Abstract
Purpose
Determine if pre-treatment biomarkers obtained from Diffuse Optical Spectroscopic Tomographic (DOST) imaging predict breast tumor response to Neoadjuvant Chemotherapy (NAC), which would have value to potentially eliminate delays in prescribing definitive local regional therapy that may occur from a standard complete 6–8 months course of NAC.
Experimental design
Nineteen patients undergoing NAC were imaged with DOST before, during and after treatment. The DOST images of total hemoglobin concentration (HbT), tissue oxygen saturation (StO2), and water (H2O) fraction at different time points have been used for testing the abilities of differentiating patients having pathologic complete response (pCR) vs. pathologic incomplete response (pIR).
Results
Significant differences (P-value<0.001, AUC=1.0) were found between pCR patients vs. pIR in outcome, based on the percentage change in tumor HbT within the first cycle of treatment. In addition, pre-treatment tumor HbT (Pre-TxHbT) relative to the contralateral breast was statistically significant (p-value=0.01, AUC=0.92) in differentiating pCR from pIR.
Conclusions
This is the first clinical evidence that DOST HbT may differentiate the two groups with predictive significance based on data acquired before NAC even begins. The study also demonstrates the potential of accelerating the validation of optimal NAC regimens through future randomized clinical trials by reducing the number of patients required and the length of time they need to be followed by using a validated imaging surrogate as an outcome measure.
Keywords: Near Infrared, Optical tomography, chemotherapy response, breast cancer
1. Introduction
Breast cancer is the most common non-skin malignancy in women worldwide, and the second leading cause of female cancer mortality in the United States.(1) A common treatment strategy is neoadjuvant chemotherapy (NAC) prior to surgery when tumor size is larger than 3 cm because of the opportunity to monitor the response of the primary disease which is expected to be representative of response of distant metastases well before they become clinically apparent.(1) Clinical studies have shown that patients with a complete pathological response (pCR) to NAC experience longer disease-free survival.(2–4) However, since the pCR rate is only about 20–30%,(4) and the delay in definitive local therapy that may occur from a complete course of NAC can be long (up to 8 months)(5), prediction of pCR before NAC and/or from early treatment response to stratify disease management is likely to improve the outcomes of patients and their long-term survival. In particular, if a prognostic marker was highly accurate of response to NAC and could successfully be utilized prior to therapy, significant benefits could occur to both the healthcare system and the patient through better disease management.
Conventional cancer imaging systems (mammography, ultrasound (5) and MRI (2, 6)) have been reported to assess response to treatment; however, the evaluations are typically based on changes in tumor volume which occur secondarily to physiological variations, and usually require at least three cycles of treatment before an accurate determination can be reached (7). Functional imaging techniques such as dynamic contrast-enhanced MRI (8), MR spectroscopy (3), BOLD MRI (9) and PET (7, 10, 11), have been used to monitor cancer response to NAC with promising initial results. While identifying prognostic biomarkers that can be imaged prior to treatment is very desirable, robust pre-treatment signatures may not exist in practice or may not be therapy and patient independent. When compared to conventional imaging modalities, Diffuse Optical Spectroscopic Tomography (DOST) is noninvasive, does not use ionizing radiation, nor does it involve costly instrumentation/facilities that are in high demand. DOST also has substantial advantages for efficient and effective longitudinal monitoring because it captures biophysical changes in tissue occurring in the vascular as well as intra- and extra-cellular matrix compartments (12–15). In studies published to date, changes in tumor total hemoglobin concentration (HbT), blood oxygen saturation (StO2) and water content (H2O) were detected before the start of the second cycle of NAC, and these changes appear to be present before morphological (size) alterations occur that can be determined from structural imaging such as x-ray mammography (12, 16–19). Additionally, tumor StO2 was recently shown to be predictive of response to therapy in a cohort of patients imaged with a sub-surface optical scanner (15).
In this paper, we expand our earlier pilot study (12) with a larger accrual of 19 patients with locally-advanced breast cancer (LABC) receiving NAC. The results show that the statistical difference between the pathological complete response (pCR) and pathological incomplete response (pIR) groups based on the percentage change in HbT within the first cycle of treatment is even stronger than before. Interestingly, and perhaps very importantly, pretreatment tumor HbT (Pre-TxHbT) relative to the contralateral breast was found to be statistically different in the two groups for the first time, and may be a prognostic indicator of response that could be assessed before NAC even begins.
2. Materials and Methods
The NIRST imaging system has been developed at Dartmouth during more than a decade of research, and was first approved for experimental breast imaging studies at Dartmouth in 2001.(20, 21) In this study, subjects provided informed consent as part of the protocol approved by the Dartmouth Institutional Review Board (IRB) to use the latest NIRST system12 which the IRB designated as an NSR (non-significant risk) device accordingly to FDA guidelines. The study was Health Insurance Portability and Accountability Act (HIPAA) compliant. Subjects enrolled in this study received NAC recommended by their medical oncologist, typically any of several Taxane/anthracycline chemotherapy regimens. More specifically the regimen frequently used at the time these patients were enrolled in this study. For women with HER-2 negative locally advanced breast cancer was TAC (docetaxel [Taxotere™], doxorubicin [Adriamycin™], and cyclophosphamide), or one of several other anthracycline/taxane combination regimens. For women with locally advanced HER-2 positive disease, patients generally were treated with AC (doxorubicin [Adriamycin™], and cyclophosphamide)/paclitaxel [Taxol]/trastuzumab (Herceptin™), given exactly as in CALGB 49909/NCCTG 9831, which was in the first large adjuvant trial demonstrating the impact of adjuvant trastuzumab (22). Table 1 lists clinical information on the 19 subjects enrolled including age, radiological breast density, menopause status, initial pathological diagnosis/receptor status, initial tumor region of interest (ROI), and surgical pathology outcome (complete or in-complete response, pCR or pIR) after treatment. The mean age of participants was 49 years old (range 27–70). The majority of subjects, 58% (11/19), were pre-menopausal and most, 74% (14/19), had mammographically dense breasts including heterogeneously dense (H) and extremely dense breasts (E) compositions. All of these women completed their NAC and surgery as part of standard-of-care breast cancer management.
Table 1.
Clinical information for the nineteen subjects enrolled including age, mammographic breast density, menopause status, initial pathological diagnosis/receptor status, initial tumor size (region of interest, ROI), and surgical pathology outcome (complete or in-complete response) after treatment. Breast Density is defined as almost entirely fat (F), scattered fibro glandular densities (S), heterogeneously dense (H), and extremely dense (E).
| Pt. Id | age | Meno-pause | Breast Density | Initial Path. (ER/PR/Her2) | Size/ROI (mm) | Treatment regimen | Surgical path. |
|---|---|---|---|---|---|---|---|
| 1 | 36 | neg | S | IDC/DCIS ER+/PR−/Her2+ |
65×37×71 | TAC | pCR |
| 2 | 51 | pos | S | IDC ER−/PR−/Her2− |
44×32×43 | TAC | pCR |
| 3 | 41 | neg | H | IDC ER+/PR+/Her2+ |
53×22×50 | TAC | pCR |
| 4 | 30 | neg | H | IDC ER−/PR−/Her2− |
36 | TAC | pCR |
| 5 | 52 | pos | H | IDC ER−/PR−/Her2+ |
100×70×50 | FEC-Herceptin/Taxol-Herceptin | pCR |
| 6 | 63 | pos | H | IDC/DCIS ER+/PR+/Her2+ |
90×70×40 | TAC | pCR |
| 7 | 60 | pos | S | IDC/DCIS ER−/PR−/Her2+ |
39×26×42 | Taxol-Herceptin/FEC-Herceptin | pCR |
| 8 | 52 | neg | E | IDC ER−/PR−/Her2− |
34×45×100 | TAC | pCR |
| 9 | 66 | pos | H | IDC/DCIS ER−/PR−/Her2+ |
59×43×53 | Taxol-Herceptin/FEC-Herceptin | pCR |
| 10 | 30 | neg | H | IDC ER−/PR−/Her2− |
40×26×40 | Taxol | pIR |
| 11 | 52 | neg | E | IDC ER+/PR+/Her2− |
58×34×45 | Taxol | pIR |
| 12 | 62 | pos | H | IDC ER+/PR+/Her2− |
70×50×50 | Adriamycin/cyclophosphamide/taxotere | pIR |
| 13 | 70 | pos | S | IDC/DCIS ER−/PR−/Her2+ |
63×28×30 | Taxol-Herceptin/FEC-Herceptin | pIR |
| 14 | 53 | neg | E | ILC ER+/PR+/Her2− |
76×45×79 | TAC | pIR |
| 15 | 27 | neg | H | IDC ER+/PR+/Her2− |
61×18×41 | TAC | pIR |
| 16 | 53 | neg | H | IDC ER+/PR+/Her2+ |
62×46×87 | Taxol-Herceptin/FEC-Herceptin | pIR |
| 17 | 50 | neg | H | IDC ER+/PR+/Her2+ |
40 | Abraxane/Avastin | pIR |
| 18 | 56 | pos | H | IDC ER+/PR+/Her2+ |
55×34×52 | TAC | pIR |
| 19 | 38 | neg | S | IDC/DCIS ER+/PR+/Her2− |
84×93×50 | TAC | pIR |
Breast Density- S: scatter; H: Heterogamous dense; E: extremely dense. Initial pathological results- IDC: invasive ductal carcinoma; DCIS: ductal carcinoma in situ.
For each subject, baseline clinical images (primarily contrast MRI) of the diseased breast were acquired as a part of standard clinical care. The details of the MRI parameters have been described previously (12). DOST was performed prior to the start of NAC (Pre-Tx, baseline), approximately days 7, 14 and 21 in cycles 1 and 2, and within 7 days before the second half of treatment was initiated (mid-point). These time points were selected because they were representative of the biological time periods during which treatment variations were most likely to be observed based upon earlier studies (13). After all treatment cycles were completed, DOST images of both breasts were obtained several days before surgery. Histopathological characteristics of the resected tissue were evaluated after surgery (17), and a complete (pCR) or incomplete (pIR) pathological response to NAC was determined and compared with the DOST results.
As described previously (12, 23), subjects were prone on a padded examination table during each DOST imaging session with the breast to be imaged pendant within the fiber-optic array. The circular fiber array was composed of 48 fiber bundles positioned in three planes that were separated vertically by a distance of 1.5 cm, and their diameters each decreased by 4 mm from chest wall towards the nipple. During the Pre-Tx imaging session, the tumor location was marked on the breast using data obtained from conventional clinical imaging (primarily contrast MRI) before the subject was positioned on the examination bed. The imaging plane on the ipsilateral breast was chosen to be in the middle of the tumor (along the direction from chest wall towards the nipple). On the contralateral side, the corresponding plane having the same distance from the chest wall was selected. A computer controlled video camera (Creative, Optia AF) was placed under the fiber array and used to position the breast in the center of the fibers, and to confirm that the fiber bundles were in contact with the breast surface. The height of the fiber bundle planes was adjusted with a vertical motor so that the middle plane of the array was placed on the breast surface mark designating the tumor location. The diameter of the fiber array was controlled by 16 motors that translated each fiber bundle radially in synchrony to ensure the bundles contacted the breast uniformly. The distance from the chest wall to the top fiber plane, and the diameter of the circular planes were recorded. In all subsequent DOST imaging sessions, the chest wall distance was kept the same for both the abnormal and contralateral breasts, whereas the diameter of the abnormal breast varied depending on the changes in tumor and breast size that occurred during treatment for each subject.
Frequency modulated near-infrared light from 6 laser diodes was delivered sequentially to illuminate the breast with an average optical power of less than 30mW. A total of 240 measurements of transmitted light amplitude and phase was acquired for each wavelength of light and all detector positions within each plane of the fiber bundle array. The data acquisition time for the three measurement planes for all six wavelengths was approximately7 minutes.
A spectrally constrained chromophore and scattering reconstruction method was used to separate the tissue absoption and scattering properties, and recover HbT, StO2 and H2O images (24). In this method, chromophore constraints were incorporated into the reconstruction algorithm to estimate oxyhemoglobin, deoxyhemoglobin, and H2O, while an empirical approximation to Mie scattering theory was used to constrain the elastic scattering properties. A series of spectral images was recovered for each plane (25), and the plane maximally overlapping the tumor was used for analysis.
For image analysis, the tumor ROI was defined by the radiologist’s interpretation of the contrast MR coronal plane (acquired prior to initiation of therapy) corresponding to the same mid-plane tumor distance from the chest wall used to position the DOST instrumentation. The entire tumor area, the entire area outside of the tumor ROI, and the entire area of the contralateral breast, in the designated plane were defined as tumor, non-tumor and contralateral ROIs, respectively. The mean values of HbT, StO2, and H2O of each ROI were found from the reconstructed images. To minimize baseline variations from inter-subject variability of breast density affecting the absolute HbT, StO2 and H2O parameters, ratios relative to the pre-Tx average of the contralateral breast (ROI/contralateral whole breast) were formed. In addition, ratios of the mean values in the ROI relative to outside the ROI were defined as tumor contrast. At each time point, a two-sample t-test was used to determine if significant changes occurred between each time point and the baseline in HbT, StO2 and H2O in the pCR and pIR patients. Receiver Operating Characteristic (ROC) curves were formed and Area Under the ROC curve (AUC) was obtained to illustrate graphically the performance of DOST at baseline, after the first cycle of NAC and after later sessions. We use 2,000 stratified bootstrap replicates to estimate the 95% confidence interval for the AUC. The Bonferroni correction was used to adjust for multiple comparisons.
3. Results
Figures 1a and 1b show a set of DOST images of a pCR case prior (same day, but before the first infusion, Pre-Tx), during (on Day 7 of cycle 1 [C1, D7]), and after NAC (Post-Tx, 7 days after the last infusion and 22 days before surgery), as well as an axial contrast MR image (30 days) before NAC was started. This 66 year old woman had a large palpable lump in the upper central portion of the left breast. MR imaging showed the lump size was 59 × 43 × 53 mm, and the biopsy results indicated it was ER(−), PR(−), HER2neu(+) (ACR Category 6) invasive ductal carcinoma (IDC) mixed with ductal carcinoma in situ (DCIS). The chemotherapy regimen this subject received was four cycles of paclitaxel (Taxol) and trastuzumab (Herceptin), followed by four cycles of FEC (5-flourouracil, epirubicin, and cyclophosphamide)/trastuzumab (Herceptin). The DOST images are shown in Fig. 1a with HbT and H2O concentrations in the ROI decreasing after the first infusion, and eventually no contrast in HbT and H2O in the RO1 was detectable after NAC was completed.
Figure 1.
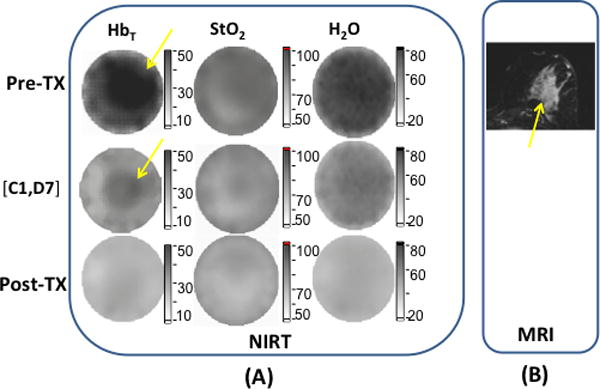
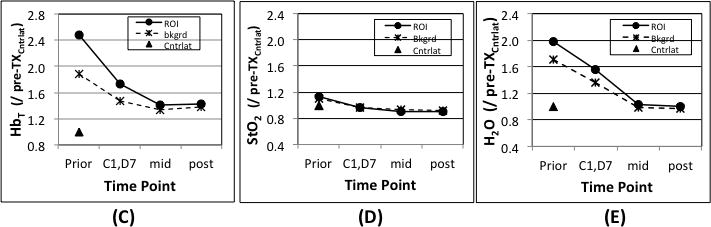
Images and graphs of a pCR case with (a) DOST images prior, during and after NAC. In (b), axial post contrast subtraction MRI before NAC shows an enhancing mass (yellow arrow) in the deep central left breast that measured 5.9 × 4.3 × 5.3 cm. In (c), (d) and (e), graphs of the tumor HbT, StO2 and H2O are shown at different time points.
Figures 1c, 1d and 1e show HbT, StO2 and H2O at different time points of NAC. The mid-point session occurred on [C4, D14], which is 7 days prior to the first infusion of the second half of the NAC regime. During the first half of NAC, HbT (Fig. 1 c), StO2 (Fig. 1d) and H2O (Fig.1e) in the ROI decreased from 2.47 to 1.41, 1.13 to 0.91 and from1.98 to 1.02, respectively, for the three parameters. Furthermore, the early percentage changes of HbT, StO2and H2O between [C1, D7] and the Pre-Tx session were −110%, −15% and −25%, respectively. The Pre-Tx contrasts in HbT, StO2 and H2O for this patient were 1.3, 1.0 and 1.2, respectively. The surgical pathology showed no residual IDC or DCIS existed in the excised specimen, and confirmed the case was a pCR.
Figures 2a and 2b contain a set of DOST images from a pIR case where Pre-Tx (on the same day, before the first infusion), during (on Day 14 of cycle 1 [C1, D14]), and Post-Tx (18 days after the last infusion and a day before the surgery), as well as contrast MR images (20 days) before the start of NAC are presented. The axial contrast MR images in Fig. 2b revealed that this 56 year old woman had an index invasive breast cancer with dimensions of 5.4 × 3.4 × 5.2 cm in the lateral right breast with rim enhancing skin metastases overlying the index tumor at 9:00 o’clock en face (upper image). Additionally, she had a subpectoral lymph node metastasis (lower image) at 12:00 o’clock measuring 2.5 × 1.9 × 3.0 cm. The biopsy results proved the lesion was an ER(+), PR(+), HER2neu(+) (ACR Category 6) mucinous IDC. The chemotherapy regimen this subject received consisted of four cycles of doxorubicin (Adriamycin)/Cyclophosphamide followed by four cycles of paclitaxel (Taxol). The subpectoral lymph node metastasis could not be imaged by DOST due to its proximity to the chest wall (See Fig. 2b, lower MR image). Since the rim enhancing skin metastases exist overlying the index tumor, (see Fig. 2b, upper MR image), the tumor ROI in DOST was evaluated as the sum in this case. In the DOST images shown in Fig. 2a, HbT and StO2 in the ROI increased, while H2O decreased in the first cycle of NAC, but residual contrast in HbT and H2O were observed in the images after NAC was completed.
Figure 2.
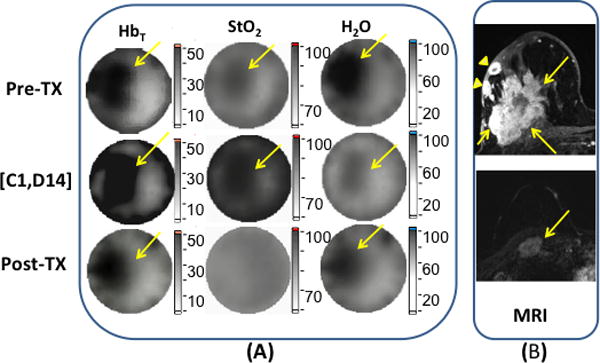
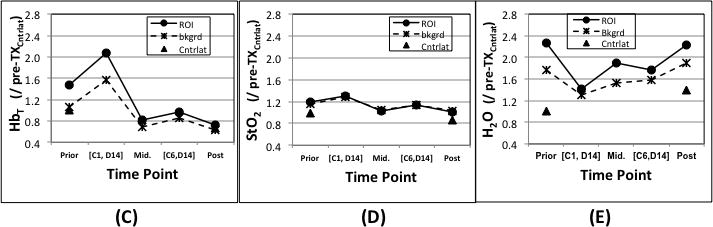
Images and graphs of a pIR case with (a) DOST images prior, during and after NAC. In (b), axial post contrast subtraction MRI before NAC shows the index invasive breast cancer (upper image) in the lateral right breast at 9:00 o’clock and measuring 5.4 × 3.4 × 5.2 cm, and a subpectoral lymph node metastasis (lower image) at 12:00 o’clock measuring 2.5 × 1.9 × 3.0 cm. The yellow arrows show the dominant tumors in each image. Additionally, rim enhancing skin metastases exist overlying the index tumor at 9:00 o’clock (arrowheads). In (c),(d) and (e), HbT, StO2 and H2O are graphed for different time points.
To quantify the HbT, StO2, and H2O changes, Figures 2c, 2d and 2e present graphs of these values at different time points relative to the NAC treatment cycles. The mid-point imaging session occurred on the same day, but prior to the first infusion of the second half of the NAC regime. During the full course of NAC, HbT (Fig. 2c), StO2 (Fig. 2d) and H2O (Fig. 2e) in the ROI fluctuated with an overall decrease from 1.47 to 0.72, 1.19 to 1.00 and from 2.26 to 2.23, respectively. However, the early percentage changes in HbT, StO2 and H2Obetween [C1, D14] and Pre-Tx were 59%, 11% and −84%, respectively. The Pre-Tx contrasts in HbT, StO2 and H2O for this patient were 1.4, 1.0 and 1.3, respectively. The pathology results on the surgical specimen revealed that residual IDC and DCIS were present in the lesions, although with some treatment effects, and confirmed the case was a pIR. In addition, IDC was identified in the tissue between the skin metastases and the index tumor; hence, the two represented one contiguous cancer with a maximum diameter of 65mm. The pathological results confirmed the validity of the ROI definition we used for the DOST image analysis.
Of the 19 patients who finished all imaging exams and were included in the analysis, 9 subjects were confirmed as pCR whereas 10 were confirmed as pIR. Figure 3 shows box plots of early percentage changes in HbT (ΔHbT) in (a), Pre-TxHbT in (b), and Pre-Tx contrasts in StO2 (cStO2) in (c) and H2O (cH2O) in (d), respectively. The early ΔHbT in Fig 3a represents the change in the pretreatment (Pre-Tx) image and the image which was acquired in the two week time-frame after the first cycle [C1, d7], and the first day of cycle 2 [C2, D1]. To allow inter-subject comparisons of HbT, StO2 and H2O, the HbT, StO2 and H2O values were normalized to the mean of the contralateral breast from the pre-treatment baseline image on a subject-by-subject basis. The red lines in the boxes represent the medians of the 9 pCR and 10pPR subjects, respectively. The two ends of the line through each box indicate the full range of each property. Means/medians of early ΔHbT% in the pCR and pIR cases were −74%/−43% and 21%/20%, respectively. The p-value for difference in these means between the pCR and pIR groups was 0.0003. As shown in Fig. 3b, means/medians of Pre-TxHbT in the pCR and pIR cases were 2.2%/2.0% and 1.5%/1.5%, respectively. The difference in mean Pre-TxHbT between the pCR and pIR groups was significant with a p-value=0.01. The box plots of Pre-TxcStO2 and cH2O in Fig. 3c and 3d showed the means/medians in pCR and pIR groups were 1.1/1.0 and 1.4/1.3, and 1.0/1.0 and 1.2/1.1, respectively. Statistical tests for differences in mean Pre-TxcStO2 and cH2O between the pCR and pIR groups indicated marginal significance (p-values of 0.07 and 0.06, respectively).
Figure 3.
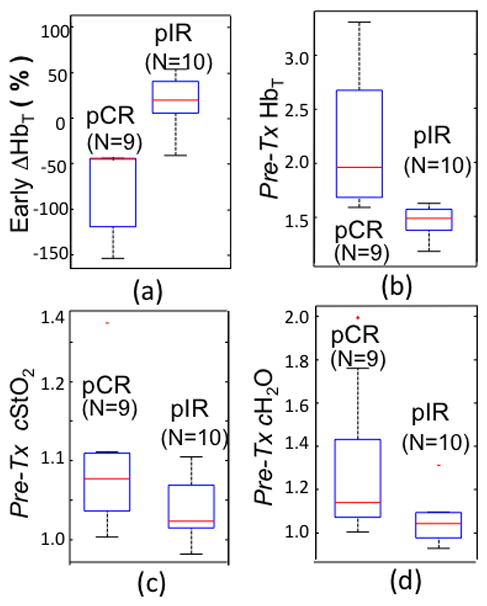
Box plots of (a) early % changes in tumor HbT between the Pre-Tx and the last imaging session in the first NAC cycle are shown with (b) Pre-TxHbT, (c) Pre-Tx contrast in StO2 and (d) Pre-Tx contrast in H2O.
Figure 4 contains Receiver Operating Characteristic (ROC) analysis of sensitivity versus specificity as quantified by the normalized Area Under Curve (AUC) for early ΔHbT% in (a), Pre-TxHbT in (b), Pre-TxcStO2in (c) and cH2O in (d). The AUC values were 1.0 and 0.92 for early ΔHbT (Fig. 4a) and Pre-TxHbT (Fig. 4b), indicating excellent potential for high sensitivity and specificity with these diagnostic measures. The p-values of Pre-TxcStO2 and cH2O were not significant in differentiating the pCR from pIR groups; however, the AUC=0.8 for StO2 and AUC=0.74 for H2O indicating fair potential for diagnostic accuracy when using these tests. To investigate the cutoff for early ΔHbT% and Pre-TxHbT, the Positive Predictive Value (PPV) was calculated. In this small patient group, less than a 40% reduction in HbT during the first NAC cycle or 1.5 in Pre-TxHbT would be needed to achieve a perfect PPV = 1.0.
Figure 4.
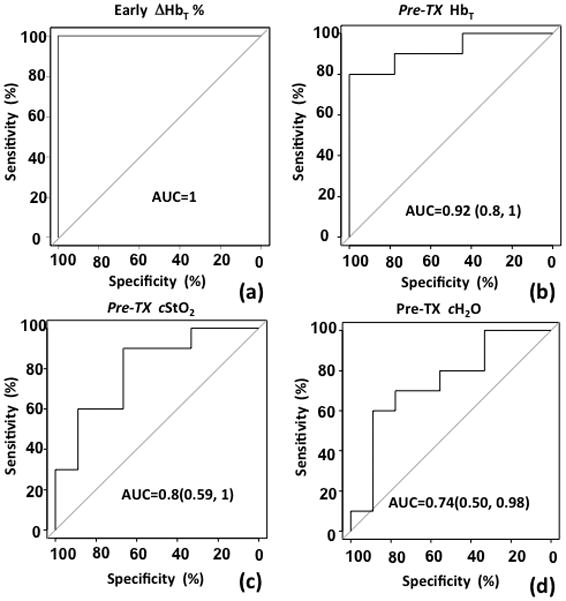
AUC curves are shown for (a) early percentage changes of tumor HbT between the Pre-Tx and the last imaging session in the first NAC cycle, and (b) Pre-TxHbT, (c) Pre-Tx contrast in StO2, and (d) Pre-Tx contrast in H2O.
Table 2 summarizes the means and standard deviations of age, early ΔHbT, and Pre-TxHbT, StO2 and H2O in the ROI and their contrast levels. The patient counts for positive and negative, p-values and AUC of menopause status, ER, PR, Her2, early ΔHbT, and Pre-TxHbT, StO2 and H2O in the ROI and their contrast levels for differentiating the pCR and pIR groups are presented in this table as well.
Table 2.
Summary of means and standard deviations for age, early ΔHbT, and Pre-TxHbT, StO2 and H2O in the ROI and their contrasts. The patient counts for positive and negative; the p-values and AUC of menopause, ER, PR, Her2, early ΔHbT, and Pre-TxHbT, StO2 and H2O in the ROI and their contrasts for differential pCR and pIR groups are shown as well.
| age | Menopause N counts (+/−) | ER (+/−) | PR (+/−) | Her2 (+/−) | Early ΔHbT | Pre-TXHbT | Pre-TXStO2 | Pre-TXH2O | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ROI | contrast | ROI | contrast | ROI | contrast | |||||||
| pCR | 50.1±3.1 | 5/4 | 3/6 | 2/7 | 6/3 | −73.6±11.7 | 2.2±0.16 | 1.8±0.25 | 1.1±0.02 | 1.1±0.02 | 1.4±0.11 | 1.4±0.08 |
| pIR | 48.3±3.3 | 3/7 | 8/2 | 8/2 | 4/6 | 20.7±10.6 | 1.5±0.03 | 1.3±0.04 | 1.3±0.01 | 1.0±0.01 | 1.4±0.13 | 1.2±0.04 |
| P-Value | 0.29 | 0.29 | 0.04* | 0.01* | 0.27 | 0.0003* | 0.01* | 0.15 | 0.29 | 0.07 | 0.57 | 0.06 |
| AUC | <0.60 | <0.60 | <0.60 | <0.60 | <0.60 | 1.00^ | 0.92^ | <0.60 | <0.60 | 0.80” | <0.60 | 0.74” |
significantly differential pCR from pIR;
the excellent accuracy of the diagnostic test;
the fair accuracy of the diagnostic test
4. Discussion
The data in Figure 3a offers improved power for statistically-valid separation of ΔHbT between subjects who had pCR versus pIR relative to our previous pilot study (12), and is encouraging given our earlier findings are reinforced with the increased number of enrollments. A statistically-significant separation of Pre-TxHbT between subjects with pCR and pIR (p-value = 0.01) was also observed for the first time, which suggests that DOST may have potential to differentiate the two groups even before NAC has begun with an easily measured parameter. Although p-values of Pre-TxcStO2 (0.07) and cH2O (0.06) did not individually yield a statistically-significant separation between the pCR and pIR groups, the AUC values of 0.8 (cStO2) and 0.74 (cH2O) suggest that the two properties can add diagnostic value to Pre-TxHbT at the start of treatment. For example, when we added these two properties to pre-TxHbT, the multi-parametric AUC increased from 0.92 to 0.93. One solution to improving the StO2 and H2O image quality, which could also improve the accuracy of the estimated HbT values, would be to acquire more data at longer wavelengths that are known to be strongly absorbed by H2O and lipids. Previous and ongoing studies (26, 27) indicate that the addition of CW data acquired from at least three longer wavelengths and the inclusion of lipid as an additional chromophore in the image reconstruction, has improved the contrast in H2O by more than 30%. Since the imaging time in the present study was about 15 min for each breast, patient movement during an exam may contribute to noise in the images. Concurrent multi-wavelength data acquisition can accomplish complete single-plane tomographic recordings in less than 1 min (28), which is expected to improve image quality by reducing/eliminating patient movement effects.
The observation that pre-treatment HbT is predictive of response to NAC concurs with the hypothesis that functional vasculature is required for adequate blood flow to the tumor, which is necessary for adequate chemotherapy distribution and cellular uptake, and that tumors which lack sufficient vascular supply will suffer from inadequate chemotherapy delivery (29, 30). While this hypothesis was not studied explicitly, the apparently prognostic indication provided by Pre-TxHbT when combined with earlier pathological analysis (17) is consistent with the theory. Specifically, our earlier study of DOST versus pathologic immunohistochemical staining of biopsy specimens and resected surgical tissues in the same breasts showed that HbT changes were directly correlated to changes in pathologically measured CD31 reduction in response to NAC (17). Here, CD31 is a pan-endothelial marker for identifying pre-existing blood vessel density and size. The results indicate that the current measure of HbT prior to NAC is an indicator of the actively perfused microvessels, and that the tumor region must be perfused to 50% above baseline in order to respond to the chemotherapy. The observation is potentially important both for fundamental understanding of NAC as well as providing a simple prognostic indicator for managing patients.
Relative to other diffuse optical spectroscopy systems for monitoring breast tumor response to NAC (16, 19), the tomographic approach applied in this study has the advantage of providing more sensitive and spatially-resolved information about tumor response, and this information is especially important when a significant amount of tumor is located deep (greater than ~3 cm) in the breast. DOST also have the potential to separate responses of each tumor component in cases involving multi-centric disease. The pIR case presented in Figure 2 is a good example. The DOST results localized the response in index tumor with rim enhancing skin metastases to NAC, whereas the response in subpectoral lymph node metastasis was excluded.
As shown in Table 2, statistically-significant separation of either ER or PR between subjects with pCR and pIR (p-value = 0.04 and 0.01, respectively) was also observed. However, analysis of the predictive power of tumor response to NAC by combining these variables with DOST properties was not considered because of the limited number of patients enrolled in the study. Nonetheless, we expect that these variables, as well as other clinical parameters such as age and radiological density, will add predictive power to DOST properties in future studies involving larger numbers of subjects.
The initial design of this NAC imaging study incorporated several therapeutic regimens that included 6–8 cycles of chemotherapy. Enrollment of women was inhibited by the requirement of breast imaging several times during the course of therapy with additional contrast MRI scans. Several women dropped out of the trial after the pretreatment imaging session because of treatment-induced fatigue from NAC or due to scheduling conflicts relative to the multiple imaging sessions that were required for participation. Imaging patients during the process of chemotherapy infusion within the oncology clinic could ease the burden of participation. Of note, a portable DOST monitor to track NAC response in subjects who are unable to return for a separately scheduled imaging session is technically feasible.
5. Conclusion
In this study of 19 patients with locally advanced breast cancer, Pre-TxHbT inside the tumor region-of-interest relative to the contralateral breast, and change in HbT after the first cycle of NAC were significant predictors of a pathologic complete response. Therefore, HbT of the involved and contralateral breasts measured before the start of NAC or a change in HbT in the involved breast within the first cycle of treatment could be used as prognostic indicators of neoadjuvant chemotherapeutic response. Given the importance of patient management in these complex, expensive and time consuming trials, validation of imaging indicators in a prospective clinical trial which can lead to wider adoption of the simple NIRS technology is needed. Identification of potential biomarkers that could lead to image-based surrogates for pCR, and accelerate the validation of optimal NAC regimens through future randomized clinical trials by reducing the number of patients required for enrollment and the length of time they need to be followed, is also critically important.
Statement of Translational Relevance.
The results of this study suggest that biomarkers obtained through Diffuse Optical Spectroscopic Tomographic imaging could be prognostic for response, potentially eliminating the delay in definitive local regional therapy that may occur from a complete 6–8 months course of Neoadjuvant Chemotherapy (NAC). The deeper implication of this information is that certain tumors are pre-disposed to responding to NAC, and that this predisposition should be known prior to choosing the therapy, similar to how immunohistochemistry is used today. The study also demonstrates the potential of accelerating the validation of optimal NAC regimens through future randomized clinical trials by reducing the number of patients required and the length of time they need to be followed by using a validated imaging surrogate as an outcome measure. In summary, both fundamental scientific insight and clinically relevant measurements about NAC result from this study.
Acknowledgments
This work has been funded by National Cancer Institute research grants PO1CA80139, R01 CA069544, and R01CA176086.
References
- 1.Gradishar WJ, Anderson BO, Blair SL, Burstein HJ, Cyr A, Elias AD, et al. Breast Cancer Version 3.2014. Journal of the National Comprehensive Cancer Network. 2014;12(4):542–90. doi: 10.6004/jnccn.2014.0058. [DOI] [PubMed] [Google Scholar]
- 2.Le-Petross CH, Hylton N. Role of Breast MR Imaging in Neoadjuvant Chemotherapy. Magnetic Resonance Imaging Clinics of North America. 2010;18:249–58. doi: 10.1016/j.mric.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Baek H-M, Chen J-H, Nie K, Yu HJ, Bahri S, Mehta RS, et al. Predicting pathologic response to neoadjuvant chemotherapy in breast cancer by using MR imaging and quantitative 1H MR spectroscopy. Radiology. 2009;251(3):653–62. doi: 10.1148/radiol.2512080553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roblyer D, Ueda S, Cerussi l, Tanamai W, Durkin A, Mehta R, et al. Optical imaging of breast cancer oxyhemoglobin flare correlates with neoadjuvant chemotherapy response one day after starting treatment. Proceedings of the National Academy of Sciences. 2011;108(35):14626–31. doi: 10.1073/pnas.1013103108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huber S, Medl M, Vesely M, Czembirek H, Zuna I, Delorme S. Ultrasonographic tissue characterization in monitoring tumor response to neoadjuvant chemotherapy in locally advanced breast cancer (work in progress) Journal of Ultrasound in Medicine. 2000;19(10):677–86. doi: 10.7863/jum.2000.19.10.677. [DOI] [PubMed] [Google Scholar]
- 6.Loo CE, Straver ME, Rodenhuis S, Muller SH, Wesseling J, Peeters M-JTFDV, et al. Magnetic resonance imaging response monitoring of breast cancer during neoadjuvant chemotherapy: relevance of breast cancer subtype. Journal of Clinical Oncology. 2011;29(6):660–6. doi: 10.1200/JCO.2010.31.1258. [DOI] [PubMed] [Google Scholar]
- 7.Rousseau C, Devillers A, Sagan C, Ferrer L, Bridji B, Campion Lc, et al. Monitoring of early response to neoadjuvant chemotherapy in stage II and III breast cancer by [18F]fluorodeoxyglucose positron emission tomography. Journal of clinical oncology. 2006;24(34):5366–72. doi: 10.1200/JCO.2006.05.7406. [DOI] [PubMed] [Google Scholar]
- 8.Pickles MD, Lowry M, Manton DJ, Gibbs P, Turnbull LW. Role of dynamic contrast enhanced MRI in monitoring early response of locally advanced breast cancer to neoadjuvant chemotherapy. Breast Cancer Research & Treatment. 2005;91(1):1–10. doi: 10.1007/s10549-004-5819-2. [DOI] [PubMed] [Google Scholar]
- 9.Tripathy D, Jiang L, Rao N, McColl R, Xie X, Weatherall P, et al. Blood oxygen level dependent (BOLD) contrast MRI and breast cancer chemotherapy response. Annual Meeting of American Society of Clinical Oncology; June 2–6; Chicago, Illinois: American Society of Clinical Oncology; 2006. p. 569S. [Google Scholar]
- 10.Dietz DW, Dehdashti F, Grigsby PW, Malyapa R, Myerson RJ, Picus J, et al. Tumor hypoxia detected by Positron Emission Tomography with 60Cu-ATSM as a predictor of response and survival in patients undergoing neoadjuvant chemoradiotherapy for rectal carcinoma: A pilot study. Diseases of the colon & rectum. 2008;51:1641–8. doi: 10.1007/s10350-008-9420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martoni AA, Zamagni C, Quercia S, Rosati M, Cacciari N, Bernardi A, et al. Early 18F-2-Fluoro-2-Deoxy-D-Glucose Positron Emission Tomography may identify a subset of patients with estrogen receptor-positive breast cancer who will not respond optimally to preoperative chemotherapy. Cancer. 2010;116(4):805–13. doi: 10.1002/cncr.24820. [DOI] [PubMed] [Google Scholar]
- 12.Jiang S, Pogue BW, Carpenter CM, Poplack SP, Wells WA, Kogel CA, et al. Evaluating tumor response to neoadjuvant chemotherapy with Diffuse Optical Spectroscopic Tomography: Case studies of tumor Region of Interest changes. Radiology. 2009;252(2):551–60. doi: 10.1148/radiol.2522081202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cerussi A, Hsiang D, Shah N, Mehta R, Durkin A, Butler J, et al. Predicting response to breast cancer neoadjuvant chemotherapy using diffuse optical spectroscopy. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(10):4014–9. doi: 10.1073/pnas.0611058104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choe R, Corlu A, Lee K, Durduran T, Konecky SD, Grosicka-Koptyra M, et al. Diffuse optical tomography of breast cancer during neoadjuvant chemotherapy: a case study with comparison to MRI. Medical Physics. 2005;32(4):1128–39. doi: 10.1118/1.1869612. [DOI] [PubMed] [Google Scholar]
- 15.Ueda S, Roblyer D, Cerussi A, Durkin A, Leproux A, Santoro Y, et al. Baseline tumor oxygen saturation correlates with a pathologic complete response in breast cancer patients undergoing neoadjuvant chemotherapy. Cancer Research. 2012;72(17):4018–328. doi: 10.1158/0008-5472.CAN-12-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerussi AE, Tanamai VW, Mehta RS, Hsiang D, Butler JA, Tromberg BJ. Frequent optical imaging during breast cancer neoadjuvant chemotherapy reveals dynamic tumor physiology in an individual patient. Academic Radiology. 2010;17:1031–9. doi: 10.1016/j.acra.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pakalniskis MG, Wells WA, Schwab MC, Froehlich HM, Jiang S, Li Z, et al. Tumor angiogenesis change estimated by diffuse optical spectroscopic tomography; demonstrated correlation in women undergoing neo-adjuvant chemotherapy for invasive breast cancer? Radiology. 2011;259(2):365–74. doi: 10.1148/radiol.11100699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choe R, Durduran T. Diffuse optical monitoring of the neoadjuvant breast cancer therapy. IEEE Journal of selected topics in quantum electronics. 2012 doi: 10.1109/JSTQE.2011.2177963. a publication of the IEEE Lasers and Electro-optics Society. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Q, DeFusco PA, Ricci AJ, Cronin EB, Hegde PU, Kane M, et al. Assessing response to neoadjuvant chemotherapy by using US-guided Near-Infrared tomography. Radiology. 2013;226(2):433–42. doi: 10.1148/radiol.12112415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pogue BW, Jiang S, Dehghani H, Kogel C, Soho S, Srinivasan S, et al. Characterization of hemoglobin, water, and NIR scattering in breast tissue: analysis of intersubject variability and menstrual cycle changes. Journal of Biomedical Optics. 2004;9(3):541–52. doi: 10.1117/1.1691028. [DOI] [PubMed] [Google Scholar]
- 21.Poplack SP, Paulsen KD, Hartov A, Meaney PM, Pogue BW, Tosteson TD, et al. Electromagnetic breast imaging: average tissue property values in women with negative clinical findings. Radiology. 2004;231(2):571–80. doi: 10.1148/radiol.2312030606. [DOI] [PubMed] [Google Scholar]
- 22.Perez EA, Romond EH, Suman VJ, Jeong J-H, Davidson NE, Geyer Charles E, Jr, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2–positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. Journal of clinical oncology. 2011;29(25) doi: 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang S, Pogue BW, McBride TO, Doyley MM, Poplack SP, Paulsen KD. Near-infrared breast tomography calibration with optoelastic tissue simulating phantoms. J Electronic Imag. 2003;12(4):613–20. [Google Scholar]
- 24.Srinivasan S, Pogue BW, Jiang S, Dehghani H, Paulsen KD. Spectrally constrained chromophore and scattering near-infrared tomography provides quantitative and robust reconstruction. Applied Optics. 2005;44(10):1858–69. doi: 10.1364/ao.44.001858. [DOI] [PubMed] [Google Scholar]
- 25.Srinivasan S, Pogue BW, Jiang S, Dehghani H, Kogel C, Soho S, et al. Interpreting hemoglobin and water concentration, oxygen saturation, and scattering measured by near-infrared tomography of normal breast in vivo. Proc Nat Acad Sci USA. 2003;100(21):12349–54. doi: 10.1073/pnas.2032822100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Jiang S, Li Z, diFlorio-Alexander RM, Barth R, Kaufman PA, et al. In vivo quantitative imaging of normal and cancerous breast tissue using broadband diffuse optical tomography. Medical Physics. 2010;37(7):3715–24. doi: 10.1118/1.3455702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Pogue BW, Jiang S, Paulsen KD. Near-Infrared Tomography of breast cancer hemoglobin, water, lipid and scattering properties using a combined frequency domain and continuous wave measurement system. Optics Letters. 2010;35(1):82–4. doi: 10.1364/OL.35.000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang S, Pogue BW, Laughney AM, Kogel CA, Paulsen KD. Measurement of pressure-displacement kinetics of hemoglobin in normal breast tissue with near-infrared spectral imaging. Applied Optics. 2009;48(10):D130–D6. doi: 10.1364/ao.48.00d130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goel S, Fukumura D, Jain RK. Normalization of the tumor vasculature through oncogenic inhibition: An emerging paradigm in tumor biology. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(20):E1214. doi: 10.1073/pnas.1203794109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bottos A, Martini M, Di Nicolantonio F, Comunanza V, Maione F, Minassi A, et al. Normalization of the tumor vasculature through oncogenic inhibition: an emerging paradigm in tumor biology. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(6):E353–9. doi: 10.1073/pnas.1105026109. [DOI] [PMC free article] [PubMed] [Google Scholar]


