Summary
Sleep is recognized to be ancient in origin, with vertebrates and invertebrates experiencing behaviorally quiescent states that are regulated by conserved genetic mechanisms[1, 2]. Despite its conservation throughout phylogeny the function of sleep remains debated. Hypotheses for the purpose of sleep include nervous system-specific functions such as modulation of synaptic strength and clearance of metabolites from the brain[3, 4], and more generalized cellular functions such as energy conservation and macromolecule biosynthesis[5]. These models are supported by the identification of synaptic and metabolic processes that are perturbed during prolonged wakefulness. It remains to be seen whether perturbations of cellular homeostasis in turn drive sleep. Here we show that under conditions of cellular stress, including noxious heat, cold, hypertonicity, and tissue damage, the nematode Caenorhabditis elegans engages a behavioral quiescence program. The stress-induced quiescent state displays properties of sleep and is dependent on the ALA neuron, which mediates the conserved soporific effect of Epidermal Growth Factor (EGF) ligand overexpression. We characterize heat-induced quiescence in detail and show that it is indeed dependent on components of EGF signaling, providing physiological relevance to the behavioral effects of EGF family ligands. We find that following noxious heat exposure, quiescence-defective animals show elevated expression of cellular stress reporter genes and are impaired for survival, demonstrating the benefit of stress-induced behavioral quiescence. These data provide evidence that cellular stress can induce a protective sleep-like state in C. elegans and suggest that a deeply conserved function of sleep is to mitigate disruptions of cellular homeostasis.
RESULTS
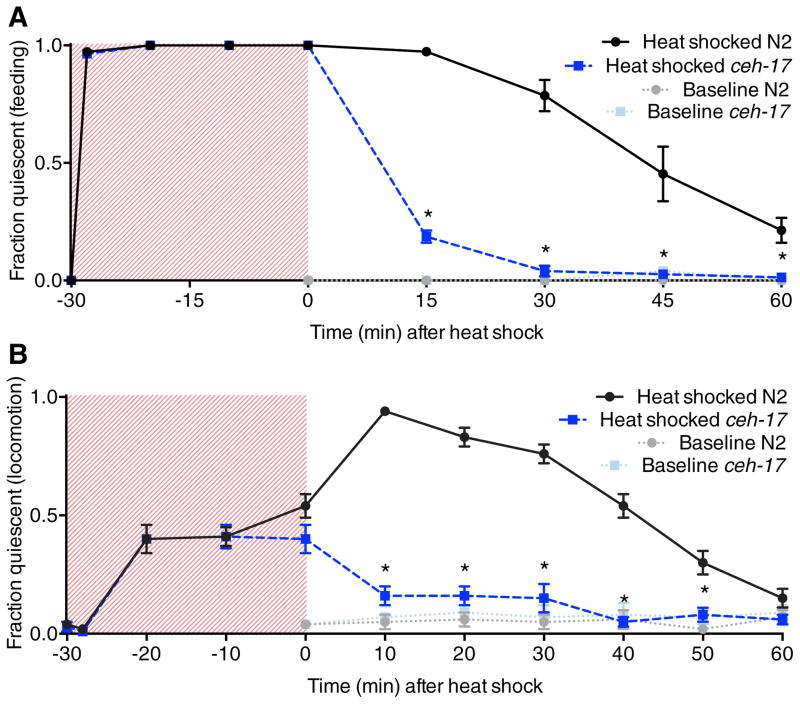
Noxious heat has several effects on C. elegans behavior. Transient exposure to temperatures above 30°C causes an engagement of avoidance and escape locomotor behaviors[7, 8] and an inhibition of pharyngeal pumping[6], a feeding behavior that is normally continuous in the presence of food. Animals removed from a brief (<1 min) heat exposure recover normal activity within minutes (Table S1). During prolonged noxious heat exposure, such as a 30 min 35°C heat shock, feeding remains completely suppressed, evasive behavior rapidly declines after 2 min of exposure, and animals experience bouts of immobility (Figures 1A and 1B, shaded regions). Unexpectedly, upon return to room temperature animals become even more quiescent, and both pharyngeal pumping and locomotion remain suppressed for up to an hour after exposure (Figures 1A and 1B). We wished to determine whether a component of this quiescent behavior was dependent on the ALA neuron, which has been characterized as both a sleep-inducing interneuron[9] and a high-threshold mechanosensor[10]. To this end, we examined animals lacking the Paired homeodomain transcription factor CEH-17, which is required for ALA neuron identity[11, 12]. We found that wild-type and ceh-17(lf) mutant animals behaved similarly during heat exposure, indicating that the effects of direct heat on behavior are ALA-independent (Figures 1A and 1B, shaded regions). However, ceh-17(lf) mutants failed to show quiescence after return to room temperature, indicating that recovery quiescence is ALA-dependent (Figures 1A and 1B). This quiescence defect is not due to a general hyperactivity of ceh-17 mutants or an inability to sense heat, as these animals show wild-type pharyngeal pumping rates[9], locomotion (Table S2), thermotaxis and thermoavoidance behaviors[11].
Figure 1.
C. elegans experiences ALA-dependent recovery quiescence following heat stress. A, Time course of feeding quiescence during and after a 30 min 35°C heat shock in wild-type and ALA neuron-defective ceh-17(np1) animals compared to untreated control animals. B, Automated (multi-worm tracker) analysis of locomotor quiescence in wild-type and ceh-17(np1) animals during and after a 30 min 35°C heat shock compared to untreated control animals. Shaded regions represent time during heat shock. Mean and SEM are shown. *p < 0.001; Fisher’s exact test (A) and Student’s t test (B).
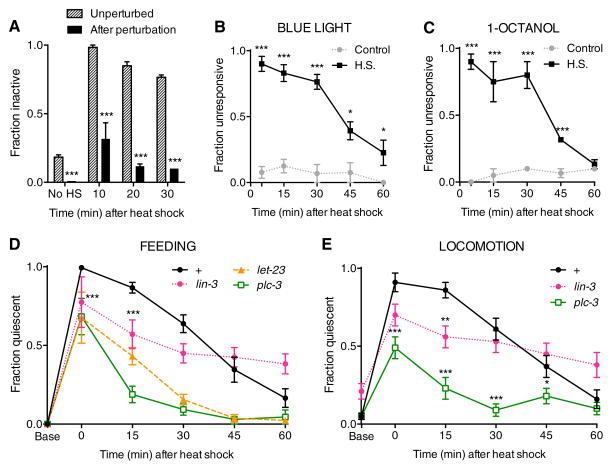
The inhibition of activity during recovery from thermal stress is quickly reversible to strong mechanical stimulation (Figure 2A and Movie S1) and is accompanied by decreased responsiveness toward the aversive stimuli blue light and 1-octanol (Figures 2B and 2C). These properties of reversible immobility and reduced sensory responsiveness are behavioral characteristics of sleep[1] and have also been observed in animals overexpressing the Epidermal Growth Factor (EGF) ligand LIN-3[9,13]. LIN-3(OE) animals show sleep-like delayed responses to sensory neuron activation, an effect that is reversed with harsh stimulation[13], and experience a cessation of feeding and locomotion[9] that is similar to that observed with EGF ligand administration to the vertebrate brain[14–16]. In C. elegans, LIN-3-induced behavioral quiescence is dependent on activation of the EGF Receptor (EGFR) LET-23 within ALA, and on the EGFR target PLC-3/PLC-γ (Phospholipase C-gamma)[9]. To determine whether EGF signaling mediates recovery quiescence, we examined mutations in lin-3, let-23, and plc-3. Because a complete loss of LIN-3 or LET-23 activity is lethal at an early stage, we examined animals harboring reduction of function mutations in these genes. lin-3(n1058) mutant animals showed reduced quiescence during recovery from thermal stress, while let-23(sy10) and plc-3(tm1340) null mutant animals were severely impaired in recovery quiescence (Figures 2D and 2E). These results indicate that thermal stress-induced recovery quiescence is a sleep-like state regulated by EGF signaling.
Figure 2.
Recovery quiescence displays sleep-like properties and requires components of EGF signaling. A, Reversibility of locomotor quiescence after perturbation by harsh touch at 10, 20, and 30 minutes after a 30 min 35°C heat shock. B and C, Fraction of heat shocked wild-type young adults that do not show locomotor response within 5 seconds after a 3 second exposure to blue light (B) or during 5 second exposure to 1-octanol (C). D and E, Time course of feeding quiescence (D) and locomotor quiescence (E) following a 30 min 35°C heat shock in wild-type, lin-3(n1058) itr-1(sy290), let-23(sy10) unc-4(e120) and plc-3(tm1340) animals. let-23 and plc-3 mutants show impaired feeding quiescence at all time points, and lin-3 mutants at time points indicated by asterisks. The failure of lin-3 mutant animals to recover activity may reflect altered kinetics of ligand release caused by the n1058 intracellular domain mutation. Locomotion data was not collected for let-23 mutant animals due to a movement defect conferred by the unc-4(e120) mutation. Mean and SEM are shown. *p < 0.05, **p < 0.01, ***p < 0.001; Fisher’s exact test (A–D) and Student’s t test (E).
On the basis of our results with heat, we reasoned that C. elegans may experience ALA-dependent behavioral quiescence during recovery from other environmental stressors. To test this prediction, we examined ALA-defective ceh-17 mutant animals for their behavioral responses under a variety of noxious conditions.
Hyperosmotic stress
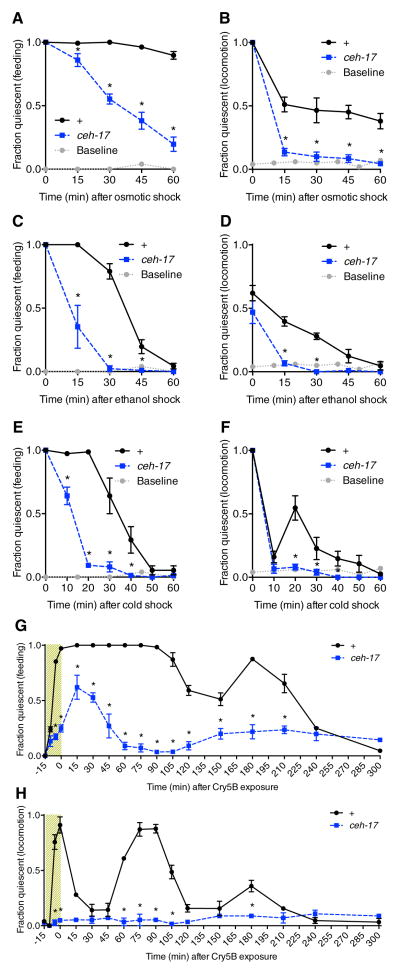
We expected that hyperosmotic disruption of the C. elegans hydrostatic skeleton should impair locomotion in an ALA-independent manner. Indeed, when exposed to a hyperosmotic solution of 500 mM NaCl for 15 minutes, both wild-type and ALA-defective ceh-17 mutant animals ceased locomotion as well as feeding. However, upon removal from osmotic shock, ceh-17 mutants recovered these behaviors earlier than wild-type controls (Figures 3A and 3B), revealing an ALA-dependent component of behavioral quiescence during recovery from hypertonic stress.
Figure 3.
Cellular stressors induce ALA-dependent recovery quiescence. A and B, Time course of feeding quiescence (A) and locomotor quiescence (B) in wild-type and ALA-defective ceh-17(np1) animals after 15 min exposure to a 500 mM NaCl solution. Grey dotted line indicates behavior of untreated wild-type animals. C and D, Time course of feeding quiescence (C) and locomotor quiescence (D) in wild-type and ceh-17(np1) animals after a 30 min exposure to 5% ethanol. E and F, Time course of feeding quiescence (E) and locomotor quiescence (F) in wild-type and ceh-17(np1) animals after a 15 min exposure to −15 °C. G and H, Time course of feeding (G) and locomotor (H) quiescence in wild-type and ceh-17(np1) animals during (shaded region) and after a 15 min exposure to Cry5B toxin. Mean and SEM are shown. *p < 0.001; Fisher’s exact test. Note that each stressor also has ALA-independent effects of varying duration on feeding and/or locomotion, observable at the earliest time points.
Alcohol stress
At high doses (>4%) of ethanol, C. elegans ceases feeding[6] and locomotion[17]. To investigate whether ethanol stress induces ALA-dependent recovery quiescence, we analyzed the behavior of wild-type and ceh-17 mutant animals in response to a 30 minute exposure to 5% ethanol. During exposure, wild-type and ceh-17 mutant animals were similarly inhibited for feeding and movement (not shown), but after removal from ethanol, ceh-17 mutants recovered locomotion and pharyngeal pumping earlier than wild-type controls (Figures 3C and 3D), indicating that a fraction of the behavioral quiescence observed following ethanol stress is ALA-dependent.
Cold stress
Exposure to noxious cold (30 min at −15°C) caused a complete cessation of feeding and locomotion in both wild-type and ceh-17 mutant animals (Figures 3E and 3F). However, ceh-17 mutants resumed feeding sooner than wild-type animals (Figure 3E), revealing ALA-dependent recovery quiescence. Interestingly, while both strains initially recovered locomotion quickly after return to room temperature, we observed a peak of ALA-dependent locomotor quiescence at 20 minutes after cold stress (Figure 3F). One interpretation of these results is that recovery quiescence is triggered by cold stress but in a delayed fashion. As the initial ALA-independent suppression of locomotion by cold is very transient (moreso than the ALA-independent effects on feeding), it is possible to clearly resolve the peak of ALA-dependent quiescence within the locomotion profile.
Tissue damage by pore-forming toxin
Cry5B is a pore-forming crystal protein produced by Bacillus thuringiensis that damages cells lining the digestive tract when ingested by C. elegans[18] and causes a cessation of feeding and locomotion within minutes [18, 19]. In response to a 15 minute exposure to Cry5B-expressing E. coli, we observed that feeding behavior in wild-type animals was completely suppressed for 90 minutes, followed by a second peak of quiescence at 3hr after exposure (Figure 3G). By contrast, ceh-17 mutant animals showed milder feeding inhibition that peaked at 15–30 min after removal from toxin (Figure 3G). Thus the initial prolonged inhibition of feeding in wild-type animals represents a combination of ALA-independent and ALA-dependent effects. Remarkably, while wild-type animals became immobile in the presence of toxin, the locomotion of ceh-17 mutant animals was unaffected (Figure 3H; Movies S2 and S3). Upon removal from the toxin, wild-type animals initially recovered locomotion but then experienced additional peaks of quiescence (Figure 3H). One interpretation of these results is that the initial bout of ALA-dependent quiescence is restricted by limiting amounts of one or more pathway components, which are up-regulated in response to stress. If disrupted cellular homeostasis persists, as may be the case with an extreme stress such as Cry5B ingestion, a second and even third bout of quiescence may occur.
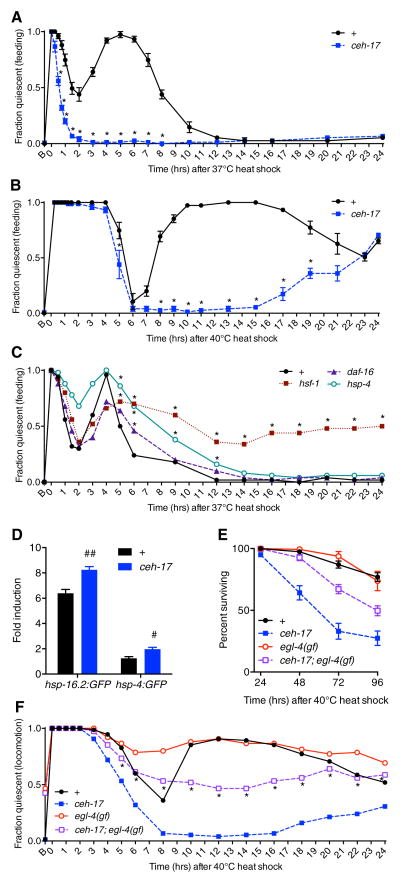
To test the hypothesis that more severe perturbations of cellular homeostasis can induce additional bouts of ALA-dependent quiescence, we examined the effect of more severe heat shocks on the behavior of wild-type and ceh-17 mutant animals. We found that a 37°C 30 minute heat shock elicited an initial ALA-dependent quiescent bout similar to that observed at 35°C, and also produced a subsequent, longer period of ALA-dependent quiescence (Figure 4A). In response to a more stringent heat shock of 40°C for 20 minutes, both wild-type and ceh-17 mutant animals were similarly quiescent for several hours (Figure 4B). After recovery from this initial ALA-independent period of inactivity, wild-type animals entered a prolonged period of ALA-dependent behavioral quiescence (Figure 4B).
Figure 4.
Recovery quiescence is associated with restoration of proteostasis and survival following noxious heat exposure. A and B, Time course of feeding quiescence in wild-type and ceh-17(np1) animals following a 30 min 37°C heat shock (A) and a 20 min 40°C heat shock (B). *p < 0.001; Fisher’s exact test. C, Time course of feeding quiescence in wild-type, hsf-1(sy441), daf-16(mu68), and hsp-4(gk514) animals following a 30 min 37°C heat shock. Peak quiescence is reduced in hsf-1 and daf-16 mutant animals compared to wild type (asterisks not shown, p = 0.0019 in both cases; Fisher’s exact test), while the duration of quiescence is significantly increased (*p < 0.05; Fisher’s exact test.) in each of the mutant strains. For panels A–C, feeding quiescence is shown but animals were similarly quiescent for locomotion. D, Fold expression of hsp-16.2:GFP and hsp-4:GFP transcriptional reporters 24 hours after a 37°C 30 min heat shock compared to untreated controls. #p < 0.01, ##p < 0.001; Student’s t test. E and F, Survival (E) and locomotor quiescence (F) among wild-type, ceh-17(np1), egl-4(ad450gf), and ceh-17(np1);egl-4(ad450gf) animals following a 20 min 40°C heat shock. ceh-17 animals are significantly impaired for survival compared to wild type (p < 0.001; log rank test). In ceh-17;egl-4(gf) mutants, locomotor quiescence is partially restored (*p < 0.05 vs. ceh-17(np1); Fisher’s exact test) and the survival defect is partially rescued (p < 0.001 vs. ceh-17(np1); log rank test). egl-4(gf) animals do not show significantly greater survival than wild-type animals (p = 0.647; log rank test) but display increased quiescence at certain time points (p < 0.05 vs. wild type at 6, 8, 22, and 24 hrs after heat shock; Fisher’s exact test). Mean and SEM are shown for all panels except C and F, which show mean only. On the X axes, “B” indicates baseline (untreated).
Because heat stress is known to disrupt protein homeostasis and trigger expression of protective protein chaperones[20], we hypothesized that the delayed ALA-dependent quiescence observed with severe heat shock is engaged to promote restoration of protein homeostasis. This hypothesis makes three predictions. First, it predicts that mutant animals that are unable to mount a proper chaperone response to cellular stress would have increased duration of behavioral quiescence after heat shock. Second, it predicts that disrupting this quiescence would result in more severe proteostatic disruption and trigger higher expression of chaperone proteins. And third, it predicts that animals defective in behavioral quiescence would show impaired survival in response to severe heat exposure.
To test the first prediction, we examined the behavior of animals defective in components of cellular proteostasis pathways. We examined animals mutant for the stress-responsive transcription factors HSF-1 and DAF-16, which, in response to heat stress, direct the synthesis of protein chaperones and suppress the synthesis of other proteins[20]. We also examined mutants for the resident endoplasmic reticulum molecular chaperone HSP-4/BiP, whose expression is elevated with increased levels of misfolded proteins[21]. In response to a 30 minute 37°C heat exposure, all three mutants showed longer second bouts of behavioral quiescence compared to wild type (Figure 4C), indicating that defects in restoration of protein folding can prolong recovery quiescence. However, we also observed that the peak of this quiescence was reduced in hsf-1(sy441) and daf-16(mu68) mutants, suggesting that these transcription factors may themselves contribute to the expression of components of the quiescent response.
To test the prediction that recovery quiescence may serve to mitigate disruptions of cellular homeostasis, we examined ceh-17 mutant animals for phenotypic defects following heat stress. In response to a mild 35°C 30 minute heat shock, we found no significant differences in survival or expression of cellular stress reporter genes (described below) in ceh-17 mutant animals compared to wild type (not shown). In response to a 37°C 30 minute heat shock, ceh-17 mutant animals showed wild-type survival (Figure S1A), but showed higher levels of cellular stress as assessed by transcriptional reporters for the molecular chaperones hsp-16.2 and hsp-4/BiP (Figure 4D). In response to a 40°C 20 minute heat shock, ceh-17 mutant animals showed significantly decreased survival compared to wild-type animals (Figure 4E). We observed a similar survival deficit in plc-3(tm1340) mutants (Figure S1B), which are quiescence-defective. Thus, conditions that produce the greatest amount of recovery quiescence, 37°C and 40°C heat shocks, are associated with more severe phenotypic consequences in quiescence-defective animals.
The survival deficit of the ceh-17 mutants in response to noxious heat is not attributable to generally reduced viability of this strain: these animals show wild-type survival in the absence of heat shock (Figure S1C) and in response to the oxidative stress-inducing agent paraquat[22] (Figure S2), which does not trigger prolonged ALA-dependent quiescence. This suggests that the impaired survival of ceh-17 mutant animals is attributable to their quiescence defect. In support of this suggestion, we found that the survival deficit of ceh-17 mutant animals can be partially rescued by a mutation that causes spontaneous bouts of inactivity. The egl-4(ad450sd) allele is a gain-of-function mutation in a protein kinase G that is required for LIN-3(OE) locomotor quiescence, and is hypothesized to act downstream of ALA function[9]. egl-4(gf) mutant animals spontaneously cease locomotion and feeding if left unperturbed[23] and show decreased sensory responses characteristic of sleep[24]. We observed that while egl-4(gf) mutant animals showed wild-type survival following noxious heat exposure, ceh-17(lf);egl-4(gf) animals showed significantly increased locomotor quiescence and survival (Figures 4F and 4E) compared to ceh-17(lf) animals. These data support the concept that behavioral quiescence promotes recovery from cellular stress.
DISCUSSION
This study reveals that perturbations of cellular homeostasis can drive behavioral quiescence in C. elegans, and that this quiescence in turn contributes to recovery from cellular stress. Consistent with our observation of a protective function of stress-induced quiescence in C. elegans, increased sleep was recently shown to promote survival during bacterial infection in D. melanogaster[25]. Behavioral quiescence may allow allocation of resources away from excitable cell function and toward engagement of cellular responses that facilitate recovery from specific stressors. In support of an adverse effect of excessive excitable cell function on cellular proteostasis, increases in C. elegans muscle excitation promote protein aggregation in muscle cells[26]. Our observation that reduced chaperone activity results in prolonged heat-induced quiescence suggests that efficient restoration of protein folding is required to minimize the duration of recovery quiescence. In Drosophila, reduction of heat shock protein activity is associated with exaggerated homeostatic responses to sleep deprivation[27]. Together these data raise the possibility that the duration and intensity of animal sleep is linked to cellular proteostasis.
C. elegans adults do not display prominent circadian sleep patterns, but we have shown here that C. elegans can display limited oscillation of stress-induced quiescence on an ultradian timescale, as seen in response to Cry5B exposure and severe heat shock. We propose a model in which stress triggers both the release of LIN-3 and the transcriptional activation of limiting pathway components, possibly by the same transcription factors that control chaperone gene expression. If proteostasis remains disrupted after synthesis of these pathway components, a second quiescent period may result, the duration of which is related to the degree of disruption of cellular homeostasis. This model is supported by our observation that mutations in the stress-responsive transcription factors hsf-1 and daf-16 are associated with decreased peak quiescence of this second bout, and that more stringent heat shock and decreased chaperone activity are each associated with longer bout duration.
How is EGF signaling initiated during heat-induced recovery quiescence? LIN-3 is expressed in a restricted pattern corresponding to its known roles in cell fate specification during development. Fluorescent in-situ hybridization[28] and GFP reporter[29] analyses indicate that LIN-3 is not expressed in sensory neurons but is expressed throughout the pharynx, and its function there has not yet been ascertained. A particular splice form, LIN-3C[9], also known as LIN-3XL[30], is exclusively expressed in the anterior region of the animal[9], in which the pharynx is the most prominent tissue. LIN-3C contains a unique juxtamembrane sequence, the region in which proteolytic processing must occur for release of the active EGF domain[31]. We hypothesize that pharyngeal LIN-3C serves as a pool of EGF that is shed in response to stress, activating EGFR on the ALA neuron in the dorsal ganglion of the head.
As vertebrate EGF family ligands have been shown to undergo stress-induced shedding[32, 33] and act as somnogens across species [14–16, 34], we posit that EGF signaling is part of a deeply conserved mechanism that contributes to sleep drive in response to cellular stress. Stress-induced quiescence in C. elegans may represent an ancestral condition in which sleep-like behavior evolved as a mechanism to promote restoration of proteostasis following environmental stress or infection. Here, we disrupted cellular homeostasis using environmental stressors to which C. elegans tissues are liable. Cells within more complex organisms may be susceptible to stresses associated with prolonged wakefulness. Indeed, elements of cellular stress have been observed after sleep deprivation in several species[35–39]. Further, clearance of metabolic waste from the mouse brain is decreased during wakefulness[4], resulting in a buildup of metabolites that may perturb cellular homeostasis. The well-documented synaptic changes that occur during sleep[3, 4, 40] may reflect processes that are optimally accomplished upon restoration of proteostasis within the nervous system.
In organisms that have circadian sleep patterns, stress-induced behavioral quiescence may have become temporally restricted through circadian regulation of pathway components. Consistent with this, transcript levels of the EGF family ligand TGF-α in the hamster suprachiasmatic nucleus show circadian oscillation, with a peak corresponding to a time of locomotor quiescence and a trough to a time of locomotor activity[15]. Rhythmic EGF ligand transcription coupled with stress-induced shedding poses a potential mechanism linking circadian and homeostatic processes, promoting sleep within the confines of the circadian clock in response to cellular stresses that have accumulated during wakefulness.
Experimental Procedures
Strains
Strains were maintained on nematode growth (NG) plates at 18–20°C and fed E. coli OP50. Assays were performed on well-fed young adults screened prior to each experiment for normal feeding behavior and locomotion.
Analysis of behavior
To assay feeding quiescence, animals were observed under a stereomicroscope for 3–4 sec and scored for contraction of the posterior pharyngeal bulb. If no contractions were observed, the animal was scored as quiescent for feeding. For Figures 1B and 2E locomotion data was collected using an automated multi-worm tracker. For subsequent panels, locomotion data was collected by direct observation. Plates were left unperturbed for 1 min, and each animal in the field of view at low magnification was observed for 3–4 sec and scored as quiescent if they showed no detectable movement. For reversibility assays (Fig 2A), inactivity was defined as less than one body length of movement in 10 sec.
Stressors
Heat: NG plates housing animals were parafilmed and placed into a water bath at the indicated temperatures, then cooled on ice for 2 min to bring to room temperature. Hypertonic stress: animals were placed in a drop of 500 mM NaCl in M9 for 15 min. Ethanol: animals were placed in a solution of 5% ethanol in M9 for 30 min. Cold shock: NG plates housing animals were immersed into a frozen bead bath in a freezer (−16°C to −14°C) for 30 min then transferred to room temperature plates. Cry5B toxin: Animals were placed for 15 min onto NG plates containing carbenicillin and 1 mM IPTG that had been seeded with JM103 bacteria containing an IPTG-inducible Cry5B expression plasmid.
Survival
Animals were scored as dead if they showed no detectable movement after prodding three times with a wire pick.
Supplementary Material
Highlights.
cellular stress induces a state of behavioral quiescence with sleep-like properties
stress-induced behavioral quiescence is mediated by EGF signaling
mutants defective in stress responses show increased duration of quiescence
quiescence-defective animals are impaired for survival following extreme stress
Acknowledgments
Many strains were provided by the CGC, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440). The authors thank Raffi Aroian for providing Cry5B toxin, David Fay for advice, Matt Nelson for sharing results and for comments, and students of the CSUN BIOL447 ‘FIRE’ lab for valuable assistance with stress assays. Research reported in this publication was supported by the National Institute of General Medical Science of the National Institutes of Health under award 1SC2GM105487 to CVB. DMR was supported by grant NS064030 from the NIH.
Footnotes
Further details of methods can be found in Supplemental Experimental Procedures.
Supplemental Information includes two figures, three tables, Supplemental Experimental Procedures, and three movies and can be found with this article online at...
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zimmerman JE, Naidoo N, Raizen DM, Pack AI. Conservation of sleep: insights from non-mammalian model systems. Trends Neurosci. 2008;31:371–376. doi: 10.1016/j.tins.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crocker A, Sehgal A. Genetic analysis of sleep. Genes Dev. 2010;24:1220–1235. doi: 10.1101/gad.1913110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, et al. Sleep Drives Metabolite Clearance from the Adult Brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mignot E. Why we sleep: the temporal organization of recovery. PLoS Biol. 2008;6:e106. doi: 10.1371/journal.pbio.0060106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones D, Candido EP. Feeding is inhibited by sublethal concentrations of toxicants and by heat stress in the nematode Caenorhabditis elegans: relationship to the cellular stress response. J Exp Zool. 1999;284:147–157. doi: 10.1002/(sici)1097-010x(19990701)284:2<147::aid-jez4>3.3.co;2-q. [DOI] [PubMed] [Google Scholar]
- 7.Wittenburg N, Baumeister R. Thermal avoidance in Caenorhabditis elegans: an approach to the study of nociception. Proc Natl Acad Sci. 1999;96:10477–10482. doi: 10.1073/pnas.96.18.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schild LC, Glauser DA. Dynamic switching between escape and avoidance regimes reduces Caenorhabditis elegans exposure to noxious heat. Nat Commun. 2013;4:2198. doi: 10.1038/ncomms3198. [DOI] [PubMed] [Google Scholar]
- 9.Van Buskirk C, Sternberg P. Epidermal growth factor signaling induces behavioral quiescence in Caenorhabditis elegans. Nat Neurosci. 2007;10:1300–1307. doi: 10.1038/nn1981. [DOI] [PubMed] [Google Scholar]
- 10.Sanders J, Nagy S, Fetterman G, Wright C, Treinin M, Biron D. The Caenorhabditis elegans interneuron ALA is (also) a high-threshold mechanosensor. BMC Neurosci. 2013;14:156. doi: 10.1186/1471-2202-14-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pujol N, Torregrossa P, Ewbank JJ, Brunet JF. The homeodomain protein CePHOX2/CEH-17 controls antero-posterior axonal growth in C. elegans. Development. 2000;127:3361–3371. doi: 10.1242/dev.127.15.3361. [DOI] [PubMed] [Google Scholar]
- 12.Van Buskirk C, Sternberg PW. Paired and LIM class homeodomain proteins coordinate differentiation of the C. elegans ALA neuron. Development. 2010;137:2065–2074. doi: 10.1242/dev.040881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho JY, Sternberg PW. Multilevel Modulation of a Sensory Motor Circuit during C. elegans Sleep and Arousal. Cell. 2014;156:249–260. doi: 10.1016/j.cell.2013.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kushikata T, Fang J, Chen Z, Wang Y, Krueger JM. Epidermal growth factor enhances spontaneous sleep in rabbits. Am J Physiol-Reg. 1998;275:509–514. doi: 10.1152/ajpregu.1998.275.2.R509. [DOI] [PubMed] [Google Scholar]
- 15.Kramer A, Yang FC, Snodgrass P, Li X, Scammell TE, Davis FC, Weitz CJ. Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signaling. Science. 2001;294:2511–2515. doi: 10.1126/science.1067716. [DOI] [PubMed] [Google Scholar]
- 16.Snodgrass-Belt P, Gilbert JL, Davis FC. Central administration of transforming growth factor-alpha and neuregulin-1 suppress active behaviors and cause weight loss in hamsters. Brain Res. 2005;1038:171–182. doi: 10.1016/j.brainres.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 17.Davies AG, Pierce-Shimomura JT, Kim H, VanHoven MK, Thiele TR, Bonci A, Bargmann CI, McIntire SL. A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans. Cell. 2003;115:655–666. doi: 10.1016/s0092-8674(03)00979-6. [DOI] [PubMed] [Google Scholar]
- 18.Wei J-Z, Hale K, Carta L, Platzer E, Wong C, Fang S-C, Aroian RV. Bacillus thuringiensis crystal proteins that target nematodes. Proc Natl Acad Sci. 2003;100:2760–2765. doi: 10.1073/pnas.0538072100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Los FCO, Ha C, Aroian RV. Neuronal Goα and CAPS regulate behavioral and immune responses to bacterial pore-forming toxins. PLoS One. 2013;8:e54528. doi: 10.1371/journal.pone.0054528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morimoto RI. The heat shock response: systems biology of proteotoxic stress in aging and disease. Cold Spring Harb Symp Quant Biol. 2011;76:91–99. doi: 10.1101/sqb.2012.76.010637. [DOI] [PubMed] [Google Scholar]
- 21.Shen X, Ellis RE, Lee K, Liu CY, Yang K, Solomon A, Yoshida H, Morimoto R, Kurnit DM, Mori K, et al. Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell. 2001;107:893–903. doi: 10.1016/s0092-8674(01)00612-2. [DOI] [PubMed] [Google Scholar]
- 22.Vanfleteren JR. Oxidative stress and ageing in Caenorhabditis elegans. Biochem J. 1993;292:605–608. doi: 10.1042/bj2920605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avery L. The Genetics of Feeding in Caenorhabditis elegans. Genetics. 1993;133:897–917. doi: 10.1093/genetics/133.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raizen DM, Zimmerman JE, Maycock MH, Ta UD, You Y, Sundaram MV, Pack AI. Lethargus is a Caenorhabditis elegans sleep-like state. Nature. 2008;451:569–72. doi: 10.1038/nature06535. [DOI] [PubMed] [Google Scholar]
- 25.Kuo T, Williams JA. Increased Sleep Promotes Survival during a Bacterial Infection in Drosophila. Sleep. 2014;37:1077–1086. doi: 10.5665/sleep.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia SM, Casanueva MO, Silva MC, Amaral MD, Morimoto RI. Neuronal signaling modulates protein homeostasis in Caenorhabditis elegans post-synaptic muscle cells. Genes Dev. 2007;21:3006–3016. doi: 10.1101/gad.1575307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw PJ, Tononi G, Greenspan RJ, Robinson DF. Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature. 2002;417:287–291. doi: 10.1038/417287a. [DOI] [PubMed] [Google Scholar]
- 28.Saffer AM, Kim DH, van Oudenaarden A, Horvitz HR. The Caenorhabditis elegans synthetic multivulva genes prevent ras pathway activation by tightly repressing global ectopic expression of lin-3 EGF. PLoS Genet. 2011;7:e1002418. doi: 10.1371/journal.pgen.1002418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang BJ, Sternberg PW. A cell-specific enhancer that specifies lin-3 expression in the C. elegans anchor cell for vulval development. Development. 2004;131:143–51. doi: 10.1242/dev.00924. [DOI] [PubMed] [Google Scholar]
- 30.Dutt A, Canevascini S, Froehli-Hoier E, Hajnal A. EGF signal propagation during C. elegans vulval development mediated by ROM-1 rhomboid. PLoS Biol. 2004;2:e334. doi: 10.1371/journal.pbio.0020334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higashiyama S, Iwabuki H, Morimoto C, Hieda M, Inoue H, Matsushita N. Membrane-anchored growth factors, the epidermal growth factor family: beyond receptor ligands. Cancer Sci. 2008;99:214–20. doi: 10.1111/j.1349-7006.2007.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischer OM, Hart S, Gschwind A, Ullrich A, Prenzel N. Oxidative and Osmotic Stress Signaling in Tumor Cells Is Mediated by ADAM Proteases and Heparin-Binding Epidermal Growth Factor. Mol Cell Biol. 2004;24:5172–5183. doi: 10.1128/MCB.24.12.5172-5183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartels H, Fraek M, Beck FX, Neuhofer W. Ectodomain shedding of pro-TGF-alpha is required for COX-2 induction and cell survival in renal medullary cells exposed to osmotic stress. Am J Physiol Cell Physiol. 2007;293:C1971–C1982. doi: 10.1152/ajpcell.00404.2007. [DOI] [PubMed] [Google Scholar]
- 34.Foltenyi K, Greenspan R, Newport J. Activation of EGFR and ERK by rhomboid signaling regulates the consolidation and maintennce of sleep in Drosophila. Nat Neurosci. 2007;10:1160–1167. doi: 10.1038/nn1957. [DOI] [PubMed] [Google Scholar]
- 35.Cirelli C, Tononi G. Gene expression in the brain across the sleep-waking cycle. Brain Res. 2000;885:303–321. doi: 10.1016/s0006-8993(00)03008-0. [DOI] [PubMed] [Google Scholar]
- 36.Shaw PJ. Correlates of Sleep and Waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 37.Terao A, et al. Differential increase in the expression of heat shock protein family members during sleep deprivation and during sleep. Neuroscience. 2003;116:187–200. doi: 10.1016/s0306-4522(02)00695-4. [DOI] [PubMed] [Google Scholar]
- 38.Naidoo N, Giang W, Galante RJ, Pack AI. Sleep deprivation induces the unfolded protein response in mouse cerebral cortex. J Neurochem. 2005;92:1150–1157. doi: 10.1111/j.1471-4159.2004.02952.x. [DOI] [PubMed] [Google Scholar]
- 39.Jones S, Pfister-Genskow M, Benca RM, Cirelli C. Molecular correlates of sleep and wakefulness in the brain of the white-crowned sparrow. J Neurochem. 2008;105:46–62. doi: 10.1111/j.1471-4159.2007.05089.x. [DOI] [PubMed] [Google Scholar]
- 40.Frank MG, Benington JH. The role of sleep in memory consolidation and brain plasticity: dream or reality? Neuroscientist. 2006;12:477–488. doi: 10.1177/1073858406293552. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.