Abstract
Purpose
To determine the prevalence of, risk factors for, and visual acuity correlations with outer retinal tubulation (ORT) seen on spectral domain optical coherence tomography (SD-OCT) in eyes with neovascular age-related macular degeneration (AMD) following anti-VEGF therapy.
Design
Prospective cohort study within a randomized clinical trial.
Participants
Patients with SD-OCT images at Week 56 and 104 in the Comparison of AMD Treatment Trials (CATT).
Methods
Participants in the CATT were randomly assigned to ranibizumab (0.5mg) or bevacizumab (1.25mg) treatment and to a monthly or PRN injection-dosing regimen. A subset of eyes were imaged with SD-OCT beginning at Week 56. Cirrus 512×128 or Spectralis 20°×20° volume cube scan protocols were used to acquire SD-OCT images. Two independent readers at the CATT OCT Reading Center graded scans, and a senior reader arbitrated discrepant grades. The prevalence of ORT, identified as a tubular structures seen on at least 3 consecutive Cirrus B scans or 2 consecutive Spectralis B scans, was determined. The associations of patient-specific and ocular features at baseline and follow-up with ORT were evaluated by univariate and multivariate analyses.
Main Outcome Measures
Outer retinal tubulations.
Results
Seven of 69 eyes (10.1%) at 56 weeks and 64 of 368 (17.4%) eyes at week 104 had ORTs. Absence of diabetes, poor visual acuity (VA), blocked fluorescence, geographic atrophy (GA), greater lesion size, and presence of subretinal hyper-reflective material at baseline were independently associated with greater risk of ORT at 104 weeks (p<0.05). Neither drug nor dosing regimen were significantly associated with ORT. The mean VA of eyes with ORT at week 104 (58.5 ETDRS letters) was worse than the mean VA of eyes without ORT (68.8 letters; p<0.0001).
Conclusion
At 2 years after initiation of anti-VEGF therapy for neovascular AMD, ORTs are present in a substantial proportion of eyes. We have identified baseline features that independently predict ORTs. It is important to identify ORTs, since eyes with ORTs have worse visual acuity outcomes than those without this finding.
Outer retinal tubulation (ORT) refers to tubular structures observed on OCT imaging within the outer retina. Photoreceptor rosettes with blue cone opsin immunoreactivity in eyes with retinitis pigmentosa are possible ORT histological correlates.1 Zweifel and associates were the first to describe these structures as ORTs, based on their optical coherence tomographic (OCT) appearance. They described ORTs as branching tubular structures located in the retinal outer nuclear layer that occurred in eyes with a variety of advanced degenerative retinal disorders. On SD-OCT B-scans, ORTs were seen as round hypo-reflective spaces with hyper-reflective borders2. Since that report, ORTs have been observed in eyes with a variety of retinal diseases, including age-related macular degeneration (AMD), pseudoxanthomaelasticum, multifocal choroiditis, central serous chorioretinopathy, and other neovascular retinal disorders.1–6
The prevalence of ORTs in eyes with neovascular AMD, and their association with ocular and non-ocular characteristics, has not been well described. We hypothesized that ORTs might be more common than previously thought in neovascular AMD, and that the visual prognosis of eyes with ORTs might differ from those without ORTs. The purpose of the present study was to determine the prevalence of ORT after anti-VEGF therapy in subjects enrolled in the Comparison of AMD Treatments Trials (CATT) and to assess whether this prevalence depended on baseline non-ocular and ocular features or on anti-VEGF drug and treatment regimen. A further aim was to evaluate the association of ORTs with other concurrent retinal morphological findings and visual acuity.
Methods
Subjects in this study were enrolled in CATT. Written informed consent was obtained from all CATT study participants and the protocol was approved by institutional review boards associated with each participating clinical center. The CATT study procedures have been previously published and can be found on ClinicalTrial.gov (study identifier, NCT00593450).7, 8 Briefly, 1185 patients with neovascular AMD were enrolled in CATT at 43 clinical centers in the United States. Patients were randomly assigned to one of four treatment groups: 1) ranibizumab monthly, 2) bevacizumab monthly, 3) ranibizumab pro re nata (PRN), or 4) bevacizumab PRN. At 52 weeks, patients originally assigned to monthly treatment were randomly assigned to continue monthly treatment or to PRN treatment of the same drug.
All patients underwent time domain (TD) OCT with a Stratus system (Carl Zeiss Meditec, Dublin, CA, USA) during year 1 of the study. Beginning in year 2 (defined as week 56), a subset of eyes were imaged with one of two spectral domain (SD) OCT machines, a Cirrus HD-OCT unit [Carl Zeiss Meditec, Dublin, CA, USA] or a Spectralis system [Heidelberg Engineering, Heidelberg, Germany]. This subset of eyes was selected based on the availability of SD-OCT machines of each participating clinical center; some eyes converted from TD OCT to SD OCT imaging at week 56 while others did not convert until later in the study period. A 512×128 macular cube and a 20°×20° 49 line high-speed macular cube, was obtained with the Cirrus and Spectralis machines, respectively.
Outer retinal tubulation grading
Two independent readers at the CATT OCT Reading Center initially graded SD-OCT scans for the presence and location of fluid, thickness of retinal layers at the foveal center point, elevation of the retinal pigment epithelium (RPE), and subretinal hyper-reflective material. Subretinal hyper-reflective material (SHRM) was defined as hyper-reflective material beneath the retina (or sub-retinal fluid, when present), and internal to the RPE, or when the RPE was disrupted and not visible, it referred to the hyper-reflective material beneath the retina (or subretinal fluid when present) and Bruch’s membrane. This material may include CNV, blood, or scar tissue. Discrepant points were arbitrated by a third, independent senior reader. In the original CATT publications the term “intraretinal fluid” was used.8,9 For these publications, readers identified “intraretinal fluid (IRF)” as a round or oval hypo-reflective cystoid space regardless of whether a hyper-reflective outer border was present. Accordingly, eyes with “intraretinal fluid” included those with ORTs, alone, or with, in addition, intra-retinal cystoid spaces. Furthermore, it is possible that round or elongated tubular structures near the outer retina boundary, may have been classified as subretinal fluid. Therefore, in the present report, to analyze ORTs at weeks 56 and 104, all SD-OCT images from 52 eyes at weeks 56 and 277 eyes at weeks 104 gradable for fluid status and with an initial finding of “intraretinal fluid” or subretinal fluid were re-graded by two independent readers in a masked fashion for presence and location of outer retinal tubulations; by definition eyes without any “intraretinal fluid” or subretinal fluid would not have had ORTs. In cases of discrepant grades, a third independent senior reader arbitrated those parameters. In the current report, to maintain consistency with previous publications, we have retained the term IRF. However, we now differentiate IRF, defined as an intraretinal cystoid structure(s) without hyper-reflective borders, from an ORT(s), which is defined as a round, ovoid, or tubular hypo-reflective area with a hyper-reflective border located in the outer retina. Furthermore, to capture the tubular nature of these structures, the hypo-reflective structure with hyper-reflective border had to appear as a contiguous lesion on more than one consecutive scan. To be considered an ORT, two consecutive scans were required for Spectralis volume cubes. Because of the higher scan density on Cirrus volume cubes, and so that ORT prevalence rates would be comparable among OCT machine types, three consecutive scans were required for Cirrus scans.
If present, the location of the ORT was indicated (within the central 1 mm region or under the foveal center), and whether the ORT was located within an area of geographic atrophy. Geographic atrophy on SD-OCT was defined as an area of RPE cell layer loss, overlying retinal thinning, and associated penetration of the light signal into the choroid. On Cirrus images, the central 1mm region was determined from the 512 X 128 volume cube, and was defined as a 1000μm× 1000μm2, which comprised 21 B scans, 10 of which were above, and 10 of which were below the most foveal-centered scan. On Spectralis images, the central 1-mm region was determined from the 49-line volume cube, and was defined as a 1000μm× 1000μm square, which comprised 11 B scans, 5 of which were above, and 5 of which were below the most foveal-centered scan. In both the Cirrus and Spectralis machines, the built-in software measurement tool was used to determine the boundaries of the 1-mm central region. In most cases, the foveal center was readily determined by the central foveal depression. In some cases, when the foveal depression was absent because of macular edema secondary to choroidal neovascularization (CNV), the foveal center was identified by the greatest outer nuclear complex thickness, and by loss of the ganglion cell complex. To evaluate change in ORTs from week 52 to 104, we correlated the OCT B-scan location of the ORT, with the corresponding location on the scanning laser ophthalmoscopic image (Spectralis), or OCT fundus image (Cirrus), to ensure that comparable locations were examined from one exam to the next.
Fluorescein angiography and fundus photography grading
Independent readers, for a variety of features, as detailed in Table 4, graded fundus photographs and fluorescein angiograms. Fibrotic and non-fibrotic scars were defined as follows: Fibrotic scars were defined as obvious white or yellow mounds of fibrous-appearing tissue that are well defined in shape and appeared solid on color stereo images. On fluorescein angiography, they were hyperfluorescent because of tissue staining or blocked fluorescence of the underlying choroid.
Non-fibrotic scars were typically flat, small, well circumscribed areas of pigmentation with varying degrees of central hypopigmentation on color fundus photographic images. The peripheral pigmentary changes in these scars often followed the outline of previously active choroidal neovascularization. On early phase fluorescein angiograms, the depigmented area were often hyperfluorescent, and this hyperfluorescence persisted or increased in intensity on late-phase fluorescein angiograms. Hypofluorescence surrounding the hyperfluorescent center corresponded to the pigmented borders apparent in the color images..
Blocked fluorescence was defined as hypofluorescence on fluorescein angiography that was contiguous with CNV and not related to hemorrhage or pigment on corresponding color fundus photographic images.
Statistical Analysis
The comparisons of features between eyes with ORT vs. those without ORT were performed using Fisher’s exact tests for comparing proportions and two group t-tests for comparing means. To assess baseline predictors of ORT prevalence at week 104, we classified predictors, determined at study enrollment and measured on a continuous scale (e.g., VA, CNV area, OCT thickness) into categories for easier clinical interpretation, based on either the normal range (as for retinal thickness), quartiles of the distribution (as for subretinal tissue complex thickness), or clinically relevant cut-points (as for baseline visual acuity). We then performed univariate analysis for each of baseline predictors including demographic characteristics (e.g., age, gender, systemic diseases), ocular characteristics (e.g., intraocular pressure, history of glaucoma, CNV features) and OCT features (e.g., subretinal, retinal, and subretinal tissue complex thickness).
The predictors with a P value less than 0.20 in the univariate analysis were included in a multivariate logistic regression so that the independent association of each baseline predictor with ORT could be assessed. The final multivariate model was created by applying a backward selection procedure that retained only those predictors with a P value less than 0.05, with the exception of drug and dosing regimen, which were included in the final multivariate model. Adjusted odds ratios (OR) and their 95% confidence intervals (95% CI) were calculated from the final multivariate logistic regression model. All statistical analyses were performed in SAS (v9.2, SAS Institute Inc., Cary, NC), and two-sided p<0.05 was considered to be statistically significant.
Results
Frequency and Location of ORTs
Three hundred and ninety-one CATT participants underwent SD-OCT at weeks 56 and 104. Of these, the fluid status could be determined in 69 of 73 (95%) eyes at week 56, and in 368 of 384 (96%) eyes at week 104. In the remaining 4 eyes at week 56 and 16 at week 104, the images were not of sufficient quality to definitively determine presence or absence of intraretinal or subretinal fluid. Subsequent analyses were performed on these 69 and 368 eyes, respectively.
Of the 69 eyes with known fluid status at week 56, 7 (10.1%) had ORTs, and of 368 eyes with known fluid status at 104 weeks, 64 (17.4%) had ORTs. There were no differences in the prevalence of ORTs seen on Cirrus and those identified on Spectralis at week 56 (12.2% and 5.0% in Cirrus and Spectralis, respectively, P=0.66) and week 104 (16.6% and 18.5% in Cirrus and Spectralis, respectively, P =0.68).
Outer retinal tubulations were located most commonly outside the central 1 mm region. At 56 weeks, only 1 (14%) of 7 eyes with ORTs was within the central 1 mm region and none were in the foveal center. At 104 weeks, 22 (34%) of 64 eyes with ORTs were within the central 1 mm region, and only 2 (3.1%) were under the foveal center. Outer retinal tubulations, were commonly found in areas of GA, particularly by 104 weeks. At 56 weeks, 2 of 7 (28%) were within a GA area, and at 104 weeks, 34 of 64 (53%) were within a GA area (Table 1 and 2).
Table 1.
Location of outer retinal tubulation by location of fluid on OCT at Week 56 (N=69)
| Location of Outer Retinal Tubulation | |||||
|---|---|---|---|---|---|
|
| |||||
| OCT fluid at Week 56 | N | Anywhere, n (%) | Central 1mm region, n (%) | Under foveal center, n (%) | In an area of GA, n (%) |
| Intraretinal only | 19 | 6 (31.6%) | 0 | 0 | 2 (10.5%) |
| Subretinal only | 16 | 0 | 0 | 0 | 0 |
| Both | 17 | 1 (5.9%) | 1 (5.9%) | 0 | 0 |
| Either intraretinal or subretinal | 52 | 7 (13.5%) | 1 (1.9%) | 0 | 2 (3.8%) |
| Total | 69 | 7 (10.1%) | 1 (1.4%) | 0 | 2 (2.9%) |
RPE = retinal pigment epithelium; GA=geographic atrophy; OCT=optical coherence tomography.
Table 2.
Location of outer retinal tubulation by location of fluid on OCT at Week 104 (N=368)
| Location of Outer Retinal Tubulation | |||||
|---|---|---|---|---|---|
|
| |||||
| OCT fluid at Week 104 | N | Anywhere, n(%) | Central 1mm region, n (%) | Under foveal center, n (%) | In an area of GA, n (%) |
| Intraretinal only | 131 | 39 (29.8%) | 15 (11.5%) | 2 (1.5%) | 27 (20.8%) |
| Subretinal only | 79 | 5 (6.3%) | 3 (3.8%) | 0 | 1 (1.3%) |
| Both | 67 | 20 (29.9%) | 4 (6.0%) | 0 | 6 (9.0%) |
| Either intraretinal or subretinal | 277 | 64 (23.1%) | 22 (7.9%) | 2 (0.7%) | 34 (12.3%) |
| Total | 368 | 64 (17.4%) | 22 (6.0%) | 2 (0.5%) | 34 (9.2%) |
RPE = retinal pigment epithelium; GA=geographic atrophy; OCT=optical coherence tomography
In some eyes, outer retinal tubulations appeared to change over time. Among the 7 eyes with ORTs at week 56, only 1 (14.3%) eye still had ORT at week 104. Among 52 eyes that did not have ORTs at week 56, 8 (15.4%) had new ORTs at week 104. When serial OCT images from a given subject were reviewed, these structures disappeared in a variable manner. For example, in two subjects, there was disorganization of the retina associated with intraretinal cysts, and subsequent disruption of the ORT. In one subject, the ORT appeared to shrink, and then disappear (Figure 1). Of the six eyes in which ORTs were seen at week 56, but not week 104, in two eyes GA was present in association with the ORTs at 56 weeks, and was also visible at week 104. In two additional eyes, there was no GA at week 54, but GA was observed at week 104, in the area previously occupied by the ORTs. Of the 8 eyes that developed new ORTs at week 104 that were not present at week 56, 6 eyes were associated with GA (Figure 2).
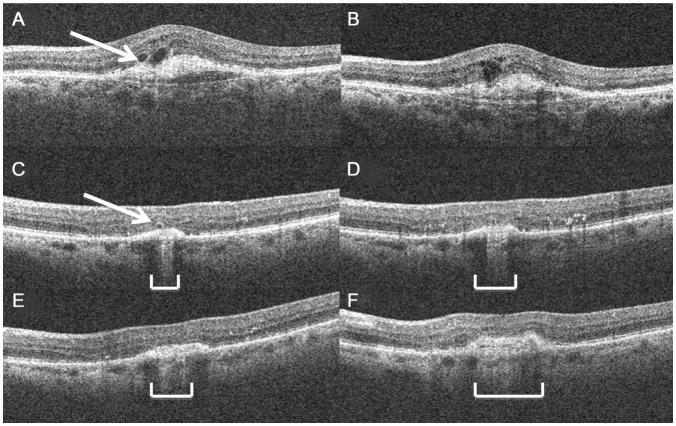
Figure 1.
Representative images to show structural changes in ORTs over time. (A) ORT (arrow) next to an intraretinal cystoid structure without a hyper-reflective rim at week 56, (B) 9 months later, the ORT and cystoid structure appear to have coalesced into a retinal area with multiple cystoid structures and disrupted layers. (C) At 56 weeks, there was a small ORT overlying an area of retinal elevation (arrow). (D) 3 months later, in this same eye as in (C) the ORT has nearly disappeared, and by (E) 9 months, and (F) 12 months, it is no longer apparent. The width of the atrophic area underlying this ORT, seen as the area with increased choroidal signal penetration, has increased over time (brackets).
*ORT = outer retinal tabulation
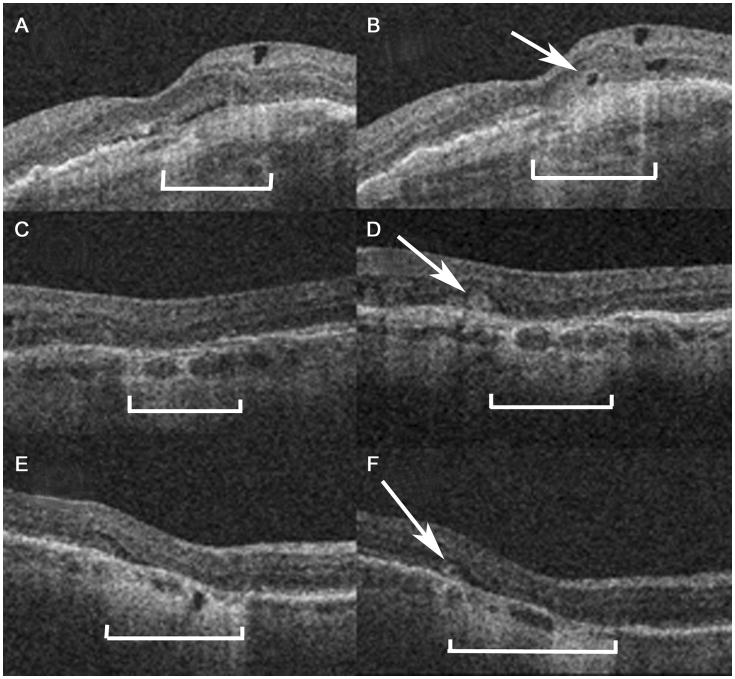
Figure 2.
Representative OCT images at week 56 (A, C, E) and week 104 (B, D, F) in three eyes without ORT at week 56 but with ORT (arrows) at week 104. ORTs are seen adjacent to areas of geographic atrophy, seen as photoreceptor layer thinning above an area of increased light penetration into the choroid (brackets).
*ORT = outer retinal tabulation
Baseline features associated with ORTs at week -104
A variety of baseline non-ocular and ocular features were evaluated for association with ORTs at week 104 (there were too few participants at week 56 for meaningful analysis). On univariate analysis, diabetes and baseline dietary supplements (e.g., beta carotene, vitamin C, vitamin E, and zinc as a combination) usage were significantly associated with lower risk of ORT (Table 3; available at www.aaojournal.org). In addition, several baseline ocular characteristics were significantly related to ORT at week 104 (Table 4; available at www.aaojournal.org). Lesions with blocked fluorescence, fibrotic or non-fibrotic scar, predominantly or minimally classic lesions, CNV without associated other lesion components (hemorrhage, blocked fluorescence, SPED, and others), hemorrhage contiguous with a lesion, hemorrhages (> one disc area), geographic atrophy, large CNV lesions, all as determined by fluorescein angiography and/or color fundus photography and eyes with relatively worse visual acuity were all significantly associated with increased risk of ORT at week 104 (Table 4; available at www.aaojournal.org). Of the eyes that developed ORTs at week 104, 17% had GA at baseline, whereas of the eyes that did not develop ORTs, only 5% had baseline GA. Furthermore, baseline OCT characteristics, that included increased sub-RPE tissue complex thickness, IRF, subretinal hyper-reflective material (SHRM) anywhere, and within the center 1mm were significantly associated with ORT (Table 5; available at www.aaojournal.org). Of note, the assigned anti-VEGF drug (bevacizumab or ranibizumab) and drug regimen (monthly or PRN) were not associated with ORTs at week 104.
To determine the factors that were independently associated with ORT, a multivariate analysis was conducted (Table 6). Diabetes was still associated with lower risk of ORTs (odds ratio [OR]: 0.17, 95% confidence interval [CI]: 0.05–0.56). Baseline ocular factors that were independently associated with increased risk of ORTs included lesions with blocked fluorescence (OR: 2.62, 95% CI: 1.12–6.13), geographic atrophy (OR: 7.01, CI: 2.27–21.7), large CNV lesions (OR: 4.62, CI: 1.82–11.7 for>4 DA as compared with 1 DA), worse baseline visual acuity (OR: 6.52, CI: 1.92–22.1 for VA 20/200 or worse as compared to 20/40 or better), and SHRM (OR: 2.50, CI: 1.01–6.17).
Table 6.
Multivariate Analysis for the baseline predictors of outer retinal tubulation (ORT) in any area at Week 104 (N=347)
| Baseline features | N† | Univariate analysis | Multivariate analysis§ | |||
|---|---|---|---|---|---|---|
|
|
||||||
| ORT (%) | OR (95% CI) | P value* | OR (95% CI) | P value** | ||
| Diabetes | ||||||
| No | 283 | 58 (20.5%) | 1.00 | 0.012 | 1.00 | 0.004 |
| Yes | 64 | 4 (6.25%) | 0.26 (0.09, 0.74) | 0.17(0.05,0.56) | ||
| Blocked fluorescence | ||||||
| No | 300 | 49 (16.3%) | 1.00 | 0.063 | 1.00 | 0.03 |
| Yes | 47 | 13 (27.7%) | 1.96 (0.96, 3.98) | 2.62(1.12,6.13) | ||
| Geographic atrophy in study eye | ||||||
| None/questionable | 325 | 52 (16.0%) | 1.00 | 0.001 | 1.00 | 0.0007 |
| Present | 22 | 10 (45.5%) | 4.38 (1.80, 10.7) | 7.01(2.27,21.7) | ||
| Baseline total area of CNV lesion (DA) | ||||||
| <=1 | 117 | 12 (10.3%) | 1.00 | 0.029 | 1.00 | 0.01 |
| >1 to <=2 | 79 | 14 (17.7%) | 1.89(0.82, 4.33) | 1.97(0.75,5.15) | ||
| >2 to <=4 | 82 | 17 (20.7%) | 2.29 (1.03, 5.10) | 2.75(1.09,6.95) | ||
| >4 | 69 | 19 (27.5%) | 3.33 (1.50, 7.38) | 4.62(1.82,11.7) | ||
| Baseline VA in study eye | ||||||
| 20/25–40 | 133 | 13 (9.77%) | 1.00 | 0.0002 | 1.00 | 0.003 |
| 20/50–80 | 126 | 20 (15.9%) | 1.74 (0.83, 3.67) | 1.38(0.61,3.10) | ||
| 20/100–160 | 67 | 20 (29.9%) | 3.93 (1.81, 8.53) | 3.22(1.36,7.63) | ||
| 20/200–320 | 21 | 9 (42.9%) | 6.92 (2.46, 19.5) | 6.52(1.92,22.1) | ||
| Subretinal hyper-reflective material | ||||||
| No | 84 | 7 (8.33%) | 1.00 | 0.012 | 1.00 | 0.047 |
| Yes | 263 | 55 (20.9%) | 2.91 (1.27, 6.66) | 2.50(1.01,6.17) | ||
| Drug | ||||||
| Lucentis | 175 | 25 (14.3%) | 1.00 | 0.08 | 1.00 | 0.08 |
| Avastin | 172 | 37 (21.5%) | 1.64 (0.94, 2.87) | 1.75(0.94,3.27) | ||
| Regimen | ||||||
| Monthly | 74 | 10 (13.5%) | 1.00 | 0.43 | 1.00 | 0.40 |
| Switched | 94 | 20 (21.3%) | 1.73 (0.75, 3.96) | 1.90(0.74,4.89) | ||
| PRN | 179 | 32 (17.9%) | 1.39 (0.65, 3.00) | 1.36(0.58,3.18) | ||
P values are from univariate logistic regression
P values are from multivariate logistic regression
The initial multivariate model includes diabetes, dietary supplement use, blocked fluorescence lesion, fibrotic or non-fibrotic scar, lesion type, hemorrhage contiguous with lesion (yes/no), geographic atrophy, baseline total area of CNV lesion (DA), baseline VA in study eye, subretinal tissue complex thickness in the foveal center, intraretinal fluid, SHRM, drug and regimen.
347 patients were included in the final multivariate model, 21 patients were excluded due to missing data in any of the baseline predictors in the final model.
VA=visual acuity; DA=disc area.
Anatomic features in eyes with ORTs at week 104
At week 104, several retinal anatomic features were compared between eyes with vs. without ORTs at that time point. Compared to eyes without ORT, eyes with ORT are more likely to have IRF, abnormally thin or thick retinas (those < 120μ or >212μ), larger CNV lesion size, fibrotic scar, and pathology in the fovea center (Table 7). They were less likely to have CNV; the proportion with ORTs among those with CNV was 10.9%, and among those without ORTs, was 18.1%. There were no eyes with ORTs at week 104 that did not also have associated fluid (intraretinal, subretinal, or sub-RPE). Conversely, among eyes without ORTs at week 104, 27.3% had fluid (intraretinal, subretinal, or sub-RPE). The incidence of de novo GA (those for whom GA was not evident at baseline, but was present at 2 years) was 7.5% among those that had ORTs, and was 15.5% among those that did not have associated ORTs. Of all eyes with GA at week 104, 10% had associated ORT, and 90% did not.
Table 7.
Comparison of Week 104 outcomes between patients with and without outer retinal tubulation (ORT) at Week 104 (N=368)
| Week 104 Outcomes | With ORT (N=64) | Without ORT (N=304) | P Value* |
|---|---|---|---|
| VA categories | <0.0001 | ||
| 20/200 or worse | 13 (20.3%) | 15 (4.9%) | |
| 20/50 – 20/160 | 22 (34.4%) | 90 (29.6%) | |
| 20/12 – 20/40 | 29 (45.3%) | 199 (65.5%) | |
| Mean VA in letters (SE) | 58.5 (3.11) | 68.8 (0.97) | <0.0001 |
| Mean VA change from baseline in letters (SE) | 3.69 (2.81) | 6.15 (0.94) | 0.31 |
| ≥ 15 letters increase from baseline (%) | 20 (31.3%) | 87 (28.6%) | 0.65 |
| Mean number of injections in Year 1 in PRN Groups (SE) † | 12.8 (1.03) | 13.7 (0.51) | 0.43 |
| Retinal thickness in microns, n (%) | 0.03 | ||
| <120 microns | 23 (35.9%) | 77 (25.4%) | |
| 120–212 microns | 30 (46.1%) | 194 (64.0%) | |
| >212 microns | 11 (17.2%) | 32 (10.6%) | |
| Mean change of total foveal thickness from baseline in microns (SE) | −168 (25.2) | −162 (11.0) | 0.83 |
| Intraretinal fluid, n (%) | 59 (92.2%) | 139 (45.7%) | <0.0001 |
| Subretinal fluid, n(%) | 25 (39.1%) | 121 (39.8%) | 1.00 |
| Sub-RPE fluid, n (%) | 25 (39.1%) | 109 (36.1%) | 0.67 |
| Leakage on angiography, n (%) | 18 (28.6%) | 83 (27.9%) | 0.88 |
| Mean lesion size at 2 years, disc areas (SE) | 4.47 (0.45) | 3.10 (0.18) | 0.002 |
| Mean change in lesion size from baseline, disc areas (SE) | 0.70 (0.29) | 0.68 (0.15) | 0.95 |
| Fibrotic scar, anywhere, n (%) | 31 (48.4%) | 68 (22.4%) | <0.0001 |
| Non-fibrotic scar, anywhere, n (%) | 12 (18.8%) | 52 (17.1%) | 0.72 |
| Geographic atrophy, anywhere, n (%) | 11 (17.2%) | 58 (19.1%) | 0.86 |
| Pathology in fovea center, n (%) | 0.03 | ||
| No pathology | 9 (14.1%) | 70 (23.0%) | |
| Fluid only | 0 (0%) | 13 (4.3%) | |
| Choroidal neovascularization | 7 (10.9%) | 55 (18.1%) | |
| Serous pigment epithelial detachment | 0 (0%) | 2 (0.7%) | |
| Scar | 27 (42.2%) | 59 (19.4%) | |
| Geographic atrophy | 3 (4.7%) | 11 (3.6%) | |
| Non-geographic atrophy | 14 (21.9%) | 58 (19.1%) | |
| Hemorrhage | 0 (0%) | 3 (1.0%) | |
| Retinal pigment epithelium tear | 0 (0%) | 5 (1.6%) | |
| Blocked fluorescence | 0 (0%) | 9 (3.0%) | |
| Other | 3 (4.7%) | 10 (3.3%) | |
| Unknown | 1 (1.6%) | 9 (3.0%) |
From Fisher exact test for comparison of proportions, and two group t-test for comparison of means.
Only patients in PRN group were included in the analysis
VA=visual acuity; SE=sphericalequivalent; OCT=optical coherence tomography.
Effect of ORTs and Fluid on Visual acuity at Week 104
Overall, eyes with ORTs at week 104 when compared to those without ORTs had worse visual acuity at week 104 (mean VA 59 letters vs. 69 letters, p<0.0001), a higher proportion with visual acuity 20/200 or worse (20% vs. 5%, p<0.0001) and a smaller percentage with visual acuity 20/40 or better (45% vs. 66%, p=0.004, Table 7). Furthermore, baseline visual acuity was worse by 6 letters in eyes with ORTs and IRF at week 104, than in eyes with IRF but no ORTs at week 104, which was worse by three letters than in eyes without IRF. The 59 eyes with both IRF and ORTs had worse visual acuity at week 104 (mean VA 57.3 letters) compared to eyes with IRF but no ORTs (mean VA 63.3 letter, p<0.0001) (Table 8). All eyes with ORTs at week 104 had associated IRF and/or SRF that were distinct from the ORTs. Furthermore, week 104 visual acuity in eyes with both IRF and ORTs was 6 letters worse than eyes with IRF and no ORTs, which was approximately 10 letters worse than in eyes without IRF (Table 8).
Table 8.
Visual acuity at baseline and at Week 104 by between eyes with and without outer retinal tubulation (ORT) at Week 104 (N=368)
| Fluid and ORT status at Week 104 | N | Visual Acuity
|
||||
|---|---|---|---|---|---|---|
| Baseline: Mean (SE) | Week 104: Mean (SE) | Change at Week 104: Mean (SE) | ≥20/40 at Week 104 n (%) | ≤20/200 at Week 104 n (%) | ||
| ORT and IRF | ||||||
| No IRF | 170 | 63.9 (1.0) | 73.5 (1.4) | 9.55 (1.33) | 127 (74.7%) | 1 (0.6%) |
| IRF with ORT | 59 | 54.8 (1.6) | 57.3 (2.33) | 2.42 (2.25) | 25 (42.4%) | 13 (22.0%) |
| IRF without ORT | 139 | 60.8 (1.1) | 63.3 (1.52) | 2.44 (1.47) | 76 (54.7%) | 14 (10.1%) |
| P value* | <0.0001 | <0.0001 | 0.0005 | <0.0001 | <0.0001 | |
| ORT and SRF | ||||||
| No SRF | 221 | 59.4 (0.8) | 64.3 (1.25) | 4.88 (1.19) | 125 (56.6%) | 23 (10.4%) |
| SRF with ORT | 25 | 60.2 (2.5) | 64.7 (3.72) | 4.44 (3.53) | 14 (56.0%) | 2 (8.0%) |
| SRF without ORT | 121 | 65.2 (1.2) | 72.5 (1.69) | 7.34 (1.60) | 89 (73.6%) | 3 (2.5%) |
| P value* | 0.0003 | 0.0004 | 0.44 | 0.005 | 0.02 | |
| ORT and IRF/SRF | ||||||
| No IRF/SRF | 91 | 61.4 (1.3) | 71.0 (1.9) | 9.64 (1.84) | 60 (65.9%) | 1 (1.1%) |
| IRF/SRF with ORT | 64 | 54.8 (1.6) | 58.5 (2.3) | 3.69 (2.19) | 29 (45.3%) | 13 (20.3%) |
| IRF/SRF without ORT | 213 | 63.2 (0.87) | 67.9 (1.27) | 4.66 (1.20) | 139 (65.3%) | 14 (6.6%) |
| P value* | <0.0001 | 0.0001 | 0.047 | 0.014 | <0.0001 | |
| ORT | ||||||
| No | 304 | 62.6 (0.73) | 68.8 (1.06) | 6.15 (1.01) | 199 (65.5%) | 15 (4.9%) |
| Yes | 64 | 54.8 (1.58) | 58.5 (2.32) | 3.69 (2.20) | 29 (45.3%) | 13 (20.3%) |
| P-value* | <0.0001 | <0.0001 | 0.31 | 0.004 | <0.0001 | |
From Fisher exact test for comparison of proportions, and two group t-test for comparison of means.
IRF = intraretinal fluid; SRF = subretinal fluid
It is conceivable that the prevalence of ORTs among subjects at clinics that had SD-OCT differed from those that had TD-OCT only. To confirm that the population evaluated in the present study was similar to the CATT study population as a whole, we did an additional analysis to compare the key baseline predictive factors, and the 2-year outcomes, in the eyes that had SD-OCT, and those in whom only TD-OCT was performed. There were no significant differences between the groups in any of these features either at baseline, or at 2 years (Table 9; available at www.aaojournal.org).
Discussion
In this study, we found that the prevalence of ORTs at week 56 and 104 was substantial in eyes with neovascular AMD that have been treated with anti-VEGF therapy. Baseline features identified at subject enrollment that were independently and positively associated with ORT prevalence included worse baseline visual acuity, presence of GA, larger CNV lesion area, blocked fluorescence on fluorescein angiography, and subretinal hyper-reflective material on OCT, while baseline diabetes was negatively associated with ORTs. Furthermore, after treatment for two years, eyes with ORTs were more likely to have abnormally thin or thick retinas, a fibrotic scar, a large CNV lesion complex, and less likely to have CNV only lesion than treated eyes without ORTs. Eyes with IRF had worse visual acuity than those without any intraretinal cystoid structures, and visual acuity was even worse in eyes that had both IRF and ORTs.
Outer retinal tubulations were identified by SD-OCT in the sub-group of CATT study participants in whom it was performed. All eyes had Stratus TD-OCT in the first year of the CATT. Initially, we attempted to identify ORTs on these TD-OCT images. However, we found that it was not possible to reproducibly identify them with this methodology. Spectral domain OCT allowed us to identify the outer retinal tubulations as tubular structures on contiguous spectral domain B scans. For this study, the requirements for visibility of ORT on multiple SD-OCT scans was conservative, and it is likely that ORT are thus more common than predicted here. It is likely that we could not identify these structures reliably on TD-OCT scans because of inadequate scan density, particularly since ORTs were typically seen distal to the foveal center, and the radial time domain OCT B scans are widely separated outside the foveal area. In addition, even in SD-OCT imaging, ORT may not always demonstrate a prominent hyper-reflective ORT rim, and decreased TD-OCT image resolution compared to spectral domain image resolution, more often precluded clear differentiation of ORT from the surrounding reflective tissue.
In our previous 1-year and 2-year CATT outcome data, we used the term “intraretinal fluid” to describe hypo-reflective intraretinal cystoid structures, but did not distinguish those intraretinal cystic structures without a hyper-reflective border from those with such a border. In the present report, we defined ORTs as those intraretinal cystic structures with a hyper-reflective border. The hyper-reflective border likely corresponds to intact photoreceptor inner segment ellipsoids that surround the photoreceptor outer segment “rosettes.” However, histologically, these rosettes also may be seen without intact surrounding ellipsoids (unpublished results, presented at the Heidelberg International Symposium, New York, 2013).
To avoid confusion, and maintain consistent terminology, we have retained the term intraretinal fluid in the present report, recognizing that some of these structures may represent outer tubulations, because it is not possible to distinguish hypo-reflective cystoid structures that originate from fluid leakage, from those that represent outer tubulations without a hyper-reflectve border on OCT. Furthermore, because it was not possible by OCT to identify outer retinal tubulations without a hyper-reflective border in eyes with neovascular AMD treated with anti-VEGF therapy, the prevalence of ORTs are likely even higher than that reported here.
It was notable that diabetic patients had lower risk of developing ORTs. The reason for the negative association between diabetes on ORTs in eyes with neovascular AMD treated with anti-VEGF agents is unclear and further studies are needed to explain this relationship.
The relationship between ORTs and neovascular AMD disease activity, and the need for anti-VEGF therapy is unknown. We have initiated a follow-up CATT study that will extend our observations to 5 years following initiation of anti-VEGF therapy to treatment-naïve patients. We will then be able to correlate ORTs at week 104 with subsequent need for treatment.
Eyes with ORTs had significantly worse visual acuity at baseline and week 104 than eyes without this finding. These results are consistent with those described in a recent study, in which ORTs were significantly associated with worse visual acuity before and after the intravitreal anti-VEGF treatment.10 Previously, we have shown that “IRF”, which included eyes with ORTs, was an independent predictor of poor visual acuity at all time points evaluated through one year.11 In the present report, we have extended, and further clarified this observation. At two years, eyes with IRF without ORTs had significantly worse visual acuity than those without any IRF, and eyes with both IRF and ORTs had even significantly worse visual acuity than eyes with IRF without ORTs. The reason for the association of ORTs with worse visual acuity is unclear, but presumably eyes with ORTs have a greater degree of photoreceptor degeneration than eyes with IRF alone.
Baseline sub-retinal hyper-reflective material and geographic atrophy predicted ORT at two years. Furthermore, in two eyes, geographic atrophy was observed at week 104 as a “footprint” of ORTs that were seen at week 56 but had disappeared by week 104. There is preliminary evidence that combination treatments such as anti-platelet-derived growth factor and anti-VEGF causes resolution of subretinal hyper-reflective tissue, an OCT-correlate of subretinal CNV tissue complex, more effectively than does anti-VEGF therapy alone, and may also result in better visual acuity outcomes.12 It will be of interest in future studies, to determine whether these types of combination therapies, or future treatments to limit geographic atrophy progression, more effectively reduce ORTs, and if so, whether the eyes with ORT resolution have better visual acuity than those without ORT resolution.
The current study investigated associations of ORT with various systemic and anatomic factors, but was not designed to determine whether anti-VEGF therapy can cause resolution of ORTs, as it does with IRF from leaking CNV, nor whether resolution influences visual acuity. Zweifel et al. suggested that the tubular arrangements seen in ORT might be a response to degenerating photoreceptors and represent a common final pathway in a variety of retinal degenerative conditions, rather than a specific response to leaking CNV.2 While this hypothesis may be correct for many conditions, data from present report indicate that once formed, ORTs are not static in neovascular AMD treated with anti-VEGF therapy. The dynamic character of ORTs is supported by the comparison of ORTs at week 56 and week 104 among 59 eyes with fluid status known at both week 56 and week 104. Among 7 eyes with ORT at week 56, only 1 eye had ORT at week 104. ORT disappearance that we observed in some eyes following anti-VEGF therapy, indicates that in some cases ORTs might respond to anti-VEGF treatment. Alternatively, ORT disappearance might be coincidental, and unrelated to anti-VEGF therapy.
The above data suggest that once formed, ORTs can resolve. To definitively state that by OCT, on a single OCT B-scan, ORTs disappeared from one time point to the next, it would be necessary to ensure that the OCT B-scan images were registered precisely on consecutive visits. However, most commonly ORTs are tubular, branching structures that would be seen on more than one consecutive OCT-B scan. We could not be certain that single B-scan OCT images were always precisely registered from one visit to the next. Therefore, to consider a structure as an ORT, we required the circular/ovoid structure with hyper-reflective border to be seen on 2 (Spectralis), or 3 (Cirrus) consecutive scans. More importantly, when we evaluated change in ORT over time, we correlated the location on OCT with the Scanning laser ophthalmoscopic (SLO) fundus image (Spectralis), or OCT fundus image (Cirrus) that accompanied it, to ensure that the same location was evaluated from one time point to the next. Accordingly, we believe that it is very unlikely that ORT appearance or disappearance could be attributed solely to registration errors.
Our results apply specifically to ORT prevalence at one and two years following anti-VEGF therapy in treatment naïve patients, and cannot be extrapolated to untreated eyes, or to eyes treated for less than one year following anti-VEGF therapy initiation. It is possible, that ORTs were present during the first year, but could not be identified by the imaging methods used. To precisely determine the time to ORT onset, and whether they can be present in treatment naïve patients, it would be necessary to obtain spectral domain OCTs at earlier time points than evaluated in the current study. The increased ORT prevalence at week 104 compared to that at week 56 suggests that in treatment naive eyes ORTs may be relatively uncommon, and supports the hypothesis that ORTs develop from degenerating photoreceptors and are a late stage of treated neovascular AMD. Further studies that evaluate ORTs at the time of anti-VEGF therapy initiation, and during the first year of treatment, will be necessary to more definitively characterize the longitudinal development and changes in appearance of ORTs, and whether or not they respond to therapy”.
Supplementary Material
Acknowledgments
The authors wish to thank Ebenezer Daniels, MD, and Juan Grunwald, MD, for their review of the manuscript
Financial Support: Supported by cooperative agreements U10 EY017823, U10 EY017825, U10 EY017826, and U10 EY017828 from the National Eye Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, Maryland.
Footnotes
Meeting Presentation: This material has been presented in part at the Retina Society meeting in Beverly Hills, CA, September, 2013
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Milam AH, Jacobson SG. Photoreceptor rosettes with blue cone opsin immunoreactivity in retinitis pigmentosa. Ophthalmology. 1990;97(12):1620–31. doi: 10.1016/s0161-6420(90)32358-8. [DOI] [PubMed] [Google Scholar]
- 2.Zweifel SA, Engelbert M, Laud K, et al. Outer retinal tubulation: a novel optical coherence tomography finding. Arch Ophthalmol. 2009;127(12):1596–602. doi: 10.1001/archophthalmol.2009.326. [DOI] [PubMed] [Google Scholar]
- 3.Tulvatana W, Adamian M, Berson EL, Dryja TP. Photoreceptor rosettes in autosomal dominant retinitis pigmentosa with reduced penetrance. Arch Ophthalmol. 1999;117(3):399–402. doi: 10.1001/archopht.117.3.399. [DOI] [PubMed] [Google Scholar]
- 4.Ellabban AA, Hangai M, Yamashiro K, et al. Tomographic fundus features in pseudoxanthoma elasticum: comparison with neovascular age-related macular degeneration in Japanese patients. Eye (Lond) 2012;26(8):1086–94. doi: 10.1038/eye.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jung JJ, Freund KB. Long-term Follow-up of Outer Retinal Tubulation Documented by Eye-Tracked and En Face Spectral-Domain Optical Coherence Tomography. Arch Ophthalmol. 2012;130(12):1618–9. doi: 10.1001/archophthalmol.2012.1902. [DOI] [PubMed] [Google Scholar]
- 6.Sergouniotis PI, Davidson AE, Lenassi E, et al. Retinal structure, function, and molecular pathologic features in gyrate atrophy. Ophthalmology. 2012;119(3):596–605. doi: 10.1016/j.ophtha.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 7.DeCroos FC, Toth CA, Stinnett SS, et al. Optical coherence tomography grading reproducibility during the Comparison of Age-related Macular Degeneration Treatments Trials. Ophthalmology. 2012;119(12):2549–57. doi: 10.1016/j.ophtha.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin DF, Maguire MG, et al. Comparison of Age-related Macular Degeneration Treatments Trials Research Group, . Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin DF, Maguire MG, et al. Comparison of Age-related Macular Degeneration Treatments Trials Research Group. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388–98. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faria-Correia F, Barros-Pereira R, Queiros-Mendanha L, et al. Characterization of neovascular age-related macular degeneration patients with outer retinal tubulations. Ophthalmologica. 2013;229(3):147–51. doi: 10.1159/000346854. [DOI] [PubMed] [Google Scholar]
- 11.Ying GS, Huang J, Maguire MG, et al. Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2013;120(1):122–9. doi: 10.1016/j.ophtha.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dugel PU. Anti-PDGF, anti-VEGF combination may be game changer in wet AMD treatment. Ocular Surgery News. 2012 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.