Abstract
Therapeutic delays in cases of external incarcerated hernias typically result in increasing morbidity, mortality, and health expenditures. We investigated the diagnostic role of blood fibrinogen level, white blood count (WBC), mean platelet volume (MPV), and platelet distribution width (PDW) in patients with incarcerated hernia. Two groups, each containing 100 patients, were studied. Group A underwent elective, and group B underwent incarcerated and urgent external hernia repair. We observed high fibrinogen and WBC levels but low MPV and PDW values for patients in group B. Contrary to our expectations, we found lower MPV and PDW values in the complicated group than in the elective group. The morbidity rate and cost burden were higher in group B, and the results were statistically significant. Early operation should be recommended for patients with incarcerated external hernias if their fibrinogen and WBC levels are high.
Key words: Incarcerated external hernia, Fibrinogen, Complete blood count
External strangulated hernia is one of the most common causes of intestinal obstruction especially in the elderly. Delay in treatment may be dangerous.1 Andrews found that the need of bowel resection rate was 27% and the mortality rate was 21% when diagnosis was delayed more than 48 hours; whereas these rates were 7% and 1.4% when diagnosis was obtained in the first 24 hours.2 In addition to increased morbidity and mortality rates, a delay in the treatment of strangulated external hernias is associated with extended hospital stays and an increased need for intensive care, thereby resulting in increased health costs. Of course, clinical signs and symptoms are the most important evidences in diagnosis of incarcerated hernias. In order to make correct diagnosis, we also have powerful weapons such as radiologic imaging methods. However, these clinical and radiologic evidences show complications, especially intestinal perforation due to incarceration. Awareness of probable complications is essential. In this regard, we sometimes need simple methods to alert us. The white blood count (WBC), mean platelet volume (MPV), platelet distribution width (PDW), and blood fibrinogen level have been shown to be valuable predictive parameters for ischemic events in different parts of the body.3–9 There are probable ischemic tissues and organs in incarcerated hernia sac. So, in this study, we aimed to investigate the effectiveness of these simple blood tests in diagnosis. Generally, there is no problem in diagnosis, but sometimes the delay in treatment results in additional morbidity such as a need for bowel resection.
Materials and Methods
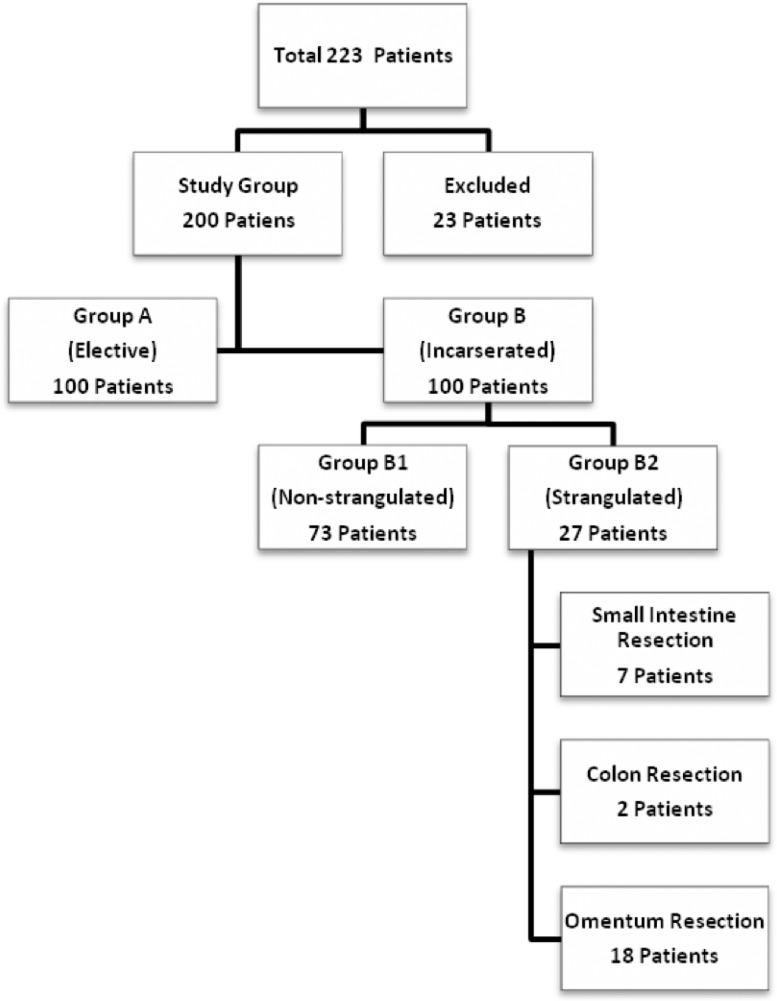
A retrospective hospital-based study was carried out on 223 consecutive patients over a period of 3 years. Clearance from the local institutional ethical committee was obtained. Consecutive patients older than 18 years who had incarcerated groin or ventral hernia were included the study. In the same period and in the same age group, consecutive patients who had nonincarcerated groin or ventral hernia were selected as the control group. Exclusion criteria were the only deficiencies in collecting knowledge from the patient files according to the study protocol. We enrolled the data of consecutive patients retrospectively until achieving the target number of 100 for each group. During sample selection, 23 patients were excluded from the study because of missing patient files. The remaining patients included in the study were divided into 2 groups for the study. Each group contained 100 patients. Group A included patients who were admitted to the general surgery clinic for elective external hernia repair. In contrast, group B included patients who were admitted to the hospital for incarcerated external hernias and referred to the general surgery clinic for treatment. Data were collected for the following parameters for each patient: age; sex; hernia type and location; duration of hernia; duration of symptoms; preoperative WBC, blood fibrinogen level, MPV, and PDW; operation type; duration of hospital stay; need for intensive care; and total cost for treatment. Hospital records of patients with fibrinogen levels were easy to find. Depending on the properties of kits used to measure preoperative coagulation parameters in the period of study, it was possible to obtain knowledge about fibrinogen levels. In group B, 27 patients had strangulated organs located in hernia sacs, while the remaining 73 patients did not. The strangulated organs involved in this subgroup of group B included 7 small intestines, 2 colons, and 18 omenta. The demographic and clinical characteristics of the patients in groups A and B are summarized in Fig. 1 and Table 1. External abdominal hernias were categorized into the following 2 main groups: groin hernias and ventral hernias. Recurrent cases were enrolled in each group separately.
Fig. 1.
Distribution of the patients in groups and types of resections in the group of patients with incarcerated external hernias.
Table 1.
Demographic and clinical characteristics of the patients
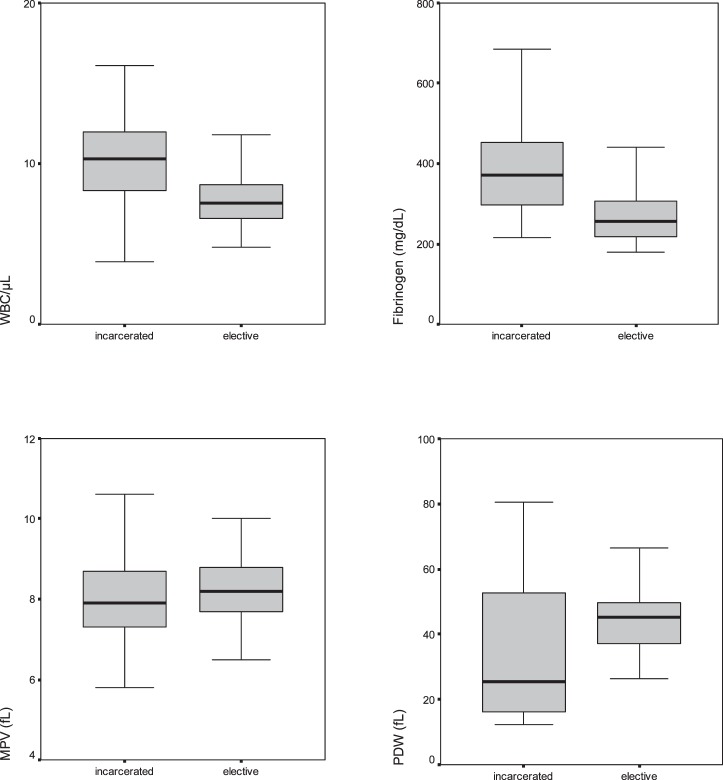
For the hematologic measurements, we used a Coulter LH 780 Hematology Analyzer (Beckman Coulter Inc, Brea, CA) and the Siemens Healthcare Diagnostics BCS XP System (Siemens AG, Berlin, Germany). Blood samples were collected at the time of admission to the emergency department in group B, and at any time during the preoperative preparation period for elective surgery in group A. Normal value ranges for WBC, fibrinogen, MPV, and PDW were 5200 to 11,400/μL, 180 to 350 mg/dL, 7.2 to 11.1 fL, and 44% to 56%, respectively. A comparison of these parameters for groups A and B is shown in Fig. 2.
Fig. 2.
Comparison of WBC, fibrinogen, MPV, and PDW levels between the groups. The horizontal line in the middle of each box indicates the median, while the top and bottom borders of the box mark the 25th and 75th percentiles, respectively. The whiskers above and below the boxes mark the maximum and minimum values, respectively.
Statistical analysis
The data analysis was performed using SPSS for Windows, Version 11.5 (SPSS Inc, Chicago, IL). The normality of the distributions of continuous variables was determined via the Shapiro-Wilk test. Levene's test was used for the evaluation of the equality of variances. Data were reported as mean ± SD or median and range where applicable. The differences between the data from the groups were compared via Student t test or Mann-Whitney U test where appropriate. When more than 2 independent groups were considered, the Kruskal-Wallis test was applied for the comparisons of the median values. When the P values from the Kruskal-Wallis test were statistically significant, Conover's nonparametric multiple comparison test was used to determine which group differed from the others. The categoric data were analyzed using Pearson's λ2 or Fisher's exact test where appropriate. Degrees of association between continuous variables were evaluated via Spearman's correlation analysis. Multiple linear regression analysis was used to assess the differences between groups in terms of WBC, fibrinogen, MPV, and PDW after adjustment for age, sex, and comorbidity. The coefficient of regression and the 95% confidence interval for each independent variable were also calculated. Moreover, a logarithmic transformation was applied for fibrinogen, MPV, and PDW because the data associated with these parameters were not normally distributed. A P value <0.05 was considered statistically significant.
Results
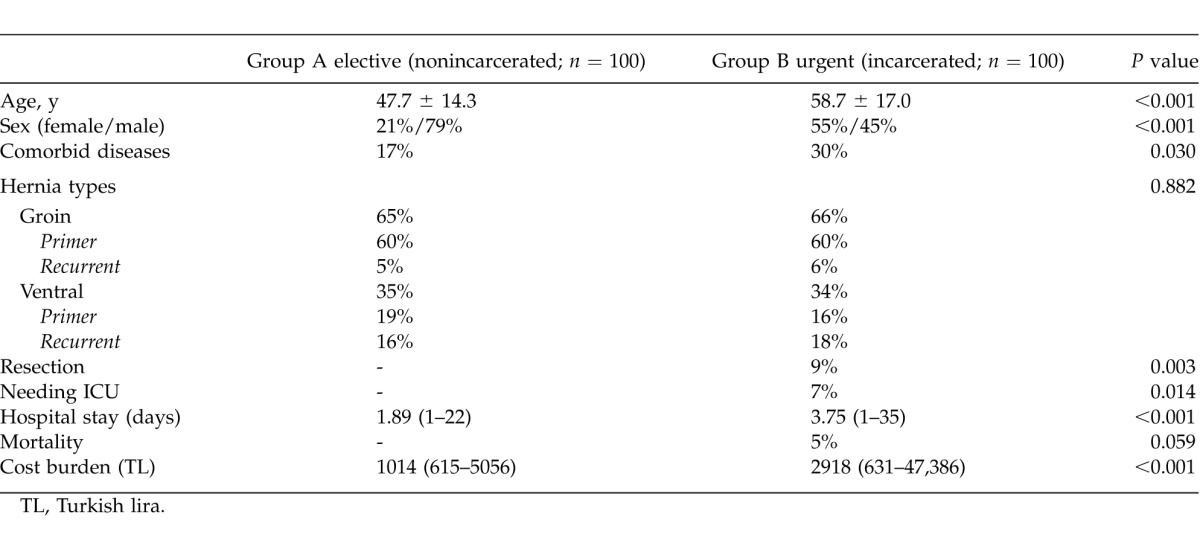
Patients in group A were younger than those in group B with mean ages of 47.7 and 58.7 years, respectively (P < 0.001). Furthermore, 87% and 57% of patients in groups A and B, respectively, were younger than 65 years old. A statistically significantly higher number of women were part of group B than group A (P < 0.001). Additionally, more comorbid diseases were observed in group B, and this finding was statistically significant as well (P = 0.030). Naturally, the mean duration of having the hernia was longer in the elective group (i.e., group A), and the mean time for symptomatic complaint was shorter in the incarcerated group (i.e., group B, P < 0.001). No statistically significant differences were observed between the groups in terms of location of the hernias (P = 0.882).
The WBC and fibrinogen levels were statistically higher in group B than in group A (P < 0.001), but the MPV and PDW values were lower in group B as compared with that of group A (P = 0.019 and P = 0.042, respectively). Also, the fibrinogen levels were statistically higher in the strangulated subgroup of group B than in the nonstrangulated subgroup of group B (P < 0.001).
Omentectomy and bowel resection rates were significantly higher, but the reduction rate was significantly lower in the incarcerated group (i.e., group B) as compared with the elective group (i.e., group A) (P < 0.001). The mean length of hospital stay was longer, and the mean cost burden was higher in group B as compared with that of group A; moreover, these results were statistically significant (P < 0.001). The ratios of group B to group A for hospital stay and costs were 3.75:1.89 days and 2918:1014 Turkish liras, respectively. The need for intensive care was found to be significantly higher in the incarcerated group (i.e., group B) as compared with the elective group (i.e., group A) (P = 0.014). Although the mortality rate was higher in group B as compared with group A, no statistically significant difference was observed between these groups for these data (P = 0.059).
In group B, no statistically significant differences were observed between the need for intensive care and the values of WBC, MPV, and PDW (P > 0.05); however, the fibrinogen level was significantly higher in the patients needing intensive care (P = 0.005). In group B, no statistically significant differences were observed between mortality and the WBC, MPV, and PDW values (P > 0.05); however, fibrinogen levels were significantly higher in the patients who died as compared with that of those who lived (P = 0.047).
When we compared cost and the WBC, MPV, PDW, and fibrinogen levels for group B, we found no significant correlations between cost and WBC, cost and MPV, or cost and PDW values (P > 0.05); however, the cost increased with increasing fibrinogen levels (r = 0.535; P < 0.001). In the patients who had bowel resections, the WBC levels and fibrinogen levels were significantly higher (P < 0.05), but no statistically significant differences were observed for MPV and PDW levels as compared with that of the other patients whose incarcerated hernias were only reduced (i.e., P values were 0.610 and 0.767, respectively).
WBC and fibrinogen levels were lower, while MPV and PDW values were higher in the elective group (i.e., group A) as compared with that of the incarcerated group (i.e., group B) in multiple linear regression analysis.
When we look at the evaluation of mortality in laboratory tests of independent factors, an increase in fibrinogen levels by 1 mg/dL was associated with a 1.010-fold increase in mortality (P = 0.004). Moreover, an increase in fibrinogen levels by 1 mg/dL was associated with a 1.010-fold increase in the need for intensive care (P < 0.001). Additionally, cost significantly increased as the fibrinogen and WBC levels increased (P < 0.001).
Discussion
Fibrinogen levels have been associated with ischemic coronary heart disorders (i.e., high fibrinogen levels are associated with a poor prognosis).4,5 Similar patterns are present in ischemic stroke events.6 Fibrinogen has a very important role in intravascular thrombosis An African study found that high fibrinogen levels are associated with thromboembolic events and poor prognosis in patients with femoral hernias.3 In our study, high fibrinogen levels were associated with incarcerated hernias. We observed positive correlations between high fibrinogen levels and the need for intensive care, an increased bowel resection rate, increased hospital stays, and increased costs (i.e., approximately 3 times higher). Increased levels of fibrinogen were also found in patients who died. The findings for WBC were also similar to these findings.
The association of high MPV and PDW values with poor prognosis and mortality rates in ischemic heart diseases were investigated in prospective and retrospective studies, and positive correlations were found.7,8 In the study by Greisenegger et al,9 high MPV values were found were associated with poor prognosis for patients with acute ischemic cerebrovascular events. MPV is considered a marker for platelet function. Big platelets have more dense granules, so they have the ability to produce high levels of thromboxane A2. Moreover, high MPV has been associated with poor clinical outcome in many kinds of thromboembolic events, especially myocardial ischemic disorders, acute ischemic cerebrovascular events, septicemia, diabetes, and congestive heart failure.9 The study by Kucukardali et al10 revealed a positive correlation between MPV and the APACHE II scores of intensive care unit patients. In another study, high MPV levels were found in marked episodes of thrombocytosis due to bacterial infection.11 A high MPV value in a patient with a bacterial infection indicates septicemia; moreover, a persistent rise or further increase indicates that the current treatment is inadequate.12,13 We expected to find high MPV and PDW values in the incarcerated hernia group. However, we found no correlation between these factors and the fibrinogen and WBC parameters. Of interest, MPV and PDW were significantly lower in the incarcerated hernia group. On the other hand, MPV and PDW values have been shown to be lower in the early phases of systemic inflammatory response syndromes, such as sepsis, and then they tend to increase as platelet counts decrease.12,14–16 Therefore, we may have seen reduced levels owing to the time at which our blood samples were collected (i.e., within this early period). To further investigate this issue, we need to conduct prospective randomized experimental studies. Complications after emergent groin hernia repair in adults can be serious. Because of the high morbidity and mortality associated with incarceration, early diagnosis and elective repair of uncomplicated groin hernias should be done whenever possible. Bowel resections are mainly needed in the treatment of femoral hernias. Overall, morbidity but not mortality is significantly affected by the bowel resection process.17 In a recent study, the factors associated with bowel resection rates were analyzed in patients with incarcerated groin hernia, and high rates were found in female patients, patients older than 65 years, and those with a femoral hernia; delay in diagnosis was found to be an important risk factor.18 Kulah et al19 found similar results in 385 consecutive adult cases with incarcerated hernia in a retrospective study. These researchers conducted another review of 189 elderly patients (i.e., over 65 years of age) who underwent emergency hernia repair and concluded that early elective surgery is preferred for elderly patients with external hernias.20 In our study, group B comprised incarcerated hernia cases that were significantly older than in group A, and women were represented in larger numbers in this population. Also, a statistically significantly high bowel resection rate was observed in the incarcerated hernia group. The mortality rate was high in this second group, but the difference was not statistically significant. In another study, male patients who were 55 years or older were randomly assigned to observation or operation; the researchers suggested repair of asymptomatic inguinal hernias due to possible potentially serious morbidity.21
Ultimately, especially in incarcerated external hernias, waiting involves such risks as increased strangulation probability, morbidity, mortality, and overall treatment costs. Early operations are recommended for these patients if their fibrinogen and WBC levels are high.
References
- 1.Heys SD, Brittenden J. Strangulated femoral hernia: the persisting clinical trap. Postgrad Med J. 1991;67(783):57–59. doi: 10.1136/pgmj.67.783.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews NJ. Presentation and outcome of strangulated external hernia in a district general hospital. Br J Surg. 1981;68(5):329–332. doi: 10.1002/bjs.1800680513. [DOI] [PubMed] [Google Scholar]
- 3.Elusoji SO, Osime C, Irhibogbe P, Egwaikhide E, Oludiran O, Elusoji C, et al. Femoral herniorrhaphy and its affect on fibrinogen levels. Afr J Biotechnol. 2009;8(4):577–579. [Google Scholar]
- 4.Moller L, Kristensen TS. Plasma fibrinogen and ischemic heart disease risk factors. Arterioscler Thromb Vasc Biol. 1991;11(2):344–350. doi: 10.1161/01.atv.11.2.344. [DOI] [PubMed] [Google Scholar]
- 5.Demiralp E, Ulusoy RE, Uslu M, Kırılmaz A, Cebeci BS, Ozmen N, et al. Fibrinogen level utilisatıon as a cardiovascular risk factor for atherosclerosis treatment and follow up [in Turkish] Gulhane Med J. 2004;46(3):232–237. [Google Scholar]
- 6.Napoli MD, Singh P. Is plasma fibrinogen useful in evaluating ischemic stroke patients? Why, How, and When. Stroke. 2009;40(5):1549–1552. doi: 10.1161/STROKEAHA.108.537084. [DOI] [PubMed] [Google Scholar]
- 7.Khandekar MM, Khurana AS, Deshmukh SD, Kakrani AL, Katdare AD, Inamdar AK. Platelet volume indices in patients with coronary artery disease and acute myocardial infarction: an Indian scenario. J Clin Pathol. 2006;59(2):146–149. doi: 10.1136/jcp.2004.025387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slavka G, Perkmann T, Haslacher H, Greisenegger S, Marsik C, Wagner OF, et al. Mean platelet volume may represent a predictive parameter for overall vascular mortality and ischemic heart disease. Arterioscler Thromb Vasc Biol. 2011;31(5):1215–1218. doi: 10.1161/ATVBAHA.110.221788. [DOI] [PubMed] [Google Scholar]
- 9.Greisenegger S, Endler G, Hsieh K, Tentschert S, Mannhalter C, Lalouschek W. Is elevated mean platelet volume associated with a worse outcome in patients with acute ischemic cerebrovascular events? Stroke. 2004;35(7):1688–1691. doi: 10.1161/01.STR.0000130512.81212.a2. [DOI] [PubMed] [Google Scholar]
- 10.Kucukardali Y, Onem Y, Terekeci H, Tangi F, Sahan B, Erikci AA, et al. Mean platelet volume (MPV) in intensive care unit (ICU) patients: is it useful parameter in assessing prediction for mortality? J Med Sci. 2010;1(3):61–64. [Google Scholar]
- 11.Robbins G, Barnard DL. Mean platelet volume changes in infection. J Clin Pathol. 1983;36(11):1320. doi: 10.1136/jcp.36.11.1320-a. [Letter to the editor.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aydemir H, Piskin N, Akduman D, Aktas E. Platelet and mean platelet volume in adult patients with sepsis. Platelets. doi: 10.3109/09537104.2012.701027. published online ahead of print June 25, 2012. doi: 10.3109/09537104.2012.701027. [DOI] [PubMed] [Google Scholar]
- 13.Van der Lelie J, Von dem Borne AK. Increased platelet volume in septicaemia. J Clin Pathol. 1983;36(6):693–696. doi: 10.1136/jcp.36.6.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taniguchi T, Tagagi D, Takeyama N, Kitazawa Y, Tanaka T. Platelet size and function in septic rats: changes in adenylate pool. J Surg Res. 1990;49(5):400–407. doi: 10.1016/0022-4804(90)90187-7. [DOI] [PubMed] [Google Scholar]
- 15.Patrick CH, Lazarchick J. The effect of bacteremia on automated platelet measurements in neonates. Am J Clin Pathol. 1990;93(3):391–394. doi: 10.1093/ajcp/93.3.391. [DOI] [PubMed] [Google Scholar]
- 16.Becchi C, Malyan MA, Fabbri LP, Marsılı M, Boddi V, Boncinelli S. Mean platelet volume trend in sepsis: is it a useful parameter? Minerva Anestesiol. 2006;72(9):749–756. [PubMed] [Google Scholar]
- 17.Alvarez JA, Baldonedo RF, Bear IG, Solis JAS, Alvarez P, Jorge JI. Incarcerated groin hernias in adults: presentation and outcome. Hernia. 2004;8(2):121–126. doi: 10.1007/s10029-003-0186-1. [DOI] [PubMed] [Google Scholar]
- 18.Kurt N, Oncel M, Ozkan Z, Bingul S. Risk and outcome of bowel resection in patients with incarcerated groin hernias: retrospective study. World J Surg. 2003;27(6):741–743. doi: 10.1007/s00268-003-6826-x. [DOI] [PubMed] [Google Scholar]
- 19.Kulah B, Kulacoglu H, Oruc MT, Duzgun AP, Moran M, Ozmen MM, et al. Presentation and outcome of incarcerated external hernias in adults. Am J Surg. 2001;181(2):101–104. doi: 10.1016/s0002-9610(00)00563-8. [DOI] [PubMed] [Google Scholar]
- 20.Kulah B, Duzgun AP, Moran M, Kulacoglu IH, Ozmen MM, Coskun F. Emergency hernia repairs in elderly patients. Am J Surg. 2001;182(5):455–459. doi: 10.1016/s0002-9610(01)00765-6. [DOI] [PubMed] [Google Scholar]
- 21.O'Dwyer PJ, Norrie J, Alani A, Walker A, Duffy F, Horgan P. Observation or operation for patients with an asymptomatic inguinal hernia a randomized clinical trial. Ann Surg. 2006;244(2):167–173. doi: 10.1097/01.sla.0000217637.69699.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]