Abstract
A 52-year-old woman was diagnosed with rectal hemangioma that had resulted in chronic bleeding. Klippel-Trenaunay syndrome was diagnosed by clinical examination. She was referred 30 years later because of progressive anemia. Colonoscopy revealed multiple bluish, polypoid nodules with severe vascular congestion and varicosis that had circumferentially spread along the wall from the dentate line to the rectosigmoid lesion. Selective abdominal angiography indicated that the hemangioma fed from the sigmoid artery and superior rectal artery. Doppler transrectal ultrasonography showed heterogeneous lesions with hypoechoic areas mostly in the submucosal layer, partly forming the mosaic pattern of the color flow signal in the intersphincteric layer on the oral side from the dentate line to 1 cm. Anterior resection and coloanal anastomosis with covering ileostomy was performed. Using a transanal approach, mucosectomy and intersphincteric resection were performed based on the Doppler transrectal ultrasonography results. There were no intraoperative complications, and her anemia resolved postoperatively.
Key words: Rectal hemangioma, Sphincter-saving resection, Transrectal ultrasonography
Hemangiomas and vascular malformations of the gastrointestinal tract can be distributed throughout the intestinal digestive system, or they can present as a singular cavernous hemangioma or malformation, 38% of which are located in the rectosigmoid region.1 Although they are uncommon, rectal hemangiomas may cause chronic bleeding and anemia. Though there are more conservative options, the treatment of choice is surgical resection. Sphincter-saving procedures involving mucosectomy with or without intersphincteric resection (ISR) were recently performed based on the results of preoperative modalities such as computed tomography (CT), magnetic resonance imaging (MRI), angiography, endoscopy, and ultrasonography in patients with syndromic/nonsyndromic rectal hemangioma.2–7 We herein report a case of proctectomy and coloanal anastomosis with partial ISR for rectal hemangioma in a patient with Klippel-Trenaunay syndrome (KTS), based on evaluations by Doppler transrectal ultrasonography (D-TRUS).
Case Report
A 52-year-old woman was diagnosed with a rectal hemangioma 30 years prior. She had suffered chronic bleeding caused by the rectal hemangioma and had received iron supplementation. Physical examination, MRI, and angiography of her lower extremity had revealed cutaneous hemangiomas of the right leg and upper trunk, hypertrophy and varicose veins of the right leg, and contracture of the right knee joint. She had been diagnosed with KTS based on the classic triad of signs. She was referred to our hospital for surgical treatment because of progressive anemia (hemoglobin of 5.3 g/dL). During a gastrointestinal study, colonoscopy revealed multiple bluish, polypoid nodules with severe vascular congestion and varicosis that had circumferentially spread along the wall from the dentate line to 20 cm from the anal verge (Fig. 1). Selective abdominal angiography indicated that the hemangioma fed from the sigmoid artery and superior rectal artery (Fig. 2). CT and MRI of the abdomen and pelvis revealed remarkable rectal wall thickening and cavernous hemangiomas in the spleen, liver, uterus, left ovary, and circumvaginal region. D-TRUS showed heterogeneous lesions with hypoechoic areas mostly in the submucosal layer, partly forming the mosaic pattern of the color flow signal in the intersphincteric layer on the oral side from the dentate line to 1 cm (Fig. 3). Anterior resection and coloanal anastomosis with covering ileostomy were performed. Using a transanal approach, the mucosa was stripped from the level of the dentate line, and ISR was begun from 1 cm above the dentate line based on the D-TRUS results (Fig. 4). There was no massive bleeding during surgery. Pathologic assessment of the excised specimen revealed that the proximal surgical margin of the sigmoid colon was not affected by the hemangioma. On the other hand, benign vascular lesions were recognized mostly in the submucosal layer and partially in the intersphincteric layer as far as the distal surgical margin; these findings were consistent with the D-TRUS results. The perioperative period was uneventful. Three months after surgery, water-soluble contrast enema as a preoperative examination of the ileostomy closure revealed a small sinus in the dorsal portion of the anastomosis. This sinus was asymptomatic and resolved without treatment in 4 months, after which ileostomy closure was performed. There was no postoperative gastrointestinal bleeding, and her anemia resolved after surgery (hemoglobin concentration 12.0 g/dL at 2 months after ileostomy closure). She achieved normal anal continence after her ileostomy closure, with an average defecation frequency of 1.5 times per day.
Fig. 1.
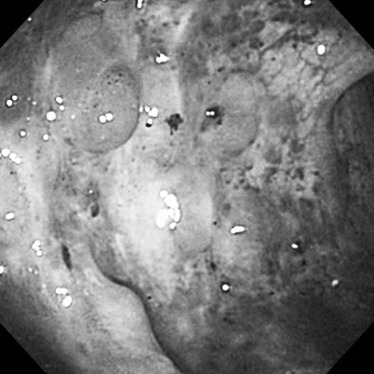
Endoscopic findings. Colonoscopy showed multiple bluish, polypoid nodules with severe vascular congestion and varicosis that had circumferentially spread along the rectum from the anal verge.
Fig. 2.
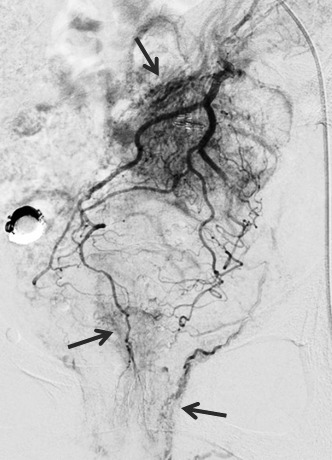
Selective abdominal angiography findings. Angiography showed that the hemangioma fed from the sigmoid artery and superior rectal artery (arrows).
Fig. 3.
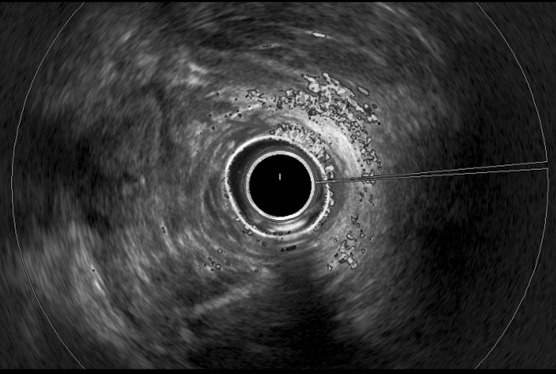
D-TRUS showed heterogeneous lesions with a mosaic pattern of the color flow signal from the submucosal layer to the intersphincteric layer on the oral side from the dentate line to 1 cm (arrow).
Fig. 4.
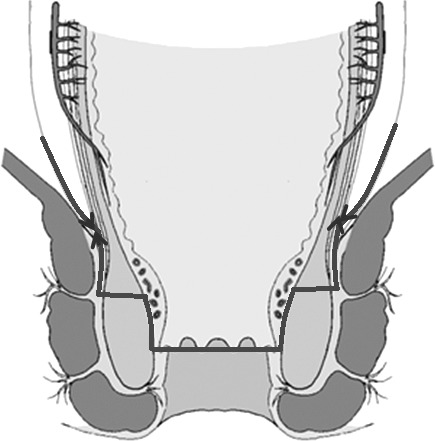
Operative scheme of intersphincteric resection. The mucosa was stripped from the level of the dentate line, and ISR was begun 1 cm above the dentate line according to the D-TRUS results.
Discussion
Hemangiomas and vascular malformations of the gastrointestinal tract are infrequently encountered entities,1 and 38% of gastrointestinal hemangiomas and malformations are located in the colon and rectum.8 They may be isolated abnormalities, or they may be associated with syndromes such as KTS, Osler-Rendu-Weber syndrome, or blue rubber bleb nevus syndrome.1
KTS is a rare congenital disease. Klippel and Trenaunay reported two cases of an extremity exhibiting a cutaneous vascular nevus (capillary malformation), varicosities, and hypertrophy of soft tissue and bone, which they termed naevus vasculosus osteohypertrophicus in 1900.9,10 Most cases of KTS are sporadic, and the condition affects males and females equally, has no racial predilection, and manifests at birth or during childhood.11 Hemangiomas in KTS involve visceral organs such as the gastrointestinal tract, liver, spleen, bladder, kidneys, lungs, and heart.10–12 The most commonly reported cause of gastrointestinal bleeding is attributed to diffuse cavernous hemangioma of the distal colon and rectum, found in an estimated 1% to 12.5% of patients with KTS.7,13
Treatment of rectal bleeding may be conservative or surgical. Conservative treatments include endoscopic hemostatic therapy with laser coagulation and clipping, and interventional radiology with embolization.14,15 However, these treatments may have a risk of recurrent bleeding and blood transfusions.14 Laser coagulation may be used for limited lesions and may be palliative or used to complement surgical treatment.6 We decided not to treat our patient by embolization because this procedure usually only results in temporary improvement and because their was a risk of rectal necrosis due to the extent of the hemangioma. Previously reported surgical treatments for rectal hemangioma extending to the anal canal16 or perianal area7 often involved abdominoperineal resection with formation of a permanent colostomy, as this was considered the safest way to avoid complications,17 including massive gastrointestinal bleeding.13 On the other hand, surgery in some cases has involved sphincter-saving procedures including proctocolectomy with mucosectomy, and several reports showed that postoperative anal function and curability were acceptable.2–7
Preoperative imaging modalities for anorectal lesions are very important when deciding on surgical management of rectal hemangioma. In previous reports, MRI, CT, angiography, and ultrasonography were performed as preoperative modalities.2–7 These modalities can facilitate determination of the extent of resection and delineation of the surgical margin because detailed information is available regarding culprit vessels and hemangioma localization. ISR for low rectal cancer was recently described as the ultimate procedure to avoid permanent colostomy with acceptable anal function.18 In rectal hemangioma, radical resection is important to reduce the risks of massive bleeding during surgery and postoperative recurrence. Hence, ISR could be an essential procedure in the treatment of hemangiomas near the anal canal. Rotholtz et al6 reported the first case of ultralow anterior resection with ISR for rectal hemangioma in a patient with KTS. However, their report did not specifically describe the location of the hemangioma in the anal canal or the extent of ISR. The location of a hemangioma in the anal canal can be preoperatively determined using D-TRUS. This modality gives detailed information about the depth and orientation of hemangiomas; thus, in the present case, we safely and definitively performed sphincter-saving surgery, including partial ISR, without massive bleeding. Although minor intermittent anal bleeding has been reported as a postoperative complication of sphincter-saving procedures,3 there was no postoperative bleeding in the present patient who underwent partial ISR with D-TRUS. Furthermore, possible preservation of the internal sphincter muscle could have a better effect on anal function. We suggest that preoperative D-TRUS to evaluate the depth and orientation of hemangiomas in the lower rectum and anal canal enables the better and safer performance of sphincter-saving surgery, if the hemangiomas do not involve the extended regions such as the perineal region and the external sphincter muscle. This case highlights the usefulness of D-TRUS for the preoperative evaluation of anorectal hemangiomas.
Conclusion
We report a case of proctectomy and coloanal anastomosis with partial ISR based on D-TRUS evaluation for rectal hemangioma. Information obtained by D-TRUS may help surgeons to perform sphincter-saving surgery, including partial ISR, and D-TRUS could be an important modality for sphincter-saving surgery in patients with rectal hemangioma.
References
- 1.Yoo S. GI-associated hemangiomas and vascular malformations. Clin Colon Rectal Surg. 2011;24(3):193–200. doi: 10.1055/s-0031-1286003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasegawa H, Teramoto T, Watanabe M, Imai Y, Muaki M, Kodaira S, et al. Diffuse cavernous hemangioma of the rectum: MR imaging with endorectal surface coil and sphincter-saving surgery. J Gastroenterol. 1996;31(6):875–879. doi: 10.1007/BF02358618. [DOI] [PubMed] [Google Scholar]
- 3.Wang HT, Tu Y, Fu CG, Meng RG, Cui L, Xu HL, et al. Diffuse cavernous hemangioma of the rectosigmoid colon. Tech Coloproctol. 2005;9(2):145–148. doi: 10.1007/s10151-005-0214-5. [DOI] [PubMed] [Google Scholar]
- 4.Sylla P, Deutsch G, Luo J, Recavarren C, Kim S, Heimann TM, et al. Cavernous, arteriovenous, and mixed hemangioma-lymphangioma of the rectosigmoid: rare causes of rectal bleeding—case series and review of the literature. Int J Colorectal Dis. 2008;23(7):653–658. doi: 10.1007/s00384-008-0466-4. [DOI] [PubMed] [Google Scholar]
- 5.Poggioli G, Marchetti F, Selleri S, Fortunato C, Laureti S, Gozzetti G. Colo-anal anastomosis with colonic reservoir for cavernous hemangioma of the rectum. Hepatogastroenterology. 1993;40(3):279–281. [PubMed] [Google Scholar]
- 6.Rotholtz N, Bun ME, Laporte M, Sandra L, Carlos P, Mezzadri N. Rectal bleeding in Klippel-Trenaunay syndrome: treatment with laparoscopic ultralow anterior resection with intersphincteric dissection. Surg Laparosc Endosc Percutan Tech. 2009;19(5):e206–e209. doi: 10.1097/SLE.0b013e3181bae76b. [DOI] [PubMed] [Google Scholar]
- 7.Wilson CL, Song LM, Chua H, Ferrara M, Devine RM, Dozois RR, et al. Bleeding from cavernous angiomatosis of the rectum in Klippel-Trenaunay syndrome: report of three cases and literature review. Am J Gastroenterol. 2001;96(9):2783–2788. doi: 10.1111/j.1572-0241.2001.04110.x. [DOI] [PubMed] [Google Scholar]
- 8.Gentry RW, Dockerty MB, Glagett OT. Vascular malformations and vascular tumors of the gastrointestinal tract. Surg Gynecol Obstet. 1949;88(4):281–323. [PubMed] [Google Scholar]
- 9.Berry SA, Peterson C, Mize W, Bloom K, Zachary C, Blasco P, et al. Klippel-Trenaunay syndrome. Am J Med Genet. 1998;79(4):319–326. [PubMed] [Google Scholar]
- 10.Kihiczak GG, Meine JG, Schwartz RA, Janniger CK. Klippel-Trenaunay syndrome: a multisystem disorder possibly resulting from a pathogenic gene for vascular and tissue overgrowth. Int J Dermatol. 2006;45(8):883–890. doi: 10.1111/j.1365-4632.2006.02940.x. [DOI] [PubMed] [Google Scholar]
- 11.Jacob AG, Driscoll DJ, Shaughnessy WJ, Stanson AW, Clay RP, Gloviczki P. Klippel-Trenaunay syndrome: spectrum and management. Mayo Clin Proc. 1998;73(1):28–36. doi: 10.1016/S0025-6196(11)63615-X. [DOI] [PubMed] [Google Scholar]
- 12.Wang ZK, Wang FY, Zhu RM, Liu J. Klippel-Trenaunay syndrome with gastrointestinal bleeding, splenic hemangiomas and left inferior vena cava. World J Gastroenterol. 2010;16(12):1548–1552. doi: 10.3748/wjg.v16.i12.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Servelle M, Bastin R, Loygue J, Montagnani A, Bacour F, Soulie J, et al. Hematuria and rectal bleeding in the child with Klippel and Trenaunay syndrome. Ann Surg. 1976;183(4):418–428. doi: 10.1097/00000658-197604000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Natterer J, Joseph JM, Denys A, Dorta G, Hohlfeld J. de Buys Roessingh AS. Life-threatening rectal bleeding with Klippel-Trenaunay syndrome controlled by angiographic embolization and rectal clips. J Pediatr Gastroenterol Nutr. 2006;42(5):581–584. doi: 10.1097/01.mpg.0000210139.14753.b7. [DOI] [PubMed] [Google Scholar]
- 15.Azizkhan RG. Life-threatening hematochezia from a rectosigmoid vascular malformation in Klippel-Trenaunay syndrome: long-term palliation using an argon laser. J Pediatr Surg. 1991;26(9):1125–1127. doi: 10.1016/0022-3468(91)90687-o. discussion 1128. [DOI] [PubMed] [Google Scholar]
- 16.Yorozuya K, Watanabe M, Hasegawa H, Baba H, Imai Y, Mukai M, et al. Diffuse cavernous hemangioma of the rectum: report of a case. Surg Today. 2003;33(4):309–311. doi: 10.1007/s005950300070. [DOI] [PubMed] [Google Scholar]
- 17.Coppa GF, Eng K, Localio SA. Surgical management of diffuse cavernous hemangioma of the colon, rectum and anus. Surg Gynecol Obstet. 1984;159(1):17–22. [PubMed] [Google Scholar]
- 18.Tytherleigh MG, Mc CMNJ. Options for sphincter preservation in surgery for low rectal cancer. Br J Surg. 2003;90(8):922–933. doi: 10.1002/bjs.4296. [DOI] [PubMed] [Google Scholar]


