Abstract
Many of the effects of dietary restriction (DR) on longevity and health span in model organisms have been linked to reduced protein and amino acid (AA) intake and the stimulation of specific nutrient signaling pathways. Studies in yeast have shown that addition of serine, threonine, and valine in media promotes cellular sensitization and aging by activating different but connected pathways. Protein or essential AA restriction extends both lifespan and healthspan in rodent models. In humans, protein restriction (PR) has been associated with reduced cancer, diabetes, and overall mortality. Thus, interventions aimed at lowering the intake of proteins or specific AAs can be beneficial and have the potential to be widely adopted and effective in optimizing healthspan.
Keywords: healthspan, longevity, CR, FMD, dietary-intervention
Basic research and understanding the effects of dietary interventions
A recent study that evaluated the major risk factors for disability and mortality caused by chronic diseases found that of the 17 major risk factors, dietary regimens formed the largest group of risk factors in the U.S. [1]. Both dietary composition and practices are among the most malleable risk factors. For example, a 30-year comprehensive lifestyle intervention in Finland resulted in an 80% reduction in risk for cardiovascular disease (CVD), of which 60% has been attributed to the reduction of common risk factors such as cholesterol, blood pressure, and smoking [2, 3].
Among the interventions associated with extended longevity and healthspan, restriction of calorie intake by 20–30% below the average (in developed countries) is the most promising due to its potent beneficial changes in risk factors and biomarkers for age-related diseases including CVD, diabetes, and cancer [4]. In model organisms ranging from yeast to rodents, calorie restriction (CR, see glossary) without malnutrition has been consistently shown to increase longevity [4]. Thus, highlighting the importance to combine basic research aimed at understanding the effects of individual dietary components on cellular and organismal response, with rodent and human studies on the effect of dietary interventions on longevity and diseases.
Among the macronutrients, proteins have the most impact on the effects of CR and dietary restriction (DR) (DR refers to the restriction of one or more macronutrients with or without reducing calories), on aging and diseases, based on the observations that restriction of proteins or certain AAs has been associated with extended longevity and reduced incidence and/or progression of multiple age-related diseases [5].
Here we provide an overview of the current understanding and impact of proteins and AAs, on health and lifespan based on numerous studies in simple organisms rodents, non-human primates, and humans. Clearly, the restriction of carbohydrate and of certain types of fats can also be beneficial, particularly in combination with PR, but because the effects of CR on health span have been covered by many reviews, we will focus on proteins, longevity and the mechanisms linking AAs to aging.
Amino acid signaling, aging and DNA damage in yeast
In all major eukaryotic model organisms, from unicellular yeast to mice, pathways that are involved in the regulation of metabolism and growth, including the target of rapamycin (TOR)/S6 kinase and adenylate cyclase-PKA pathways, also promote aging and increase mortality [4]. In most instances, CR has been demonstrated to not only extend lifespan, but also promote healthspan, suggesting that the evolutionarily conserved pathways responsible for the protective effects of CR have been naturally selected for in ancient organisms as a mechanism to protect against extrinsic and intrinsic damage in times of energy scarcity. Among the model organism to study aging the yeast Saccharomyces cerevisiae is perhaps the most amenable to a combination of genetics and biochemical studies aimed at understanding the link between specific components of the diet, such as sugars and AAs, to cellular protection and aging [6, 7]. Aging in yeast is studied by measuring replicative senescence (the replicative lifespan), and chronological aging (the chronological lifespan). Unlike higher organisms, prototrophic yeast cells can synthesize all AAs but auxotrophic strains that lack the ability to synthesize specific AAs can be easily generated, allowing researchers to regulate the levels of AAs in the media and intracellularly.
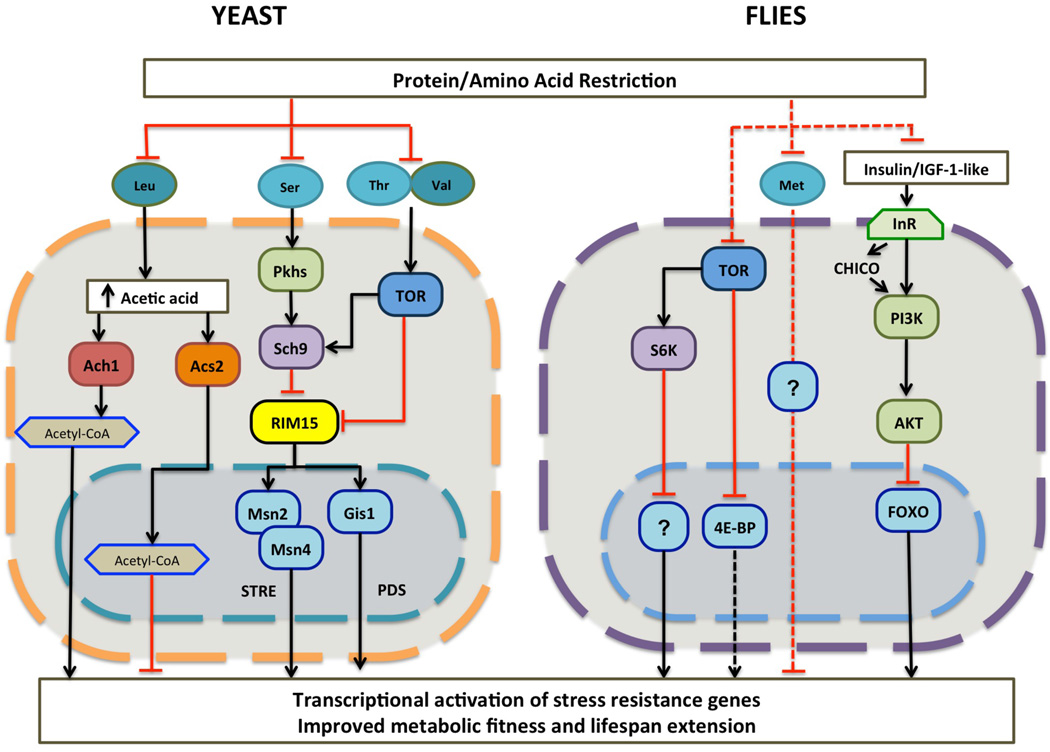
In S. cerevisiae multiple pathways represent the central pro-growth and pro-aging signaling network activated by nutrients [4]. The TOR1-Sch9 pathway is primarily activated by AAs (Fig. 1), while the Ras2-cAMP-PKA pathway is turned on predominantly by glucose [4]. The activation of these pathways by nutrients inhibits Rim15 activity and consequently the activity of stress resistance transcription factors Msn2/Msn4 and Gis1, which play critical roles in the lifespan extension caused by CR [8, 9]. Genetic disruption of TOR1-Sch9, Ras2, or both, extends lifespan in this organism by up to 5-fold [10]. In agreement with an effect of nutrient signaling on both aging and mortality, yeast cells deficient in Sch9-TOR1 or Ras2-cAMP-PKA signaling have reduced genome instability phenotype and show greater resistance to superoxide [6, 10, 11].
Figure 1. Schematic of nutrient signaling pathways regulated by protein and amino acid restriction.
Left: Yeast nutrient signaling pathway is directly affected by the availability of certain amino acids. In addition, production of acetyl-CoA can be modulated by branched chain amino acid Leu, together these pathways make up the pro-aging and growth pathways affecting longevity. Right: An oversimplified representation of the same evolutionary conserved pathway in flies that are affected by restriction of proteins and essential amino acids, in particular, Met. These restrictions result in reduced signaling activity of the TOR network and decreased activation of the IIS pathway.
The availability of strains lacking or overexpressing most of the genes in the nutrient signaling pathways combined with the use of biochemically defined media has allowed researchers to evaluate the impact of specific AAs on the pro-aging signaling described above and their subsequent role on macromolecular damage and lifespan [12–14]. Both glucose and AA restriction are sufficient to extend the yeast lifespan [13, 14]. AA scarcity drives the accumulation of uncharged tRNAs resulting in activation of general control nonderepressible 2 (GCN2), a protein kinase and important regulator of the general AA control pathway, leading to slow growth [14]. Thus, GCN2 plays a role in restoring and maintaining metabolic homeostasis and growth adaptation in response to culture conditions where AAs are scarce [14].
The levels of specific AAs can also affect lifespan. Wu et al., have shown that restriction in methionine (Met) or an increase in glutamic acid results in lifespan extension in yeast [15]. However, a screen for the ability of each AA to sensitize cells to oxidative stress, a phenotype consistently correlated with reduced longevity, three amino acids previously not associated with aging were shown to have the most potent effect on cellular protection, DNA damage and lifespan [18]. When cells were sensitized to oxidative stress by glucose in a Ras-dependent manner, AAs serine (Ser), threonine (Thr) and valine (Val) were the only three that caused a strong sensitization of cells to oxidative stress and promoted aging [13]. In particular, serine activated the Pkh1/2 (PDK1/2 orthologs) while threonine and valine activated TOR signaling, both of which converged to activate the S6 kinase (S6K) ortholog, Sch9 (Fig. 1). The lack of these AAs was sufficient to extend lifespan but also to decrease age-dependent DNA damage [13, 16]. Notably, in yeast, glucose has a pro-aging effect that is more pronounced than that caused by AAs but also cooperates with AAs to accelerate cell damage and death.
In yeast, catabolism of the branched chain amino acid (BCAA), leucine (Leu), results in production of acetic acid, which can subsequently be utilized to generate acetyl-CoA in a mitochondrial Ach1-dependent or nucleo-cytosolic Acs2-dependent manner (Fig. 1) [12, 17]. Depending on which pathway is utilized for acetyl-CoA production, changing the acetylation state of histones can have a major impact on cellular longevity. Histone acetylation is affected by accumulation of cytosolic or nuclear acetyl-CoA. Thus, histone acetylation and cellular protein expression profile in response to internal and external stimuli is indirectly affected by catabolism of BCCA [12, 17]
In summary, the biochemical and genetics studies in yeast indicate that specific AAs and glucose promote aging and stress sensitization through the activation of an interconnected network of signaling enzymes and down-regulation of several stress resistance transcription factors (Fig. 1).
Dietary restriction and lifespan in worms and Flies
Studies of the nematode Caenorhabditis together with those in yeast and flies, established the evolutionary conservation of nutrient-sensing pathways, and in particular those of the insulin/ insulin-like growth factor (Igf-like, IIS) and TOR-S6K signaling pathways. Reduction in IIS as well as TOR and S6K promotes lifespan extension in this organism. TOR activity is reduced by decreased availability of AA and growth factors [4, 18–20]. Furthermore, inhibition of the TOR amino acid-sensing pathway under DR conditions extends lifespan (Fig. 1) [4, 21, 22].
The major method for DR in C. elegans involves reducing the availability of E. coli, or replacing the bacterial food source with a synthetic medium [20, 24, 25]. However, given the lack of purified diets and that the role of specific components of the diet on longevity has not been investigated, it is currently difficult to elucidate the relative contributions of specific AAs and protein relative to reduced calorie intake in worms [26].
Well-studied worms such as the eat-2 (ad465) mutants live longer, when maintained on decreased food uptake [27, 28]. The effect of this mutation on longevity has been linked to inhibition of TOR network signaling, including reduced phosphorylation of S6K (which regulates development), mRNA translation, lipid storage, and autophagy [19, 28]. Another pro-longevity process that is likely to be regulated in part by AA availability is the increased autophagy observed in daf-2/Inr and tor mutants, which is partially mediated by PHA-4/FoxA, a forkhead box transcription factor [19, 29]. In fact, a recent study shows that GCN2, the evolutionarily conserved kinase responsive to AA deficiency, may be able to mediate lifespan extension under DR conditions by converging on TOR/S6K signaling via PHA-4/FoxA transcription factors and its downstream target genes [28, 30]. These nematode studies indicate that AAs, through the interplay of nutrient-sensing pathways and IIS, play critical role in longevity and aging, but also underlines the need to study them further and to describe the connection between each AAs and the various pathways implicated in aging.
In flies, DR can be achieved by changing the content of sugar and yeast (protein source) in the food media without affecting the diet’s calorie content. Experiments testing the independent effect of AAs show that in comparison to nonessential AAs (NEAAs), adding back all essential amino acids (EAAs) decreases survival. Moreover, while omission of Met extends lifespan, omission of tryptophan (Trp) does not increase survival, thus indicating the impact of Met availability on lifespan extension [34]. The impact of DR and AA restriction in flies has recently been reviewed extensively elsewhere [23].
Recent studies indicate that longevity in Drosophila is influenced not only by protein intake but also by the interplay between carbohydrate and protein ingestion [35–38]. A study using Queensland fruit fly (Batrocera tryoni) and chemically defined diets that allowed for the manipulation of the ratio of protein (free AAs) to carbohydrates (sucrose) while keeping other nutrients consistent, found that caloric effects are dependent upon the protein to carbohydrate ratio and noted that lifespan decreased monotonically as diets became more protein rich [36]. Similar observations were made in Drosophila studies designed to obtain accurate measurements of nutrient intake by use of radioisotope labeling, or individually housed flies fed diets varying in both the ratio of yeast to sucrose and the total concentration of yeast and sugar combined [35, 37]. Taken together, studies in Drosophila indicate that, individual nutrients and particularly the levels of proteins/AAs rather than calories alone are the key mediators of lifespan, in agreement with the findings in S. cerevisiae (Fig. 1).
Amino acids, proteins, longevity and healthspan in rodents
Mice represent perhaps the most commonly used model to explore the role of genes and processes associated with lifespan extension in mammals. As in non-mammalian models, reduction in IIS and TOR signaling extends lifespan [4]. Remarkably, insulin/IGF-I signaling deficient mice have a delayed occurrence of fatal neoplasms, increased insulin sensitivity, and a reduction in age-dependent cognitive impairment, including protection from Alzheimer’s disease (AD)-like associated pathologies, as shown in a model of AD [4, 31]. Similarly, inhibition of mTOR/S6K signaling results in increased lifespan and reduction of age-associated pathologies [4, 21].
In rodents, low protein diets are associated with improved healthspan, increased lifespan, and inhibition of hepatitis B virus expression and consequent development of hepatocellular carcinoma, amongst other neoplasms [32, 33].
Although earlier studies attributed increases in lifespan to reduced calories rather than to the reduction of individual nutrients [34], recent research indicates that varying the proportions and quality of individual dietary components can regulate aging independently of caloric intake [34, 35]. To date, several studies have shown decreased age-related pathologies and lifespan extension through the modulation of protein intake [26, 34–36]. Low protein diets have been demonstrated to reduce spontaneous tumor formation, as well as in mimicking the effects of CR in improving renal function [34]. In the past few decades it has also been demonstrated that PR or restriction in tryptophan or methionine, can extend longevity [26, 37–39].
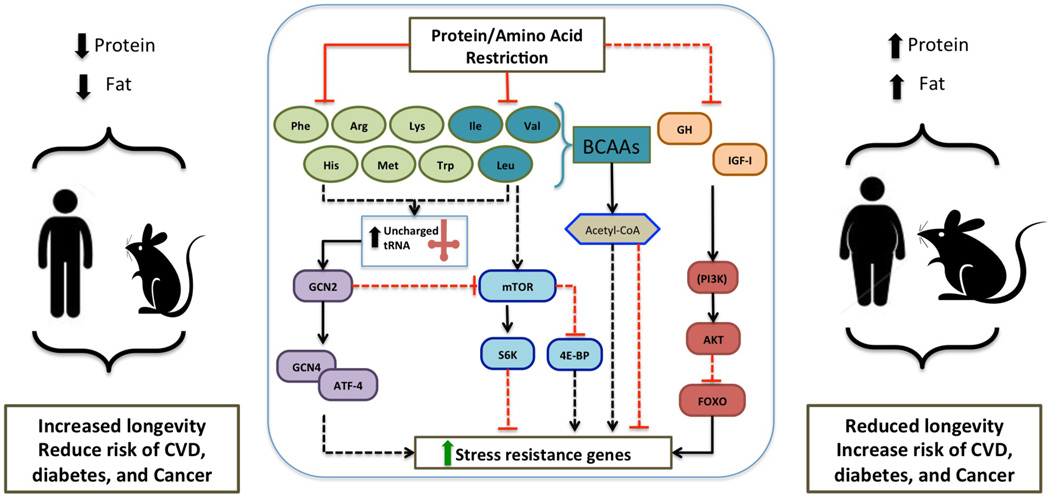
The effect of AAs on the activation of the IGF-I and TOR-S6K pathways is likely to be an important factor in explaining the lifespan extension in AA restricted organisms and previous studies have noted benefits related to the restriction of particular AAs (Fig. 2). In rodents, restricting Trp intake delayed sexual maturation and tumor onset, improved hair growth, coat condition, protected against ischaemia/reperfusion injury to both kidney and liver, and increased maximum health span [26, 34, 40]. The protective effects of Met restriction instead may be caused in part by decreased mitochondrial ROS, serum glucose, insulin, and IGF-I levels, and altered lipogenic/lyipolytic balance contributing to loss of adiposity [26, 34]. Restriction of Leu, may also contribute to longevity by improving insulin sensitivity [26, 41]. However, to date, only Trp and Met restriction have been shown to extend longevity in mice (Fig. 2) [26].
Figure 2. Model of pro-aging nutrient signaling pathways altered by protein and amino acid restriction in mammals.
Intake of high levels of protein and fats, especially from animal sources has been associated with decreased healthspan in both humans and mouse models. In contrast low levels of dietary protein, in particular protein derived from plants, show an inverse correlation to risk of cardiovascular disease (CVD), diabetes, and some types of cancers as observed in human and mouse models. Pro-aging pathways are inhibited through restriction of protein or amino acids via amino acid sensing mTOR and GCN2 pathways. Restriction of essential amino acids results in elevated uncharged tRNAs, which in turn activates the amino acid deficiency sensing GCN2 pathway yielding reduced mTOR network activity. Branched chain amino acids, Ile, Val, Leu, affect production of acetyl-CoA, which in turn plays a role in regulation of lifespan by modulating activity of autophagy response. Direct role of GH/IGF-I signaling remains to be elucidated. Cytoplasmic availability of acetyl-CoA differentially regulates autophagy and subsequently modulates longevity.
Much like the observed effect of CR on longevity via regulation of IGF-I signaling, the TOR and GCN2 AA-response pathways control processes that contribute to the benefits observed with PR, including, autophagy, energy metabolism, immune function, and food intake [26]. Evidence from murine models suggests that crosstalk between TOR and GCN2 occurs upstream of mTORC1. Activation of mTORC1 is possible through the cooperation between leucine and glutamine which enhance glutaminolysis and 2-oxoglutarate (alpha-ketoglutarate) production [42]. Cell culture experiments with rodent-derived cell lines indicate that the presence of AAs stimulates S6K phosphorylation, whereas their absence results in a rapid dephosphorylation of S6K and eIF4E-binding protein (4E-Bp1), and arrests the growth factor-dependent stimulation of S6K activity. The AA deficiency-dependent activation of GCN2 results from an increase in uncharged tRNAs formed from the lack of specific AAs [26]. Although few studies have investigated the connection between AA deprivation, GCN2, and longevity, deficiency of protein is expected to promote longevity in part by repressing mTOR and possibly by activating GCN2.
AAs are known to accumulate in the lysosome after extracellular addition, and this organelle is the end-point of catabolic processes like autophagy, and is also where mTORC1 signaling occurs. The presence of AAs causes mTORC1 to translocate from the cytoplasm to the lysosomal surface where it possibly interacts with the Rheb GTPase. Recent evidence has shown that Rag GTPases, which lie downstream of AAs, are necessary for mTORC1 lysosomal translocation to occur, by communicating availability of AAs to mTORC1, and thus are critical to the understanding of AA-sensing pathways. However, further studies are required to determine how they are modulated during CR or PR [43]. Tissue culture and mouse model experiments have demonstrated that autophagy, as a mechanism to restore cellular homeostasis, is in part regulated by availability of acetyl-CoA. In mammals, three different pathways are utilized to produce acetyl-CoA including the catabolism of BCAA’s. Thus availability of these AAs in the cellular environment directly affects acetyl-CoA production, which in turn is involved in epigenetic modification of histones and regulation of autophagy, affecting lifespan (Fig. 2) [44, 45]. This observation is in line with findings in yeast, discussed above, underlining the conserved role of BCAA in longevity regulation (Fig. 1) [12, 17].
A potential artifact associated with PR studies in rodents is the argument that both protein and AA restriction decrease food consumption, which raises the possibility that some of the effects of PR may, in fact, be due to CR [34, 46]. The use of diets designed to restrict protein by removing an isocaloric amount of protein or sucrose while maintaining calories from fat and complex carbohydrates constant, has helped address this concern. In the case of AA restriction, EAAs or NEAAs known to influence IGF-I, TOR, and GCN2 signaling were removed and replaced with isocaloric levels of other AAs in order to maintain an equal level of calorie and nitrogen intake (Fig. 2).
In light of these confounders, a study by Solon-Biet and colleagues used a method (Geometric Framework) to distinguish between the interactive effects of dietary macronutrients on appetite, total energy intake, metabolic health, and longevity outcomes in ad libitum-fed mice [47, 48]. Their results indicate that protein and carbohydrates dominate the intake feedbacks. Meaning that protein and carbohydrates but not fat are the driving factors to consume food under these conditions (i.e. compensatory feeding to meet target requirements). Interestingly, leptin feedback from body fat was not associated with lower energy intake. The overall interpretation of these results is that intake was passive for fat when in excess, in order to facilitate reaching the target levels of protein and carbohydrate ingestion [48].
As suggested by recent studies in non-mammalian models, the balance between protein and carbohydrate consumption can influence lifespan. Given that both IGF-I and mTOR are activated by AAs, and that inhibition of growth hormone receptor (GHR)-IGF-I and TOR-S6K signaling cause major longevity and/or healthspan extension [4, 21, 26], these effects may be replicated through reduced protein intake. In fact, in a rodent study testing various combinations of different sources of macronutrients in the diet, the longest lifespan was achieved by a low protein high carbohydrate diet [48]. Furthermore, an increase in protein:carbohydrate ratio was accompanied by an increase in hepatic mTOR activation in agreement with the finding that mTOR activation is regulated by changes in BCAAs and glucose levels [48]. Since BCAAs and insulin activate mTOR and are required to activate downstream pathways, the authors concluded that the low protein and high carbohydrate longevity diet resulted in both low mTOR activation and low insulin levels while also accounting for compensatory feeding driven to meet protein and carbohydrate requirements [48]. These rodent studies point to protein and AA-dependent effect on aging via the stimulation of GHR-IGF-I and TOR-S6K signaling, but also underline the need to explore how lifespan is further affected by the quality and composition of different sources of proteins, fats and carbohydrates.
Effects of CR in Non-human primates
While a multitude of studies have shown that CR extends lifespan in most model organisms, studies in non-human primate models have not been conclusive. Two prominent studies, the Wisconsin National Primate Research Center (WNPRC) from the University of Wisconsin and one from the National Institute on Aging (NIA), have evaluated the effects of CR (caloric intake reduced by 30%) on rhesus monkeys [49–51]. The two-decade longitudinal adult-onset WNPRC investigation reported 13% mortality in the CR group in comparison to the 37% mortality in the control diet group [49]. In contrast, the 25-year study of a CR regimen implemented in young and older age rhesus monkeys at the NIA, showed no difference in lifespan between control and CR animals [51]. A follow-up report from the WNPRC study extended their original findings by demonstrating that despite different observation in regards to lifespan extension, both WNPRC and NIA investigations support the beneficial impact of CR on healthspan in non-human primates [50].
Comparison of the two studies reveals the following differences: the NIA monkeys were fed a diet containing natural ingredients with 17.3% of calorie intake from protein and 5% from fat. In contrast, animals in the WNPRC study were fed a semi-purified, nutritionally fortified diet providing 15% of calorie intake from proteins and 10% from fat. Moreover, in the NIA diet, protein was derived from wheat, corn, soybean, fish and alfalfa meal, whereas the WNPRC diet used only lactalbumin as the protein source. In both studies, the diets contained 57–61% carbohydrates by weight. However, the source of carbohydrates in the WNPRC diet was from corn, starch, and 28.5% sucrose, whereas the NIA diet was primarily comprised of ground wheat and corn with only 3.9% sucrose. Lastly, the control animals in the WNPRC investigation were truly fed ad libitum whereas in the NIA investigation control animals were not. Considering the variations in food ingredients in the two studies, one explanation for the different outcomes in regards to lifespan could be due to the different carbohydrate sources as well as differences in protein source and AA composition between plant-based proteins sources (NIA) and lactalbumin (WNPRC). As highlighted in this review, there is increasing amount of evidence supporting unfavorable effects of high protein diets and in particular dietary protein derived from animal sources in contrast to plant sources, as discussed in the following section. [16, 52]. Thus it is possible that in the NIA study, the control animals were at a lower risk for aging-related disease and this subsequently resulted in decreased mortality due to the mostly plant-based sources of proteins.
Protein Intake Aging and Disease in Humans
In the last decade, a number of cohort studies have independently evaluated the impact of protein intake on health and the prevalence of diseases, including CVD, diabetes, and cancer. A comparative review of all major US and Swedish cohort studies indicates a positive correlation between the high intake of animal-derived protein and adverse long-term side effects that manifest as chronic and aging-related disease. A recent article proposed this possibility, based on the US NHANES III database, which includes dietary intake data. When the entire 50 and older population of over 6,000 people was considered, protein intake was not associated with increased mortality. However, when the population was divided into 50- to 65 and 65 and older, the 65 and younger group reporting consumption of over 20% of calories from proteins had a 4-fold increased risk for cancer mortality and a 75% increase in overall mortality, compared to subjects reporting consuming less than 10% of calories from proteins [16]. Interestingly, in a plant derived protein source diet, the association between high protein intake and mortality was abolished whereas that on cancer mortality was attenuated [16]. Because the higher protein intake group also had a higher level of IGF-I, a factor that decreases with aging, the authors proposed that whereas individuals younger than 65 may benefit from reduced protein intake and the reduced levels of growth factors, the older groups did not. In fact, subjects over 65 who reported consuming a low protein diet displayed increased mortality compared to subjects reporting a moderate to high protein intake. These results were supported by animal experiments showing that young but not old mice could maintain normal weight after being placed on a very low protein diet, indicating that protein and AA absorption, processing or utilization are negatively affected by aging. Experiments in mice also supported the role of high protein consumption on increasing IGF-I and decreasing the IGF-I inhibitor protein IGFBP1 and in promoting both the incidence and progression of both melanoma and breast cancer [16].
Although not directly focused on protein intake and mortality a 26-year follow-up of the “Nurses’ Health Study” (NHS) cohort and a 20-year follow-up of the “Health Professionals’ Follow-up Study” (HPFS) cohort provided important evidence for the association between dietary proteins and health. In these studies, a different score was assigned to each of the macronutrients based on the estimated energy intake, thus enabling the investigators to distinguish between the impact of each macronutrient group on health and mortality. This allowed for the division of individuals into decile groups ranging from 1 to 10. Among the 85,168 women included in the NHS cohort and the 44,548 men included in the HPFS cohort, a total of 5,204 deaths from CVD and 8,740 deaths from cancer were observed from the 21,233 documented cumulative deaths [52]. When the intake was mostly from animal-based products the lowest carbohydrate intake score was associated with higher all-cause mortality in both men and women, with a hazard ratio (HR) of 1.23 (confidence interval (CI): of 1.11–1.37) [52]. In contrast, a vegetable-based low-carbohydrate regimen was associated with both lower all-cause mortality and CVD mortality rates in both cohorts (HR, 0.80, CI: 0.75–0.85) [52]. The study concluded that a low carbohydrate diet was beneficial when it was part of a diet rich in animal-based food, but in fact, the study also showed that the same low carbohydrate intake group had the highest protein intake with over 22% of daily calories derived from proteins.
An independent investigation regarding protein intake among men in the HPFS cohort and its impact on stroke incidence and ischemic heart disease (IHD) indicated no statistically significant associations between the 1,057 incidents of stroke and the 2,959 incidents of IHD with respect to total protein intake. However, when comparing the top and bottom quintile group of protein source for either an animal or plant-based diet, there was an inverse correlation between higher plant-based protein intake and stroke or IHD, but a positive correlation between higher animal protein intake and these cardiovascular diseases [53, 54].
Similarly, in a multivariable analysis of the NHS cohort, which recorded 2,210 cases of nonfatal infarctions and 952 deaths from coronary heart disease investigators found a direct correlation between the risk of IHD among women and an increase in red meat and high-fat dairy intake [55]. In contrast, a lower risk was associated with high intake of nuts, beans, and low fat dairy [55]. Meta-data analysis of the NHS cohort also suggests a correlation between high consumption of red or processed meat and increased risk for colorectal cancer [56]. Furthermore, a study evaluating a multiethnic cohort of 29,759 Caucasian, 35,244 Japanese-American, and 10,509 Native Hawaiian men and women in Hawaii, aged 45–75 years at baseline, indicated a positive correlation between red and processed meat intake and increased risk for diabetes [57].
Similar to the observations for US cohorts, in a cohort of 43,396 Swedish women, a 1/10 decrease in carbohydrate intake or a 1/10 increase in protein intake was associated with statistically significant increases in incidences of CVD. For this study, a 1/10 change in carbohydrate or protein intake corresponded to a 5g increase in protein intake or 20g reduction in carbohydrate intake resulting in a 5% increase in CVD risk [58]. The study found that individuals often substituted carbohydrates with animal protein, thus resulting in the overall increase in protein intake. In contrast, in a northern Swedish population-based cohort, one study found no general association between a low-carbohydrate, high-protein score and mortality [59]. An advantage of the Swedish cohorts over the American cohorts is that the nationwide data linkage in Sweden allows for virtually complete follow-up and objective ascertainment of cardiovascular outcomes.
Although the overwhelming majority of studies point to a negative effect of high protein intake, the lack of an association between protein intake and diseases in a few studies may be explained by the existence of two or more groups in which protein intake may have positive or negative effects depending on the age range, as shown by Levine et al. Similar to rodent studies, human clinical studies have demonstrated that reduction in protein intake results in reduced IGF-I concentration [60, 61]. In agreement with this observation, a study evaluating refeeding of undernourished Bangladeshi children, compared to control age-matched healthy American children, showed significantly higher increase in IGF-I in the group receiving high protein diet, versus normal protein diet [60]. Although long term CR by itself does not affect IGF-I levels, when it is combined with reduced protein intake it results in significant reduction in total IGF-I concentration [61].
Rodent studies have shown that inhibition of the protein-activated GHR-IGF-I axis results in a 40% longevity extension, and causes remarkable reduction in age-related pathologies [62]. Similarly, a 22-year investigation of the inhabitants of a small town in Ecuador who are deficient in GHR and IGF-I, also demonstrated that GHR-IGF-I deficiency is linked to protection against chronic and aging-related pathologies, including cancer and diabetes [63]. Therefore, both mouse and human studies indicate that protein intake accelerates aging and increases the incidence of age-related diseases, at least in part by activating the GHR-IGF-I axis.
Effects of High Protein Intake in the Elderly
One of the limitations of the studies listed above is the combined analysis of all age groups. While decreased protein intake has been shown to reduce detrimental aging-related phenotypes in middle-aged adults, it is important to understand what the role of proteins is for groups of different ages. An important consideration is that protein intake is critical for a number of physiological pathways including maintaining a positive nitrogen balance by providing essential AAs to counter muscle loss due to catabolic pathways and growth in individuals over 65 years of age.
Over the past two decades many investigators have attempted to assess the protein needs of the elderly population. Some studies proposed that the optimal protein dose can be established by calculating the short-term protein intake required to replenish the nitrogen that is lost, to achieve nitrogen homeostasis. However, these studies have been mostly inconclusive, with some reports supporting the current Recommended Dietary Allowance (RDA), and others suggesting that a higher protein intake is needed in healthy older adults [64].
High-quality protein is defined by AA composition, as measured by EAA score, digestibility, absorption, and regulatory roles of specific AAs in cellular processes [65]. Although muscle protein synthesis is stimulated by increased protein consumption, protein intake of more than 30 g per serving does not further stimulate this response [66]. Furthermore, muscle protein synthesis is attenuated in the elderly upon limitation of EAAs, specifically leucine, and when protein intake is accompanied by glucose intake [64, 66, 67]. The current RDA recommendation for protein intake is 0.8 g/kg per day of quality protein for healthy adults. However, growing evidence suggests that this amount of protein may not be sufficient for at least some individuals over the age of 65 [16].
In contrast to the RDA recommendation, the PROT-AGE Study Group that represents the European Union Geriatric Medical Society (EUGMS), the International Association of Gerontology and Geriatrics (IAGG), the International Academy on Nutrition and Aging (IANA), and the Australian and New Zealand Society for Geriatric Medicine (ANZSGM), recommends a daily protein intake of 1.0–1.2 g/kg a day for older adults in order to maintain and regain muscle loss from sarcopenia. PROT-AGE also advocates that the elderly should consume 25 to 30 g of protein per meal, containing about 2.5–2.8 g of leucine [65, 68]. In further support of these findings, a meta-analysis study showed that a multi-nutrient oral supplement of high protein concentration significantly improved handgrip strength test and resulted in significant overall reduction in a range of complications in elderly adults [69]. This study evaluated a randomized controlled trial community of patients older than 65 years of age who suffered from chronic obstructive pulmonary disease, gastrointestinal disease, and hip fracture. Exceptions to this recommendation are for older individuals with severe kidney disease (not on dialysis), who require a reduced protein regimen [65]. However, biological age is different from chronological age and therefore, it is likely that a high and even a moderate protein intake (over 20% and between 10–20% of calories from proteins, respectively) can provide some benefits in subjects suffering from specific pathologies, but may still promote aging, cancer and overall mortality in individuals older than 65 but otherwise healthy. Because a moderate protein intake from plant based sources after age 65 was not associated with increased mortality, a 1.0 g/kg/day of plant-derived proteins is expected to be the most effective in providing adequate nourishment without excess activation of GHR-IGF-I signaling which is expected to promote cancer and overall mortality. This protein intake is also consistent with the recommendation by geriatricians, although it uses the lower point of the recommended range and plant-derived proteins instead of any protein source. It will be important to determine whether elderly individuals may benefit from a high intake of animal-based proteins and also to determine how each source of proteins may affect IGF-I levels and aging.
Okinawa, Southern Italy, centenarians, and prevalence of disease
In the areas of Giuliana, Bisacquino, and Chiusa Sclafani in Western Sicily, the prevalence of centenarians per inhabitants is 6-fold higher than the national average and 14-fold higher than the national average for male centenarians. An evaluation of life style and diet of the inhabitants of this region revealed a strong adherence to the Mediterranean diet [70, 71]. Their lifestyle can be summarized by moderate physical activity and three small meals comprised of small amounts of carbohydrates and meat and large amounts of seasonal fruits and vegetables, beans, olives and olive oil. This life style and diet practices are associated with better health status as determined by cardiovascular risk factors, obesity, diabetes, and hypertension and subsequently a lower rate of mortality [70–72].
Similar to the centenarians in southern Italy, studies of one of the longest lived populations in the world, the Okinawans, during the 1950’s, reveals a dietary regimen rich in vegetables, fruits, grains, and soy products with only 9% of calories coming from proteins, mostly plant-based [73]. Although the diets of the Okinawans is changing and becoming more westernized, many of the centenarians still maintain dietary habits similar to those adopted in the 50’s, when they were young adults. Thus, the diets of some of the longest lived populations from around the world, are mostly plant based low protein diets [74–77], in agreement with the animal and epidemiological studies discussed above and also in agreement with the hypotheses that protein intake is at the center of many diseases [32]. However, the potentially negative effect of low protein intake in the elderly population underlines the need for multi-disciplinary approaches that take into consideration biogerontology, epidemiology, centenarian studies, and clinical trials in order to identify dietary recommendations that optimize healthspan.
Concluding remarks and future perspectives
In conclusion, we have presented evidence ranging from invertebrate, mammalian, and primate model organisms, epidemiological findings, and human studies that show a clear link between protein and AA intake, the activation of pro-aging and disease promoting pathways (Fig. 1 and 2), and the incidence of age-related diseases and reduced lifespan. In particular, high consumption of proteins from red meat and other animal sources appear to be associated with a wide range of diseases. Although this review focuses on the detrimental effects of proteins, it does not diminish the potentially negative effects of certain types of carbohydrates and fats, which have been reviewed elsewhere. Studies comparing healthspan and mortality in groups randomized to either a low or high protein diet together with additional basic research on the mechanisms of protein-dependent damage are necessary to identify diets that minimize the burden on the population while maximizing protective effects.
Highlights.
Protein or amino acid (AA) restriction has been shown to be as potent as calorie restriction in extending healthspan in multiple model organisms.
AA restriction affects lifespan partly through modulation of the amino acid sensing pathways TOR and GCN2
Human epidemiological studies highlight the detrimental effects of high protein diets in particular animal derived protein sources in contrast to plant base sources.
Epidemiological studies indicate that low protein diets are associated with lower risk of chronic and age-related diseases such as cardio vascular diseases, diabetes, and cancer.
Acknowledgments
This study was funded in part by NIH/NIA grants AG20642, AG025135, and P01 AG034906. V.D.L. has equity interest in L-Nutra, a company that develops medical food.
Glossary
- Branched chain amino acid (BCAA)
are essential amino acids that have an aliphatic side chain, which is a carbon atom bound to at least two other carbon atoms. Three most common BCAAs are leucine, isoleucine and valine.
- Caloric restriction (CR)
is the reduction of calorie intake by 20–40% without malnutrition.
- Dietary restriction (DR)
is a dietary regimen in which specific food groups or micronutrients are reduced or removed from the diet. DR also includes CR.
- General control nonderepressible 2 (GCN2)
is a serine/threonine-protein kinase that plays a key role in modulating amino acid metabolism as a response to nutrient deprivation. It senses amino acid deficiency through binding to uncharged transfer RNA (tRNA) and represses general protein synthesis.
- Insulin-like growth factors (IGFs)
proteins that share high sequence similarity to insulin. IGFs are part of the IGF axis, a complex system composed of two cell-surface receptors (IGF1R and IGF2R), two ligands (Insulin-like growth factor 1 (IGF-I) and Insulin-like growth factor 2 (IGF-2)), six high-affinity IGF-binding proteins (IGFBP-1 to IGFBP-6), and IGFBP degrading enzymes.
- Target of rapamycin (TOR)
an evolutionarily conserved kinase that regulates cell cycle progression and cell growth and as such cell, organ, and organismal size by integrating signals from nutrients such as amino acids and growth factors (in higher eukaryotes) to. In mammals, TOR regulates translation through the ribosomal protein S6 kinases (S6Ks) and the eukaryotic translation initiation factor 4E-binding proteins.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Collaborators, U.S.B.o.D. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valsta LM, et al. Explaining the 25-year decline of serum cholesterol by dietary changes and use of lipid-lowering medication in Finland. Public Health Nutr. 2010;13(6A):932–938. doi: 10.1017/S1368980010001126. [DOI] [PubMed] [Google Scholar]
- 3.Vartiainen E, et al. Thirty-five-year trends in cardiovascular risk factors in Finland. Int J Epidemiol. 2010;39(2):504–518. doi: 10.1093/ije/dyp330. [DOI] [PubMed] [Google Scholar]
- 4.Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328(5976):321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Couzin-Frankel J. Nutrition. Diet studies challenge thinking on proteins versus carbs. Science. 2014;343(6175):1068. doi: 10.1126/science.343.6175.1068. [DOI] [PubMed] [Google Scholar]
- 6.Fabrizio P, et al. Chronological aging-independent replicative life span regulation by Msn2/Msn4 and Sod2 in Saccharomyces cerevisiae. FEBS Lett. 2004;557(1–3):136–142. doi: 10.1016/s0014-5793(03)01462-5. [DOI] [PubMed] [Google Scholar]
- 7.Longo VD, et al. Replicative and chronological aging in Saccharomyces cerevisiae. Cell Metab. 2012;16(1):18–31. doi: 10.1016/j.cmet.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longo VD, Finch CE. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 2003;299(5611):1342–1346. doi: 10.1126/science.1077991. [DOI] [PubMed] [Google Scholar]
- 9.Wei M, et al. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet. 2008;4(1):e13. doi: 10.1371/journal.pgen.0040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fabrizio P, et al. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292(5515):288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- 11.Madia F, et al. Longevity mutation in SCH9 prevents recombination errors and premature genomic instability in a Werner/Bloom model system. J Cell Biol. 2008;180(1):67–81. doi: 10.1083/jcb.200707154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu J, et al. Tor-Sch9 deficiency activates catabolism of the ketone body-like acetic acid to promote trehalose accumulation and longevity. Aging Cell. 2014 doi: 10.1111/acel.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirisola MG, et al. Serine- and threonine/valine-dependent activation of PDK and Tor orthologs converge on Sch9 to promote aging. PLoS Genet. 2014;10(2):e1004113. doi: 10.1371/journal.pgen.1004113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaborske JM, et al. Selective control of amino acid metabolism by the GCN2 eIF2 kinase pathway in Saccharomyces cerevisiae. BMC Biochem. 2010;11:29. doi: 10.1186/1471-2091-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Z, et al. Independent and additive effects of glutamic acid and methionine on yeast longevity. PLoS One. 2013;8(11):e79319. doi: 10.1371/journal.pone.0079319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levine ME, et al. Low Protein Intake Is Associated with a Major Reduction in IGF-1, Cancer, and Overall Mortality in the 65 and Younger but Not Older Population. Cell Metab. 2014;19(3):407–417. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenberg T, et al. Nucleocytosolic depletion of the energy metabolite acetyl-coenzyme a stimulates autophagy and prolongs lifespan. Cell Metab. 2014;19(3):431–444. doi: 10.1016/j.cmet.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Depuydt G, et al. Reduced insulin/insulin-like growth factor-1 signaling and dietary restriction inhibit translation but preserve muscle mass in Caenorhabditis elegans. Mol Cell Proteomics. 2013;12(12):3624–3639. doi: 10.1074/mcp.M113.027383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lapierre LR, Hansen M. Lessons from C. elegans: signaling pathways for longevity. Trends Endocrinol Metab. 2012;23(12):637–644. doi: 10.1016/j.tem.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou KI, Pincus Z, Slack FJ. Longevity and stress in Caenorhabditis elegans. Aging (Albany NY) 2011;3(8):733–753. doi: 10.18632/aging.100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kapahi P, et al. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010;11(6):453–465. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Partridge L, et al. Ageing in Drosophila: the role of the insulin/Igf and TOR signalling network. Exp Gerontol. 2011;46(5):376–381. doi: 10.1016/j.exger.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tatar M, Post S, Yu K. Nutrient control of Drosophila longevity. Trends Endocrinol Metab. 2014 doi: 10.1016/j.tem.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8(2):113–127. doi: 10.1111/j.1474-9726.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cypser JR, Kitzenberg D, Park SK. Dietary restriction in C. elegans: recent advances. Exp Gerontol. 2013;48(10):1014–1017. doi: 10.1016/j.exger.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 26.Gallinetti J, Harputlugil E, Mitchell JR. Amino acid sensing in dietary-restriction-mediated longevity: roles of signal-transducing kinases GCN2 and TOR. Biochem J. 2013;449(1):1–10. doi: 10.1042/BJ20121098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tissenbaum HA. Genetics, life span, health span, and the aging process in Caenorhabditis elegans. J Gerontol A Biol Sci Med Sci. 2012;67(5):503–510. doi: 10.1093/gerona/gls088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rousakis A, et al. The general control nonderepressible-2 kinase mediates stress response and longevity induced by target of rapamycin inactivation in Caenorhabditis elegans. Aging Cell. 2013;12(5):742–751. doi: 10.1111/acel.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lapierre LR, et al. Autophagy and lipid metabolism coordinately modulate life span in germline-less C. elegans. Curr Biol. 2011;21(18):1507–1514. doi: 10.1016/j.cub.2011.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhong M, et al. Genome-wide identification of binding sites defines distinct functions for Caenorhabditis elegans PHA-4/FOXA in development and environmental response. PLoS Genet. 2010;6(2):e1000848. doi: 10.1371/journal.pgen.1000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parrella E, et al. Protein restriction cycles reduce IGF-1 and phosphorylated Tau, and improve behavioral performance in an Alzheimer's disease mouse model. Aging Cell. 2013;12(2):257–268. doi: 10.1111/acel.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell TC, Campbell TM. 1st BenBella Books ed. Dallas, Tex: BenBella Books; 2005. The China study : the most comprehensive study of nutrition ever conducted and the startling implications for diet, weight loss and long-term health. xviii, 417 p. [Google Scholar]
- 33.Cheng Z, et al. Inhibition of hepatocellular carcinoma development in hepatitis B virus transfected mice by low dietary casein. Hepatology. 1997;26(5):1351–1354. doi: 10.1002/hep.510260538. [DOI] [PubMed] [Google Scholar]
- 34.Minor RK, et al. Dietary interventions to extend life span and health span based on calorie restriction. J Gerontol A Biol Sci Med Sci. 2010;65(7):695–703. doi: 10.1093/gerona/glq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez-Torres M, Barja G. Lowered methionine ingestion as responsible for the decrease in rodent mitochondrial oxidative stress in protein and dietary restriction possible implications for humans. Biochim Biophys Acta. 2008;1780(11):1337–1347. doi: 10.1016/j.bbagen.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell JR, et al. Reducing elective vascular surgery perioperative risk with brief preoperative dietary restriction. Surgery. 2013;153(4):594–598. doi: 10.1016/j.surg.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perrone CE, et al. Metabolic adaptations to methionine restriction that benefit health and lifespan in rodents. Exp Gerontol. 2013;48(7):654–660. doi: 10.1016/j.exger.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Segall PE, Timiras PS. Patho-physiologic findings after chronic tryptophan deficiency in rats: a model for delayed growth and aging. Mech Ageing Dev. 1976;5(2):109–124. doi: 10.1016/0047-6374(76)90012-9. [DOI] [PubMed] [Google Scholar]
- 39.Miller RA, et al. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4(3):119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng W, et al. Surgical stress resistance induced by single amino acid deprivation requires Gcn2 in mice. Sci Transl Med. 2012;4(118):118ra11. doi: 10.1126/scitranslmed.3002629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao F, et al. Leucine deprivation increases hepatic insulin sensitivity via GCN2/mTOR/S6K1 and AMPK pathways. Diabetes. 2011;60(3):746–756. doi: 10.2337/db10-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duran RV, et al. Glutaminolysis activates Rag-mTORC1 signaling. Mol Cell. 2012;47(3):349–358. doi: 10.1016/j.molcel.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 43.Bar-Peled L, Sabatini DM. Regulation of mTORC1 by amino acids. Trends Cell Biol. 2014 doi: 10.1016/j.tcb.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marino G, et al. Regulation of autophagy by cytosolic acetyl-coenzyme a. Mol Cell. 2014;53(5):710–725. doi: 10.1016/j.molcel.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 45.Shimazu T, et al. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339(6116):211–214. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koehnle TJ, Russell MC, Gietzen DW. Rats rapidly reject diets deficient in essential amino acids. J Nutr. 2003;133(7):2331–2335. doi: 10.1093/jn/133.7.2331. [DOI] [PubMed] [Google Scholar]
- 47.Piper MD, et al. Dietary restriction and aging: a unifying perspective. Cell Metab. 2011;14(2):154–160. doi: 10.1016/j.cmet.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Solon-Biet SM, et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19(3):418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colman RJ, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colman RJ, et al. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun. 2014;5:3557. doi: 10.1038/ncomms4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mattison JA, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489(7415):318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fung TT, et al. Low-carbohydrate diets and all-cause and cause-specific mortality: two cohort studies. Ann Intern Med. 2010;153(5):289–298. doi: 10.1059/0003-4819-153-5-201009070-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Preis SR, et al. Lack of association between dietary protein intake and risk of stroke among middle-aged men. American Journal of Clinical Nutrition. 2010;91(1):39–45. doi: 10.3945/ajcn.2009.28060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Preis SR, et al. Dietary protein and risk of ischemic heart disease in middle-aged men. American Journal of Clinical Nutrition. 2010;92(5):1265–1272. doi: 10.3945/ajcn.2010.29626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bernstein AM, et al. Major dietary protein sources and risk of coronary heart disease in women. Circulation. 2010;122(9):876–883. doi: 10.1161/CIRCULATIONAHA.109.915165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alexander DD, et al. Meta-analysis of prospective studies of red meat consumption and colorectal cancer. Eur J Cancer Prev. 2011;20(4):293–307. doi: 10.1097/CEJ.0b013e328345f985. [DOI] [PubMed] [Google Scholar]
- 57.Steinbrecher A, et al. Meat consumption and risk of type 2 diabetes: the Multiethnic Cohort. Public Health Nutr. 2011;14(4):568–574. doi: 10.1017/S1368980010002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lagiou P, et al. Low carbohydrate-high protein diet and incidence of cardiovascular diseases in Swedish women: prospective cohort study. BMJ. 2012;344:e4026. doi: 10.1136/bmj.e4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nilsson LM, et al. Low-carbohydrate, high-protein score and mortality in a northern Swedish population-based cohort. Eur J Clin Nutr. 2012;66(6):694–700. doi: 10.1038/ejcn.2012.9. [DOI] [PubMed] [Google Scholar]
- 60.Pucilowska JB, et al. The effect of dietary protein supplementation on insulin-like growth factors (IGFs) and IGF-binding proteins in children with shigellosis. J Clin Endocrinol Metab. 1993;77(6):1516–1521. doi: 10.1210/jcem.77.6.7505287. [DOI] [PubMed] [Google Scholar]
- 61.Fontana L, et al. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell. 2008;7(5):681–687. doi: 10.1111/j.1474-9726.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bartke A, Sun LY, Longo V. Somatotropic Signaling: Trade-Offs between Growth, Reproductive Development, and Longevity. Physiological Reviews. 2013;93(2):571–598. doi: 10.1152/physrev.00006.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guevara-Aguirre J, et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3(70):70ra13. doi: 10.1126/scitranslmed.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Volpi E, et al. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J Gerontol A Biol Sci Med Sci. 2013;68(6):677–681. doi: 10.1093/gerona/gls229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bauer J, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013;14(8):542–559. doi: 10.1016/j.jamda.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 66.Symons TB, et al. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J Am Diet Assoc. 2009;109(9):1582–1586. doi: 10.1016/j.jada.2009.06.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Glynn EL, et al. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J Nutr. 2010;140(11):1970–1976. doi: 10.3945/jn.110.127647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morley JE, et al. Nutritional recommendations for the management of sarcopenia. J Am Med Dir Assoc. 2010;11(6):391–396. doi: 10.1016/j.jamda.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cawood AL, Elia M, Stratton RJ. Systematic review and meta-analysis of the effects of high protein oral nutritional supplements. Ageing Res Rev. 2012;11(2):278–296. doi: 10.1016/j.arr.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 70.Tyrovolas S, Panagiotakos DB. The role of Mediterranean type of diet on the development of cancer and cardiovascular disease, in the elderly: a systematic review. Maturitas. 2010;65(2):122–130. doi: 10.1016/j.maturitas.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 71.Vasto S, et al. Mediterranean diet and longevity in Sicily: survey in a Sicani Mountains population. Rejuvenation Res. 2012;15(2):184–188. doi: 10.1089/rej.2011.1280. [DOI] [PubMed] [Google Scholar]
- 72.Grosso G, et al. Protective role of the Mediterranean diet on several cardiovascular risk factors: Evidence from Sicily, southern Italy. Nutr Metab Cardiovasc Dis. 2013 doi: 10.1016/j.numecd.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 73.Willcox BJ, et al. Caloric restriction, the traditional Okinawan diet, and healthy aging: the diet of the world's longest-lived people and its potential impact on morbidity and life span. Ann N Y Acad Sci. 2007;1114:434–455. doi: 10.1196/annals.1396.037. [DOI] [PubMed] [Google Scholar]
- 74.Willcox BJ, Willcox DC. Caloric restriction, caloric restriction mimetics, and healthy aging in Okinawa: controversies and clinical implications. Curr Opin Clin Nutr Metab Care. 2014;17(1):51–58. doi: 10.1097/MCO.0000000000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Willcox DC, Scapagnini G, Willcox BJ. Healthy aging diets other than the Mediterranean: A focus on the Okinawan diet. Mech Ageing Dev. 2014 doi: 10.1016/j.mad.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Robine JM, et al. Accuracy of the centenarian numbers in Okinawa and the role of the Okinawan diet on longevity: responses to Le Bourg about the article "Exploring the impact of climate on human longevity". Exp Gerontol. 2013;48(8):840–842. doi: 10.1016/j.exger.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 77.Appel LJ. Dietary patterns and longevity: expanding the blue zones. Circulation. 2008;118(3):214–215. doi: 10.1161/CIRCULATIONAHA.108.788497. [DOI] [PubMed] [Google Scholar]