Abstract
The correlation between the amount of peritoneal fluid and clinical parameters in patients with perforated peptic ulcer (PPU) has not been investigated. The authors' objective was to derive a reliable formula for determining the amount of peritoneal fluid in patients with PPU before surgery, and to evaluate the correlation between the estimated amount of peritoneal fluid and clinical parameters. We investigated 62 consecutive patients who underwent emergency surgery for PPU, and in whom prediction of the amount of accumulated intraperitoneal fluid was possible by computed tomography (CT) using the methods described by Oriuchi et al. We examined the relationship between the predicted amount of accumulated intraperitoneal fluid and that measured during surgery, and the relationship between the amount of fluid predicted preoperatively or measured during surgery and several clinical parameters. There was a significant positive correlation between the amount of fluid predicted by CT scan and that measured during surgery. When patients with gastric ulcer and duodenal ulcer were analyzed collectively, the predicted amount of intraperitoneal fluid and the amount measured during surgery were each associated with the period from onset until CT scan, perforation size, the Mannheim peritoneal index, and the severity of postoperative complications according to the Clavien–Dindo classification. Our present results suggest that the method of Oriuchi et al is useful for predicting the amount of accumulated intraperitoneal fluid in patients with PPU, and that this would be potentially helpful for treatment decision-making and estimating the severity of postoperative complications.
Key words: Peptic ulcer, Perforation, Peritoneal fluid, Gastric ulcer, Duodenal ulcer, Computed tomography
Perforated peptic ulcer (PPU) remains a life-threatening condition, especially in elderly patients for whom treatment is often delayed, with reported mortality rates of 10–40%.1–3 Surgical treatment for PPU that includes simple closure or placement of an omental patch using a conventional open approach or laparoscopy has become widely accepted.4 A patient age of >60 years, delayed treatment, or shock at presentation are reported to be factors that predict an unfavorable outcome after surgery.5,6 Computed tomography (CT) is reported to be a more sensitive diagnostic modality than conventional X-ray examination for detection of extra-luminal free air, recognition of the presence of peritoneal fluid, or even the perforation site.7–13 Although the amount of accumulated peritoneal fluid may correlate with the severity of peritonitis caused by PPU, no adequate formula for predicting the amount of ascites before surgery has yet been reported. Oriuchi et al14 have proposed a simple method for calculating the amount of peritoneal fluid in gastric cancer patients with peritoneal carcinomatosis from CT scan images. In the present study, we calculated the amount of peritoneal fluid using Oriuchi's method, and compared it with the actual amount of peritoneal fluid collected during surgery. In addition, we investigated the correlation between the amount of peritoneal fluid predicted preoperatively or that measured during surgery and several clinical parameters or operative outcome.
Materials and Methods
This study was approved by the local ethics committee of Saitama Medical Center, Saitama Medical University.
Patients
Sixty-two consecutive patients [perforation of gastric ulcer (PGU): 9 cases, perforation of duodenal ulcer (PDU): 53 patients] who underwent emergency surgery at our institution between April 2005 and June 2011 were analyzed retrospectively. All patients underwent CT examination within 6 hours before surgery.
Evaluation of the estimated amount of peritoneal fluid (EAPF)
As described above, EAPF was calculated according to the method proposed by Oriuchi et al14 In brief, on conventional CT images, the thickness of ascites in centimeters was measured in 3 planes: the bilateral subphrenic space (A and B), the bilateral paracolic space (C and D) and the pre-bladder space (E). The average thickness, (A + B + C + D + E)/5, was then multiplied by the area of a standard abdominal cavity in the anterior projection, assumed to be 1000 cm2, to yield the volume of ascites as: (A + B + C + D + E) × 200 mL (Fig. 1).
Fig. 1.

Formula for deriving the estimated amount of peritoneal fluid (EAPF) using Oriuchi's method: EAPF = (A + B + C + D + E) cm × 200 mL.
Surgical procedure and evaluated clinical parameters
Seven patients underwent distal gastrectomy, and 45 underwent simple closure with or without an omental patch. A conventional open approach was used in 17 patients, and a mini-laparotomy (a skin incision of less than 7 cm) in 45.15 Just after encountering the abdominal cavity, peritoneal fluid was collected before washing the abdominal cavity with saline, and the actual amount of peritoneal fluid (AAPF) was recorded. Peritoneal fluid was collected using an aspiration system routinely employed in our institution, and its volume determined using a graduated container (Fitfix, Daiken-iki Co, Osaka, Japan). The period from onset of abdominal pain to CT examination, diameter of perforation, Mannheim peritonitis index (MPI),16 and postoperative complications after surgery (Clavien–Dindo classification)17 were retrieved from the medical charts.
We evaluated the correlation between the EAPF and AAPF, and furthermore we analyzed the correlation between each of EAPF and AAPF and clinical parameters.
Statistical analysis
Variables were expressed as median and range. Pearson's simple regression analysis was applied for examining correlations between variables. Differences between continuous variables were analyzed by MannWhitney U test and chi-squared test. Differences at P < 0.05 were considered significant.
Results
Patient characteristics and clinical parameters are shown in Table 1. There were 42 men and 20 women with a median age of 60.5 years (range 20–94 years). There were no significant differences between PGU and PDU with regard to age, diameter of perforation, surgical procedure employed, systolic blood pressure on admission, MPI, and period from onset of abdominal pain until surgery. Gender (Mann-Whitney U test: P = 0.02) and the degree of severity based on the Clavien–Dindo classification (chi-squared test: P = 0.04) showed significant differences between the PGU and PDU groups.
Table 1.
Patient's characteristics and clinical parameters

A total of 25 patients developed one or more postoperative complications, including wound infections in 14, intra-abdominal abscess in 10, disseminated intravascular coagulopathy in three, anastomotic leakage in 2, pneumonia in 2, and renal failure in 1. We performed re-intervention (defined as Clavien–Dindo classification Grade 3) successfully in 4 of the patients who developed intra-abdominal abscess using surgical (n = 1) or percutaneous echo-guided (n = 3) drainage.
Correlation between EAPF and AAPF
For the PPU cases overall, the median (range) EAPF was 800 (0–10,000) mL, whereas the median (range) AAPF was 440 (0–7000) mL, and thus the 2 parameters were strongly correlated (r = 0.919, P < 0.01; Fig. 2a). Separate analysis of the PGU and PDU groups showed that the median (range) EAPF and AAPF in the former were 720 (220–3000) mL and 420 (100–3000) mL, respectively, whereas those in the latter were 800 (0–10,000) mL and 460 (0–7000) mL, respectively. There were also strong correlations between EAPF and AAPF in both the PGU (r = 0.980, P < 0.01) and PDU (r = 0.920, P < 0.01) groups (Fig. 2b, 2c).
Fig. 2.
(a) Correlation between the estimated and actual amounts of peritoneal fluid in patients with perforated peptic ulcer. n = 62, r = 0.919, Y = 45.753 + 0.678X, P < 0.01. (b) Correlation between the estimated and actual amounts of peritoneal fluid in patients with perforated gastric ulcer. n = 9, r = 0.980, Y = −264.939 + 1.057X, P < 0.01. (c) Correlation between the estimated and actual amounts of peritoneal fluid in patients with perforated duodenal ulcer. n = 53, r = 0.920, Y = 54.973 + 0.663X, P < 0.01.
Correlation between EAPF and clinical parameters (Table 2)
Table 2.
Correlation between EAPF and clinical parameters
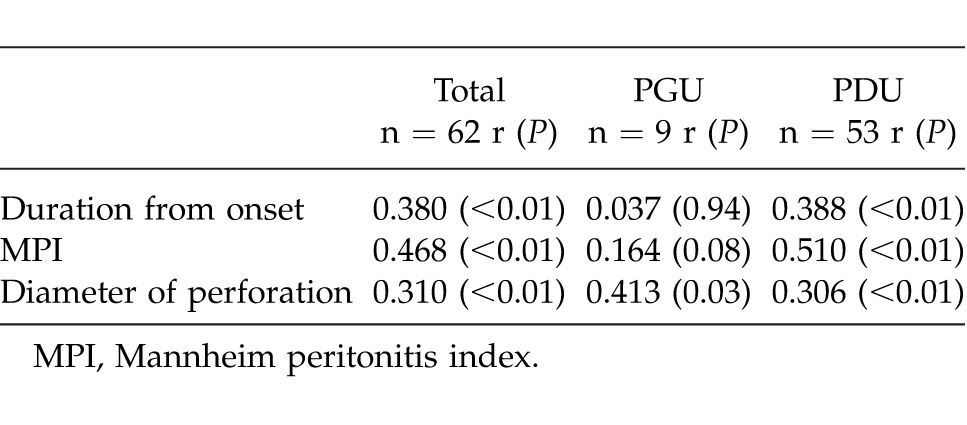
Analysis of the patients as a whole revealed a significant correlation between EAPF and the period from onset until CT examination (r = 0.380, P < 0.01). When the groups were analyzed separately, PDU patients showed a significant correlation between EAPF and the period from onset until CT examination (r = 0.388, P < 0.01). However, the corresponding correlation in PGU patients was not significant (r = 0.037, P = 0.94). Weak but significant correlations between EAPF and MPI were observed in both the patients as a whole (r = 0.468, P < 0.01) and in PDU patients (r = 0.510, P < 0.01). However, no significant correlation was evident in PGU patients (r = 0.164, P = 0.08). Weak but significant correlations between EAPF and diameter of perforation were also observed in the patients as a whole (r = 0.310, P < 0.01), PGU patients (r = 0.413, P = 0.03), and PDU patients (r = 0.306, P < 0.01).
Table 3 shows correlations between AAPF or EAPF and complications after surgery (Clavien–Dindo classification) for both the PDU and PGU patients as a whole.
Table 3.
Correlation between actual or estimated amount of peritoneal fluid and Clavien–Dindo classification of perforated peptic ulcer patients
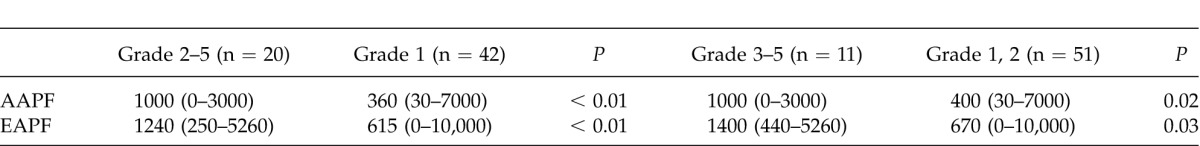
Among PPU patients, the median AAPF in those with a Clavien–Dindo Grade of 2 or over was significantly greater than that in Grade 1 patients (Mann-Whitney U test: P < 0.01), and a similar result was obtained for EAPF (P < 0.01). Similarly, when PPU patients were divided into those with Clavien–Dindo Grades of worse than 3 and better than 2, median AAPF or EAPF was significantly greater in the former than in the latter (Mann-Whitney U test: P = 0.02 and P = 0.03, respectively).
Discussion
We clearly demonstrated in patients with PPU, that there was a significant positive correlation between the amount of fluid predicted by CT scan and that measured during surgery, regardless of the site of perforation (stomach or duodenum). In addition, when patients with gastric ulcer and duodenal ulcer were analyzed collectively, the predicted amount of intraperitoneal fluid and the amount measured during surgery were each associated with the period from onset until CT scan, perforation size, the Mannheim peritoneal index, and the severity of postoperative complications according to the Clavien–Dindo classification.
In a clinical setting, ultrasonography makes it possible to detect peritoneal fluid.18 Ultrasonography has an obvious benefit in comparison with CT in terms of cost efficiency or exposure of patients to radiation. However, there is some concern that the results of ultrasonography may differ according to the proficiency of the examiner. From this viewpoint, CT examination is a more objective examination than ultrasonography. Oriuchi's method for calculation of the EAPF was originally established to assess the response of malignant ascites to chemotherapy. Our present study revealed a strong correlation between EAPF calculated using Oriuchi's method and the AAPF collected from PPU patients during surgery. This correlation was also maintained when we analyzed PGU and PDU patients separately. Previous reports have mentioned the usefulness of CT scan for pretreatment diagnosis of PPU in terms of detection of both pneumoperitoneum and the site of perforation.7–13 However, the correlation between the amount of peritoneal fluid and the general condition of patients has never been examined. Some Japanese reports have indicated the importance of the amount of peritoneal fluid as a parameter for treatment decision-making.19–20 The Japanese guidelines for selection of PGU patients for conservative treatment have proposed that the amount of peritoneal fluid can be used as a parameter for treatment planning.21 However, no reliable method for calculation or prediction of the amount of peritoneal fluid has yet been devised. Our present study is the first to have proposed a reliable method for predicting the actual amount of peritoneal fluid in PPU patients.
We also confirmed that there were significant correlations between the EAPF and the period from onset until CT examination, MPI, and perforation diameter in PPU patients. In addition, with regard to the severity of postoperative complications according to the Clavien–Dindo classification, both AAPF and EAPF were significantly greater in patients who were classified as Grade 3 or worse (requiring surgery) than in those who were classified as Grade 1 and 2 (requiring conservative treatment). Furthermore, AAPF and EAPF were significantly greater in patients who were classified as Grade 2 or worse than in those who were classified as Grade 1. Considering these findings, the EAPF calculated from CT findings is considered to be a useful parameter for prediction of postoperative complications in patients with PPU.
Using multivariate analysis, it will be necessary to confirm that EAPF (or AAPF) is an independent factor that can be used to predict the severity of postoperative complications in PPU patients. Furthermore, a reliable cut-off value of EAPF should be established in order to predict each category of postoperative complication according to the Clavien–Dindo classification, and for this purpose further accumulation of cases is warranted. One limitation of this study is that we were unable to clarify whether PGU and PDU patients can be treated equally in terms of treatment planning or choice of surgical procedure, as only 9 PGU patients (15%) were enrolled. Therefore, further accumulation of PGU cases is also needed.
In conclusion, our present results suggest that the method of Oriuchi et al is useful for predicting the amount of accumulated intraperitoneal fluid in patients with PPU, and that this would be potentially helpful for treatment decision-making and estimating the severity of postoperative complications. Future prospective studies should be conducted for standardization and stratification of PPU treatment using the EAPF calculated from preoperative CT scan data.
References
- 1.Thorsen K, Glomsaker TB, von Meer A, Soreide K, Soreide JA. Trends in diagnosis and surgical management of patients with perforated peptic ulcer. J Gastrointest Surg. 2011;15(8):1329–1335. doi: 10.1007/s11605-011-1482-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hemmer PH, de Schipper JS, van Etten B, Pierie JP, Bonenkamp JJ, de Graaf PW, et al. Results of surgery for perforated gastroduodenal ulcers in a Dutch population. Dig Surg. 2001;28((5–6)):360–366. doi: 10.1159/000331320. [DOI] [PubMed] [Google Scholar]
- 3.Thorsen K, Søreide JA, Kvaløy JT, Glomsaker T, Søreide K. Epidemiology of perforated peptic ulcer: Age- and gender-adjusted analysis of incidence and mortality. World J Gastroenterol. 2013;19(3):347–354. doi: 10.3748/wjg.v19.i3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertleff MJ, Lange JF. Perforated peptic ulcer disease: a review of history and treatment. Dig Surg. 2010;27(3):161–169. doi: 10.1159/000264653. Epub 2010 Jun 22. Review. [DOI] [PubMed] [Google Scholar]
- 5.Zittel TT, Jehle EC, Becker HD. Surgical management of peptic ulcer disease today: indication, technique and outcome. Langenbecks Arch Surg. 2000;385(2):84–96. doi: 10.1007/s004230050250. [DOI] [PubMed] [Google Scholar]
- 6.Sarosi GA, Jr, Jaiswal KR, Nwariaku FE, Asolati M, Fleming JB, Anthony T. Surgical therapy of peptic ulcers in the 21st century: more common than you think. Am J Surg. 2005;190(5):775–779. doi: 10.1016/j.amjsurg.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 7.Kim SH, Shin SS, Jeong YY, Heo SH, Kim JW, Kang HK. Gastrointestinal tract perforation: MDCT findings according to the perforation sites. Korean J Radiol. 2009;10(1):63–70. doi: 10.3348/kjr.2009.10.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeung KW, Chang MS, Hsiao CP, Huang JF. CT evaluation of gastrointestinal tract perforation. Clin Imaging. 2004;28(5):329–333. doi: 10.1016/S0899-7071(03)00204-3. [DOI] [PubMed] [Google Scholar]
- 9.Tsujimoto H, Yaguchi Y, Hiraki S, Sakamoto N, Kumano I, Matsumoto Y, et al. Peritoneal computed tomography attenuation values reflect the severity of peritonitis caused by gastrointestinal perforations. Am J Surg. 2011;202(4):455–460. doi: 10.1016/j.amjsurg.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 10.Thorsen K, Glomsaker TB, von Meer A, Søreide K, Søreide JA. Trends in diagnosis and surgical management of patients with perforated peptic ulcer. J Gastrointest Surg. 2011;15(8):1329–1335. doi: 10.1007/s11605-011-1482-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hainaux B, Agneessens E, Bertinotti R, De Maertelaer V, Rubesova E, Capelluto E, et al. Accuracy of MDCT in predicting site of gastrointestinal tract perforation. Am J Roentgenol. 2006;187(5):1179–1183. doi: 10.2214/AJR.05.1179. [DOI] [PubMed] [Google Scholar]
- 12.Furukawa A, Sakoda M, Yamasaki M, Kono N, Tanaka T, Nitta N, et al. Gastrointestinal tract perforation: CT diagnosis of presence, site, and cause. Abdom Imaging. 2005;30(5):524–534. doi: 10.1007/s00261-004-0289-x. Review. [DOI] [PubMed] [Google Scholar]
- 13.Pun E, Firkin A. Computed tomography and complicated peptic ulcer disease. Australas Radiol. 2004;48(4):516–519. doi: 10.1111/j.1440-1673.2004.01364.x. [DOI] [PubMed] [Google Scholar]
- 14.Oriuchi N, Nakajima T, Mochiki E, Takeyoshi I, Kanuma T, Endo K, et al. A new, accurate and conventional five-point method for quantitative evaluation of ascites using plain computed tomography in cancer patents. Jpn J Clin Oncol. 2005;35(7):386–390. doi: 10.1093/jjco/hyi109. [DOI] [PubMed] [Google Scholar]
- 15.Ishida H, Ishiguro T, Kumamoto K, Ohsawa T, Sobajima J, Ishibashi K, et al. Minilaparotomy for perforated duodenal ulcer. Int Surg. 2011;96(3):194–200. doi: 10.9738/1403.1. [DOI] [PubMed] [Google Scholar]
- 16.Linder MM, Wacha H, Feldmann U, Wesch G, Streifensand RA, Gundlach E. The Mannheim peritonitis index. An instrument for the intraoperative prognosis of peritonitis. Chirurg. 1987;58(2):84–92. [PubMed] [Google Scholar]
- 17.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coppolino F, Gatta G, Di Grezia G, Reginelli A, Iacobellis F, Vallone G. Gastrointestinal perforation: ultrasonographic diagnosis. Crit Ultrasound J. 2013;5(Suppl 1):S4. doi: 10.1186/2036-7902-5-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kyokane T, Iyomasa S, Sawasaki N, Tojima Y, Goto H, Oshiro T, et al. Retrospective study of therapeutic limits to laparoscopic omental patch repair for perforated gastroduodenal peptic ulcer. Jpn J Gastroenterol Surg. 2010;43(1):1–9. [Written in Japanese with English abstract] [Google Scholar]
- 20.Okamura Y, Harada A, Inokawa Y, Sugae T, Shirota T, Takase T, et al. The utility of abdominal CT to judge the operative indication for upper gastrointestinal tract ulcer perforation. Jpn J Gastroenterol Surg. 2007;40(5):529–535. [Written in Japanese with English abstract] [Google Scholar]
- 21.The Japanese Society of Gastroenterology. Evidence-based clinical practice guidelines for peptic ulcer disease [in Japanese] Tokyo, Japan: Nankodo; 2009. [Google Scholar]



