma-chain (γc) cytokine superfamily that has well documented roles in lymphocyte development and homeostasis. The interleukin-7 receptor α chain (IL-7Rα) gene was identified as a top non-major histocompatibility complex-linked risk locus for MS. The objective of this study was to test biological function of IL-7/IL-7Rα on NK cells in MS patients. We observed markedly lower IL-7 levels in MS sera, and relatively higher IL-7Rα expression in NK cells of MS. Upon IL-7 stimulation, IL-7Rα on NK cells from MS patients was significantly down-regulated compared with healthy controls (HC). IL-7 induced a higher increase of IFN-γ production in CD56bright NK cells and a pronounced enhancement of cytotoxicity in NK cells from MS. IL-7 does not impact the proliferation of NK cells differently in MS and HC. In contrast, IL-7 promotes a higher survival of CD56bright NK cells in MS and inhibits their apoptosis by increasing Bcl-2 expression, but has no effect on CD56dim NK cell survival in MS. In conclusion, MS patients have lower serum IL-7 and a higher membrane IL-7Rα expression on CD56bright NK cells. The skew at the IL-7 and IL-7Rα level influences functional responsiveness of NK cells in MS.
Keywords: IL-7, IL-7Rα, Natural killer cells, CD56bright, CD56dim, Multiple sclerosis
1. Introduction
Natural killer (NK) cells are one component of the innate immune system and have the ability to both lyse target cells and provide an early source of immunoregulatory cytokines such as interferon (IFN)-γ (French and Yokoyama, 2004). Two distinct populations of human NK cells could be identified based upon their cell-surface density of CD56 (Cooper et al., 2001). The majority (~90%) of human NK cells have low-density expression of CD56 (CD56dim) and express high levels of Fc γ receptor III (Fc γ RIII, CD16), whereas ~10% of NK cells are CD56brightCD16dim or CD56brightCD16−. The CD56dim NK-cell subset is more naturally cytotoxic and the CD56bright subset has the capacity to produce abundant cytokines following activation. Administration of daclizumab (a humanized monoclonal antibody directed against the IL-2α chain) resulted in the expansion of CD56bright NK cells in MS patients in association with clinical and radiological improvement (Bielekova et al., 2006). Furthermore, NK cells inhibit the inflammatory and autoimmune responses against myelin antigens and genesis of experimental autoimmune encephalomyelitis (EAE), an animal model for MS (Zhang et al., 1997). These findings imply that NK cells may serve as an active disease player and therapeutic target in MS (Shi and Zhou 2011).
Recent genetic studies strongly implicate the IL-7Rα-related immune response pathway in the etiology of MS (Gregory et al., 2007). The impact of IL-7/IL-7Rα signaling on CD4+ T cells (Kreft et al., 2012a), Treg cells (Haas et al., 2011), and CD8+ T cells (Kreft et al., 2012b) in MS has been reported to demonstrate that this pathway may subsequently enhance the pathogenicity of these immune cells. The importance of this pathway for NK cell homeostasis is underlined by several studies in both healthy human and animals (Lundstrom et al., 2012; Mazzucchelli and Durum, 2007; Michaud et al., 2010a); however, its impact on NK cell function in MS has not yet been reported.
The present study attempted to decipher in detail the impact of IL-7/IL-7Rα signaling components on NK homeostasis and function in MS. We show that the serum IL-7 level is markedly lower and the expression of IL-7Rα on NK cells is significantly higher in MS patients. Upon IL-7 stimulation, IL-7Rα on NK cells from MS patients was significantly down-regulated compared with those from healthy controls (HC). Moreover, IL-7 stimulation resulted in a higher enhanced cytotoxicity in NK cells and higher increased survival and interferon-gamma (IFN-γ) production in CD56bright NK subsets in MS patients. Our findings indicate that IL-7/IL-7Rα axis alters activation of NK cells in MS, which sheds new light for the potential new therapies targeting NK cells in this disease.
2. Materials and methods
2.1. Subjects
Patients were recruited from the Neurology Department at Tianjin Medical University General Hospital in China and St. Joseph’s Hospital and Medical Centre in USA. Collection of human blood was conducted using the protocol BNI-005: MS and Healthy Subject Tissue Acquisition for Immunological Studies, which is approved by the institutional review board. Inclusion criteria included males and females between 18 and 55 years of age with clinically defined MS, as determined by 2010 McDonald criteria and an Expanded Disability Status Scale score between 0.5 and 6.5. Healthy subject as controls were recruited with best efforts to match MS patients for age, gender and demography. Most controls were patients’ close friends, spouses, siblings, etc. Detailed information on all patients and controls is depicted in Table 1. Peripheral blood mononuclear cells (PBMCs) and sera were isolated from blood samples collected from MS patients and HCs. None of the patients received immunomodulatory therapies three month prior to sampling.
Table 1.
Clinical and demographic features of patients with MS and healthy controls.
| MS | HC | |
|---|---|---|
| Number | 26 | 26 |
| Sex ratio (female to male) | 21:5 | 20:6 |
| Relapsing-remitting disease course | 14 (54%) | NA |
| Secondary progressive disease course | 5 (19%) | NA |
| Primary progressive disease course | 7 (27%) | NA |
| Disease duration at time of samples (mean, range in years) | 5.4 (0.5–20) | NA |
| EDSS score (mean, range in years) | 4.6 (1.5–6) | NA |
| Total number of attacks in last 2 years | 1.7 (0–5) | NA |
| Treatment at time of sample | 0/26 (0%) | NA |
EDSS, extended disability status scale.
2.2. Sera preparation and cytokine quantification
The whole blood sample was collected and left for 1 hour at 37°C to allow it to clot and subsequently, overnight at 4°C for clot contraction. The clot was released from the sides of the tube and centrifuged at 4,000 rpm for 20 minutes at 4°C. The serum from the clot was gently removed with a Pasteur pipette into a clean tube and stored at -80°C until tested. Secreted cytokine IL-7 in sera was measured by enzyme linked immunosorbent assay (ELISA) kits (R&D system, Minneapolis, MN) according to the manufacturer’s instructions.
2.3 Peripheral blood mononuclear cell isolation and NK cell purification
PBMCs were isolated by centrifugation on a Ficoll-Hypaque density gradient. Cells were diluted to 35 mL of suspension on 15 mL of Ficoll-Paque in a 50 mL conical tube and centrifuged at 400xg for 30 minutes at 20°C. The upper layer was aspirated leaving the mononuclear cell layer (lymphocytes, monocytes, and thrombocytes) undisturbed at the interphase. The mononuclear cell layer was transferred to a new 50 mL conical tube. The tube was filled with buffer and centrifuged at 300xg for 10 minutes at 20°C. The supernatant containing the platelets and PBMCs was resuspended with buffer. The NK cell isolation kit (Miltenyi Biotec, 130-092-657) was used for purification of NK cells. The cell number of PBMCs was determined and cells were stained with NK cell biotin-antibody cocktail, mixed and incubated for 5 minutes at 4°C. Cells were then washed with buffer and mixed with a NK cell microbead cocktail and kept in the refrigerator for 10 minutes. The cells were washed again and magnetically sorted using the MACS separator with MS columns.
2.4. Cell culture
PBMCs were cultured in RPMI-1640 medium, 10% fetal bovine serum, 20 mM L-glutamine, 100 U/mL of penicillin G, 100 μg/mL of streptomycin sulphate, and 0.25 μg/mL of amphotericin at 37°C at 5% of CO2 supplemented with IL-7 (50 ng/ml) or without any cytokine stimulation for 72h..
2.5. Flow cytometry analysis
A total of 1 × 106 PBMCs were stained with the following anti-human mAbs (BD Pharmingen, San Diego, CA): CD3-Pacific Blue (558117), CD56-APC (555518) and CD127-PE (557938) for surface staining. Nonspecific binding was prevented by a short incubation with fetal bovine serum before the addition of specific antibodies. For intracellular cytokine staining, PBMCs were incubated at 37°C for 72h in a 24-well plate (1 X 106 cells/well) with or without IL-7 (50 ng/ml) and followed by a mixture of PMA (20 ng/ml), Ionomycine (1 μg/ml) and GolgiPlug (1μg/ml) for another 5h at 37°C. After harvesting, cells were stained for surface markers CD3-Pacific Blue (558117) and CD56-APC (555518) followed by fixation and permeabilization with the Cytofix/Cytoperm kit (BD). They were then respectively stained with anti-IFN-γ-PE (BD, 340452) or anti-Bcl-2-PE (BD, 340651) as designed. After staining, all samples were acquired on a FACSAria™ flow cytometer and analyzed with FACSDiva software 6.1.3 or FCS Express 4 software (BD, Mountain View, CA).
2.6. Cytotoxicity assay
NK cell cytotoxicity towards K562 cells was measured using the lactate dehydrogenase (LDH)-based CytoTox96-non-radioactive cytotoxicity assay kit (Sigma, TOX7) in accordance with the manufacturer’s protocol. LDH is a stable cytosolic enzyme that is released into the media upon cell lysis. Purified NK cells were cultured with 50 ng/mL IL-7 at 37°C for 72 hours. For NK cell cytotoxicity assays, K562 cells were plated in a round bottom 96-well plate (1X104 cells per well). By using 2.5:1, 5:1 and 10:1 effector cell to target cell ratios, NK cells were added to the wells and the plate was incubated at 37°C for 4h. Cells were pelleted by centrifugation at 250xg for 4 minutes. The supernatant was transferred to a new 96-well plate and the reconstituted substrate mix was added. The absorbance was measure at 490 nm. Each assay was tested in triplicate. Percentage of specific lysis was determined as follows: (Experimental release-effector spontaneous release-target spontaneous release)/(target maximum release-target spontaneous release) x100). The experimental release was determined by detecting the absorbance of the effector with target cell suspension. The spontaneous release was determined by detecting the absorbance of the effector or target cell suspension, whereas the maximum release was determined by detecting the absorbance of the target cells lysed with LDH assay lysis solution.
2.7. Cell proliferation assay by Bromodeoxyuridine (BrdU)
PBMCs from MS patients and HCs were cultured in 24-well-plates (1X106 cells/well) with or without IL-7 (50 ng/mL). 10 μM BrdU (BD Pharmingen, 550891) was added into the culture medium prior to 72 hour incubation at 37°C. After harvesting, cells were stained for surface markers CD3-Pacific Blue (558117) and CD56-APC (555518) followed by fixation and permeabilization with the Cytofix/Cytoperm kit (BD), with 300ng/mL DNase (Sigma, D4513) incubation for 1 hour at 37°C. Anti-BrdU-PE (eBioscience, 12-5071-42) was added for flow cytometry analysis.
2.8. Apoptosis assays by 7-AAD and AnnexinV
Apoptosis was assessed by Annexin V and 7-amino-actinomycin D staining (7-AAD) using the BD Biosciences apoptosis kit (559763). PBMCs from MS and HCs were cultured in 24-well-plates (1X106 cells/well) with or without IL-7 (50 ng/mL) at 37°C for 72 hours. The cells were harvested and stained for surface markers CD3-Pacific Blue (558117), CD56-APC (555518), followed by 7AAD and Annexin V-PE staining. The samples were analyzed by flow cytometry within 1 hour.
2.9. Statistical analysis
Data are presented as mean ± SEM and tested for statistical significance by the two-tailed unpaired Student’s t test for comparisons of difference between two groups. Multiple comparisons were performed with Two-way ANOVA accompanied by Bonferroni post-hoc test. p < 0.05 was considered statistically significant.
3. Results
3.1. Decreased serum IL-7 in MS
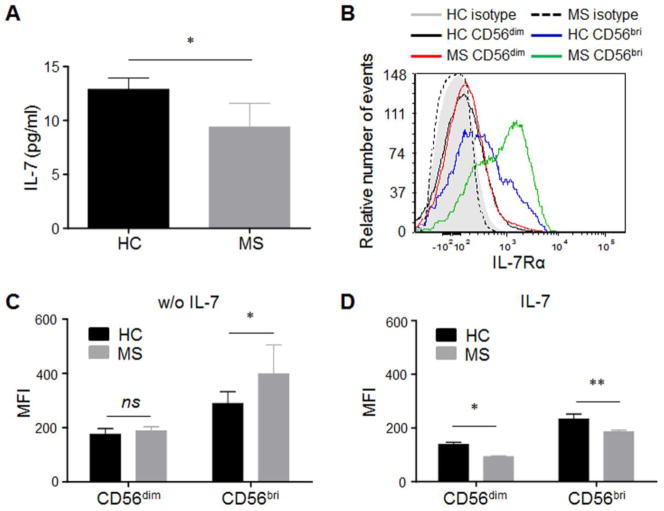
To evaluate IL-7/IL-7Rα signaling differences between MS patients and HCs, we assessed the concentration of IL-7 in sera of MS patients (n=26) and HC (n=26) (Table 1). MS patients had significantly lower levels of IL-7 (9.387 ± 2.196 ng/ml) compared with HC (12.89 ± 1.039 ng/ml; p < 0.05. Fig. 1A), which is consistent with several previous findings (Kreft et al., 2012a; Kreft et al., 2012b).
Fig. 1. Decreased serum IL-7 level and increased IL-7Rα expression on NK cells in MS patients.
(A) Serum IL-7 level is significantly decreased in MS patients. IL-7 in sera from MS patients and health control (HC) subjects were measured by ELISA kits (R & D system, Minneapolis, MN). *p < 0.05. n = 26 per group. (B) Representative IL-7Rα (CD127) staining on CD56bright and CD56dim NK cells from one HC and one MS is shown in histogram analyzed by flow cytometry. Gray filled area is HC isotype control, and dashed line is MS isotype control. (C) Level of IL-7Rα expression (MFI) corrected for background signal was compared between MS patients and HC at baseline. (D) Comparison of IL-7Rα expression (MFI) on NK cells after IL-7 50ng/ml stimulation for 72 hours. For C and D, HC, n = 15; MS, n = 15. 10 relapsing remitting patients, 3 secondary progressive and 2 primary progressive patients were included in this study. HC were relatives of MS patients. *p < 0.05, **p < 0.01. IL-7Rα expression was analyzed by flow cytometry and presented as mean fluorescence intensity (MFI). CD56bri indicates CD56bright.
3.2. Increased IL-7Rα expression on NK cells in MS patients
Signaling from IL-7 occurs through the heterodimeric IL-7R, which consists of two chains, the IL-7R α-chain (IL-7Rα; also known as CD127) and the common cytokine-receptor γ-chain (γc; also known as CD132). The availability of membrane-bound IL-7Rα (mIL-7Rα) is the limiting factor in IL-7 receptor formation hence its signaling (Mazzucchelli and Durum, 2007). Next, we assessed the concentration of mIL-7Rα on NK cells from MS patients and HC. Flow cytometry analysis of lymphocytes from HC and MS patients confirmed that IL-7Rα is expressed on human peripheral NK cells (Fig. 1B). CD56bright NK cells tend to have higher expression of IL-7Rα than CD56dim population both in controls and MS. The expression of IL-7Rα on CD56bright NK cells is significantly higher in MS. (Fig. 1C). After IL-7 stimulation in vitro, decreased IL-7Rα expression was observed in both groups, with a significantly lower IL-7Rα expression on CD56bright and CD56dim populations in MS patients (Fig. 1D). This finding is consistent with previous findings of down-regulation of IL-7R by IL-7 (Michaud et al., 2010b; Park et al., 2004), which suggests a specific regulator role of IL-7 on IL-7Rα.
The frequency and expression of IL-7Rα in both CD56bright and CD56dim NK cells were comparable between the different MS disease courses. Additionally, no correlation was found between the expression of IL-7Rα and disease duration (data not shown). Therefore, all data were pooled and are shown together.
3.3. IL-7 stimulation leads to a higher increase of IFN-γ production in CD56bright NK cells of MS patients
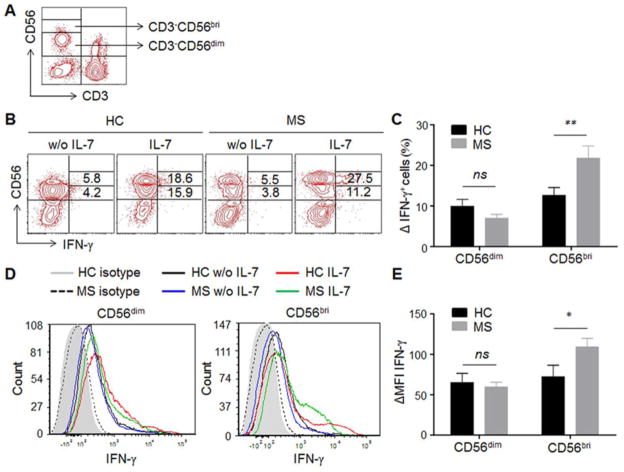
Next, we questioned whether the significantly increased IL-7Rα expression in CD56bright NK cells would impact their IFN-γ production. NK subsets were analyzed using flow cytometry and gated according to CD56+ phenotypical analysis (Fig. 2A). Subsequently, the frequency of IFN-γ+ cells within each subset (Fig. 2B) and expression level of IFN-γ per cell (mean fluorescence intensity, MFI) were assessed (Fig. 2D) with the illustrated gating strategy. At baseline, the percentage of IFN-γ+ NK cells within different NK cell subsets between MS patients and HC was similar (Fig. 2B); no differences in MFI of IFN-γ were observed between MS and HC (Fig. 2D). After stimulation of PBMCs with 50ng/ml IL-7 for 72 hours, in MS patients a significantly higher percentage of IFN-γ+CD56bright NK cells were found compared with those of HC (p < 0.01, Fig. 2C). Changes of MFI of IFN-γ were also calculated, and a significant increase in IFN-γ production (ΔMFI) in CD56bright NK cells of MS upon IL-7 stimulation was also observed (p < 0.05, Fig. 2E). These data indicate the differential responsiveness in IFN-γ production by NK subpopulations from MS patients.
Fig. 2. IL-7 stimulation leads to a higher increase of IFN-γ production in NK cells of MS patients.
(A) Gating strategy for NK subpopulations. NK cells in PBMCs are analyzed by flow cytometry and gated based on surface markers CD3 and CD56, which express CD3−CD56dim or CD3−CD56bright. (B) Representative IFN-γ expressing NK cells from one MS patient and one HC with or without IL-7 stimulation are shown. PBMCs from MS patients and HCs were cultured with or without IL-7 for 72 hour. Intracellular staining of IFN-γ gating on CD3−CD56dim and CD3−CD56bright NK cells were analyzed by flow cytometry. (C) Increase in the percentage of IFN-γ-expressing CD56dim and CD56bright cells was calculated as the difference between unstimulated and IL-7 stimulation. (D) Representative intracellular IFN-γ intensity on NK cells from one MS patient and one HC is shown. (E) The changes in IFN-γ MFI on CD56dim and CD56bri cells were calculated as the difference between unstimulated and IL-7 stimulation. Data from 10 HCs and 10 MS are summarized. 5 relapsing remitting patients, 3 secondary progressive and 2 primary progressive patients were included in this study. HC were relatives of MS patients. *p < 0.05; **p < 0.01. ns, not significant. MFI, mean fluorescence intensity. CD56bri indicates CD56bright.
3.4. IL-7 stimulation induces a higher enhancement of cytotoxicity in NK cells of MS patients
Cytolytic function is one of two major NK cell-mediated effects. We tested whether IL-7 stimulation in vitro influences the cytolytic effect of NK cells from MS patients. NK cells purified by Magnetic sorting were cultured in the presence of IL-7 for 72 hours. We used a standard lactate dehydrogenase (LDH) release assay to measure the ability of NK cells to lyse K562 targets. Though killing K562 cells does not significantly differ between MS and HC prior to IL-7 stimulation, NK cells from MS patients cultured with IL-7 show higher enhanced cytotoxicity effects at effector to target ratios of 2.5:1, 5:1 and 10:1 (p < 0.05 or p < 0.01, Fig. 3).
Fig. 3. IL-7 stimulation induces a higher enhancement of cytotoxicity in NK cells of MS patients.
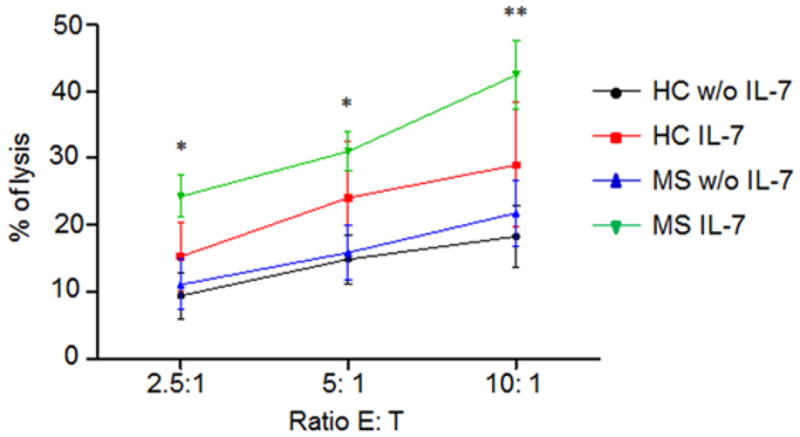
NK cells from MS patients and HCs were purified with magnetic sorting and cultured with or without IL-7 for 72 hours. Cytotoxic activity was measured by the lactate dehydrogenase (LDH)-based assay. Three ratios [effector: target cells (E: T)] were tested and lysis (in %) is indicated. Compared to HC, NK cells from MS patients cultured with IL-7 show a higher enhancement of cytotoxicity effects at effector to target ratio of 2.5:1, 5:1 and 10:1. Data from five independent experiments were statistically analyzed. 5 relapsing remitting patients, 3 secondary progressive and 2 primary progressive patients were included in this study. 10 HC were relatives of MS patients. *p < 0.05; **p < 0.01.
3.5. No differences in IL-7-induced proliferation
IL-7 is reportedly required for the development of thymic (CD127+) and mucosal NK cells (Cella et al., 2010; Vosshenrich et al., 2006). Given decreased serum IL-7 level and increased receptor expression on NK subsets in MS patients, we next questioned whether this altered axis influences the proliferative response of NK cells in MS. Proliferation of PBMCs from MS patients and HCs was tested using the BrdU incorporation assay. After 72 hour incubation, no significant difference in IL-7-induced proliferation in NK cells was found between MS patients and HCs, though NK cells from both groups proliferated upon IL-7 stimulation (Fig. 4). The result suggests that IL-7/IL-7Rα does not impact proliferation of NK cells in MS patients differently from HCs. Other factors must contribute to IL-7-mediated NK cell proliferation. The finding is supported by several previous studies (He and Malek, 1996; Mazzucchelli and Durum, 2007).
Fig. 4. No differences in IL-7-induced proliferation.
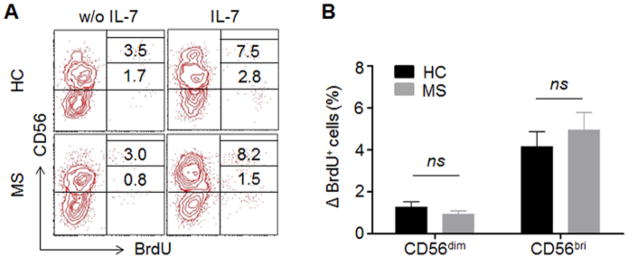
PBMCs from MS patients and HCs were cultured with cytokine IL-7 or left unstimulated without IL-7 for 72 hours. Proliferation of NK cells were analyzed by BrdU incorporation assay. (A) Representative fluorescence-activated cell sorting (FACS) plot showing proliferating NK cells (BrdU+) from one MS patient and one HC. (B) The changes on the percentage of BrdU+CD56dimand BrdU+CD56bright cells were calculated between unstimulated and IL-7 stimulation. n = 10 per group. 5 relapsing remitting patients, 3 secondary progressive and 2 primary progressive patients were included in this study. HC were relatives of MS patients. CD56bri indicates CD56bright. ns, not significant.
3.6. IL-7 stimulation promotes increased survival in NK cells of MS patients
One of the major roles of IL-7 in lymphopoiesis and homeostasis is to promote cell survival by inhibiting apoptosis. IL-7 was reported enhancing the survival of human mature CD56bright NK cells (Michaud et al., 2010a). Here we investigate whether and how IL-7 stimulation impacts the survival of NK cells in MS patients. The levels of apoptotic NK cells were measured by flow cytometry using 7-AAD and Annexin V staining. In MS patients, in vitro stimulation with IL-7 significantly increased the ratio of live cells (7-AAD−Annexub V−) in the CD3−CD56bright population, whereas it did not impact survival of CD56dim NK cells (Fig. 5A). The increased survival upon IL-7 stimulation is higher in CD56bright NK cells (p < 0.05) and lower in CD56dim NK cells (p < 0.01) of MS patients compared to HC (Fig. 5B). One significant consequence of IL-7Rα signaling is the maintenance of cell survival by promoting a favorable balance of B-cell lymphoma 2 (Bcl-2)-family members by increasing expression of the survival proteins Bcl-2 and Mcl-1 (myeloid-cell leukemia sequence 1), and by redistributing the cell-death proteins BAX (Bcl-2-associated X protein) and BAD (Bcl-2-antagonist of cell death) (Al-Rawi et al., 2003; Hofmeister et al., 1999). In our study, a significant increase in expression (MFI) of Bcl-2 upon IL-7 stimulation was observed in CD56bright NK cells of both MS and HC and in CD56dim NK cells of HC (Fig. 5C). The increase of Bcl-2 (ΔMFI) is significantly higher in CD56bright NK cells (p < 0.05) while dramatically lower in CD56dim NK cells (p < 0.01) in MS patients when compared with HC (Fig. 5D). The increased live cells positively correlate with enhanced Bcl-2 levels, indicating that IL-7/IL-7Rα specifically maintains NK cells by inhibiting apoptotic cell death.
Fig. 5. IL-7 promotes an increased survival in NK cells of MS patients by up-regulating Bcl-2 expression.
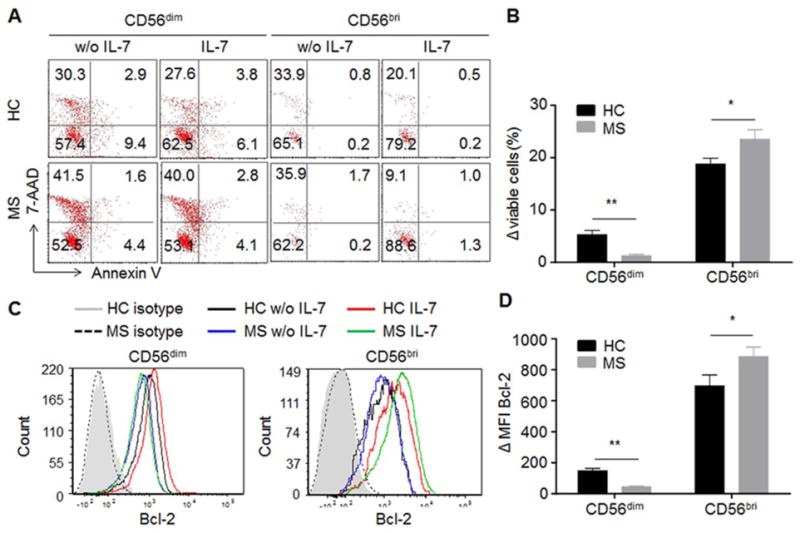
(A) Representative FACS plot showing viable NK cells with or without IL-7 stimulation. PBMCs from MS patients and HCs were cultured with cytokine IL-7 or left unstimulated without IL-7 for 72 hours. Apoptosis of CD56dim and CD56bright NK cells were examined by Annexin V and 7-AAD staining and analyzed by flow cytometry. (B) Increased viable NK cells upon IL-7 stimulation were compared between HC and MS. Viable cells were negative for both Annexin V and 7-AAD. The changes on the percentage of viable CD56dim and CD56brigt cells were calculated between unstimulated and IL-7 stimulation. (C) Representative FACS histogram showing Bcl-2 expression on NK cells with or without IL-7 stimulation. (D) Changes of Bcl-2 MFI response to IL-7 stimulation were compared between control and MS. *p < 0.05. **p < 0.01. n = 15. 10 relapsing remitting patients, 3 secondary progressive and 2 primary progressive patients were included in this study. HC were relatives of MS patients. 7-AAD, 7-amino-actinomycin D. CD56bri indicates CD56bright.
4. Discussion
In this study, we show that serum IL-7 levels are decreased and the expression levels of IL-7Rα on NK cells are increased in MS patients. We demonstrate that IL-7/IL-7Rα signaling differentially impacts the proliferation, survival, and function of circulating NK cells in MS.
Our result is consistent with previous findings in which slightly but significantly decreased systemic IL-7 and a higher membrane to soluble IL-7Rα ratio on CD4+ and CD8+ cells were reported (Kreft et al., 2012a; Kreft et al., 2012b). However, these findings appear in contrast with the up-regulated IL-7 plasma levels observed by Hass et al (Haas et al., 2011). It is not clear how IL-7 expression is regulated in MS. The reciprocal relation between mIL-7Rα and plasma concentrations of IL-7 detected in our study and others underlines the tight balance between the components of this signaling pathway. Mounting evidence indicates that receptor mediated consumption is a primary mechanism but not the sole regulation of IL-7 levels (Hodge et al., 2011). As speculated by others, whether IL-7 levels are altered during different phases of disease activity in MS patients (i.e., during relapses versus in remissions) (Kreft et al., 2012a) is of interest and deserves to be further studied. Moreover, what remains to be determined is whether the onset of the autoimmune disease triggers altered IL-7 levels among MS patients or whether aberrant IL-7 levels in MS patients predate the onset of the disease. The IL-7 mediated suppression of mIL-7Rα has been previously demonstrated in the subpopulations of T cells at the different stages and illustrated at transcriptional level (Mazzucchelli and Durum, 2007). It is also evident in our study by a down-regulation of IL-7Rα upon IL-7 stimulation in NK cells from both MS and HC, with a more pronounced down-regulation in MS.
The availability of mIL-7Rα is the limiting factor in IL-7 signaling. Hence, the altered IL-7/IL-7Rα levels putatively lead to varied downstream biological function of NK cells in MS compared with HC. In the current study, upon IL-7 stimulation, CD56bright NK cells from MS demonstrated a higher increase of IFN-γ production compared with HC. CD56dim NK cells are often referred to as cytolytic cells. Though IL-7Ra expression is comparable on CD56dim NK cells from MS patients and HC, it is interesting to note that heterogeneous mixture of CD56bright and CD56dim NK cells from MS showed a higher enhancement of cytotoxicity after IL-7 stimulation. The difference might result from IL-7-promoted CD56bright cytotoxicity. Indeed, NK cells are recently reported to possess killing function (Wiendl and Gross, 2013). Another speculative reason is that CD56dim NK cells from MS have lower threshold in response to IL-7. It is conceivable that the lower serum concentrations of IL-7 in conjunction with the increased/unaltered membrane-bound IL-7Rα found in NK cells of MS patients, lead to an overall increased IL-7 responsiveness (low threshold to IL-7 stimulation) when IL-7 binds to the membrane-bound receptor. In line with our data, increased IL-7Rα signaling capacities were observed in CD4+ and CD8+ T cells of MS patients. The intralesional production of IL-7 in combination with the lower threshold for IL-7–induced cytotoxicity in MS may enhance the pathogenicity of these T cells (Kreft et al., 2012a; Kreft et al., 2012b). Low expression of IL-7Rα by Treg cells suggests that impaired suppression of an autoimmune mechanism may be important in MS (Haas et al., 2011). Taken together, our study and that of others (Kreft et al., 2012a; Kreft et al., 2012b) demonstrate the altered function of IL-7/IL-7Rα in IL-7Rα-expressing lymphocytes including CD4, CD8, Treg, and NK cells in MS, which may enhance their pathogenicity and exacerbate the disease in a collaborative manner.
IL-7 is reportedly required for the development of thymic (CD127+) and mucosal NK cells (Cella et al., 2010; Vosshenrich et al., 2006) and enhancement of survival of human mature CD56bright NK cells (Michaud et al., 2010a). In the current study, the proliferation of NK cells in response to IL-7 stimulation was observed in NK cells from both groups; however, it does not differ between patients with MS and HC. Compared with healthy adults, IL-7 promotes a higher survival of CD56bright NK cells, but has little effect on CD56dim NK cells survival in MS. As previously observed (Armant et al., 1995; Michaud et al., 2010a), the inhibition of apoptosis by IL-7 is mediated by the up-regulation of Bcl-2, especially in CD56bright NK cells. Results presented here establish that IL-7Rα pathway is dysregulated in the survival and apoptosis of the NK subsets in MS.
To conclude, this work suggests a tight interplay between the IL-7/IL-7Rα pathway and NK cell function in MS. It is possible that abnormally low levels of systemic IL-7 may suppress NK cell function in MS patients. And it is conceivable that CD56bright NK cells with high levels of IL-7Rα from MS have enhanced activity and survival upon IL-7 stimulation. As previously reported that administration of daclizumab resulted in the expansion of CD56bright NK cells in MS patients in association with clinical and radiological improvement (Bielekova et al., 2006), targeting the IL-7/IL-7Rα pathway on CD56bright NK cells might be a compelling potential for therapeutic intervention of MS. Of note, PBMC are used as surrogate tissues in most experiments presented here that may mimic effects occurring in vivo, whether the observed IL-7 biological effects are exclusively mediated by the IL-7R signaling on NK cells is worthy of further investigation.
Highlights.
Serum IL-7 level is decreased and expression of IL-7Rα (CD127) on NK cells is increased in MS patients.
IL-7 stimulation leads to higher increase in cytokine production by CD56bright NK cells and cytotoxicity in NK cells of MS patients.
IL-7 induced proliferation in NK cells does not differ between MS patients and healthy controls.
IL-7 promotes an increased survival in CD56bright NK cells of MS patients by up-regulating Bcl-2 expression.
Acknowledgments
The authors thank Dr. Gay Samuelson for her assistance of manuscript improvement and language editing. The study was supported in part by the National Basic Science Program of China (81230028), National Science Foundation of China (2013CB966900), and US National Institutes of Health (R01AI083294).
Footnotes
Disclosures
The authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Rawi MA, Mansel RE, Jiang WG. Interleukin-7 (IL-7) and IL-7 receptor (IL-7R) signalling complex in human solid tumours. Histol Histopathol. 2003;18:911–23. doi: 10.14670/HH-18.911. [DOI] [PubMed] [Google Scholar]
- Armant M, Delespesse G, Sarfati M. IL-2 and IL-7 but not IL-12 protect natural killer cells from death by apoptosis and up-regulate bcl-2 expression. Immunology. 1995;85:331–7. [PMC free article] [PubMed] [Google Scholar]
- Bielekova B, Catalfamo M, Reichert-Scrivner S, Packer A, Cerna M, Waldmann TA, McFarland H, Henkart PA, Martin R. Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci U S A. 2006;103:5941–6. doi: 10.1073/pnas.0601335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Otero K, Colonna M. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1beta reveals intrinsic functional plasticity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:10961–6. doi: 10.1073/pnas.1005641107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–40. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- French AR, Yokoyama WM. Natural killer cells and autoimmunity. Arthritis Res Ther. 2004;6:8–14. doi: 10.1186/ar1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory SG, Schmidt S, Seth P, Oksenberg JR, Hart J, Prokop A, Caillier SJ, Ban M, Goris A, Barcellos LF, Lincoln R, McCauley JL, Sawcer SJ, Compston DA, Dubois B, Hauser SL, Garcia-Blanco MA, Pericak-Vance MA, Haines JL. Interleukin 7 receptor alpha chain (IL7R) shows allelic and functional association with multiple sclerosis. Nature genetics. 2007;39:1083–91. doi: 10.1038/ng2103. [DOI] [PubMed] [Google Scholar]
- Haas J, Korporal M, Schwarz A, Balint B, Wildemann B. The interleukin-7 receptor alpha chain contributes to altered homeostasis of regulatory T cells in multiple sclerosis. European journal of immunology. 2011;41:845–53. doi: 10.1002/eji.201041139. [DOI] [PubMed] [Google Scholar]
- He YW, Malek TR. Interleukin-7 receptor alpha is essential for the development of gamma delta + T cells, but not natural killer cells. The Journal of experimental medicine. 1996;184:289–93. doi: 10.1084/jem.184.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge JN, Srinivasula S, Hu Z, Read SW, Porter BO, Kim I, Mican JM, Paik C, Degrange P, Di Mascio M, Sereti I. Decreases in IL-7 levels during antiretroviral treatment of HIV infection suggest a primary mechanism of receptor-mediated clearance. Blood. 2011;118:3244–53. doi: 10.1182/blood-2010-12-323600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmeister R, Khaled AR, Benbernou N, Rajnavolgyi E, Muegge K, Durum SK. Interleukin-7: physiological roles and mechanisms of action. Cytokine Growth Factor Rev. 1999;10:41–60. doi: 10.1016/s1359-6101(98)00025-2. [DOI] [PubMed] [Google Scholar]
- Kreft KL, Verbraak E, Wierenga-Wolf AF, van Meurs M, Oostra BA, Laman JD, Hintzen RQ. Decreased systemic IL-7 and soluble IL-7Ralpha in multiple sclerosis patients. Genes and immunity. 2012a;13:587–92. doi: 10.1038/gene.2012.34. [DOI] [PubMed] [Google Scholar]
- Kreft KL, Verbraak E, Wierenga-Wolf AF, van Meurs M, Oostra BA, Laman JD, Hintzen RQ. The IL-7Ralpha pathway is quantitatively and functionally altered in CD8 T cells in multiple sclerosis. Journal of immunology. 2012b;188:1874–83. doi: 10.4049/jimmunol.1102559. [DOI] [PubMed] [Google Scholar]
- Lundstrom W, Fewkes NM, Mackall CL. IL-7 in human health and disease. Seminars in immunology. 2012;24:218–24. doi: 10.1016/j.smim.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nature reviews. Immunology. 2007;7:144–54. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- Michaud A, Dardari R, Charrier E, Cordeiro P, Herblot S, Duval M. IL-7 enhances survival of human CD56bright NK cells. Journal of immunotherapy. 2010a;33:382–90. doi: 10.1097/CJI.0b013e3181cd872d. [DOI] [PubMed] [Google Scholar]
- Michaud A, Dardari R, Charrier E, Cordeiro P, Herblot S, Duval M. IL-7 enhances survival of human CD56bright NK cells. J Immunother. 2010b;33:382–90. doi: 10.1097/CJI.0b013e3181cd872d. [DOI] [PubMed] [Google Scholar]
- Park JH, Yu Q, Erman B, Appelbaum JS, Montoya-Durango D, Grimes HL, Singer A. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Vosshenrich CA, Garcia-Ojeda ME, Samson-Villeger SI, Pasqualetto V, Enault L, Richard-Le Goff O, Corcuff E, Guy-Grand D, Rocha B, Cumano A, Rogge L, Ezine S, Di Santo JP. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nature immunology. 2006;7:1217–24. doi: 10.1038/ni1395. [DOI] [PubMed] [Google Scholar]
- Wiendl H, Gross CC. Modulation of IL-2Ralpha with daclizumab for treatment of multiple sclerosis. Nature reviews. Neurology. 2013;9:394–404. doi: 10.1038/nrneurol.2013.95. [DOI] [PubMed] [Google Scholar]
- Zhang B, Yamamura T, Kondo T, Fujiwara M, Tabira T. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J Exp Med. 1997;186:1677–87. doi: 10.1084/jem.186.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]