Summary
Among the most important decisions an animal makes is whether to engage in active movement and feeding behavior or to become quiescent. The molecular signaling mechanisms underlying this decision remain largely unknown. The nematode Caenorhabditis elegans displays sleep-like quiescence following exposures that result in cellular stress [1]. The neurosecretory ALA neuron is required for this stress-induced recovery quiescence [1] but the mechanisms by which ALA induces quiescence have been unknown. We report here that quiescence induced by heat stress requires ALA depolarization and release of FMRFamide-like neuropeptides encoded by the flp-13 gene. Optogenetic activation of ALA reduces feeding and locomotion in a FLP-13-dependent manner. Over expression of flp-13 is sufficient to induce quiescent behavior during normally active periods. We have here identified a major biological role for FMRFamide-like neuropeptides in nematodes, and suggest that they may function in a similar capacity in other organisms.
Keywords: neuropeptides, FLP-13, ALA neuron, behavioral quiescence, sleep, heat shock, FMRFamide
Results and Discussion
ALA depolarization is necessary for heat-induced recovery quiescence
In C. elegans, environmental exposures such as heat that result in cellular stress cause an adaptive sleep-like behavioral response [1]. Among the 302 neurons, a single neuron called ALA is required for the quiescence response [1]. ALA, which also is a nociceptive neuron [2], has been proposed to be peptidergic based on the presence of dense core vesicles in electron micrographs [3], but the neuropeptide(s) it uses to induce behavioral quiescence have been unknown. Recovery quiescence is dependent on components of epidermal growth factor (EGF) receptor signaling [1], and elevation of EGF signaling in the ALA neuron causes behavioral quiescence [4]. However, since the typical signaling roles of EGF are not known to involve membrane potential changes, it is unclear how EGF signaling activates a peptidergic neuron.
To test the role of ALA membrane voltage, we used the Drosophila melanogaster histamine-gated chloride channel Ort as a tool to chemically silence neurons in vivo in a time-controlled fashion. Activation of Ort, as well as other histamine-gated chloride channels in both C. elegans [5] and Drosophila [6] neurons causes reduced excitability. Histamine is not known to act as a neurotransmitter in C. elegans [7], allowing its use in conjunction with Ort without interference with endogenous processes.
Since histamine-gated chloride channels have only recently been introduced as a tool for neural manipulation [5, 6] we first confirmed that Ort is effective in silencing C. elegans neurons. To do this, we expressed Ort in the well-characterized HSN neurons (Figure S1A), which are required for egg laying behavior in a manner that involves membrane potential changes [8, 9]. We transferred wild-type and Ort-expressing animals onto the surface of agar with or without 50 mM histamine. After 6 hours, we counted the eggs retained in the uterus of each adult. Transgenic animals expressing Ort in the HSN neurons and exposed to histamine retained more eggs than wild-type animals exposed to histamine as well as more eggs than transgenic sisters that were not exposed to histamine (Figure S1B). Thus, as expected, Ort activation in the HSNs inhibited egg laying.
To test if ALA membrane depolarization is required for heat-induced quiescence, we expressed Ort in ALA. Animals grown in the absence of histamine were transferred to the surface of agar that either contained or lacked histamine and were then subjected to a heat shock of 35°C for 30 minutes. We assessed quiescence following removal from the heat stress. Histamine exposure did not affect the wild-type feeding quiescence response to heat stress (Figure 1A). In contrast, animals expressing Ort in the ALA neuron and exposed to histamine showed a reduction of the feeding quiescence after heat stress (Figure 1B). Like feeding quiescence, locomotor quiescence after heat stress was also attenuated by activation of Ort in ALA (Figure 1C, 1D).
Figure 1. ALA depolarization is required for heat-induced quiescence.
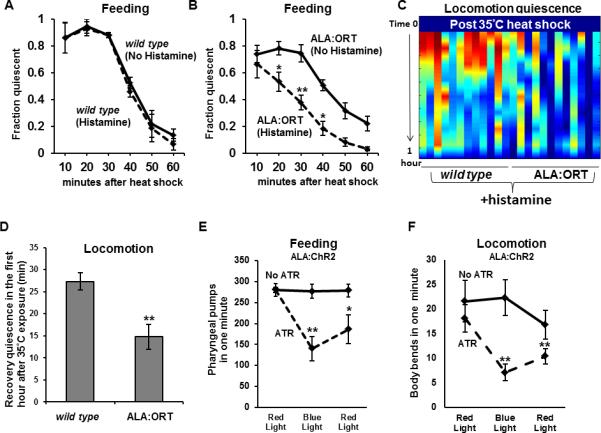
(A) After a 30-minute 35°C heat shock (Protocol 1), wild-type animals cultivated either with (dashed line) or without (solid line) histamine showed equivalent degrees of feeding quiescence (N=18-20 worms per trial, 3 trials, Student's t-test. Error bars are SEM in this and all subsequent figures). (B) After a 30-minute 35°C heat shock (Protocol 1), worms that expressed Ort in the ALA neuron cultivated in the presence of histamine (dashed line) displayed reduced feeding quiescence than their transgenic sisters that were not exposed to histamine (N=18-20 worms per trial, 8 trials, 3 extrachromosomal lines analyzed, *p<.05, **p<.005, Students t-test). (C) Heat map showing quiescence (red is most quiescent) in the hour after a 35°C heat shock (protocol 1) of 12 individual wild-type worms and 12 individual worms expressing ORT in ALA. (D) Average locomotion quiescence is reduced in animals expressing ORT in ALA in comparison to wild-type animals, all cultivated on histamine (N=12 for each genotype, **p<.005, Students t-test). (EF) Activation of ChR2 in the ALA neuron causes a reduction in feeding (E) and locomotion (F) in a manner that depends on the wavelength of light and on the ChR2 essential cofactor all trans retinal (ATR). (No ATR condition N=10, ATR condition N=12, *p<.05 **p<.005, Wilcoxon rank sum test, comparing the initial red light condition to the blue light condition, and comparing the initial to the terminal red light conditions). Note that in A-D, we are reporting quiescence measures whereas in E and F, we are reporting activity measures. See also Figure S1.
To verify that a) ALA was present and had normal process morphology after histamine exposure, and that b) neural activity was reduced following exposure to histamine, we simultaneously expressed both Ort and the genetically encoded calcium indicator GCaMP6 [10] in the ALA neuron. The morphology of the ALA neuron appeared normal both in the presence and absence of histamine. Furthermore, GCaMP6 fluorescence was reduced in the presence of histamine (Figure S1C-D) indicating reduced ALA excitation. Thus, ALA membrane depolarization is required for heat-induced recovery quiescence.
Depolarization of ALA reduces locomotion and feeding behaviors
Our Ort experiments indicate that depolarization of the ALA neuron is required to induce behavioral quiescence under conditions (heat stress) that activate ALA in an EGF-dependent fashion. We wished to determine whether ALA depolarization alone, in the absence of heat stress, is sufficient to promote behavioral quiescence. To this end, we expressed the light activated cation channel Channelrhodopsin-2 (ChR2) [11] in the ALA neuron and then used blue light to depolarize ALA. We observed a reduction of both feeding (Figure 1E) and locomotion (Figure 1F) behaviors in response to ChR2 activation in ALA. The lack of complete behavioral quiescence may be because EGF activation of ALA, in addition to depolarization, is required for the full quiescence-inducing effects of this neuron, perhaps by transcriptional induction of ALA effectors. This possibility is supported by our analysis of the effects of heat shock on flp-13 mRNA (see below).
FLP-13 is required in ALA for LIN-3/EGF-induced quiescence
The identities of the molecular signals released from ALA that trigger behavioral quiescence are unknown but have been hypothesized to be peptidergic [4].
Previously, ALA was known to express only one neuropeptide encoding gene, flp-7 [12]. A null mutation in flp-7 does not prevent the LIN-3/EGF-induction of quiescence [4], suggesting that other peptides are released from ALA to induce quiescence. To identify other ALA-expressed neuropeptides, we made use of an analysis performed in Ascaris suum, a parasitic nematode much larger than C. elegans. Ascaris has a similar neural anatomy to C. elegans, allowing in many cases the identification of the Ascaris equivalent to a C. elegans neuron. In particular, the Ascaris equivalent of the ALA neuron, located in the dorsal ganglion in the anterior end of the animal, can be identified [13]. Jarecki and colleagues identified several neuropeptides encoded by the gene flp-13 to be expressed in the Ascaris ALA [13].
In C. elegans, the FLP-13 preproprotein is processed into seven peptides (Figure 2A), each containing 9-10 amino acids with a C-terminus of the sequence PLIRF (six peptides) or PFIRF (one peptide). To test whether in C. elegans, as in Ascaris, flp-13 is expressed in ALA, we constructed fluorescent reporters that contained >5kb of regulatory sequence upstream of the flp-13 coding region. We generated transgenic animals containing this construct as well as a fluorescent transcriptional reporter for the gene ida-1, known to be expressed in ALA [14]. We observed expression of flp-13 in ALA, evident by the morphology of the neurons and by colocalization with the ida-1 reporter, which marks ALA (Figure 2B). We also observed flp-13 expression in pharyngeal neurons, as previously reported [12].
Figure 2. flp-13 is expressed in the ALA neuron and is required for ALA-induced quiescence.
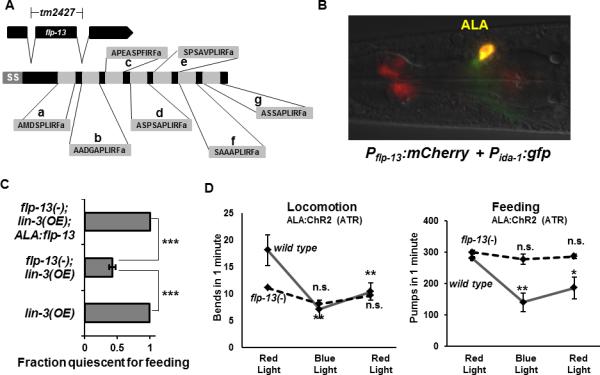
(A) The gene flp-13 encodes for a preproprotein that is processed into 7 distinct neuropeptides, FLP-13a-g, each of which is amidated at the C-terminus. The tm2427 deletion removes all of exon 2, which results in a frame shift mutation. “ss” denotes signal sequence. (B) flp-13 is expressed in the ALA neuron. mCherry is expressed under the control of the flp-13 promoter and GFP is expressed in the ALA neuron under the control of the ida-1 promoter. (C) Over-expression of LIN-3/EGF in the wild-type genetic background results in a feeding quiescence phenotype two hours after induction of the lin-3 transgene. In the flp-13(tm2427) background, fewer animals are quiescent during lin-3 over-expression (N=18-20 worms per trial, 4 trials, ***P<.0005, Student's t test). flp-13 expressed in ALA using the ida-1 promoter restores the ability of LIN-3 to induce quiescence (N=21 animals per trial, 2 trials, ***P<.0005, Student's t test). (D) flp-13(tm2427) mutation impairs the suppression of locomotion (left) and feeding (right) caused by activating ChR2 in the ALA neuron with blue light in the presence of all trans retinal. (N=10 animals per condition, *p<.05 **p<.005, Wilcoxon rank sum test, comparing initial red to blue and initial red to final red conditions). See also Figure S2.
To test the hypothesis that FLP-13 peptides are used by ALA to mediate its quiescence-promoting effects, we activated the ALA neuron in adult animals by over-expressing LIN-3/EGF in a strain containing the flp-13 deletion mutation tm2427. tm2427 eliminates the second exon of the gene, resulting in an early frame shift and thus the elimination of all seven FLP-13 peptides (Figure 2A). In contrast to the strong quiescence induced by LIN-3/EGF over-expression in a wild-type background, the LIN-3/EGF-induced quiescence was attenuated in a strain containing the flp-13(tm2427) mutation (Figure 2C). flp-13(tm2427) suppressed the behavioral but not the developmental effects of LIN-3/EGF over-expression [15], as evident by the presence of the multi-vulva phenotype in 49% of adult animals after heat shock during larval development (N=37). This result indicates that the flp-13(tm2427) mutation did not generally affect transgene expression, the heat shock transcriptional response, or EGF signaling, but rather, specifically affected the behavioral-quiescence consequence downstream of EGF signaling.
flp-13 expressed in ALA was sufficient to restore flp-13(tm2427) mutant animals the quiescence-inducing effects of LIN-3/EGF over-expression (Figure 2C). These results show that FLP-13 from ALA can function to regulate EGF-induced sleep-like behavior and suggest that peptides encoded by the flp-13 gene are a major quiescence-promoting output of ALA in response to EGF. In addition, the flp-13 mutation blocked the suppressive effects of ALA optogenetic depolarization on feeding and locomotion (Figure 2D), indicating that FLP-13 peptides are also required for ALA depolarization-induced behavioral outputs.
FLP-13 is required in ALA for normal heat-induced recovery quiescence
In addition to cellular-stress induced quiescence in the adult stage studied here and reported elsewhere [1], EGF signaling within the ALA neuron contributes to behavioral quiescence during lethargus, a larval transition stage [4]. To test if FLP-13 peptides are required for the regulation of lethargus quiescence, we compared total locomotion quiescence during L4 lethargus in flp-13(tm2427) mutant animals to that of wild-type animals. There was no change in total quiescence during L4 lethargus or in the duration of L4 lethargus (Figure S2), suggesting that FLP-13 peptides are either not involved in the regulation of lethargus quiescence or that redundancy masks their role.
To test the hypothesis that FLP-13 peptides mediate the ALA-induced quiescence in response to cellular stress, we subjected wild-type and flp-13(tm2427) mutant animals to a 30-minute heat shock treatment at temperatures ranging from 27 to 37 degrees Celsius (See Protocol 2 in Supplemental Experimental Procedures). We observed locomotion and feeding quiescence to occur during the first hour after the heat exposure and the degree of quiescence was a function of the temperature of the heat exposure (Figure 3A). The ALA neuron was required for recovery locomotion and feeding quiescence after exposure to heat, as evident by our analysis of ceh-17 mutants, which have defective ALA function [16, 17] (Figure 3A). We compared the quiescence between flp-13 mutants and wild-type control animals in the first hour after heat exposure. flp-13 mutants had reduced quiescence in both feeding and locomotion during recovery from exposures to heat stress (Figure 3A, S3A), though the defect in recovery quiescence in flp-13 mutants was less severe than that observed for ceh-17 mutants. Neither ceh-17 nor flp-13 were defective in their ability to sense and respond to heat as both mutants increased locomotion in response to acute heat exposure (Figure S3C). Restoration of flp-13 in ALA partially restored the feeding quiescence defects of flp-13 mutants following exposure to a temperature of 35°C (Figure S3B). The incomplete restoration of feeding quiescence following heat stress could be explained by the fact that the ALA:flp-13 transgene was carried as a mitotically-unstable extrachromosomal array and may have been lost from ALA in some animals. Alternatively, it is possible that the ida-1 promoter used to express flp-13 in ALA does not promote expression to the same level as the endogenous flp-13 promoter after heat shock. In support of the latter explanation, we observed up-regulation in response to heat shock of flp-13 mRNA expressed from the endogenous genomic flp-13 gene (Figure 3B).
Figure 3. flp-13 is required for heat shock-induced behavioral quiescence and is transcriptionally-induced by heat shock.
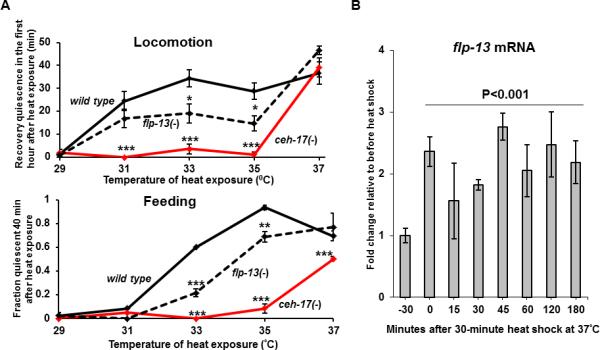
(A) ceh-17 (red line) and flp-13 (dashed line) are required for quiescence of locomotion (top) and of feeding (bottom) after exposure to heat at various temperatures (Protocol 2—preheated plates). (B) The level of flp-13 mRNA is increased in response to a heat shock of 37°C. (N=3 biological replicates, one way ANOVA). See also Figure S3.
In summary, FLP-13 peptides are required in the ALA neuron for normal recovery quiescence following heat stress and appear to be regulated both transcriptionally and post-transcriptionally.
flp-13 over-expression induces behavioral quiescence
We predicted that if FLP-13-derived neuropeptides are secreted from ALA to promote quiescence, then expression of flp-13 in a temporal and spatial ectopic fashion would induce quiescence much like LIN-3/EGF. To test this prediction, we used an inducible heat-shock promoter to drive expression of flp-13 in wild-type animals in all somatic cells during the normally active adult stage (Figure 4A). We subjected the animals to a mild 33°C heat-shock for 30 minutes and then assessed feeding and locomotion behavior for several hours after the heat exposure. Importantly, the assessment period extended far beyond the endogenous period of recovery quiescence (Movie S1) that occurs within the first hour in response to the heat stress. After the first hour, any quiescence observed is due to activation of transgene expression rather than to the acute heat stress recovery quiescence. While wild-type animals were not quiescent beyond the first hour post heat-shock (Figure 4A-B and Movie S2), animals over expressing flp-13 became quiescent, as evident by a lack of feeding and movement (Figure 4A-B and Movie S3). The behavioral quiescence was not a consequence of injury to the animals because the quiescence was reversible to strong stimulation (Movie S3) and because eight hours following inducible over-expression all animals recovered normal movement and feeding behaviors (Figure 4A-B). Animals over-expressing flp-13 (Movie S3) were more quiescent than wild-type animals observed 20-minutes after heat stress (Movie S1), suggesting that flp-13 derived peptides were indeed over-expressed relative to their levels during physiological activation of ALA. Thus, flp-13 over-expression is sufficient to promote behavioral quiescence and further supports the hypothesis that FLP-13 peptides are major quiescence-promoting outputs of the ALA neuron.
Figure 4. flp-13 over-expression is sufficient to induce behavioral quiescence.
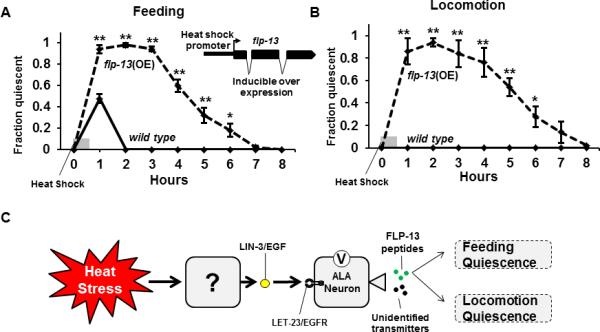
The flp-13 gene is placed under the control of the inducible heat-shock promoter of the gene hsp-16.2. (A) Wild-type animals (solid line) display feeding quiescence in response to a 30-minute 33°C heat shock but recover after an hour. Animals that over express flp-13 (dashed line) stop feeding and do not fully recover until eight hours after the start of the heat shock (average of 5 trials, N≥10 animals/trial, *p<.05, **p<.005, Student's t-test). (B) Animals that over express flp-13 (dashed line) stop moving and do not fully recover until eight hours after the start of the heat shock (average of 5 trials, N≥10 animals/trial, *p<.05, **p<.005, Student's t-test). (C) A model for the regulation of behavioral quiescence in response to heat stress. Heat exposure causes an unknown cell to release LIN-3/EGF, which signals through its receptor, LET-23, on the ALA neuron. This, at least in part, leads to a membrane depolarization and the release of FLP-13 neuropeptides as well as unidentified co-transmitters. The FLP-13 neuropeptides then promote feeding and locomotion quiescence.
Our results, together with those of Hill et al. [1] support a model (Figure 4C) in which cellular stress triggers the release of diffusible LIN-3/EGF, which activates its receptor LET-23/EGFR [18] on the ALA neuron [4]. Activation of ALA by EGF results in membrane depolarization, release of FLP-13 neuropeptides, and FLP-13 transcript up regulation. Because neither the recovery quiescence observed after acute heat stress nor the quiescence observed with EGF over-expression was fully eliminated in flp-13 mutants, there are likely to be additional, as yet undiscovered, neurotransmitters used by ALA.
flp-13 encodes for peptides in the FMRFamide family, a neuropeptide family characterized by the amino acids Arginine (R), and Phenylalanine (F) at their C-termini. While FMRFamide-like peptides are found throughout phylogeny, only in few cases have their roles in animal physiology been understood [19]. Interestingly, recent reports showed a strong sleep-promoting effect of Drosophila FMRFamide-like peptide short Neuropeptide F (sNPF) [20]. Similar to four FLP-13 peptides, four sNPF peptides have a Serine-Proline (SP) or SPS motif at or close to the N-terminus of the peptides [21]. Thus, it is possible that a quiescence-promoting function was present in an evolutionary ancestor to FLP-13 and sNPF.
Experimental Procedures
All experimental procedures are in the Supplemental Information section.
Supplementary Material
Acknowledgments
We acknowledge Colin Smith and Julia George-Raizen for contributing to the discovery that flp-13 over-expression induces quiescence. We thank Nicholas Trojanowski, Hilary Debardeleben, Tom Janssen, and Lilliane Schoofs for helpful discussions and critiques. This work was supported by NIH T32HL07713 (M.D.N., PI, Allan Pack), 1SC2GM105487 (C.V.B), R01NS084835 (C.F.-Y.), R01NS064030 (D.M.R.), the Ellison Medical Foundation (C.F.-Y.), Alfred P. Sloan Foundation (C.F.-Y.) , and a NARSAD Young Investigator Award (D.M.R.). Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). The strains flp-13(tm2427) was provided by the National BioResource Project (PI, Shohei Mitani). The plasmid pLR304 was provided by Luis Rene Garcia and the plasmid pUAST-Ort was provided by Chi-Hon Lee.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hill A, Mansfield R, Lopez JM, Raizen DM, Van Buskirk C. Cellular Stress Induces a Protective Sleep-like State in C. elegans. Current biology : CB. doi: 10.1016/j.cub.2014.08.040. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanders J, Nagy S, Fetterman G, Wright C, Treinin M, Biron D. The Caenorhabditis elegans interneuron ALA is (also) a high-threshold mechanosensor. BMC neuroscience. 2013;14:156. doi: 10.1186/1471-2202-14-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 4.Van Buskirk C, Sternberg PW. Epidermal growth factor signaling induces behavioral quiescence in Caenorhabditis elegans. Nature neuroscience. 2007;10:1300–1307. doi: 10.1038/nn1981. [DOI] [PubMed] [Google Scholar]
- 5.Pokala N, Liu Q, Gordus A, Bargmann CI. Inducible and titratable silencing of Caenorhabditis elegans neurons in vivo with histamine-gated chloride channels. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:2770–2775. doi: 10.1073/pnas.1400615111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu WW, Wilson RI. Transient and specific inactivation of Drosophila neurons in vivo using a native ligand-gated ion channel. Current biology : CB. 2013;23:1202–1208. doi: 10.1016/j.cub.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chase DL, Koelle MR. Biogenic amine neurotransmitters in C. elegans. WormBook. 2007:1–15. doi: 10.1895/wormbook.1.132.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sulston JE, Horvitz HR. Abnormal cell lineages in mutants of the nematode Caenorhabditis elegans. Developmental biology. 1981;82:41–55. doi: 10.1016/0012-1606(81)90427-9. [DOI] [PubMed] [Google Scholar]
- 9.Leifer AM, Fang-Yen C, Gershow M, Alkema MJ, Samuel AD. Optogenetic manipulation of neural activity in freely moving Caenorhabditis elegans. Nat Methods. 2011;8:147–152. doi: 10.1038/nmeth.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagel G, Brauner M, Liewald JF, Adeishvili N, Bamberg E, Gottschalk A. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol. 2005;15:2279–2284. doi: 10.1016/j.cub.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 12.Kim K, Li C. Expression and regulation of an FMRFamide-related neuropeptide gene family in Caenorhabditis elegans. J Comp Neurol. 2004;475:540–550. doi: 10.1002/cne.20189. [DOI] [PubMed] [Google Scholar]
- 13.Jarecki JL, Andersen K, Konop CJ, Knickelbine JJ, Vestling MM, Stretton AO. Mapping neuropeptide expression by mass spectrometry in single dissected identified neurons from the dorsal ganglion of the nematode Ascaris suum. ACS Chem Neurosci. 2010;1:505–519. doi: 10.1021/cn1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai T, Fukushige T, Notkins AL, Krause M. Insulinoma-Associated Protein IA-2, a Vesicle Transmembrane Protein, Genetically Interacts with UNC-31/CAPS and Affects Neurosecretion in Caenorhabditis elegans. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:3115–3124. doi: 10.1523/JNEUROSCI.0101-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katz WS, Hill RJ, Clandinin TR, Sternberg PW. Different levels of the C. elegans growth factor LIN-3 promote distinct vulval precursor fates. Cell. 1995;82:297–307. doi: 10.1016/0092-8674(95)90317-8. [DOI] [PubMed] [Google Scholar]
- 16.Van Buskirk C, Sternberg PW. Paired and LIM class homeodomain proteins coordinate differentiation of the C. elegans ALA neuron. Development. 2010;137:2065–2074. doi: 10.1242/dev.040881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pujol N, Torregrossa P, Ewbank JJ, Brunet JF. The homeodomain protein CePHOX2/CEH-17 controls antero-posterior axonal growth in C. elegans. Development. 2000;127:3361–3371. doi: 10.1242/dev.127.15.3361. [DOI] [PubMed] [Google Scholar]
- 18.Hill RJ, Sternberg PW. The gene lin-3 encodes an inductive signal for vulval development in C. elegans. Nature. 1992;358:470–476. doi: 10.1038/358470a0. [DOI] [PubMed] [Google Scholar]
- 19.Peymen K, Watteyne J, Frooninckx L, Schoofs L, Beets I. The FMRFamide-Like Peptide Family in Nematodes. Frontiers in endocrinology. 2014;5:90. doi: 10.3389/fendo.2014.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shang Y, Donelson NC, Vecsey CG, Guo F, Rosbash M, Griffith LC. Short neuropeptide F is a sleep-promoting inhibitory modulator. Neuron. 2013;80:171–183. doi: 10.1016/j.neuron.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nassel DR, Winther AM. Drosophila neuropeptides in regulation of physiology and behavior. Progress in neurobiology. 2010;92:42–104. doi: 10.1016/j.pneurobio.2010.04.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


