Abstract
Colorectal primary signet ring cell carcinoma (PSRCCR) is a rare entity with a dismal prognosis, mainly because of delayed diagnosis. The objective of this study was to investigate the clinicopathologic features and prognostic factors for PSRCCR. This is a retrospective study including the data of 22 patients with PSRCCR who underwent surgery. Patients were categorized by age, sex, tumor site, and stage. Fifteen patients were male. Median age was 40 years. Sites for metastases were lymph nodes (86.4%), peritoneum (40.9%), and liver (9.1%). Most of the patients (91%) had stage III or IV tumors. The rates of curative and palliative resections performed were equal. Mean overall survival and mean progression-free survival times were found to be 33.3 ± 7.1 months (95% confidence interval, 19.4–47.2 months) and 11.8 ± 3.5 months (95% confidence interval, 4.9–18.7 months), respectively. It was concluded that site of the tumor, presence of bowel obstruction, peritoneum and lung metastases, adjacent organ infiltration, TNM stage, and efficiency of surgery have significant effects on survival. All in all, these aggressive tumors are generally diagnosed at advanced stages. Depending on the situation, survival is shorter. A high degree of vigilance is required for these patients to avoid the negative impact of late diagnosis on survival.
Key words: Signet ring cell, Colorectal cancer, Histopathology, Survival
Primary signet ring cell carcinoma is a tumor most commonly located in the stomach, and less frequently in the breast, gallbladder, bladder, and pancreas.1 Primary signet ring cell carcinoma of the colon and rectum (PSRCCR) is a rare entity, with a reported incidence of less than 1%.2 It has a markedly poor prognosis.3 Because symptoms often develop late, it is usually diagnosed at an advanced stage.4 Furthermore, it typically appears in young adults.5
Macroscopically, PSRCCR shows the characteristic appearance of linitis plastica, as a shrunken, rigid structure.1–5 Histologically, the neoplastic cells resemble signet rings because they contain abundant intracytoplasmic mucin, which pushes the nuclei to the periphery.1–5 The presence of mucus secretion in microscopic examinations of the tumor is one of the most important parameters determining the biologic behavior of colorectal carcinomas; other factors are age, sex, tumor location, tumor diameter, grade, stage, lymphatic and vascular invasion, periserosal overgrowth, and distant metastasis.6,7
So far, only a limited number of case reports have been published on this subject. Most publications have reported on a small number of patients and have presented controversial results. The objective of this study was to investigate the characteristic clinicopathologic features of colorectal signet ring cell carcinomas and the parameters affecting prognosis within our patient group.
Patients and Methods
Data collection
The study was conducted according to the recommendations of the Declaration of Helsinki on Biomedical Research involving Human Subjects. This retrospective clinical study was performed in the Baskent University Department of General Surgery after obtaining the approval of the university ethics committee (KA08/188). In November 2006, a colorectal cancer database was set up containing clinicopathologic information for each patient and corresponding data. Eligible cases were selected from this database. From March 2007 to June 2013 a total of 842 cases of primary colorectal cancer were diagnosed and treated at our centers. Signet cell carcinoma of the colon or rectum was observed in 22 of these patients. The pathologic diagnosis was made by specially trained pathologists, and curative resection was the preferred approach for treating colorectal cancers when possible. Curative surgery was defined as radical resection including lymphatic dissection with surgically clean margins upon microscopic examinations.
Patient demographics and tumor characteristics
Demographic records for each patient included age, sex, and family history of malignancy, as well as presence of bowel obstruction, surgical treatment, tumor presence at surgical margins, recurrence, and mortality. Cancer-specific information included tumor location, size, grade and depth of infiltration, and the presence of metastatic lymph nodes, adjacent organ invasion, hepatic metastasis, and peritoneal dissemination.
In accordance with the definition of the World Health Organization, signet ring cell carcinoma is diagnosed when a colorectal tumor contains at least 50% signet ring cells.8 In addition, tumors were staged according to the new (American Joint Committee on Cancer 7th edition) TNM classification.9 Complete pathologic staging was available only in patients after tumor resection.
Survival and statistical analysis
Follow-up controls were routinely scheduled at 3-month intervals for the first 2 years, every 6 months for the subsequent 3 years, and annually thereafter. Data were collected and analyzed digitally using the SPSS version 17.0 (Statistical Software for Social Sciences Inc, Chicago, Illinois) program for statistics. Cumulative event rates were calculated using the Kaplan-Meier method, and survival curves were compared using the log-rank (Mantel-Cox) test. Adjusted hazard ratios and 95% confidence intervals (95% CIs) were used for estimation. The 12 factors thought to influence mortality are age (≤40 years or >40 years), sex, family history of malignancy, site of primary tumor (right hemicolon, left hemicolon, and rectum), presence of bowel obstruction, tumor size (≤5 cm; >5 cm to ≤10 cm; >10 cm), stage of the tumor, site of distant metastasis (peritoneum, lung, and liver), depth of invasion, presence of adjacent organ infiltration, surgical treatment type, and presence of additional therapies. Overall survival was determined as time interval between histologic diagnosis and death or the last follow-up. In addition, progression-free survival time was calculated from the date of histologic diagnosis until tumor progression or death. The results of both the overall survival and the progression-free survival times are presented as mean values for their continuous variables. For all tests, P < 0.05 was considered to be statistically significant.
Results
Of the 842 patients with primary colorectal cancers who underwent surgery, 2.6% (n = 22) presented with histologically verified signet ring cell carcinomas (Figs. 1 and 2). Only one of these patients exhibited a synchronous primary malignant disease (gastric cancer). This gastric cancer, which was determined during preoperative examinations by chance, was an early-stage adenocarcinoma (T1N0M0). As for signet ring cell colon cancer, it was at stage IIIC. Because of its detection at an early stage and because the patient received curative treatment, this gastric cancer is thought not to have an effect on prognosis. Because colon cancer was considered to determine the survival, this patient was included in the study. The demographics and perioperative clinicopathologic features of these 22 PSRCCR patients are detailed in Table 1. Many of these patients (54.5%; n = 12) with signet ring cell carcinoma were younger than 40 years at the time of the diagnosis. In addition, 59% of the signet ring cell carcinomas (n = 13) were located in the rectum. The sites of the primary tumors are detailed in Table 1.
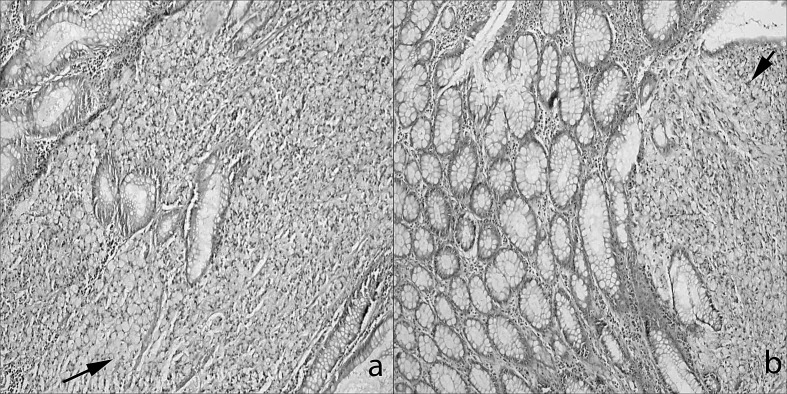
Fig. 1.
Two cases in which the signet ring cells have invaded the mucosa of the (a) left colon and (b) rectum, as seen in these 2 different colon specimens (hematoxylin and eosin, ×100).
Fig. 2.
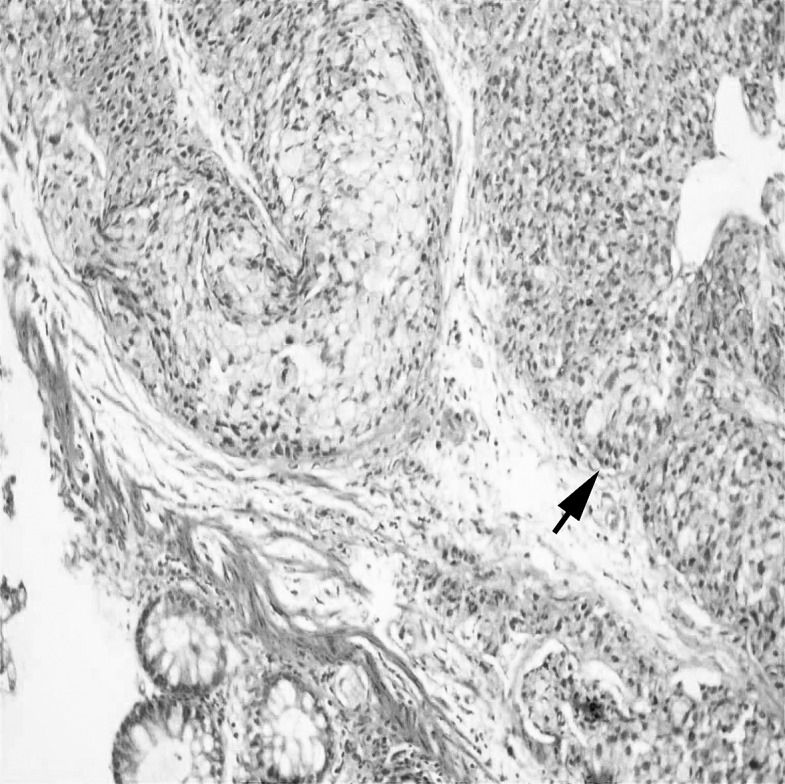
Using endoscopic biopsy prior to surgery, the signet ring cells can clearly be seen to have invaded the rectal mucosa (hematoxylin and eosin, ×100).
Table 1.
Analysis of factors that may affect mortality/survival
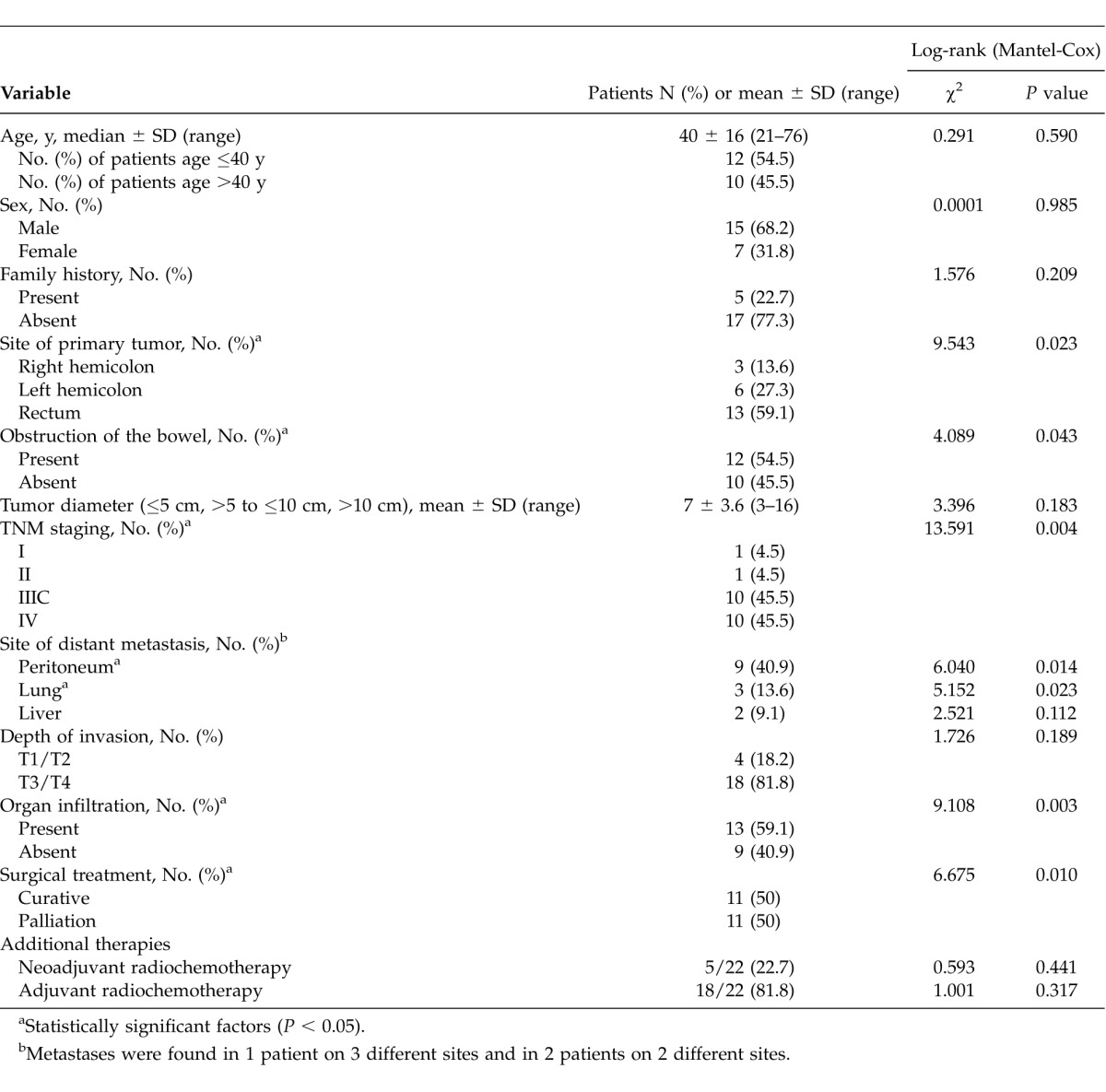
A curative resection (R0 resection with related radical lymphatic dissection) was achieved in 11 of the 22 patients (50%). Palliative resections were performed on the remaining 11 patients. The mean survival time was calculated as 48.4 ± 10.8 months (95% CI, 27.2–69.5 months) for the patients who underwent curative resection and 19.2 ± 7.3 months (95% CI, 4.9–33.4 months) for those who underwent palliative resection. Emergency procedures had to be performed in 54.5% of patients (n = 12) with PSRCCR because of signs of bowel obstruction. Diverting colostomy or ileostomy was applied for 14 patients (63.6%). As for the risk of metastasis and local invasion according to preoperative examinations (magnetic resonance imaging and/or computed tomography), neoadjuvant radiochemotherapy was applied for 5 patients. A total of 18 patients were exposed to postoperative adjuvant therapies. The applications of adjuvant and neoadjuvant therapies are shown in Table 1.
The tumor stage according to TNM classification is presented in Table 1. There was a tendency toward advanced tumor stage at the time of presentation (91%; n = 20; stages III and IV). A total of 10 of the 22 patients (45.5%) with signet ring cell carcinomas presented with distant metastasis at the time of diagnosis. The most common site for metastases was peritoneum in 9 patients (40.9%); less common was lung in 3 patients (13.6%) and liver in 2 patients (9.1%). In addition, the depth of invasion was T3 or T4 in 18 of the 22 patients (81.8%). In addition to this, tumor invasion of the lymphatic vessels occurred in 19 patients (86.4%). None of the patients had inflammatory bowel disease.
The mean survival time observed for the entire group of patients with signet ring cell carcinoma was 25.9 ± 25.6 months (range, 1–81 months), and the mean overall survival was found to be 33.3 ± 7.1 months (95% CI, 19.4–47.2 months; Fig. 3A).
Fig. 3.
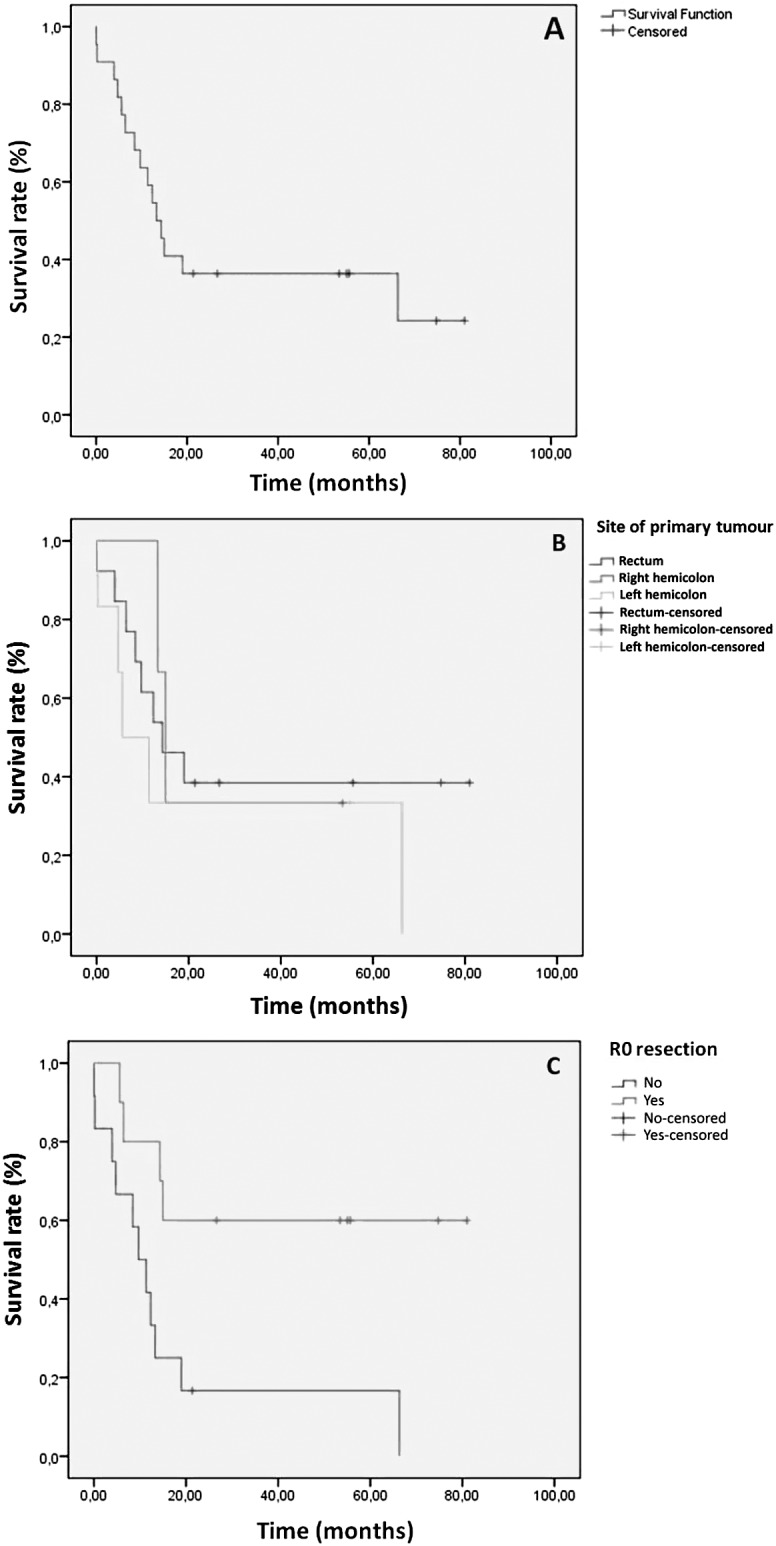
Kaplan-Meier overall survival curve (A); survival analysis according to the location of the tumor (B); and survival analysis of the patients who could or could not have R0 resection performed upon them (C).
There was a statistically significant difference for the survival rates between the group that underwent emergency surgery (bowel obstruction) and patients who underwent elective surgery; the rate of those who underwent emergency surgery was 20.1 ± 6.6 months (95% CI, 7.1–33.1 months) compared with an average rate of 48.2 ± 10.2 months (95 CI%, 28.2–68.2 months) for those who underwent elective surgery (P = 0.043). Similarly, it was determined that the site of the primary tumor (right hemicolon, left hemicolon, and rectum), TNM stage, adjacent organ infiltration, peritoneum, and lung metastasis status, as well as efficacy of surgery (palliative or curative), all showed statistically significant difference (P < 0.05; Table 1) in terms of survival. Right colon and rectum tumors had a worse prognosis compared with left colon tumors [left colon, 25.8 ± 13.2 months (95% CI, 0–51.6 months); right colon, 27.2 ± 10.7 months (95% CI, 6.3–48.2 months); and rectum 36.8 ± 9.7 months (95% CI, 17.7–56 months); Fig. 3B]. High TNM stage with the presence of adjacent organ infiltration, along with peritoneum and lung metastasis, correlated with an unfavorable prognosis. The patients for whom curative surgical treatment was performed were more likely to have a favorable prognosis than those who underwent palliative treatment.
It was found that age (≤40 years or >40 years), sex, family history, the size of the tumor, presence of liver metastasis, depth of invasion, and addition of adjuvant as well as neoadjuvant therapies, had no statistically significant effect on the survival period (P > 0.05).
The mean survival time was calculated as 52.7 ± 11 months (95% CI, 31.2–74.2 months) in patients who underwent R0 resection and 18 ± 6.7 months (95% CI, 4.8–31.2 months) in the others (Fig. 3C). Of these 22 patients, 15 died during the follow-up period. Of these 15 patients, 10 had stage IV disease (all of the patients with stage IV disease) and 5 patients had stage IIIC disease. The mean progression-free survival time of these patients was 11.8 ± 3.5 months (95% CI, 4.9–18.7 months).
Discussion
PSRCCR is a rare type of colorectal cancer that accounts for 0.1% to 2.6% of all colorectal cancer cases.10 This rate rises to 19% in different series.6 In an analysis of PubMed articles, when “signet ring cell carcinoma,” “colon,” and “Turkey” are used as key words in the investigation of related literature, there are 6 articles, most of which are case reports. No information about the rate of PSRCCR is available. In our series, patients who underwent an operation for PSRCCR accounted for 2.6% of all primary colorectal cancers. This percentage is close to the upper value mentioned in the literature. This relatively high frequency may be due to the specialties and experience of our pathologists and close sections obtained from specimens in order to not overlook disease.
It is emphasized in this study that PSRCCR is an aggressive tumor that is commonly seen around the age 40 years and generally has a poor prognosis. In a study where signet ring cell adenocarcinoma of the rectum was compared with non–signet ring cell mucinous adenocarcinoma of the rectum in addition to nonmucinous adenocarcinoma of the rectum. The mean age of patients at onset with signet ring cell adenocarcinoma was 48.1 years while it was 57.4 years for patients with non–signet ring cell mucinous adenocarcinoma of the rectum and 62.6 years for nonmucinous adenocarcinoma of the rectum.11 It was suggested in the same study that PSRCCR had male predominancy, with a ratio of 67.2%.11,12 Concordant with our data, in data presented by Bittorf et al,12 patients who underwent operations for signet ring cell carcinoma were younger at the time of diagnosis, with a median age of 40. Similar to previous publications, we observed a male predominance (68.2%) for PSRCCR patients.10,13,14
Parallel with the previous studies, in the current study age and sex were shown not to have any significant effect on survival.12,15,16 As opposed to a study suggesting that female sex had a worse effect on survival, it was found in the current study that there were no differences in survival between sexes. Although there are various data concerning the size of tumors in the literature, it was suggested that well- and moderately-differentiated carcinomas might be diagnosed at larger sizes (5.5–6.2 cm).6,7,11 However, in our study the tumors were even larger, with a mean diameter of 7 cm. As our center is commonly considered to be a referral hospital, many problematic patients are admitted to our hospital. Thus, the diameters of the tumors might be larger than those mentioned in literature at the time of diagnosis. In a study it was suggested that there were no effects of the size of the tumor on survival15; in a similar fashion, we obtained an identical result.
Most publications suggest that in these cases it is found that the right colon and rectum are the most frequent sites for PSRCCR.7,17 In a study performed in Japan, it was suggested that signet ring cell carcinoma of colon clearly existed in a proximal location.15 On the contrary, in our study rectum was found to be prominent, with 59%, and the left colon after it, with 27.3%. Recently, it has been found that there has been an increase in the ratio of tumors in the right colon and a decrease in the left for well- and moderately-differentiated carcinomas.18 On the contrary, PSRCCR tumors, which have aggressive characteristics, were shown to be more evident in rectum and left hemicolon in our series.
In the survival analysis, it was confirmed that tumor site had a significant effect on survival. In this study, rectal tumors were detected to have the best prognosis according to tumor site. As opposed to our results, in a previous study in which PSRCCR tumors were divided into two groups according to their localization, proximal tumors had a better prognosis.15
Hereditary nonpolyposis colorectal cancers usually develop in the right colon. However, signet ring cell carcinomas can also develop in the right colon and should not be underestimated. Hereditary nonpolyposis colorectal cancer generally presents with mucinous carcinoma in young patients.19 In our series, 1 patient had colon cancer related to hereditary nonpolyposis colorectal cancer, which was located in the right colon.
Patients with ulcerative colitis have an increased long-term risk for developing signet ring cell carcinomas and mucinous carcinomas compared with the general population.2,7 In contrast, in our series no patients were observed to have ulcerative colitis.
In a study in which patients with the diagnosis of signet ring cell carcinoma of the rectum were examined, according to the TNM classification 10% were at stages I and II, 30% were at stage III, and 41% were at stage IV.11 In another study, in which 154 patients were studied, 78.2% of patients were identified as having stage III or IV disease. In addition, in this study lymph node involvement was determined in 77.4% of patients, peritoneal metastasis in 38.7%, and liver metastasis in 2.9%.17 In our study the rates were 9% for stages I to II, 45.5% for stage III, and 45.5% for stage IV. In line with the former publications, we diagnosed most signet ring cell cancers at advanced stages.7,11–17 Data published concerning lymphatic and vascular invasions are generally heterogeneous. In accordance with Tung et al13 and Makino et al,17 in our series metastasis tended to develop in lymph nodes and on peritoneal surfaces rather than in the liver. In a study in which carcinoma metastases were examined, it was reported that the rate for peritoneum metastases was 38%, and the rate for metastases that spread out to other sites (including liver, lung, brain, and bone metastases) through hematogenous route was 18.5%.11 In other studies, 92.8% of the patients with a PSRCCR diagnosis were at stages III and IV, 64.3% of them had peritoneum metastases, and 14.3% of them had liver metastases7 Parallel with those rates, 81.8% of our patients had T3 to T4 tumor, 40.9% had peritoneum metastases, 13.6% had lung metastases, and 9.1% had liver metastases. It was determined that 91% of our patients were at stages III and IV. In the survival analysis, TNM stage and presence of peritoneum and lung metastases, as well as adjacent organ infiltration, had significant effects on prognosis. However, it was found that depth of invasion and presence of liver metastasis had no effects on survival. In the studies performed it was reported that TNM stage had a significant effect on survival.11 Accordingly, there were no relevant studies available performed on the parameters mentioned above.
PSRCCR is the result of a tumor with diffuse infiltration of the intestines and a circular thickening of the bowel wall, and thus it can be recognized as the solidification is established; therefore, it is called “colonic linitis plastica.” However, larger tumors with mass effect were observed in our series. This phenomenon may be due to the arrival of the cases at relatively later stages. It should be considered that PSRCCRs at advanced stages may form more vegetating and large masses.
Curative resection was achieved with the appropriate surgical approach in half of the patients in our study. This rate was lower in different studies.7,17 This can be explained by the advanced stage of the tumor at the time of diagnosis and the underlying biologic behavior. Most published studies argue that it is difficult to diagnose this histologic type of cancer with a small amount of tissue obtained by an endoscopic biopsy done before surgery. However, 4 of the patients in our study group were given the correct diagnosis by using endoscopic biopsy prior to surgery (Fig. 2). Because endoscopic biopsy samples represented limited pieces of tumors, final results were reported by their definitive diagnoses (e.g., malignant tumor containing signet ring cells). To be defined as PSRCC, demonstration of more than 50% signet ring cells is recommended.
It was found in the survival analysis that the presence of bowel obstruction and having only undergone palliative surgery were correlated with a worse prognosis. However, additional therapies (neoadjuvant radiochemotherapy and adjuvant radiochemotherapy) had no effects according to survival analysis. It seems that there are no studies related to PSRCCR that examine the effect of bowel obstruction and palliative surgical treatment on survival. On the other hand, there are various studies claiming that the presence of bowel obstruction and palliative surgical treatment in patients with well- and moderately-differentiated colorectal carcinomas had negative effects on survival.20
PSRCCR generally has a poor prognosis. A relatively high proportion of these cases receive a delayed diagnosis, leading to advanced tumor stage and shortened lifespan. Survival rates over a 5-year period in previous studies ranged between 9% and 36%.10–17,21 Average survival time was reported to be between 20 and 45 months.4,17,21 Similar to the results in our study, the mean overall survival and mean progression-free survival times were found to be 33.3 ± 7.1 months (95% CI, 19.4–57.2 months) and 11.8 ± 3.5 months (95% CI, 4.9–18.7 months), respectively.
Conclusion
Overall, we demonstrated that PSRCCR was mostly seen in the early fifth decade of life, with male predominance, in rectum. Furthermore, it can also be concluded that survival rates were influenced by the site of the tumor, the presence of bowel obstruction as well as peritoneum and lung metastases, the TNM stage, and the efficiency of the surgery implemented. However, tumor size, liver metastasis, depth of invasion, and additional therapies seem to have no significant impact on survival. In general, the prognosis for patients with PSRCCR is extremely poor; this can only be recovered by early diagnosis and aggressive surgical intervention. All in all, it is clear that there is room for further evaluation, because our study had a limited number of patients presenting with PSRCCR. It is suggested that there is a lot to gain by conducting relevant multicenter studies in the future.
Acknowledgments
We are happy to acknowledge Cagla Sariturk, the statistician of Adana Baskent Medical and Research Center, who performed and checked the statistical analysis, for the statistical processing of this study, making this article possible.
References
- 1.Almagro UA. Primary signet-ring carcinoma of the colon. Cancer. 1983;52(8):1453–1457. doi: 10.1002/1097-0142(19831015)52:8<1453::aid-cncr2820520819>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 2.Kang H, O'Connell JB, Maggard MA, Sack J, Ko CY. A. 10-year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Dis Colon Rectum. 2005;48(6):1161–1168. doi: 10.1007/s10350-004-0932-1. [DOI] [PubMed] [Google Scholar]
- 3.Min BS, Kim NK, Ko YT, Baek SH, Lee KY, Sohn SK. Clinicopathological features of signet-ring cell carcinoma of the colon and rectum: a case-matched study. Hepatogastroenterology. 2009;56(93):984–988. [PubMed] [Google Scholar]
- 4.Casavilca Zambrano S, Sanchez Lihon J, Zavaleta A. Colon and rectum signet-ring cell carcinoma in the National Institute of Neoplastic Diseases. Rev Gastroenterol Peru. 2004;24(3):234–237. [PubMed] [Google Scholar]
- 5.Kaw LL, Jr, Punzalan CK, Crisostomo AC, Bowyer MW, Wherry DC. Surgical pathology of colorectal cancer in Filipinos: implications for clinical practice. J Am Coll Surg. 2002;195(2):188–195. doi: 10.1016/s1072-7515(02)01186-9. [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim NK, Abdul-Karim FW. Colorectal adenocarcinoma in young Lebanese adults: the American University of Beirut-Medical Center experience with 32 patients. Cancer. 1986;58(3):816–820. doi: 10.1002/1097-0142(19860801)58:3<816::aid-cncr2820580335>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 7.Psathakis D, Schiedeck TH, Krug F, Oevermann E, Kujath P, Bruch HP. Ordinary colorectal adenocarcinoma vs. primary colorectal signet-ring cell carcinoma: study matched for age, gender, grade, and stage. Dis Colon Rectum. 1999;42(12):1618–1625. doi: 10.1007/BF02236218. [DOI] [PubMed] [Google Scholar]
- 8.Jass JR, Sobin LH. Histological Typing of Intestinal Tumours. 2nd ed. Berlin, Germany: Springer;; 1989. [Google Scholar]
- 9.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Handbook. 7th ed. New York, NY: Springer;; 2010. [Google Scholar]
- 10.Anthony T, George R, Rodriguez-Bigas M, Petrelli NJ. Primary signet-ring cell carcinoma of the colon and rectum. Ann Surg Oncol. 1996;3(4):344–348. doi: 10.1007/BF02305663. [DOI] [PubMed] [Google Scholar]
- 11.Chen JS, Hsieh PS, Hung SY, Tang R, Tsai WS, Changchien CR. Clinical significance of signet ring cell rectal carcinoma. Int J Colorectal Dis. 2004;19(2):102–107. doi: 10.1007/s00384-003-0515-y. [DOI] [PubMed] [Google Scholar]
- 12.Bittorf B, Merkel S, Matzel KE, Wein A, Dimmler A, Hohenberger W. Primary signet-ring cell carcinoma of the colorectum. Langenbecks Arch Surg. 2004;389(3):178–183. doi: 10.1007/s00423-004-0474-y. [DOI] [PubMed] [Google Scholar]
- 13.Tung SY, Wu CS, Chen PC. Primary signet ring cell carcinoma of colorectum: an age- and sex-matched controlled study. Am J Gastroenterol. 1996;91(10):2195–2199. [PubMed] [Google Scholar]
- 14.Kawabata Y, Tomita N, Monden T, Ohue M, Ohnishi T, Sasaki M. Molecular characteristics of poorly differentiated adenocarcinoma and signet-ringcell carcinoma of colorectum. Int J Cancer. 1999;84(1):33–38. doi: 10.1002/(sici)1097-0215(19990219)84:1<33::aid-ijc7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 15.Ishihara S, Watanabe T, Akahane T, Shimada R, Horiuchi A, Shibuya H. Tumor location is a prognostic factor in poorly differentiated adenocarcinoma, mucinous adenocarcinoma, and signet-ring cell carcinoma of the colon. Int J Colorectal Dis. 2012;27(3):371–379. doi: 10.1007/s00384-011-1343-0. [DOI] [PubMed] [Google Scholar]
- 16.Chang DT, Pai RK, Rybicki LA, Dimaio MA, Limaye M, Jayachandran P. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: an adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod Pathol. 2012;25(8):1128–1139. doi: 10.1038/modpathol.2012.61. [DOI] [PubMed] [Google Scholar]
- 17.Makino T, Tsujinaka T, Mishima H, Ikenaga M, Sawamura T, Nakamori S. Primary signet-ring cell carcinoma of the colon and rectum: report of eight cases and review of 154 Japanese cases. Hepatogastroenterology. 2006;53(72):845–849. [PubMed] [Google Scholar]
- 18.Omranipour R, Doroudian R, Mahmoodzadeh H. Anatomical distribution of colorectal carcinoma in Iran: a retrospective 15-yr study to evaluate rightward shift. Asian Pac J Cancer Prev. 2012;13(1):279–282. doi: 10.7314/apjcp.2012.13.1.279. [DOI] [PubMed] [Google Scholar]
- 19.Green SE, Bradburn DM, Varma JS, Burn J. Hereditary non-polyposis colorectal cancer. Int J Colorectal Dis. 1998;13(1):3–12. doi: 10.1007/s003840050123. [DOI] [PubMed] [Google Scholar]
- 20.National Cancer Institute. Colon Cancer Treatment PDQ. Accessed July 2014. Available at: http://www.cancer.gov/cancertopics/pdq/treatment/colon/HealthProfessional. [Google Scholar]
- 21.Song W, Wu SJ, He YL, Cai SR, Zhang CH, Zhang XH. Clinicopathologic features and survival of patients with colorectal mucinous, signet-ring cell or non-mucinous adenocarcinoma: experience at an institution in southern China. Chin Med J (Engl) 2009;122(13):1486–1491. [PubMed] [Google Scholar]