Abstract
Purpose
To examine the relations of maternal pre-pregnancy body mass index (ppBMI) and gestational weight gain (GWG) with offspring cardiometabolic health.
Design
We studied 1,090 mother-child pairs in Project Viva, a Boston-area pre-birth cohort. We measured overall (DXA total fat; BMI z-score) and central adiposity (DXA trunk fat), and SBP in offspring at 6–10 years. Fasting bloods (n=687) were assayed for insulin and glucose (for calculation of HOMA-IR), triglycerides, leptin, adiponectin, hsCRP and IL-6. Using multivariable linear regression, we examined differences in offspring outcomes per 1 SD maternal ppBMI and GWG.
Results
After adjustment for confounders, each 5 kg/m2 higher ppBMI corresponded with 0.92 (95% CI: 0.70, 1.14) kg higher total fat, 0.27 (0.21, 0.32) BMI z-score, and 0.39 (0.29, 0.49) kg trunk fat. ppBMI was also positively associated with HOMA-IR, leptin, hsCRP, IL-6, and SBP; and lower adiponectin. Each 5 kg of GWG predicted greater adiposity (0.33 [0.11, 0.54] kg total fat; 0.14 [0.04, 0.23] kg trunk fat) and higher leptin (6% [0%, 13%]) in offspring after accounting for confounders and ppBMI.
Conclusions
Children born to heavier mothers have more overall and central fat and greater cardiometabolic risk. Offspring of women with higher GWG had greater adiposity and higher leptin.
Keywords: prenatal, gestational weight gain, dual x-ray absorptiometry, adiposity, cardiometabolic health, childhood obesity, lean mass
INTRODUCTION
Childhood obesity has reached epidemic proportions - even among infants (1), suggesting that the perinatal environment plays a role in ‘programming’ excess adiposity. In rodents, maternal obesity prior to and during pregnancy induces dysregulated feeding behavior and altered adipose tissue cellularity in offspring, resulting in obesity and related metabolic derangements later in life (2–5). Although epidemiologic studies in humans indicate that higher maternal pre-pregnancy body mass index (ppBMI) (6) and greater gestational weight gain (GWG) (7–10) are both associated with offspring obesity risk, there are gaps in literature that need to be addressed.
First, most studies on maternal peripartum weight and offspring health have been conducted in children <3 years of age (6). Excess weight during the school-age years is more strongly related to later risk of coronary heart disease (11), diabetes (12), and metabolic syndrome (13); thus, identifying modifiable predictors of adiposity during this timeframe is critical. Second, body mass index (BMI), a crude indicator of body size, is often used as the only measure of offspring adiposity (6). Because early accrual of visceral adipose tissue is particularly pernicious and predicts adverse cardiometabolic outcomes in adulthood (14), it is important to consider not only the amount but also the distribution of body fat. Finally, although a handful of investigations examined how maternal peripartum weight relates to biomarkers of cardiometabolic risk in offspring (15–17), only one study was in children (17). Considering that subclinical markers of cardiovascular risk, such as insulin resistance, dyslipidemia, and high blood pressure begin in childhood and track into adulthood (18), elucidating their relations with modifiable prenatal characteristics could enhance preventive efforts.
In this study, we investigated the extent to which maternal ppBMI and GWG influenced offspring total and central adiposity and established cardiometabolic risk biomarkers during mid-childhood in a longitudinal pre-birth cohort.
METHODS
Study population
This study included participants in Project Viva, a prospective cohort of pregnant women and their children. Details on recruitment and eligibility are described elsewhere (19). All mothers and children originally enrolled in Project Viva and had not subsequently disenrolled were eligible to attend the mid-childhood visit. Of the 2,128 live singleton births, 420 disenrolled before the mid-childhood visit, leaving 1,708 mother-child pairs, of whom 65% (n=1116) attended an in-person visit at age 6–10 years. We measured anthropometry in 1,084 children, and 848 of them completed a dual X-ray absorptiometry (DXA) scan. We excluded mother-child pairs with a prenatal history of type 1 or type 2 diabetes (n=16), and 45 pregnancies with gestation length<34 weeks. The final analytic sample comprised 1,090 mother-child dyads with information on maternal ppBMI or GWG, and anthropometry (n=1084) or a blood specimen (n=687) from the child during mid-childhood. The 1,090 children in the study population were similar those not included due to loss of follow-up and exclusion of those with maternal diabetes (n=1,037) in terms of early life sociodemographic characteristics, and dietary and lifestyle factors during mid-childhood. Mothers who were not included had slightly higher BMI (0.6 kg/m2), were approximately 0.5 years younger, were more likely to smoke during pregnancy (15.6% vs. 9.8%), and had a lower attained education level (24.5% vs. 33.9% college education). Mothers of the subsample of children who provided a blood specimen (n=687) were similar to the original participants, except they were less likely to be married/cohabiting (89.2% vs. 91.4%).
All mothers provided written informed consent at recruitment and at outcome assessment. All children provided verbal assent. The institutional review board of Harvard Pilgrim Health Care approved all study protocols. All procedures were conducted in accordance with established ethical standards.
Exposures: maternal pre-pregnancy BMI and GWG
We calculated pre-pregnancy BMI (ppBMI; kg/m2) using self-reported pre-pregnancy weight collected at enrollment and clinically-measured height. We categorized ppBMI as underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), or obese (≥30.0 kg/m2) (20) We determined total gestational weight gain (GWG) as the difference between the last clinically-measured weight prior to delivery and self-reported pre-pregnancy weight, and categorized it as inadequate, adequate, or excessive according to the 2009 Institute of Medicine (IOM) recommendations (21).
Anthropometric outcomes
During mid-childhood, research assistants (RAs) measured the children’s weight using an electronic scale (Tanita, Arlington Heights, IL) and standing height with a calibrated stadiometer (Shorr Productions, Olney, MD). We calculated age- and sex-specific BMI and height z-scores and percentiles using the Centers for Disease Control (CDC) growth reference (22). RAs measured subscapular (SS) and triceps (TR) skinfold thicknesses with Holtain calipers (Cross-well, UK), from which we calculated the sum (SS+TR) and the ratio (SS/TR) of the skinfold thicknesses. Waist circumference was measured using a non-stretchable measuring tape (Hoechstmass Balzer GmbH, Sulzbach, Germany).
Whole body DXA scans were measured using the Hologic model (Bedford, MA). A single RA checked scans for positioning and defined body regions for analysis. Intra-rater reliability for body compartments and bony landmarks was high (r=0.99). We assessed total fat mass (kg) and trunk fat mass (kg) and calculated fat mass index (kg/m2).
Cardiometabolic outcomes
At the mid-childhood visit, 687 children provided a blood specimen, 93% of whom were fasting for at least 8 hours. Samples were protected from sunlight and transported on ice for processing within 24 hours. We centrifuged all samples and stored plasma aliquots at −80°C. We measured fasting insulin using an electrochemiluminescence immunoassay. Fasting glucose was measured enzymatically (Roche Diagnostics, Indianapolis, IN). We calculated insulin resistance in using the homeostasis model assessment (HOMA-IR). Triglycerides were measured enzymatically with correction for endogenous glycerol. We measured plasma concentrations of leptin and adiponectin with a radioimmunoassay (Linco Research Inc, St Charles, MO). We used an immunoturbidimetric high-sensitivity assay on a Hitachi 911 analyzer to determine C-reactive protein (hsCRP) concentrations (Roche Diagnostics, Indianapolis, IN). Plasma interleukin-6 (IL-6) was measured by ELISA.
We measured blood pressure with an automated oscillometric recorder. For each child, we obtained 5 blood pressure measurements taken 1 minute apart. We calculated systolic blood pressure (SBP) as the average since the intra-class coefficient was high (ICC=0.74).
Covariates
Mothers provided information on their race/ethnicity, age, education, parity, date of last menstrual period, household income, and paternal weight and height via interviews and questionnaires administered at enrollment. Prenatal medical records provided information on prenatal glucose tolerance status (23), child’s sex, birthweight, and delivery date. We calculated gestational age at birth from the date of the last menstrual period (LMP) or from a second trimester ultrasound if the estimated delivery date differed by >10 days (n=127); correlation between GWG calculated with LMP vs. ultrasound was high (r=0.80). We determined birthweight-for-gestational-age-and-sex z-scores (‘fetal growth’) based on U.S. reference data (24). We defined small-for-gestational-age-and-sex (SGA) as <10th percentile, and large-for-gestational-age-and-sex (LGA) as ≥90th percentile. Mothers reported breastfeeding duration in postpartum questionnaires. At the mid-childhood visit, mothers provided information on the child’s dietary and lifestyle habits.
Statistical analysis
We first examined the distributions of ppBMI and GWG across categories of early life sociodemographic characteristics to identify confounders. Associations with GWG were adjusted for ppBMI since weight gain during pregnancy is strongly related to weight entering pregnancy (25).
In multivariable analyses, we examined ppBMI and GWG as exposures in separate linear regression models. We started by evaluating ppBMI in weight status categories to assess for non-linear associations with the offspring outcomes based on the direction, magnitude, and precision of the estimates, and a test for linear trend where the ordinal variable was entered into the model as continuous. Since associations were monotonic across categories, we examined ppBMI continuously. We employed the same approach for GWG. Associations with outcomes were generally linear across the IOM categories, although we noted nonlinear associations with adiponectin and discuss those results separately.
To investigate the extent to which ppBMI and GWG each influenced offspring health, we constructed a series of multivariable models based on bivariate associations and prior knowledge of childhood cardiometabolic health determinants. In the basic model where ppBMI was the exposure of interest (Model 1), we accounted for the child’s sex and age. In the main model (Model 2), we included Model 1 covariates, plus potential confounders (mother’s race/ethnicity, parity, smoking habits; father’s BMI; household income). We then included GWG to evaluate the extent to which ppBMI influenced offspring health through a pathway independent of weight gain during pregnancy (Model 3). We used the same models to assess associations with the cardiometabolic biomarkers. We natural log −(ln)-transformed all biomarkers prior to regression analyses due to non-normal distributions. Estimates from models with a ln-transformed outcome may be exponentiated and interpreted as a % difference. Models where SBP was the outcome also included height z-score, a strong predictor of blood pressure. Because relations of peripartum weight with the cardiometabolic biomarkers could be mediated by offspring adiposity, we also examined associations after accounting for DXA fat mass index (Model 4). The multivariable models for GWG were similar to those for ppBMI, except they also included ppBMI as a covariate starting from Model 1.
Additional adjustment for maternal age, gestational glucose tolerance, physical activity prior to and during pregnancy, delivery mode, fetal growth, and breastfeeding duration did not change associations. We also examined relations after including maternal postpartum weight retention and physical activity measured at the mid-childhood visit to account maternal-child shared lifestyle behaviors; as well as child’s intake of sugar-sweetened beverages, television viewing, sleep habits, and physical activity. Inclusion of these behaviors did not change findings either; thus, they were not included in the final models. In sensitivity analyses, we further restricted the sample by excluding 109 infants born ≥34 weeks and <37 weeks (in addition to the 45 infants born <34 weeks) and saw no difference in the results, so we opted to exclude only those born <34 weeks to maximize power.
We found no indication of effect modification by sex for ppBMI or GWG, so we present results for both sexes combined. We also assessed for an interaction between ppBMI and GWG. There was some evidence that GWG modified the relation of ppBMI with central adiposity indicators (P-interaction<0.05), so we also examined the associations of ppBMI with the anthropometric outcomes within strata of IOM categories.
Because some participants were missing covariate information, we performed multiple imputation using chain equations (26). We generated 50 imputed datasets and obtained estimates using PROC MIANALYZE. We present results from the imputed analysis throughout the manuscript unless otherwise indicated. All 2,128 participants were used in the imputation process, but only those with observed ppBMI and GWG were included in the analysis. For the offspring anthropometric outcomes, we imputed missing values for BMI z-score, SS+TR, waist circumference, SS/TR, and SBP if the child participated at a mid-childhood visit (n=1084). All children who attended a DXA visit had scan results (n=848); thus, DXA measurements were not imputed. We imputed values for the cardiometabolic biomarkers if the child had a blood draw during the visit (n=687) but was missing biomarker data. Results from imputed data were similar to those of original data (available upon request).
All analyses were performed with use of the SAS 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Our sample of 1,090 mother-child pairs included 38 underweight (3.5%), 667 normal weight (61.2%), 232 overweight (21.3%), and 153 obese mothers (14.0%); mean(SD) ppBMI was 24.6(5.2) kg/m2. Over half (57.7%) of the mothers gained excessive weight during pregnancy (21). Women with higher ppBMI were more likely to be single, multiparous, black or Hispanic, smoke during pregnancy, have lower income and an overweight partner (Table 1). As expected, overweight and obese women gained less weight during pregnancy than normal weight women. After accounting for ppBMI, women with higher GWG were younger, had fewer previous births, and were more likely to smoke during pregnancy. Median age of children in our sample was 7.7 years (range 6.6–10.9); 49.7% (n=542) were boys. Details on the mid-childhood offspring outcomes are presented in Table 2.
Table 1.
Maternal pre-pregnancy BMI and gestational weight gain according to characteristics among Project Viva mother-child pairs
| N (%) | Pre-pregnancy BMI (kg/m2) Mean(SD) |
Pb | Gestational weight gaina (kg) Mean (SE) |
Pb | |
|---|---|---|---|---|---|
| Overall | 1090 | 24.8 (0.12) | 15.5 (5.3) | -- | |
| Maternal & family characteristics | |||||
| Mother's age at enrollment | 0.12 | <0.0001 | |||
| 15–24 years | 106 (9.7) | 25.2 (5.4) | 15.5 (0.5) | ||
| 25–34 years | 647 (59.4) | 24.7 (5.3) | 15.8 (0.2) | ||
| 35–44 years | 337 (30.9) | 24.3 (4.9) | 14.9 (0.3) | ||
| Maternal race/ethnicity | <0.0001 | 0.14 | |||
| Black | 178 (16.4) | 26.9 (6.7) | 15.1 (0.4) | ||
| Hispanic | 72 (6.7) | 25.6 (5.1) | 15.0 (0.6) | ||
| White | 736 (67.5) | 24.1 (4.6) | 15.7 (0.2) | ||
| Asian | 53 (4.9) | 21.9 (3.7) | 14.5 (0.7) | ||
| Other | 50 (4.6) | 25.5 (5.2) | 16.7 (0.8) | ||
| Mother's marital status | 0.001 | 0.71 | |||
| Married/cohabiting | 993 (91.1) | 24.5 (5.0) | 15.5 (0.2) | ||
| Single | 97 (8.9) | 26.2 (6.5) | 15.3 (0.5) | ||
| Annual household incomec | <0.0001 | 0.83 | |||
| < $20,000 | 58 (5.3) | 26.3 (7.0) | 14.7 (0.8) | ||
| $20,000 – $39,999 | 132 (12.2) | 26.4 (6.9) | 16.0 (0.5) | ||
| $40,000 – $69,999 | 237 (21.7) | 25.2 (5.9) | 15.4 (0.4) | ||
| > $70,000 | 663 (60.8) | 23.9 (4.4) | 15.5 (0.2) | ||
| Maternal education | <0.0001 | 0.52 | |||
| Primary | 104 (9.6) | 27.1 (6.5) | 14.6 (0.5) | ||
| Secondary | 617 (56.6) | 24.9 (5.3) | 15.7 (0.2) | ||
| University | 369 (33.8) | 23.5 (4.1) | 15.4 (0.3) | ||
| Maternal smoking habits | 0.01 | 0.20 | |||
| Never | 772 (70.8) | 24.4 (5.1) | 15.3 (0.2) | ||
| Quit before pregnancy | 211 (19.4) | 24.6 (5.0) | 15.7 (0.4) | ||
| Smoked in early pregnancy | 106 (9.8) | 26.0 (5.9) | 16.6 (0.5) | ||
| Parity | 0.0008 | <0.0001 | |||
| 0 | 516 (47.3) | 24.0 (4.6) | 16.1 (0.2) | ||
| 1 | 390 (35.8) | 25.2 (5.4) | 15.0 (0.3) | ||
| ≥ 2 | 184 (16.9) | 25.2 (6.1) | 14.9 (0.4) | ||
| Mother's pre-pregnancy BMI | <0.0001 | ||||
| Underweight (<18.5 kg/m2) | 38 (3.5) | -- | -- | 15.9 (5.3) | |
| Normal (18.5 to 24.9 kg/m2) | 667 (61.2) | -- | -- | 16.1 (5.2) | |
| Overweight (25.0 – 29.9 kg/m2) | 232 (21.3) | -- | -- | 16.0 (5.2) | |
| Obese (≥ 30.0 kg/m2) | 153 (14.0) | -- | -- | 12.3 (5.2) | |
| Paternal BMI | <0.0001 | 0.90 | |||
| Not overweight (< 25 kg/m2) | 393 (36.0) | 23.4 (4.5) | 15.5 (0.3) | ||
| Overweight (≥ 25 kg/m2) | 697 (64.0) | 25.3 (5.4) | 15.5 (0.2) | ||
| Perinatal & early life characteristics | |||||
| Gestational weight gaind | <0.0001 | ||||
| Inadequate | 133 (12.3) | 24.1 (5.8) | -- | -- | |
| Adequate | 323 (29.9) | 23.5 (4.9) | -- | -- | |
| Excessive | 623 (57.5) | 25.4 (5.0) | -- | -- | |
| Gestational glucose tolerance | <0.0001 | 0.01 | |||
| Normoglycemic | 909 (83.4) | 24.4 (5.1) | 15.7 (0.2) | ||
| Isolated hyperglycemia | 95 (8.7) | 24.9 (5.0) | 14.8 (0.5) | ||
| Impaired glucose tolerance | 37 (3.4) | 25.9 (5.0) | 15.5 (0.9) | ||
| Gestational diabetes | 49 (4.5) | 27.6 (6.4) | 13.4 (0.8) | ||
| Delivery method | 0.0002 | 0.21 | |||
| Caesarean section | 242 (22.2) | 25.7 (6.1) | 15.9 (0.3) | ||
| Vaginal | 848 (77.8) | 24.3 (4.9) | 15.4 (0.2) | ||
| Childs sex | 0.74 | <0.0001 | |||
| Male | 542 (49.7) | 24.7 (5.0) | 16.0 (0.2) | ||
| Female | 548 (50.3) | 24.6 (5.3) | 15.0 (0.2) | ||
| Fetal growthe | 0.002 | <0.0001 | |||
| SGA (< 10th percentile) | 64 (5.9) | 23.7 (5.8) | 14.1 (0.6) | ||
| AGA (10 – ≤ 90th percentile) | 875 (80.3) | 24.5 (5.1) | 15.3 (0.2) | ||
| LGA (≥ 90th percentile) | 151 (13.9) | 25.8 (5.4) | 17.1 (0.4) | ||
| Duration of any breastfeeding | <0.0001 | ||||
| < 1 month | 200 (18.4) | 26.4 (6.5) | 15.9 (0.4) | ||
| 1–6 months | 374 (34.3) | 24.9 (5.4) | 15.4 (0.3) | ||
| 7–11 months | 213 (19.5) | 23.9 (4.8) | 15.3 (0.4) | ||
| ≥ 12 months | 302 (27.7) | 23.6 (4.2) | 15.6 (0.3) | ||
BMI: body mass index; AGA: appropriate for gestational age; LGA: large for gestational age; SGA: small for gestational age
Estimates are adjusted for continuous pre-pregnancy BMI, with the exception of the 'overall' estimate and those for pre-pregnancy BMI, which is mean (SD).
Represents a test for linear trend in which the ordinal predictor is entered into the model as a continuous variable for all variables except race/ethnicity, marital status, child’s sex, smoking status, and delivery method (Wald test).
Reported at enrollment.
According to the Insitute of Medicine (IOM) 2009 Guidelines.
Based on gestational age- and sex-specific U.S. national reference data.
Table 2.
Descriptive statistics of the offspring outcome variables assessed in mid-childhood (median 7.7 years)
| N | Mean ± SD | |
|---|---|---|
| Overall Adiposity | ||
| DXA total fat (kg) | 848 | 7.4 ± 3.8 |
| BMI-for-age Z-scorea | 1078 | 0.39 ± 1.00 |
| SS+TR sum (mm)b | 1077 | 19.9 ± 9.8 |
| Central Adiposity | ||
| DXA trunk fat (kg) | 848 | 2.5 ± 1.7 |
| Waist circumference (cm) | 1081 | 60.0 ± 8.3 |
| SS/TR ratioc | 1077 | 0.7 ± 0.2 |
| Lean mass | ||
| DXA fat-free mass (kg) | 848 | 21.7 ± 4.3 |
| Height-for-age Z-scorea | 1084 | 0.24 ± 0.97 |
| Cardiometabolic Risk Biomarkers | ||
| Fasting Insulin (µU/mL) | 593 | 7.9 ± 6.4 |
| HOMA-IR (mmol/L) | 544 | 1.9 ± 1.8 |
| Triglycerides (mg/dL) | 615 | 59.1 ± 38.0 |
| Leptin (ng/mL) | 602 | 6.1 ± 7.4 |
| Adiponectin (µg/mL) | 602 | 15.6 ± 8.8 |
| hsCRP (mg/L) | 620 | 0.9 ± 2.9 |
| IL-6 (pg/mL) | 602 | 1.0 ± 1.4 |
According to the sex- and age- specific CDC 2–20 y growth reference.
The sum of the subscapular (SS) and triceps (TR) skinfold thicknesses.
The ratio of the SS to TR skinfold thicknesses.
We found positive associations of ppBMI with offspring overall and central adiposity (Table 3). In the main model (Model 2), every 5 kg/m2 of ppBMI corresponded to 0.92 (95% CI: 0.70, 1.14) kg higher total fat mass, and 0.39 (0.29, 0.49) kg higher trunk fat mass. Similar trends were observed for the other adiposity indicators. Higher ppBMI was also directly related to most measures of cardiometabolic risk. Each 5 kg/m2 increment in BMI was related to 0.10 (0.04, 0.16) ln-transformed HOMA-IR, which corresponds to 11% (4%, 17%) higher HOMA-IR. Greater ppBMI was also related to higher offspring leptin, hsCRP, IL-6, and SBP; and lower adiponectin (Table 3 Model 2). Accounting for GWG did not change the associations (Table 3 Model 3). Inclusion of total fat mass index attenuated estimates for all biomarkers to the null (Table 3 Model 4).
Table 3.
Associations of offspring adiposity and cardiometabolic risk biomarkers with maternal pre-pregnancy BMI among 1084 mother-child pairs in Project Vivaa
| Difference (95% CI) per 5 kg/m2 maternal pre-pregnancy BMI | ||||||||
|---|---|---|---|---|---|---|---|---|
| N | Model 1 | N | Model 2 | N | Model 3 | N | Model 4 | |
| Anthropometric outcomes | ||||||||
| Overall adiposity | ||||||||
| DXA fat mass (kg) | 848 | 1.08 (0.87, 1.30) | 848 | 0.92 (0.70, 1.14) | 837 | 1.02 (0.79, 1.25) | ||
| BMI-for-age z-score | 1084 | 0.32 (0.27, 0.38) | 1084 | 0.27 (0.21, 0.32) | 1073 | 0.30 (0.24, 0.35) | ||
| SS + TR sum (mm) | 1084 | 2.76 (2.25, 3.27) | 1084 | 2.29 (1.76, 2.82) | 1073 | 2.49 (1.95, 3.03) | ||
| Central adiposity | ||||||||
| DXA trunk fat mass (kg) | 848 | 0.46 (0.36, 0.55) | 848 | 0.39 (0.29, 0.49) | 837 | 0.43 (0.33, 0.54) | ||
| Waist circumference (cm) | 1084 | 2.43 (2.01, 2.85) | 1084 | 2.08 (1.64, 2.52) | 1073 | 2.29 (1.84, 2.74) | ||
| SS/TR skinfold ratio | 1084 | 0.03 (0.02, 0.04) | 1084 | 0.02 (0.01, 0.03) | 1073 | 0.02 (0.01, 0.03) | ||
| Cardiometabolic risk outcomesb | ||||||||
| HOMA-IR | 687 | 0.12 (0.06, 0.17) | 687 | 0.10 (0.04, 0.16) | 677 | 0.10 (0.04, 0.16) | 592 | 0.01 (−0.05, 0.07) |
| Triglycerides (mg/dL) | 687 | −0.01 (−0.04, 0.02) | 687 | −0.01 (−0.04, 0.03) | 677 | 0.00 (−0.04, 0.03) | 592 | −0.03 (−0.06, 0.00) |
| Leptin (ng/mL) | 687 | 0.11 (0.05, 0.17) | 687 | 0.10 (0.04, 0.16) | 677 | 0.11 (0.05, 0.18) | 592 | 0.01 (−0.05, 0.07) |
| Adiponectin (µg/mL) | 687 | −0.04 (−0.09, 0.00) | 687 | −0.04 (−0.09, 0.00) | 677 | −0.03 (−0.08, 0.01) | 592 | −0.03 (−0.08, 0.02) |
| hsCRP (mg/L) | 687 | 0.28 (0.17, 0.40) | 687 | 0.23 (0.11, 0.35) | 677 | 0.24 (0.12, 0.37) | 592 | 0.04 (−0.09, 0.17) |
| IL-6 (pg/mL) | 687 | 0.10 (0.04, 0.16) | 687 | 0.09 (0.02, 0.15) | 677 | 0.09 (0.02, 0.16) | 592 | 0.07 (0.00, 0.14) |
| SBP (mmHg)c | 1084 | 0.81 (0.34, 1.29) | 1084 | 0.77 (0.27, 1.27) | 1073 | 0.74 (0.22, 1.25) | 837 | 0.29 (−0.28, 0.86) |
Model 1: Adjusted for child's sex and age at mid-childhood examination.
Model 2: Model 1 + maternal race/ethnicity, parity, and smoking habits during pregnancy, father's BMI, and annual household income.
Model 3: Model 2 + gestational weight gain.
Model 4: Model 3 + DXA total fat mass index.
Estimates are derived from imputed data.
All cardiometabolic risk outcomes except SBP were natural log transformed because of non-normal distributions.
All models for SBP are additionally adjusted for height z-score.
After adjustment for ppBMI, higher GWG was independently related to greater overall and central fat mass in offspring (Table 4). In Model 2, every 5 kg GWG was associated 0.33 (0.11, 0.54) kg higher total fat, and 0.14 (0.04, 0.23) kg higher trunk fat. GWG was also positively related to BMI z-score, SS+TR, and waist circumference, but not SS/TR (0.00 [−0.01, 0.01] per 5 kg). GWG was not associated with any biomarkers except leptin (0.06 [0.00, 0.12] higher ln-transformed leptin), although the association was attenuated after accounting for total fat mass index (Table 4 Model 3). GWG was weakly positively associated with SBP in Model 1 and 2, but adjustment for fat mass index in Model 3 produced a stronger inverse relation, suggesting that greater GWG is related to lower blood pressure among offspring with similar levels of adiposity. We also noted a slight positive association with adiponectin in all linear exposure models; however, when we assessed GWG in the IOM categories, the association was inverse J-shaped, with the lowest levels among offspring of mothers who gained inadequate weight (Supplemental Table 1). Neither ppBMI nor GWG was associated with offspring triglyceride levels.
Table 4.
Associations of offspring adiposity and cardiometabolic risk biomarkers with gestational weight gain among 1073 mother-child pairs in Project Vivaa
| Difference (95% CI) per 5 kg maternal gestational weight gain | ||||||
|---|---|---|---|---|---|---|
| N | Model 1 | N | Model 2 | N | Model 3 | |
| Anthropometric outcomes | ||||||
| Overall adiposity | ||||||
| DXA fat mass (kg) | 837 | 0.33 (0.11, 0.55) | 837 | 0.33 (0.11, 0.54) | ||
| BMI-for-age z-score | 1073 | 0.12 (0.06, 0.17) | 1073 | 0.11 (0.06, 0.17) | ||
| SS+TR sum (mm) | 1073 | 0.66 (0.15, 1.17) | 1073 | 0.65 (0.15, 1.16) | ||
| Central adipositya | ||||||
| DXA trunk fat mass (kg) | 837 | 0.14 (0.04, 0.24) | 837 | 0.14 (0.04, 0.23) | ||
| Waist circumference (cm) | 1073 | 0.85 (0.43, 1.28) | 1073 | 0.80 (0.38, 1.23) | ||
| SS/TR ratio | 1073 | 0.00 (−0.01, 0.01) | 1073 | 0.00 (−0.01, 0.01) | ||
| Cardiometabolic outcomesb | ||||||
| HOMA-IR | 677 | 0.01 (−0.05, 0.07) | 677 | 0.01 (−0.05, 0.07) | 592 | −0.02 (−0.07, 0.04) |
| Triglycerides (mg/dL) | 677 | 0.00 (−0.03, 0.02) | 677 | −0.01 (−0.04, 0.02) | 592 | −0.01 (−0.04, 0.02) |
| Leptin (ng/mL) | 677 | 0.06 (0.00, 0.12) | 677 | 0.06 (0.00, 0.12) | 592 | 0.02 (−0.04, 0.07) |
| Adiponectin (µg/mL) | 677 | 0.05 (0.01, 0.09) | 677 | 0.05 (0.01, 0.09) | 592 | 0.05 (0.00, 0.09) |
| hsCRP (mg/L) | 677 | 0.04 (−0.08, 0.16) | 677 | 0.04 (−0.08, 0.17) | 592 | −0.04 (−0.16, 0.08) |
| IL-6 (pg/mL) | 677 | 0.00 (−0.06, 0.07) | 677 | 0.01 (−0.05, 0.07) | 592 | −0.01 (−0.07, 0.06) |
| SBP (mmHg)c | 1073 | 0.06 (−0.42, 0.54) | 1073 | −0.11 (−0.59, 0.38) | 837 | −0.47 (−0.99, 0.04) |
Model 1: Adjusted for maternal pre-pregnancy BMI, child's sex, age at examination.
Model 2: Model 1 + maternal race/ethnicity, parity, and smoking habits during pregnancy, father's BMI, and annual household income.
Model 3: Model 2 + DXA fat mass index.
Estimates are derived from imputed data.
All cardiometabolic risk outcomes except SBP were natural log transformed because of non-normal distributions.
All models for SBP are additionally adjusted for height z-score.
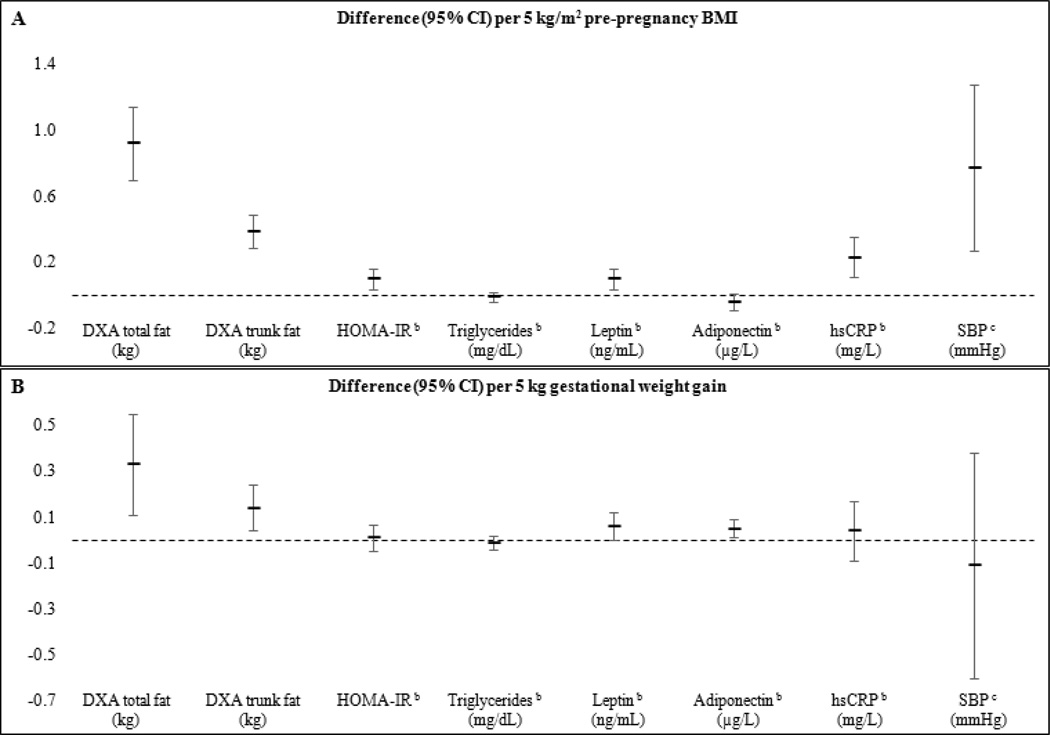
Figure 1 summarizes findings for select offspring outcomes with respect to ppBMI (panel A), and GWG (panel B) from the main model (Model 2).
Figure 1. Associations of offspring adiposity and cardiometabolic risk biomarkers according to maternal pre-pregnancy BMI and gestational weight gaina.
Footnotes:
a Estimates are derived from imputed data. Associations for pre-pregnancy BMI are adjusted for mother’s race/ethnicity, parity, and smoking habits during pregnancy, father’s BMI, annual household income, and child’s sex and age (Model 2).
b Cardiometabolic outcomes are natural log transformed due to non-normal distributions.
c Estimates for SBP are additionally adjusted for height z-score.
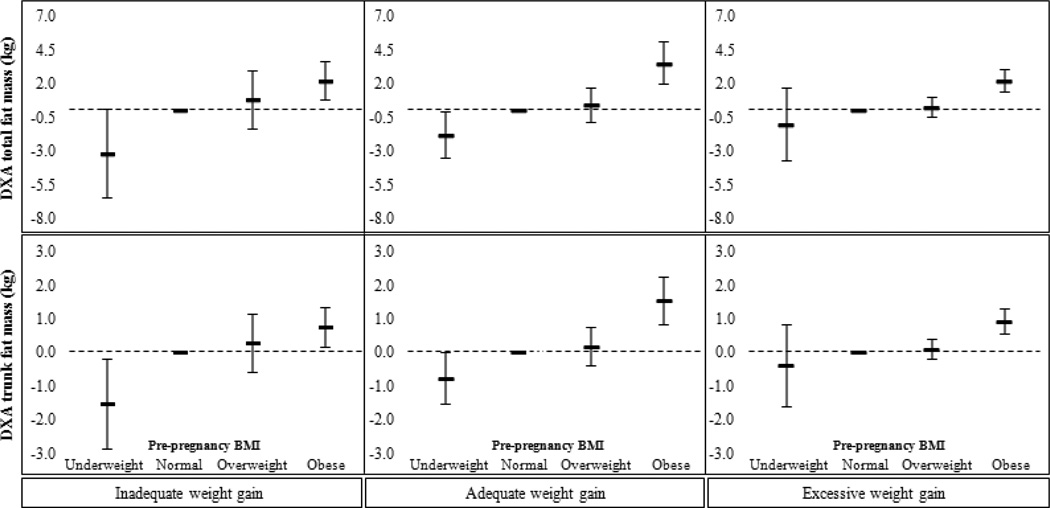
Higher ppBMI was corresponded with greater total and trunk fat in all three GWG categories (Figure 2 and Table 5). Associations were similar for all other adiposity indicators.
Figure 2. Associations of overall and central adiposity indicators for children of underweight, overweight, and mothers, versus those of normal weight mothers, stratified by Institute of Medicine 2009 gestational weight gain guidelinesa.
Footnotes:
a Estimates are derived from imputed data. Associations are adjusted for mother’s race/ethnicity, parity, and smoking habits during pregnancy, father’s BMI, annual household income, and child’s age and sex (Model 2).
Table 5.
Associations of pre-pregnancy BMI with offspring anthropometry during mid-childhood, within categories of gestational weight gain
| Mean difference (95% CI) in offspring anthropometry a per maternal pre-pregnancy BMI category |
||||
|---|---|---|---|---|
| Inadequate gestational weight gain n = 133 |
Adequate gestational weight gain n = 323 |
Excessive gestational weight gain n = 623 |
||
| Pre-pregnancy BMI | ||||
| Overall adiposity | ||||
| DXA fat mass (kg) | Underweight | −3.19 (−6.46, 0.08) | 1.84 (−3.59, −0.09) | −1.03 (−3.74, 1.68) |
| Normal | 0.00 (Reference) | 0.00 (Reference) | 0.00 (Reference) | |
| Overweight | 0.79 (−1.35, 2.93) | 0.42 (−0.85, 1.69) | 0.25 (−0.45, 0.94) | |
| Obese | 2.18 (0.74, 3.62) | 3.50 (1.93, 5.07) | 2.21 (1.37, 3.05) | |
| P trend | 0.0008 | <0.0001 | <0.0001 | |
| BMI z-score | Underweight | −1.16 (−1.94, −0.37) | −0.68 (−1.11, −0.26) | −0.37 (−1.00, 0.27) |
| Normal | 0.00 (Reference) | 0.00 (Reference) | 0.00 (Reference) | |
| Overweight | 0.13 (−0.61, 0.86) | 0.41 (0.08, 0.73) | 0.20 (0.03, 0.37) | |
| Obese | 0.92 (0.45, 1.39) | 0.65 (0.23, 1.08) | 0.57 (0.37, 0.78) | |
| P trend | <0.0001 | <0.0001 | <0.0001 | |
| SS + TR sum (mm) | Underweight | −9.24 (−15.80, −2.68) | −3.26 (−7.02, 0.49) | −0.97 (−7.25, 5.32) |
| Normal | 0.00 (Reference) | 0.00 (Reference) | 0.00 (Reference) | |
| Overweight | 4.12 (−1.96, 10.20) | 2.32 (−0.57, 5.21) | 1.85 (0.21, 3.50) | |
| Obese | 5.50 (1.57, 9.44) | 8.61 (4.83, 12.39) | 5.26 (3.27, 7.25) | |
| P trend | 0.0002 | <0.0001 | <0.0001 | |
| Central adiposity | ||||
| DXA trunk fat mass (kg) | Underweight | −1.54 (−2.87, −0.21) | −0.76 (−1.53, 0.01) | −0.40 (−1.61, 0.81) |
| Normal | 0.00 (Reference) | 0.00 (Reference) | 0.00 (Reference) | |
| Overweight | 0.27 (−0.60, 1.15) | 0.18 (−0.38, 0.74) | 0.09 (−0.22, 0.40) | |
| Obese | 0.75 (0.16, 1.34) | 1.54 (0.85, 2.24) | 0.91 (0.54, 1.29) | |
| P trend | 0.003 | <0.0001 | <0.0001 | |
| Waist circumference (cm) | Underweight | −4.28 (−9.25, 0.69) | −2.61 (−5.92, 0.70) | −1.73 (−6.88, 3.42) |
| Normal | 0.00 (Reference) | 0.00 (Reference) | 0.00 (Reference) | |
| Overweight | 1.52 (−3.09, 6.13) | 0.89 (−1.65, 3.44) | 1.27 (−0.08, 2.61) | |
| Obese | 6.16 (3.22, 9.09) | 7.45 (4.13, 10.77) | 5.32 (3.68, 6.95) | |
| P trend | <0.0001 | <0.0001 | <0.0001 | |
| SS/TR ratio | Underweight | −0.01 (−0.18, 0.15) | −0.03 (−0.11, 0.04) | 0.16 (0.04, 0.29) |
| Normal | 0.00 (Reference) | 0.00 (Reference) | 0.00 (Reference) | |
| Overweight | −0.07 (−0.22, 0.09) | −0.03 (−0.08, 0.03) | 0.05 (0.01, 0.08) | |
| Obese | 0.01 (−0.08, 0.11) | 0.09 (0.01, 0.16) | 0.06 (0.02, 0.10) | |
| P trend | 0.89 | 0.08 | 0.007 | |
Estimates are derived from imputed data and represent mean differences and 95% CI adjusted by mother's race/ethnicity, parity, and smoking habits during pregnancy; father's BMI; annual household income; and child's age and sex.
DISCUSSION
In this U.S. study of mother-child pairs with prospectively-collected information on perinatal characteristics, research-quality measures of body composition and cardiometabolic biomarkers in mid-childhood, and rich data on covariates, children of heavier mothers had more overall and central fat, greater insulin resistance, dysregulated adipocytokines, elevated inflammation, and higher blood pressure. Independent of ppBMI, greater GWG was associated with higher adiposity and leptin.
Our findings that ppBMI and GWG are each associated with offspring overall and central adiposity make two key contributions to literature. First, with the exception of a study in ALSPAC (17), most investigations examining associations of maternal peripartum weight with offspring adiposity used BMI as the only adiposity indicator (6), which may be misleading since it depends on both fat and lean mass and provides no information on fat distribution. In addition to the anthropometric measurements examined in ALSPAC (17), we also assessed trunk fat mass, an accurate proxy for intra-abdominal adipose tissue (27,28). The fact that both ppBMI and GWG were directly related to offspring trunk fat is particularly salient because abdominal adiposity is strongly correlated with cardiometabolic biomarkers and is also an independent predictor of adverse metabolic outcomes in later life (14). Second, our findings are directly relevant to the current U.S. population. ALSPAC recruitment dates back to the early 1990s; thus, their findings reflect a leaner population (21% overweight/obese mothers) with lower gestational weight gain (. excessive GWG) (17). Considering recent reports that >2/3 of reproductive-aged women in the U.S. are overweight or obese (29) and that approximately half experience excess GWG, (21) Project Viva mothers are more representative of the U.S. population.
While research on maternal peripartum weight and offspring health has generally shown that obese mothers and women who gain excessive GWG have heavier children (6), little is known of how these factors relate to offspring cardiovascular risk. Understanding how modifiable perinatal characteristics correlate with cardiometabolic health during childhood is critical because many subclinical risk factors like hypertension (30), dyslipidemia (31), and inflammation (32) begin early in life, and could be present in normal weight children (33). In accordance with findings from ALSPAC (17), we found that greater ppBMI corresponded with higher hsCRP, IL-6, and SBP during mid-childhood. Additionally, higher ppBMI predicted insulin resistance, which is corroborated by findings in adults (16). Children of heavier women also had higher leptin and lower adiponectin. These associations were attenuated after accounting for the child’s fat mass, suggesting mediation by adiposity. However, because the biomarkers and body composition were measured contemporaneously, we are not able to untangle temporality. It is also possible that metabolic derangements lead to weight gain. For example, researchers have traditionally regarded altered adipocytokines and inflammation as consequences of excess adiposity, yet recent evidence indicates that elevated leptin (34,35) and CRP (36) precede weight gain in children. Such findings highlight the importance of elucidating associations with cardiometabolic biomarkers as they not only persist from childhood to adulthood (18), but may also be indicative of future weight gain.
GWG was not related to any of the biomarkers with the exceptions of leptin, and a slight positive relation with adiponectin that was primarily driven by the low levels among offspring whose mothers gained inadequate weight during pregnancy. In ALSPAC, greater GWG was associated with higher leptin, as well as higher CRP, IL-6, and SBP in offspring (17). Since the direction and magnitude of those associations were similar to those we observed, the discrepancy could be due to limited power since the number of children with data on biomarkers in Project Viva was ~1/4 of that in ALSPAC.
This study has some limitations. First, we were not able to directly assess the influence of genetics; however, adjusting for paternal BMI, and mother’s postpartum weight change through the mid-childhood examination did not change findings. Second, there may be attrition bias, although baseline characteristics were similar between those in the study sample versus those who were not. Third, there could be self-report bias of pre-pregnancy weight, as many women tend to under-report weight which would result in lower estimates of ppBMI and higher estimates of GWG. However, we conducted a validation study comparing self-reported pre-pregnancy weight with clinically-measured weight in the medical records within 3 months before the last menstrual period (n=343); correlation between self-reported and objectively-measured BMI was high (r=0.99), and mean underreporting of weight (~1 kg) did not differ by race/ethnicity, gestational age at study enrollment, or weight itself (9). Thus, the degree of self-report bias likely had minimal effect on either measure of peripartum weight, or the results. Finally, Project Viva participants all had health insurance and were recruited from one region of the U.S., so the results may not be generalizable to other populations.
In conclusion, higher maternal ppBMI was associated with greater offspring overall and central adiposity, and an adverse cardiometabolic profile. GWG was directly related to offspring adiposity and leptin levels, independently of ppBMI. The magnitude of effects we observed has important implications at the population level. In a retrospective study of 276,835 Danish adults, each unit of BMI z-score at 7 years corresponded with 10% and 7% higher risk of fatal coronary heart disease in males and females, respectively (11). Furthermore, the association increased with age such that the relative risk was twice as high by age 13 for both sexes. While additional work is warranted to determine causality and modifiability, our findings serve as additional fodder for obstetricians to raise vigilance and make recommendations for women to achieve a healthy weight prior to conception and moderate weight gain during pregnancy to benefit health of offspring and reduce risk of consequent chronic disease.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Dr. Mandy Belfort and Dr. Susannah Huh for their contributions to the laboratory assays. We are also grateful to Sheryl Rifas-Shiman for data management and preparation. We are indebted to the mothers and children of Project Viva for their generous participation, and appreciate the invaluable assistance of past and present Project Viva staff.
FUNDING: This work was supported by the US National Institutes of Health (K24 HD069408, R37 034568, P30 DK092924). Dr. Perng received support from the Thomas O. Pyle Fellowship of the Harvard Pilgrim Health Care Institute.
Abbreviations
- ppBMI
pre-pregnancy body mass index
- DXA
dual x-ray absorptiometry
- HOMA-IR
homeostatic model assessment of insulin resistance
- hsCRP
high sensitivity C-reactive protein
- IL-6
interleukin-6
- IOM
Institute of Medicine
- SS
subscapular skinfold
- SBP
systolic blood pressure
- TR
triceps skinfold
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLAIMER: The funders were not involved in the design and conduct of the study; collection, management, analysis, or interpretation of the data; and preparation, review, or approval of the manuscript.
CONFLICT OF INTEREST: The authors have no conflict of interest.
REFERENCES
- 1.Kim J, Peterson KE, Scanlon KS, et al. Trends in overweight from 1980 through 2001 among preschool-aged children enrolled in a health maintenance organization. Obesity. 2006;14:1107–1112. doi: 10.1038/oby.2006.126. [DOI] [PubMed] [Google Scholar]
- 2.Begum G, Davies A, Stevens A, et al. Maternal undernutrition programs tissue-specific epigenetic changes in the glucocorticoid receptor in adult offspring. Endocrinology. 2013 doi: 10.1210/en.2013-1693. [DOI] [PubMed] [Google Scholar]
- 3.Samuelsson AM, Matthews PA, Argenton M, et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008;51:383–392. doi: 10.1161/HYPERTENSIONAHA.107.101477. [DOI] [PubMed] [Google Scholar]
- 4.Bringhenti I, Moraes-Teixeira JA, Cunha MR, Ornellas F, Mandarim-de-Lacerda CA, Aguila MB. Maternal obesity during the preconception and early life periods alters pancreatic development in early and adult life in male mouse offspring. PLoS One. 2013;8:e55711. doi: 10.1371/journal.pone.0055711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howie GJ, Sloboda DM, Kamal T, Vickers MH. Maternal nutritional history predicts obesity in adult offspring independent of postnatal diet. J Physiol. 2009;587:905–915. doi: 10.1113/jphysiol.2008.163477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PloS one. 2013;8:e61627. doi: 10.1371/journal.pone.0061627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stamnes Kopp UM, Dahl-Jorgensen K, Stigum H, Frost Andersen L, Naess O, Nystad W. The associations between maternal pre-pregnancy body mass index or gestational weight change during pregnancy and body mass index of the child at 3 years of age. Int J Obes. 2012;36:1325–1331. doi: 10.1038/ijo.2012.140. [DOI] [PubMed] [Google Scholar]
- 8.Oken E, Rifas-Shiman SL, Field AE, Frazier AL, Gillman MW. Maternal gestational weight gain and offspring weight in adolescence. Obstet Gynecol. 2008;112:999–1006. doi: 10.1097/AOG.0b013e31818a5d50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol. 2007;196:322, e321–e328. doi: 10.1016/j.ajog.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olson CM, Strawderman MS, Dennison BA. Maternal weight gain during pregnancy and child weight at age 3 years. Matern Child Health J. 2009;13:839–846. doi: 10.1007/s10995-008-0413-6. [DOI] [PubMed] [Google Scholar]
- 11.Baker JL, Olsen LW, Sorensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. The New England journal of medicine. 2007;357:2329–2337. doi: 10.1056/NEJMoa072515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhargava SK, Sachdev HS, Fall CH, et al. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. New Engl J Med. 2004;350:865–875. doi: 10.1056/NEJMoa035698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sachdev HP, Osmond C, Fall CH, et al. Predicting adult metabolic syndrome from childhood body mass index: follow-up of the New Delhi birth cohort. Arch Dis Child. 2009;94:768–774. doi: 10.1136/adc.2008.140905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt MD, Dwyer T, Magnussen CG, Venn AJ. Predictive associations between alternative measures of childhood adiposity and adult cardio-metabolic health. Int J Obes. 2011;35:38–45. doi: 10.1038/ijo.2010.205. [DOI] [PubMed] [Google Scholar]
- 15.Mamun AA, O'Callaghan M, Callaway L, Williams G, Najman J, Lawlor DA. Associations of gestational weight gain with offspring body mass index and blood pressure at 21 years of age: evidence from a birth cohort study. Circulation. 2009;119:1720–1727. doi: 10.1161/CIRCULATIONAHA.108.813436. [DOI] [PubMed] [Google Scholar]
- 16.Hochner H, Friedlander Y, Calderon-Margalit R, et al. Associations of maternal prepregnancy body mass index and gestational weight gain with adult offspring cardiometabolic risk factors: the Jerusalem Perinatal Family Follow-up Study. Circulation. 2012;125:1381–1389. doi: 10.1161/CIRCULATIONAHA.111.070060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser A, Tilling K, Macdonald-Wallis C, et al. Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation. 2010;121:2557–2564. doi: 10.1161/CIRCULATIONAHA.109.906081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camhi SM, Katzmarzyk PT. Tracking of cardiometabolic risk factor clustering from childhood to adulthood. Int J Pediatr Obes. 2010;5:122–129. doi: 10.3109/17477160903111763. [DOI] [PubMed] [Google Scholar]
- 19.Oken E, Baccarelli AA, Gold DR, et al. Cohort profile: Project Viva. Int J Epidemiol. doi: 10.1093/ije/dyu008. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. BMI Classification. [Accessed 5/21, 2012];Global Database on Body Mass Index. http://apps.who.int/bmi/index.jsp?introPage=intro_3.html.
- 21.Institute of Medicine National Research Council Committee to Reexamine I. O. M. Pregnancy Weight Guidelines. The National Academies Collection: Reports funded by National Institutes of Health. In: Rasmussen KM, Yaktine AL, editors. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington (DC): National Academies Press (US) National Academy of Sciences; 2009. [Google Scholar]
- 22.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital and health statistics. Series 11, Data from the national health survey. 2002:1–190. [PubMed] [Google Scholar]
- 23.Regnault N, Gillman MW, Rifas-Shiman SL, Eggleston E, Oken E. Sex-Specific Associations of Gestational Glucose Tolerance With Childhood Body Composition. Diabetes Care. 2013 doi: 10.2337/dc13-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fraser A, Tilling K, Macdonald-Wallis C, et al. Associations of gestational weight gain with maternal body mass index, waist circumference, and blood pressure measured 16 y after pregnancy: the Avon Longitudinal Study of Parents and Children (ALSPAC) Am J Clin Nutr. 2011;93:1285–1292. doi: 10.3945/ajcn.110.008326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 27.Goran MI, Gower BA, Treuth M, Nagy TR. Prediction of intra-abdominal and subcutaneous abdominal adipose tissue in healthy pre-pubertal children. Int J Obes Relat Metab Disord. 1998;22:549–558. doi: 10.1038/sj.ijo.0800624. [DOI] [PubMed] [Google Scholar]
- 28.He Q, Ramirez A, Gidwani S, Heymsfield S, Heshka S, Gallagher D. Measurement of central fat in prepubertal children: MRI, DXA and waist circumference. FASEB J. 2007;21 [Google Scholar]
- 29.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 30.Sun SS, Grave GD, Siervogel RM, Pickoff AA, Arslanian SS, Daniels SR. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119:237–246. doi: 10.1542/peds.2006-2543. [DOI] [PubMed] [Google Scholar]
- 31.Khalil A, Huffman MD, Prabhakaran D, et al. Predictors of carotid intima-media thickness and carotid plaque in young Indian adults: the New Delhi birth cohort. Int J Cardiol. 2013;167:1322–1328. doi: 10.1016/j.ijcard.2012.03.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zieske AW, Tracy RP, McMahan CA, et al. Elevated serum C-reactive protein levels and advanced atherosclerosis in youth. Arterioscler Thromb Vasc Biol. 2005;25:1237–1243. doi: 10.1161/01.ATV.0000164625.93129.64. [DOI] [PubMed] [Google Scholar]
- 33.Mokha JS, Srinivasan SR, Dasmahapatra P, et al. Utility of waist-to-height ratio in assessing the status of central obesity and related cardiometabolic risk profile among normal weight and overweight/obese children: the Bogalusa Heart Study. BMC Pediatr. 2010;10:73. doi: 10.1186/1471-2431-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fleisch AF, Agarwal N, Roberts MD, et al. Influence of serum leptin on weight and body fat growth in children at high risk for adult obesity. J Clin Endocrinol Metab. 2007;92:948–954. doi: 10.1210/jc.2006-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boeke CE, Mantzoros CS, Hughes MD, et al. Differential associations of leptin with adiposity across early childhood. Obesity. 2013;21:1430–1437. doi: 10.1002/oby.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nappo A, Iacoviello L, Fraterman A, et al. High-sensitivity C-reactive protein is a predictive factor of adiposity in children: results of the identification and prevention of dietary- and lifestyle-induced health effects in children and infants (IDEFICS) study. J Am Heart Assoc. 2013;2:e000101. doi: 10.1161/JAHA.113.000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.