Abstract
This study aimed to assess the pathogenic causes, clinical conditions, surgical procedures, in-hospital mortality, and operative death associated with emergency operations at a high-volume cancer center. Although many reports have described the contents, operative procedures, and prognosis of elective surgeries in high-volume cancer centers, emergency operations have not been studied in sufficient detail. We retrospectively enrolled 28 consecutive patients who underwent emergency surgery. Cases involving operative complications were excluded. The following surgical procedures were performed during emergency operations: closure in 3 cases (10.7%), diversion in 22 cases (78.6%), ileus treatment in 2 cases (7.1%), and hemostasis in 1 case (3.6%). Closure alone was performed only once for peritonitis. Diversion was performed in 17 cases (77.3%) of peritonitis, 4 cases (18.2%) of stenosis of the gastrointestinal tract, and 1 case (4.5%) of bleeding. There was a significant overall difference (P = 0.001). The frequency of emergency operations was very low at a high-volume cancer center. However, the recent shift in treatment approaches toward nonoperative techniques may enhance the status of emergency surgical procedures. The results presented in this study will help prepare for emergency situations and resolve them as quickly and efficiently as possible.
Key words: High-volume cancer center, Emergency operations
In high-volume cancer centers, surgical operations are usually elective treatments. Accordingly, many reports have been published describing the contents, operative procedures, and prognosis of elective surgeries.1 In contrast, emergency operations are scarce. However, in recent years, the growing administration of anticancer drugs, especially for colorectal cancer, has given rise to instances of bowel perforation, ileus, and bleeding. Since such adverse effects often require emergency surgery, understanding the present status of emergency operations is crucially important.
Materials and Methods
We enrolled 28 consecutive patients who underwent emergency surgery without postoperative complications. All subjects gave their informed consent and this manuscript is a retrospective study. The surgeries were performed by the colorectal team in the Department of Gastroenterological Surgery, Aichi Cancer Center Hospital, Nagoya, Japan, between January 2004 and December 2012. The hospital records were reviewed to obtain clinicopathologic information about the patients, including sex (15 men, 13 women) and age (median, 63 years), as well as assess pathogenic causes, clinical conditions, surgical procedures, in-hospital mortality, and operative death. The pathogenic causes included iatrogenic events, adverse events associated with chemotherapy, and adverse events after adjuvant radiation therapy. The iatrogenic events consisted of peritonitis caused by perforation in three colonoscopy cases and two upper endoscopy cases. The adverse events during chemotherapy, which included administration of folinic acid, oxaliplatin, fluorouracil, and bevacizumab for the recurrence of colorectal cancer, were peritonitis caused by bowel perforation, stenosis of the gastrointestinal tract because of adhesion, and bleeding. Finally, the adverse events after adjuvant radiation therapy consisted of peritonitis and stenosis of the gastrointestinal tract resulting from fibrosis in three cases of gynecologic disorders and one case of adjuvant therapy for rectal cancer.
Surgical procedures were classified into four groups: closure alone, diversion, treatment of ileus, and hemostasis. For the purpose of this study, diversion refers to treatment of intra-abdominal abscesses, including: closure with drainage, colostomy alone, colostomy with drainage, and drainage alone.
The Fisher exact probability test was used to identify factors that might influence the operative procedure. All data are expressed as mean ± SD. Statistical significance was set at P < 0.05.
Results
The frequency of emergency operations was 0.89% during the time of the study (i.e., 9 years between January 2004 and December 2012). The total number of operations performed in this period was 3151.
Table 1 shows the causes of emergency operations. All the adverse iatrogenic events were due to peritonitis resulting from a colonoscopic perforation. Chemotherapy-associated adverse events accounted for 13 cases (68.4%) of peritonitis, 4 cases (21.1%) of stenosis of the gastrointestinal tract, and 2 cases (10.5%) of bleeding. The adverse events after adjuvant radiation therapy consisted of 2 cases (50.0%) of peritonitis and 2 cases (50.0%) of stenosis of the gastrointestinal tract. Overall, no significant differences were found (P = 0.355).
Table 1.
Clinicopathologic causations
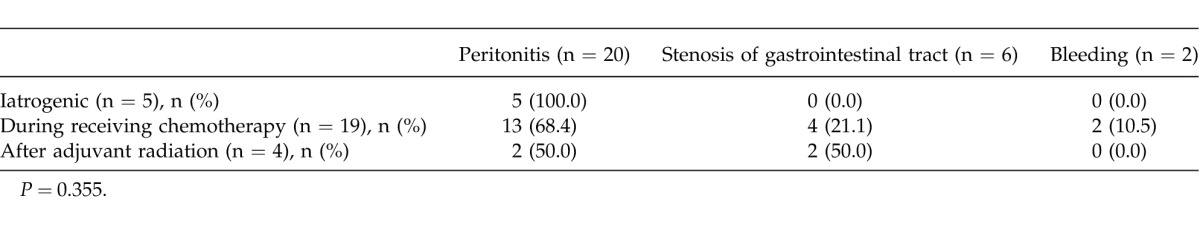
Table 2 lists surgical procedures that were performed during the emergency operations [i.e., 3 cases (10.7%) of closure alone, 22 cases (78.6%) of diversion, 2 cases (7.1%) of ileus treatment, and 1 case (3.6%) of hemostasis]. Closure alone was performed only once for peritonitis. Diversion was performed in 17 cases (77.3%) of peritonitis, 4 cases (18.2%) of stenosis of the gastrointestinal tract, and 1 case (4.5%) of bleeding. There was a significant overall difference (P = 0.001).
Table 2.
Surgical procedure
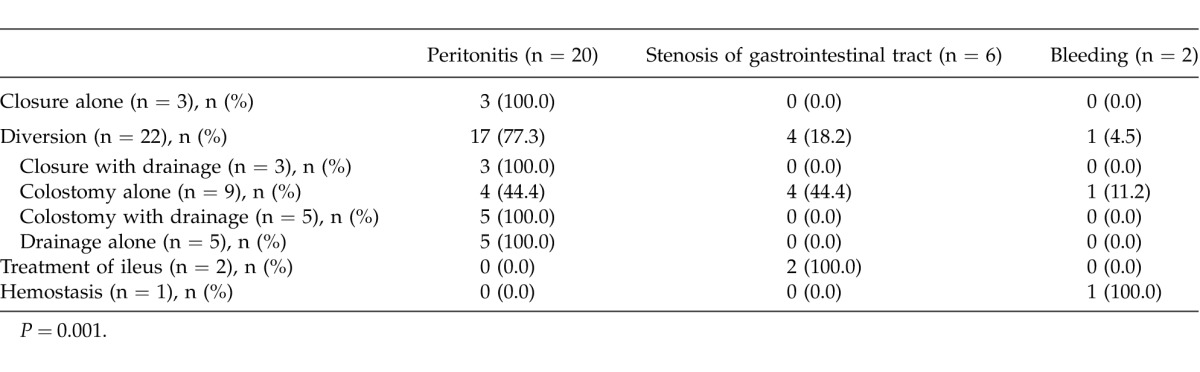
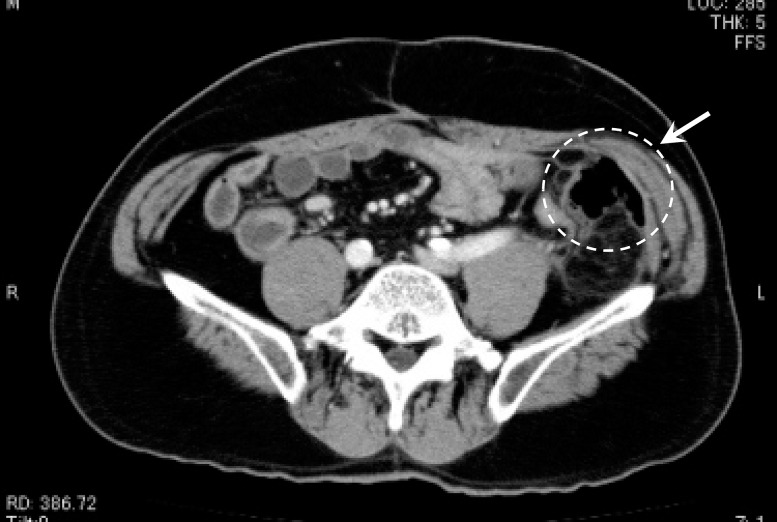
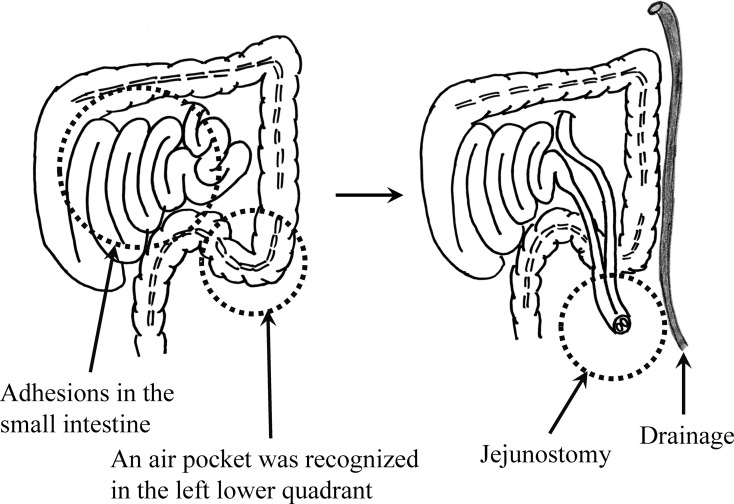
Figure 1 illustrates an air pocket detected in the left-lower quadrant by computed tomography (CT). This patient experienced sudden severe abdominal pain in the left-lower quadrant during the chemotherapy course for recurrence of rectal cancer, and emergency laparotomy was performed. The corresponding operative findings are depicted in Fig. 2. Although adhesion in the small intestine interfered with identification of the site of perforation, free air was recognized in the left-lower quadrant. Accordingly, jejunostomy and drainage were performed.
Fig. 1.
Free air detected in the left-lower quadrant with CT.
Fig. 2.
Operative findings. Left: The site of perforation could not be clearly identified because of adhesions in the small intestine. Nevertheless, an air pocket was recognized in the left lower quadrant. Right: Illustration of the jejunostomy and drainage procedure.
A detailed analysis identified 3 cases (10.7%) of closure with drainage, 9 cases (32.1%) of colostomy only, 5 cases (17.9%) of colostomy with drainage, and 5 cases (17.9%) of drainage only. Closure with drainage was performed only once for peritonitis. Colostomy was performed in 4 cases (44.4%) of peritonitis, 4 cases (44.4%) of stenosis of the gastrointestinal tract, and 1 case (11.2%) of bleeding. Both colostomy with drainage and drainage alone were performed once for peritonitis. Ileus treatment was required in 1 case of stenosis of the gastrointestinal tract, and hemostasis was performed once for bleeding.
Although no instances of perioperative death were encountered, there was a single case of in-hospital death resulting from peritonitis during chemotherapy.
Discussion
Little is currently known about the characteristics and outcomes of emergency operations in high-volume cancer centers. In the meantime, impressive advances in the development of nonoperative treatments, in particular anticancer drugs and radiation therapies, also resulted in a substantial increase of adverse effects. Among anticancer drugs, bevacizumab is frequently associated with adverse effects.2 Specifically, Isobe et al.3 reported that bevacizumab administration led to gastrointestinal bleeding in 16.7 and 8.7% of patients with and without residual primary tumor lesions, respectively. Furthermore, Saito et al.4 described risk factors associated with a high incidence of gastrointestinal perforation during chemotherapy that included bevacizumab.
Perforation and stenosis of the intestine are relatively common after radiation therapy.5 Moreover, Birgisson et al.6 showed that small bowel obstruction was more common in patients with rectal cancer treated with preoperative radiation therapy. According to the study of Sagawa et al.,7 the perforation risks associated with diagnostic and therapeutic colonoscopy reached 0.15 to 3% at a tertiary referral hospital. Importantly, new therapeutic approaches involving endoscopic submucosal dissection techniques, which have become more popular in the recent years, are associated with a higher risk of perforation compared with that of conventional techniques such as polypectomy and endoscopic mucosal resection. Accordingly, an increase in the frequency of iatrogenic perforation may also be expected. However, it has to be noted that endoscopic clipping is emerging as a highly effective nonsurgical approach for treatment of perforations.8
In conclusion, although the frequency of emergency operations was very low at Aichi Cancer Center Hospital, the recent shift in treatment approaches toward nonoperative techniques may enhance the status of emergency surgical procedures. The results presented in this study will help prepare for emergency situations and resolve them as quickly and efficiently as possible.
References
- 1.Osler M, Iversen LH, Borglykke A, Martensson S, Daugbjerg S, Harling H, et al. Hospital variation in 30-day mortality after colorectal cancer surgery in Denmark: the contribution of hospital volume and patient characteristics. Ann Surg. 2011;253(4):733–738. doi: 10.1097/SLA.0b013e318207556f. [DOI] [PubMed] [Google Scholar]
- 2.Gordon MS, Cunningham D. Managing patients treated with bevacizumab combination therapy. Oncology. 2005;69((suppl 3)):25–33. doi: 10.1159/000088481. [DOI] [PubMed] [Google Scholar]
- 3.Isobe T, Uchino K, Makiyama C, Ariyama H, Arita S, Tamura S, et al. Analysis of Adverse Events of Bevacizumab-containing Systemic Chemotherapy for Metastatic Colorectal Cancer in Japan. Anticancer Res. 2014;34(4):2035–2040. [PubMed] [Google Scholar]
- 4.Saito S, Hayashi N, Sato N, Iwatsuki M, Baba Y, Sakamoto Y, et al. Chemotherapy with bevacizumab for metastatic colorectal cancer: a retrospective review of 181 Japanese patients. Int J Clin Oncol. 2013;18(4):689–695. doi: 10.1007/s10147-012-0426-4. [DOI] [PubMed] [Google Scholar]
- 5.Komori K, Kimura K, Kinoshita T, Sano T, Ito S, Abe T, et al. Complications associated with postoperative adjuvant radiation therapy for advanced rectal cancer. Int Surg. 2014;99(2):100–105. doi: 10.9738/INTSURG-D-13-00200.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birgisson H, Pahlman L, Gunnarsson U, Glimelius B. Late gastrointestinal disorders after rectal cancer surgery with and without preoperative radiation therapy. Br J Surg. 2008;95(2):206–213. doi: 10.1002/bjs.5918. [DOI] [PubMed] [Google Scholar]
- 7.Sagawa T, Kakizaki S, Iizuka H, Onozato Y, Sohara N, Okamura S, et al. Analysis of colonoscopic perforations at a local clinic and a tertiary hospital. World J Gastroenterol. 2012;18(35):4898–4904. doi: 10.3748/wjg.v18.i35.4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taku K, Sano Y, Fu KI, Saito Y. Iatrogenic perforation at therapeutic colonoscopy: should the endoscopist attempt closure using endoclips or transfer immediately to surgery? Endoscopy. 2006;38(4):428. doi: 10.1055/s-2006-925248. [DOI] [PubMed] [Google Scholar]