Abstract
Objectives
Retrospective and cross-sectional studies of seasonal variation of depressive symptoms in unipolar major depression have yielded conflicting results. We examined seasonal variation of mood symptoms in a long-term prospective cohort – the Collaborative Depression Study (CDS).
Methods
The sample included 298 CDS participants from five academic centers with a prospectively derived diagnosis of unipolar major depression who were followed for at least ten years of annual or semi-annual assessments. Generalized linear mixed models were utilized to investigate the presence of seasonal patterns. In a subset of 271 participants followed for at least 20 years, the stability of a winter depressive pattern was assessed across the first two decades of follow-up.
Results
A small increase in proportion of time depressed was found in the months surrounding the winter solstice, although the greatest symptom burden was seen in December through April with a peak in March. The relative burden of winter depressive symptoms in the first decade demonstrated no relationship to that of the second decade. The onset of new episodes was highest October through January, peaking in January.
Conclusions
There exists a small but statistically significant peak in depressive symptoms from the month of the winter solstice to the month of the spring equinox. However, the predominance of winter depressive symptoms did not appear stable over the long-term course of illness.
Keywords: Seasonal Affective Disorder, Seasonal Mood Disorder, Major Depressive Disorder, Seasonal Pattern, Seasonality, Stability, Prospective Studies, Longitudinal Studies
1. Introduction
Documentation of seasonal variation in mood states dates back to the time of Hippocrates. Evidence for this phenomenon ranges from prospective symptom tracking to retrospective interrogation with the Seasonal Patterns of Affective Disorders Questionnaire (SPAQ) to global internet search patterns – all of which have reported seasonal mood patterns in patients and in the general population [1–6]. This “seasonality” of mood seems to lie on a spectrum of severity [7], with the more extreme cases falling under the description of “seasonal affective disorder,” or SAD, as established by Rosenthal et al. in 1984 [8]. It has been estimated that in any given year, 5% of the U.S. population and up to 9.7% of the population in other countries may suffer from SAD [9, 10], while the prevalence of SAD in patients with major depression has been estimated at between 10–20% [10], suggesting greater seasonal mood fluctuation in those with unipolar major depressive disorder (MDD).
As the first criterion in Rosenthal’s proposed definition of SAD is “A history of major affective disorder, according to the RDC” (Research Diagnostic Criteria) [8], an increased incidence of SAD within those with MDD is not only to be expected, but by at least this definition must be the case since those with syndromal depression cannot meet the Rosenthal criteria. However, much of the epidemiological data on SAD to date has been generated using the SPAQ, which assays seasonality independent of a mood disorder diagnosis. High scores could thus reflect seasonal variation in specific symptoms rather than changes in point prevalence of SAD, and the SPAQ may therefore overestimate the prevalence of MDD, seasonal pattern (MDD-SP) [1, 11, 12]. In fact, one study found that neither the Global Seasonality Score (GSS) nor the report of season change as a problem on the SPAQ predicted longitudinal mood ratings [13]. Other key limitations of the SPAQ include its retrospective and seasonality-specific nature, which subjects it to recall and measurement biases, respectively. Indeed, the SPAQ has been shown to exaggerate seasonal mood differences as compared to prospective assessments in certain populations [1].
To address these limitations in studying the seasonality of major depressive episodes, some have administered non-seasonality-specific mood assessments to depressed patient populations in a cross-sectional manner throughout the different seasons. The evidence of seasonality from these investigations – even within single studies – has been inconclusive. For example, data from the U.S. National Comorbidity Survey showed that 10–20% of people with MDD had symptoms that recurred at consistent times each year [11], but only 0.4% met strict Diagnostic and Statistical Manual (DSM) criteria for MDD-SP. Similarly, a study of 2,225 general practice patients in London showed that while those with RDC major depression had significant peaks for episode onset in the winter and recovery in the summer [14], corresponding winter and summer changes in General Health Questionnaire scores did not cross the threshold of statistical significance.
Retrospective chart review was utilized to construct course of illness by Faedda et al., who applied DSM-III-R MDD-SP criteria to clinical records of 557 outpatients with recurrent depression. Over an average of 12 years of documented illness course, 75 (13.5%) with recurrent depression had a seasonal pattern [15], with high intra-individual stability in timing of depressive episode onset and remission. Based upon the large sample, longitudinal nature and substantial “follow-up” period, this study offers the most compelling current evidence of seasonal patterns in unipolar depression.
This finding was not replicated, however, in a similar setting. Posternak et al. retrospectively examined presentation patterns of 1,500 consecutive patients at a Rhode Island outpatient psychiatric clinic and reported no significant seasonal changes in the rate of depressive symptoms or proportion of patients diagnosed with MDD [16]. Similarly, a large cross-sectional study in the Netherlands found no significant effect of season of administration on overall scores on the Inventory of Depressive Symptoms (IDS), though atypical and melancholic IDS scores were heightened during the winter [17]. Additionally, a study by Hardin et al. reported no significant difference between depressed patients and controls on SPAQ global seasonality scores [18].
The existing literature on seasonality in MDD is problematic not only due to these discordant results, but in that much of the research is cross-sectional or based on patterns of patient presentation or admission to health care facilities, and not on systematic, prospective follow-up. Those studies which have examined the same patients over a number of years are either retrospective or focused on individuals already diagnosed with SAD or recurrent depression-seasonal pattern to study illness course and diagnostic stability [19–22]. Prospective studies are further limited by inadequate duration, small sample sizes and confounding effects of treatment. For example, Sakamoto et al., which offers the longest prospective look at SAD patients with a mean follow-up of 6 years, did not systematically control for treatment and included only 25 patients, analyzing those with bipolar disorder and unipolar major depression together [19].
In sum, the degree to which patients with unipolar major depression experience varying symptom severity coincidental with the changing seasons has not been adequately examined in a prospective manner over an extended period using a standardized, non-seasonality-specific assessment. We sought to assess the monthly burden of clinically significant depressive symptoms over long-term follow-up in a clinical sample with unipolar MDD. Although some studies report spring and/or fall peaks in depression onset [15, 23], most of the literature on MDD-SP and SAD suggests that depressive symptoms are worst in the winter (variably defined in different studies as spanning November or December through January or February), at least in certain subgroups [1–3, 14, 24–28]; this encompasses a wide range of data, including hospital admission rates, SPAQ responses and standardized mood assessments. Thus, we hypothesized that those with MDD would have a peak in depressive symptomatology in the months surrounding the winter solstice (e.g. November, December, January), which would be consistent with our findings in bipolar disorder [29]. Lastly, we attempted to identify whether this pattern would persist over 20 years of follow-up.
2. Methods
2.1. Participants
The CDS included individuals with mood disorders from the following academic centers: Harvard University (Boston), Rush Presbyterian-St. Luke’s Medical Center (Chicago), University of Iowa (Iowa City), New York State Psychiatric Institute and Columbia University (New York City), and Washington University School of Medicine (St. Louis). Participants were European-American (genetic hypotheses were tested), spoke English, had an IQ score of at least 70, and no evidence of terminal medical illness at intake or a mood disorder due to a primary medical condition. All centers are in temperate regions of the contiguous United States (38.75 – 42.37 degrees latitude), although participants did not necessarily reside in these regions throughout follow-up. The institutional review boards of all sites approved the study and all participants provided written informed consent.
All participants included in this study underwent an initial assessment with the use of the Schedule of Affective Disorders and Schizophrenia (SADS) scale to determine if they met Research Diagnostic Criteria (RDC) for MDD, schizoaffective disorder, or manic disorder [30, 31]. Treatment was neither required nor administered by study staff in this observational study.
We included individuals with a prospectively derived diagnosis of unipolar MDD as described previously [32, 33]. These individuals had major depression at intake and did not develop mania or hypomania over follow-up [34, 35]. The CDS used RDC criteria (the progenitor of DSM III) in which MDD is similar to DSM-5 MDD [36]. One difference in the criteria is that RDC schizoaffective disorder, mainly affective, depressed is consistent with a DSM 5-defined MDD. We restricted the sample to include only those participants with at least ten years of follow-up to facilitate a 10-year prospective analysis and to maximize our ability to identify seasonal patterns over long-term follow-up. These inclusion and exclusion criteria limited our sample to 298 individuals from an original sample of 472 with unipolar MDD.
2.2. Follow-up Course
Individuals were interviewed every six months for the first five years of follow-up and then annually by trained raters who used the Longitudinal Interval Follow-up Evaluation (LIFE), a system for assessing longitudinal course including an instruction booklet, coding sheet, and training materials to guide the interview [37]. Semi-structured interviews were the primary source of information used for the LIFE, and raters assessed weekly symptom severity on ordinal scales. Patient interviews used chronological memory prompts (e.g., holidays) to determine changes in mood symptoms. Medical records along with data obtained from interviews were quantified using the LIFE Psychiatric Status Rating scales (Table 1 & Table 2), which directly correlate to the diagnostic thresholds of the RDC [38]. Major depression was based on LIFE ratings for major depression or schizoaffective disorder, depressive type (see Table 1). Clinically significant symptoms could also be registered on the scales for intermittent depressive disorder, minor depressive disorder and hypomania (Table 2).
Table 1.
Legend: This table represents the scale raters used during follow-up to quantify symptom severity for major depression and mania. Clinically significant symptomatology was based on a rating of 3 or higher.
LIFE Psychiatric Status Scale for Episodic Affective Disorders
| Code | Term | Definition |
|---|---|---|
| 6 | Definite criteria severe |
Meets RDC criteria for definite and either prominent psychotic symptoms or extreme impairment in functioning |
| 5 | Definite criteria | Meets RDC criteria for definite but no prominent psychotic symptoms and no extreme impairment in functioning |
| 4 | Marked | Does not meet definite RDC criteria but has major symptoms or impairment from this disorder |
| 3 | Partial remission | No more than moderate impairment in functioning, but still has obvious evidence of the disorder. |
| 2 | Residual | Either patient claims not to be completely back to “usual self” or rater notes the presence of one or more symptoms of this disorder in no more than a mild degree |
| 1 | Usual self | Patient returns to “usual self” without any residual symptoms of this disorder. |
Table 2.
This table represents the scale raters used to quantify symptom severity for minor depression, intermittent depression, or hypomania. Clinically significant symptomatology was based on a rating of 3 on these scales.
LIFE Psychiatric Status Scale for all other conditions (Chronic minor depression, intermittent depression and hypomania)
| Code | Term | Definition |
|---|---|---|
| 3 | Definite Criteria Severe |
Meets definite RDC criteria for this disorder |
| 2 | Probable Criteria Mild | Previously met RDC criteria for chronic minor/intermittent depression, minor depression, intermittent depressive features, or hypomania and now has some minor manifestations of one of these disorders. |
| 1 | Not Present | Previously met RDC criteria for the disorder but currently there is no evidence of this disorder. |
2.3. Data Analysis
We examined the seasonality of symptom burden in MDD, based on previously defined thresholds of 3 or greater on the major depression LIFE scale (Table 1) or 3/3 on the minor depression LIFE scale (Table 2) [29, 32–35, 39–41]. The ordinal LIFE scales were collapsed into an indicator (dichotomous) variable with a value of one or zero assigned for each day a patient did or did not meet, respectively, at least one of the above thresholds for clinically significant symptomology. The proportion of time depressed by month was calculated as the simple average of the daily indicator variable value.
SAS 9.3 (SAS Institute, Cary, NC) was used for statistical analysis and graphs made in SigmaPlot 12.0 (Systat Software, San Jose, CA). In order to ascertain whether a significant seasonal pattern was present in the aggregate symptom burden data, we ran separate generalized linear mixed models with season indicators for November-January and December-April (though our a priori hypothesis was that symptom burden would peak in November-January, the December-April indicator was selected after reviewing descriptive data summaries). To define the outcome, major depression LIFE scores were collapsed from six into three categories – well to minimal (1–2), mild to moderate (3–4) and severe (5–6) illness – to facilitate stability and convergence of the fitted models. The models were based on a multinomial distribution with a cumulative logit link, and included the covariates age, gender and treatment. The use of any antidepressant treatment in a given month was identified with an indicator variable as in previous studies [29, 32–35, 39–41].
The model is configured so that positive coefficient estimates occur when increases in an explanatory variable lead to increases in the log odds that the patient will fall into a more favorable LIFE category, and negative coefficient estimates occur when increases in an explanatory variable lead to decreases in the log odds that the patient will fall into a more favorable LIFE category. In particular, the effect estimates corresponding to the season indicators represent the difference between the log odds within the time periods of interest and the log odds outside these periods. Therefore, negative effect estimates would provide evidence consistent with our hypothesis.
In addition to the covariates, random effects were included for both the subject and the subject / season indicator interaction. The former accounts for between-subject heterogeneity in categorized LIFE score; the latter were included to account for potential deviations between a subject’s typical categorized LIFE score and the population baseline during the periods of interest.
In order to judge stability of the seasonal pattern over time, a seasonality index was created by dividing the weighted average monthly symptom burden in December-April (the post hoc observed peak months) by the weighted average monthly symptom burden for May-November. The Spearman’s rank correlation between seasonal indices for study years 1–10 and 11–20 in the 271 patients with at least 20 years of follow-up data was assessed. To assess potential confounding of treatment, this analysis was repeated on a sub-group with 147 patients who had periods of at least two years without treatment in each of the first two decades of follow-up.
Akin to a prior analysis of participants with bipolar disorder from this sample [29], the timing of relapse over the entire duration of follow-up was additionally explored with the unit of analysis being mood episodes rather than the 298 individual participants constituting the sample. The calendar month of onset of any new depressive episodes (major or minor) was determined following any periods of recovery, defined as eight consecutive weeks with no or only residual symptoms.
3. Results
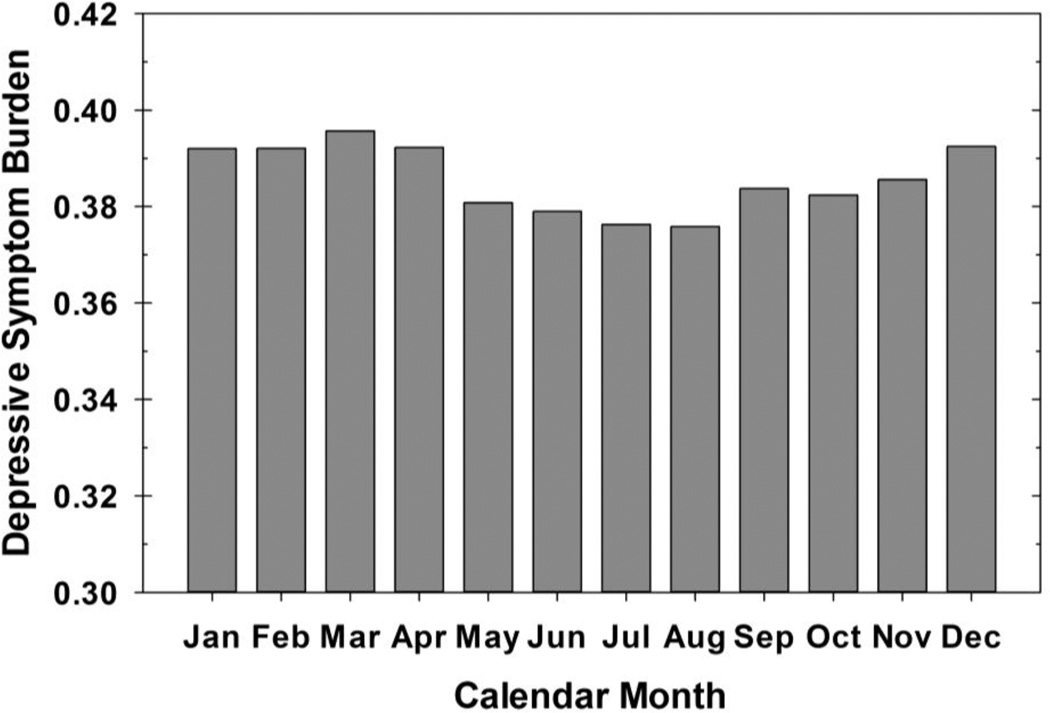
Our sample included 298 participants with unipolar major depression and was predominantly female (63%) as shown in Table 3. When contrasted to the 174 participants with a prospective diagnosis of unipolar major depression who did not complete 10 years of follow-up, our sample was more likely to be female (χ2=6.2, df=1, p=0.01) and less likely to have a diagnosis of alcoholism at intake (χ2=8.0, df=1, p<0.01). They also tended to have a younger age of onset (26.7 vs. 32.1 years, Wilcoxon Z=3.4, p<0.001) and a lesser persistence of depressive symptoms. They did not differ in married status, college graduation, inpatient status on intake, anxiety disorder co-morbidity, or drug use. Figure 1 shows the mean proportion of weeks with clinically significant depressive symptoms in each calendar month over the 10-year follow-up period. A small increase in depressive symptom burden seemed to occur in the months surrounding the winter solstice (e.g., Dec-Feb) as expected, although peak symptomatology was noted in March. Lower symptom burden was noted in the months surrounding the summer solstice (e.g., May-July).
Table 3.
Sociodemographic and Clinical Characteristics for Sample at Study Entry
| Characteristics | Entire Sample (n=298) |
|
|---|---|---|
| Sex | n(%) | |
| Male | 109 (37%) | |
| Female | 189 (63%) | |
| Marital Status | ||
| Married/Partnered | n(%) | 146 (49%) |
| Divorced/Separated | 47 (16%) | |
| Single | 95 (32%) | |
| Widowed | 10 (3%) | |
| Education | ||
| Without diploma | n(%) | 58 (19%) |
| High school graduate | 92 (31%) | |
| Some college | 82 (28%) | |
| College graduate | 66 (22%) | |
| Age at study intake | Mean (S.D.) | 37.8 (14.1) |
| Median | 34 (47–27) | |
| (IQR) | ||
| Age at onset of 1st lifetime affective episode | Mean (S.D.) | 26.7 (12.7) |
| Median | 24 (33–18) | |
| (IQR) | ||
| Number of depressive episodes prior to intake | n(%) | |
| None | 102 (34%) | |
| One | 75 (25%) | |
| Two | 44 (15%) | |
| Three or More | 77 (26%) | |
| Inpatient status at intake | 226 (76%) | |
| Comorbid conditions at intake (Research Diagnostic Criteria)† |
n(%) | |
| Generalized anxiety disorder | 20 (7%) | |
| Panic disorder | 16 (5%) | |
| Phobic disorder | 26 (9%) | |
| Obsessive compulsive disorder | 4 (1%) | |
| Alcohol use disorder | 67 (22%) | |
| Drug use disorder | 17 (6%) |
Ever met criteria for disorder in lifetime.
Figure 1.
This figure depicts the mean proportion of days per calendar month each subject spent with clinically significant depressive symptoms as operationally defined by a score of ≥3/6 on the major depression scale or 3/3 on the minor depression LIFE scale.
Estimates of fixed effects from the generalized linear mixed models are given in Table 4. The fixed effect of our a priori seasonal indicator (Nov-Jan) did not cross the threshold of statistical significance (p=0.096), while the indicator derived from our post hoc analysis (Dec-Apr) did display a significant though small effect (p=0.011), which is best illustrated by the varying symptomatology across months graphically displayed in Figure 1. Additionally, the effect of age on depressive symptom severity was highly significant (p<0.0001), as higher LIFE scores were associated with younger age. The random effect estimates for the subject / season indicator interaction exhibited negligible variability, and thereby did not facilitate delineation of a seasonal subgroup.
Table 4.
This table gives estimates of fixed effects for the a priori (Nov-Jan) and post hoc (Dec-Apr) seasonal indicators and pertinent covariates derived from generalized linear mixed models.
| TABLE 3: Generalized Linear Mixed Models - Solutions for Fixed Effects | |||
|---|---|---|---|
| Variable | Effect size | 95% C.I. | p-value |
| Model 1: Seasonal Indicator Nov-Jan | |||
| Season | −0.048 | −0.105 to 0.0086 | 0.096 |
| Female gender | −0.259 | −0.755 to 0.238 | 0.31 |
| Age | 0.052 | 0.044 to 0.0592 | <.0001† |
| Treatment Use | −0.945 | −1.015 to −0.875 | <.0001† |
| Model 2: Seasonal Indicator Dec-Apr | |||
| Season | −0.068 | −0.121 to −0.016 | 0.011† |
| Female gender | −0.259 | −0.755 to 0.237 | 0.31 |
| Age | 0.051 | 0.043 to 0.0585 | <.0001† |
| Treatment Use | −0.945 | −1.016 to −0.875 | <.0001† |
Significant
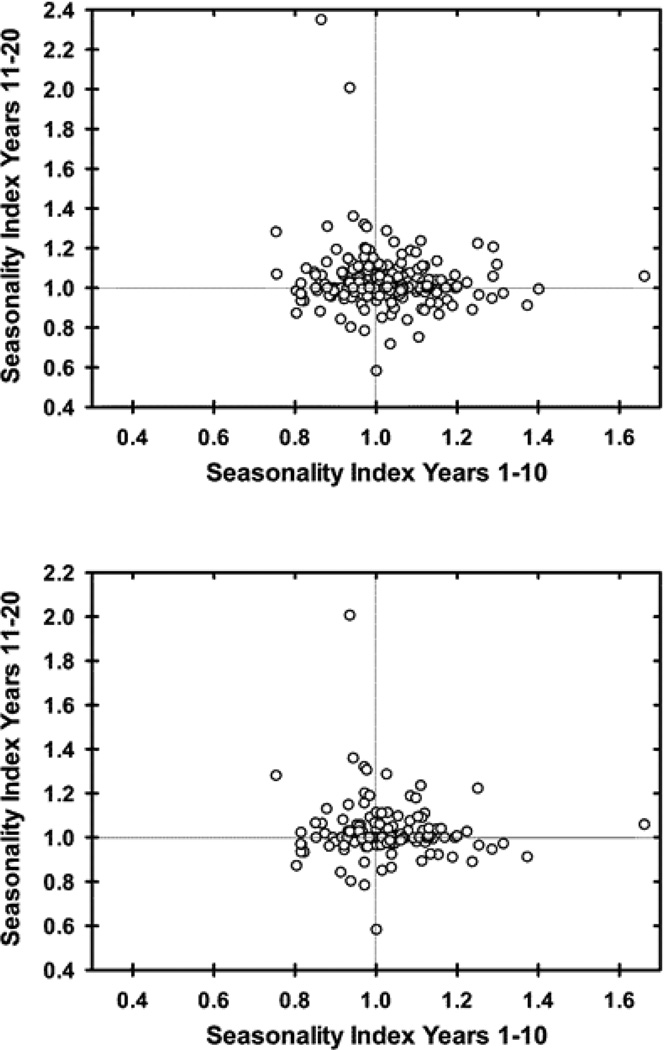
For the 271 participants with at least two decades of follow-up, the correlation between seasonal indices (December-April relative to remainder of the year) for years 1–10 and 11–20 was −0.03 (Spearman’s rho, p=0.59), essentially no relationship between seasonal indices in the two decades. Figure 2 shows the relationship between seasonal indices for each patient. Analysis of the less treated sub-group, who received treatment a mean (median;SD) of only 12.0 (7.7; 13.6)% of the two decades of follow-up, resulted in a similar rho of −0.01 (p=0.90). Results did not substantively differ when using November-January as the reference season for the seasonal indices.
Figure 2.
Scatter plots of seasonal indices for the first and second decades of follow-up for each participant with at least 20 years of follow-up (N=271). The seasonal index represents the ratio of the proportion of weeks with clinically significant symptoms during the observed peak (December-April) relative to the remainder of the year. A seasonal index of 1 would suggest an equal proportion of time spent with clinically significant depressive symptoms during the winter months relative to the rest of the year. The top panel includes the entire sample (rho= −0.03, p=0.59) and the lower panel includes the mostly untreated sample (N=147, rho= −0.01, p=0.90).
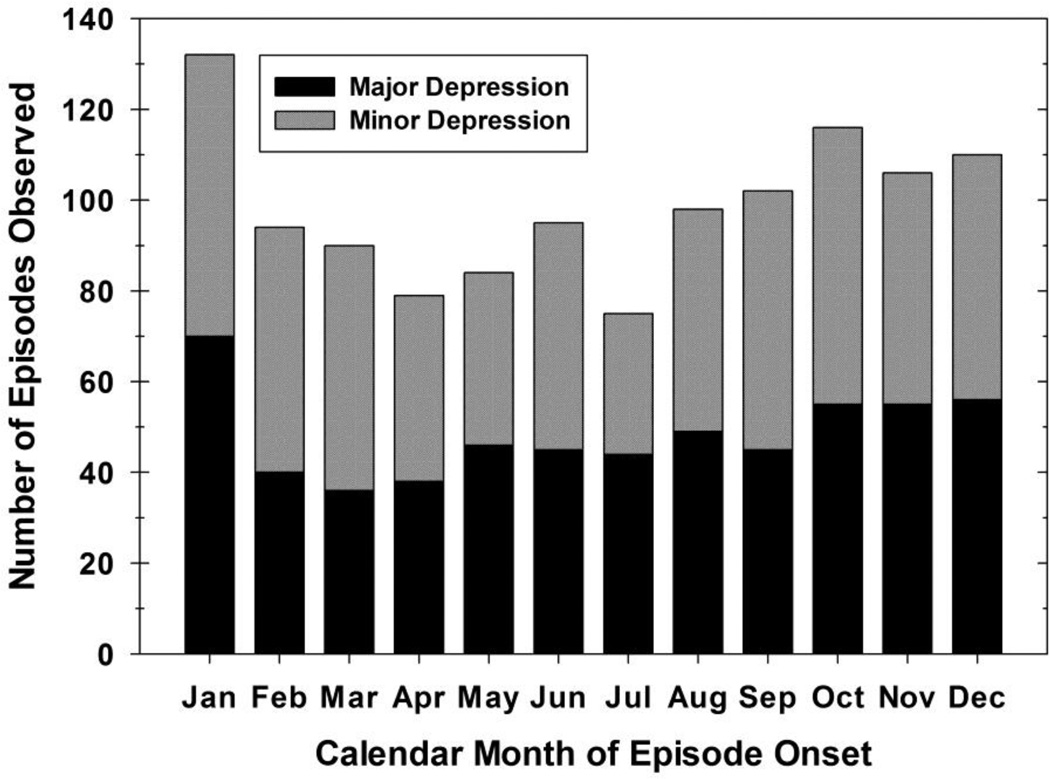
Over a mean (SD) of 22.7 (6.4) and up to 31 years of follow-up, these 298 participants had an onset of 1,181 depressive episodes following an 8-week remission. The timing of these episodes is illustrated in Figure 3. Relapses into depression (major or minor) were most common in October (9.8% of all episodes), November (9.0%), December (9.3%), and January (11.2%) and least common in July (6.4%). The 579 major depressive episodes were similarly more likely to begin in January (12.1%), but least likely to occur in March (6.2%).
Figure 3.
Timing of relapse into minor or major depressive episodes in persons with unipolar major depression. There were a total of 602 new episodes of minor depression and 579 new episodes of major depression in the sample. Depressive episodes were most likely to begin in January (11.2%) and least likely to begin in July (6.4%).
4. Discussion
In the present study we found that, on average, participants with unipolar major depression spent a greater proportion of time depressed in the months surrounding the winter solstice, though this difference only reached significance when using the post hoc December-April seasonal indicator. Statistics for the December-April indicator are subsequently best framed as hypothesis-generating and the observed peak was considered most appropriate for the assessment of the long-term stability of seasonality in those with unipolar MDD. This observed peak is partially consistent with several cross-sectional and retrospective studies showing winter – most often defined as Dec-Feb – peaks in depressive symptoms, episode onsets and/or hospital admissions [1–3, 14, 24–28]. Our analysis of new episode onset suggested an October to January peak (highest in January), preceding the peak for symptom burden.
Not all studies of depressed populations have demonstrated such seasonal patterns. Possible explanations for these inconsistencies include use of different assessment tools or criteria for establishing diagnoses, differences in latitude or cultural values (e.g., individualism and power distance, or how accepting lower social classes are of unequal power distribution [42]) among nations in which the studies were done, and divergent patient demographics. For example, the lower prevalence of MDD-SP found by Blazer et al. [11] as compared to Levitt et al. [12] can be largely attributed to a higher ratio of seasonal: non-seasonal depressive episodes required for inclusion in the “seasonal pattern” group. Additionally, studies of hospital admissions records have shown a winter peak for depression that is only significant in females and Asians [26].
The relatively small effect size observed in our sample is most likely due to diminution of substantial season fluctuations in a subset of participants when averaged over the group as a whole. Previous research has provided prevalence estimates of 0.4–13.5% for DSM seasonal pattern among depressed patients [11, 12, 24], most often with winter worsening. Thus, most with MDD do not display clear or the same seasonality, and even within the seasonal group there may be a sizeable subset of participants with summer symptom peaks [15], distinguished not only by their worst season, but by symptom profile, including more endogenous vegetative symptoms, decreased appetite and insomnia [43]. Another potential explanation for the small effect size is that some more chronically depressed participants experience seasonal worsening of mood and continue to have subsyndromal symptoms across other seasons. Some cases of SAD, then, may result from individuals with a higher baseline mood and somatic symptomology that is close enough to a disordered state and/or slightly more labile so that these minor seasonal shifts – particularly winter worsening of mood – can push them across the diagnostic threshold.
The observed March peak in proportion of time depressed is unexpected, but not without potential explanation. Inter-individual variability in exact timing of depressive episode onset undoubtedly exists even among subjects with predominantly winter depression. Thus, as the median depressive episode duration among CDS participants was found to be 23 weeks [44], the March peak may simply reflect the maximum coincident illness burden for participants with winter episode onset before remission outweighs onset in April. This is supported by the timing for onset of new episodes peaking being greatest from October until ultimately peaking in January. Sensitivity to weather changes common to springtime has been linked to higher seasonality scores on the SPAQ [2, 3], and Postolache et al. found a direct correlation between depressive symptom ratings and severity of allergic symptoms in patients with bipolar and recurrent depression [45]. Thus, the March peak could also be due, in part, to environmental sensitivity in the sub-group of participants with the most significant seasonality.
We could not reliably identify any distinct subset of participants with a seasonal pattern. One way to substantiate a diagnosis is to confirm its stability over time. Interestingly, in the present study there was no correlation between seasonal indices calculated for the first and second decades of follow-up, and this persisted after examining only the least-treated individuals. Additionally, within-subject morbidity has been shown to be consistent over time in this sample [46], so the lack of correlation cannot be ascribed to a general change in symptom burden. These results seem to suggest that seasonal worsening of depressive symptom burden is not a stable phenomenon over follow-up periods of greater than a decade. Some evidence for limited stability exists in the prior literature. Previous studies on both SAD (Rosenthal criteria) and MDD-SP (DSM criteria) have found that 26–38% of patients maintain a stable seasonal pattern of recurrence over mean follow-ups of up to 10 years [19–22]. In a mixed retrospective chart review and prospective cohort of 41 clinic patients with SAD, Sakamato et al. found that 9/28 with a fall-winter seasonal pattern maintained this over a mean of 8 years while 11 patients with non-seasonal mood disorders developed a fall-winter pattern [19]. Two studies that did single follow-up interviews had strikingly similar results. Thompson et al. found that after 5–8 years, 38% of the 93 participants assessed continued to meet criteria for MDD-SP [21]. Schwartz et al. found that after a mean of 8.8 years, 42% of their 59 participants with SAD remained seasonal [22]. Leonardt et al. employed weekly ratings of 26 patients with SAD over 2.5–8.25 years and 9 participants (35%) maintained a seasonal pattern [20]. Our study assayed stability prospectively in all participants with 20 years of follow-up rather than individually tracking those with the highest seasonality index values over time. Further, we prospectively tracked only those participants with unipolar MDD, while many previous studies on diagnostic stability are based on retrospective interviews and include those with bipolar disorder without controlling for treatment. From Figure 2 it is apparent that a sub-set of participants had index values greater than 1 in both decades – indicating a persistent seasonal worsening of depressive symptoms – although as evident by no greater representation in this quadrant, this appears a chance finding. We attempted to identify and track this high-seasonality subgroup both through modeling the effects of seasonal indicators in each individual and with our seasonal index, but the models did not converge (due to great complexity/number of parameters) and the validity and meaning of an arbitrarily-chosen index threshold were unclear at best.
Interpretation of our results must acknowledge key limitations of the study. First, although the overall study design was prospective, mood ratings were obtained twice a year during the first five years then once a year thereafter. Thus, the weekly symptom ratings obtained through these surveys may not accurately reflect the exact temporal course of each participant’s symptomatology. Second, we did not keep records of participant residence throughout the course of the study, so effects of latitude on mood symptoms could not be ascertained. Third, participants received a variety of somatic therapies during follow-up, which could influence individual symptomatology. Although an indicator for treatment for each month was included in statistical models, the potential for residual confounding persists. Additionally, treatment data only included psychiatric medications and psychotherapy, which precluded us from analyzing effects of light therapy or examining the potential link between severity of depressive symptoms and allergies (e.g., allergy visits, antihistamine use). It is also important to recognize that this was not exclusively a sample of individuals with SAD and the seasonal patterns observed apply to this clinical sample recruited for the presence of a mood disorder, which may not represent of the general population with major depression. One comparison found those with non-seasonal depression had earlier hospitalization than those with seasonal depression [47], but another found no differences in likelihood of hospitalization [48]. If those with seasonal depression are less likely to be admitted during the course of their depression, our sample, which initially recruited many as inpatients, may have underrepresented those with seasonal patterns. Finally, stability of a seasonal pattern was based on a dimensional rather than categorical formulation of seasonality, which makes implicit assumptions about the ideal conceptualization of this construct, although as previously mentioned we could find no point of rarity to support a categorical cutoff.
To our knowledge, this is the first study to use data from prospectively followed individuals with unipolar major depression over 10 or more years using a standardized assessment with the express intent of characterizing seasonal variations in depressive symptoms. Participants were not recruited with the intent to study seasonality and were consented prior to Rosenthal’s seminal paper on the topic [8], thereby minimizing differential participation based on the degree of any seasonality, and much of the data were collected before SAD was a prominent mental health topic, minimizing any recall bias. This is important because having heard of SAD has been shown to be associated with higher reported seasonality [2].
In sum, we examined a prospective cohort of 298 individuals with MDD over 10 years of follow-up and found a significant peak in proportion of time depressed in the months between the winter solstice and spring equinox (i.e., December-April). We also analyzed an additional 10 years of data in 271 participants and observed a near-zero correlation between the above-mentioned seasonal mood shifts in the first and second decades of follow-up. Several studies – including the present – have reported seasonal changes in symptomatology in depressed patient populations, and despite the lack of stability demonstrated herein, existing research shows that some patients do exhibit persistent seasonal patterns of depressive symptom burden and/or episode onset and remission. Important goals for subsequent studies include uncovering genetic underpinnings and clinically measurable physiological correlates of seasonality which would illuminate pathophysiological mechanisms and potentially allow for identification of a more homogenous subgroup.
Highlights.
Clinically significant depressive symptoms are more likely to occur between the months of December and April in persons with unipolar major depression.
Depressive symptoms appear less frequently between May and July for persons with unipolar major depression.
The pattern of greater depressive symptomatology in Winter and the following months does not appear to be stable across decades within individuals.
Acknowledgements
This study was funded by NIMH grants 5R01MH025416–33 (W Coryell), 5R01MH023864–35 (J Endicott), 5R01MH025478–33 (M Keller), 5R01MH025430–33 (J Rice), and 5R01MH029957–30 (WA Scheftner). Dr. Fiedorowicz is supported by the National Institutes of Health (1K23MH083695–01A210). Dr. Solomon serves as Deputy Editor to UpToDate.com. Dr. Keller has served as a consultant or received honoraria for CENEREX, Medtronic, and Sierra Neuropharmaceuticals. He has received grant/research support from Pfizer. He has also served on an advisory board for CENEREX.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All other authors have no potential conflicts of interest to report.
Conducted with current participation of the following investigators: M.B. Keller, M.D. (Chairperson, Providence), W. Coryell (Co-Chairperson, Iowa City); D.A. Solomon, M.D. (Providence); W.A. Scheftner, M.D. (Chicago); W. Coryell, M.D. (Iowa City); J. Endicott, Ph.D., A.C. Leon, Ph.D.,* J. Loth, M.S.W. (New York); J. Rice, Ph.D., (St. Louis). Other current contributors include: H.S. Akiskal, M.D., J. Fawcett, M.D., L.L. Judd, M.D., P.W. Lavori, Ph.D., J.D. Maser, Ph.D., T.I. Mueller, M.D. The data for this manuscript came from the National Institute of Mental Health (NIMH) Collaborative Program on the Psychobiology of Depression-Clinical Studies (Katz and Klerman, 1979). The Collaborative Program was initiated in 1975 to investigate nosologic, genetic, family, prognostic and psychosocial issues of Mood Disorders, and is an ongoing, long-term multidisciplinary investigation of the course of Mood and related affective disorders. The original Principal and Co-principal investigators were from five academic centers and included Gerald Klerman, M.D.* (Co-Chairperson), Martin Keller, M.D., Robert Shapiro, M.D.* (Massachusetts General Hospital, Harvard Medical School), Eli Robins, M.D.,* Paula Clayton, M.D., Theodore Reich, M.D.,* Amos Wellner, M.D.* (Washington University Medical School), Jean Endicott, Ph.D., Robert Spitzer, M.D. (Columbia University), Nancy Andreasen, M.D., Ph.D., William Coryell, M.D., George Winokur, M.D.* (University of Iowa), Jan Fawcett, M.D., William Scheftner, M.D. (Rush-Presbyterian-St. Luke’s Medical Center). The NIMH Clinical Research Branch was an active collaborator in the origin and development of the Collaborative Program with Martin M. Katz, Ph.D., Branch Chief as the Co-Chairperson and Robert Hirschfeld, M.D. as the Program Coordinator. Other past contributors include: J. Croughan, M.D., M.T. Shea, Ph.D., R. Gibbons, Ph.D., M.A. Young, Ph.D., D.C. Clark, Ph.D.
*deceased
References
- 1.Nayyar K, Cochrane R. Seasonal changes in affective state measured prospectively and retrospectively. Br J Psychiatry. 1996;168(5):627–632. doi: 10.1192/bjp.168.5.627. [DOI] [PubMed] [Google Scholar]
- 2.Kasper S, et al. Epidemiological findings of seasonal changes in mood and behavior. A telephone survey of Montgomery County Maryland. Arch Gen Psychiatry. 1989;46(9):823–833. doi: 10.1001/archpsyc.1989.01810090065010. [DOI] [PubMed] [Google Scholar]
- 3.Mersch PP, et al. The prevalence of seasonal affective disorder in The Netherlands: a prospective and retrospective study of seasonal mood variation in the general population. Biol Psychiatry. 1999;45(8):1013–1022. doi: 10.1016/s0006-3223(98)00220-0. [DOI] [PubMed] [Google Scholar]
- 4.Harmatz MG, et al. Seasonal variation of depression and other moods: a longitudinal approach. J Biol Rhythms. 2000;15(4):344–350. doi: 10.1177/074873000129001350. [DOI] [PubMed] [Google Scholar]
- 5.Murray G, Allen NB, Trinder J. A longitudinal investigation of seasonal variation in mood. Chronobiol Int. 2001;18(5):875–891. doi: 10.1081/cbi-100107522. [DOI] [PubMed] [Google Scholar]
- 6.Yang AC, et al. Do seasons have an influence on the incidence of depression? The use of an internet search engine query data as a proxy of human affect. PLoS One. 2010;5(10):13728. doi: 10.1371/journal.pone.0013728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wehr TA, Rosenthal NE. Seasonality and affective illness. Am J Psychiatry. 1989;146(7):829–839. doi: 10.1176/ajp.146.7.829. [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal NE, et al. Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry. 1984;41(1):72–80. doi: 10.1001/archpsyc.1984.01790120076010. [DOI] [PubMed] [Google Scholar]
- 9.Kurlansik SL, Ibay AD. Seasonal affective disorder. Am Fam Physician. 2012;86(11):1037–1041. [PubMed] [Google Scholar]
- 10.Magnusson A. An overview of epidemiological studies on seasonal affective disorder. Acta Psychiatr Scand. 2000;101(3):176–184. [PubMed] [Google Scholar]
- 11.Blazer DG, Kessler RC, Swartz MS. Epidemiology of recurrent major and minor depression with a seasonal pattern. The National Comorbidity Survey. Br J Psychiatry. 1998;172:164–167. doi: 10.1192/bjp.172.2.164. [DOI] [PubMed] [Google Scholar]
- 12.Levitt AJ, et al. Estimated prevalence of the seasonal subtype of major depression in a Canadian community sample. Can J Psychiatry. 2000;45(7):650–654. doi: 10.1177/070674370004500708. [DOI] [PubMed] [Google Scholar]
- 13.Murray G. The Seasonal Pattern Assessment Questionnaire as a measure of mood seasonality: a prospective validation study. Psychiatry Res. 2003;120(1):53–59. doi: 10.1016/s0165-1781(03)00147-1. [DOI] [PubMed] [Google Scholar]
- 14.Blacker CV, Thomas JM, Thompson C. Seasonality prevalence and incidence of depressive disorder in a general practice sample: identifying differences in timing by caseness. J Affect Disord. 1997;43(1):41–52. doi: 10.1016/s0165-0327(96)00102-4. [DOI] [PubMed] [Google Scholar]
- 15.Faedda GL, et al. Seasonal mood disorders. Patterns of seasonal recurrence in mania and depression. Arch Gen Psychiatry. 1993;50(1):17–23. doi: 10.1001/archpsyc.1993.01820130019004. [DOI] [PubMed] [Google Scholar]
- 16.Posternak MA, Zimmerman M. Lack of association between seasonality and psychopathology in psychiatric outpatients. Psychiatry Res. 2002;112(3):187–194. doi: 10.1016/s0165-1781(02)00235-4. [DOI] [PubMed] [Google Scholar]
- 17.Winthorst WH, et al. Seasonality in depressive and anxiety symptoms among primary care patients and in patients with depressive and anxiety disorders; results from the Netherlands Study of Depression and Anxiety. BMC Psychiatry. 2011;11:198. doi: 10.1186/1471-244X-11-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardin TA, et al. Evaluation of seasonality in six clinical populations and two normal populations. J Psychiatr Res. 1991;25(3):75–87. doi: 10.1016/0022-3956(91)90001-q. [DOI] [PubMed] [Google Scholar]
- 19.Sakamoto K, et al. A longitudinal follow-up study of seasonal affective disorder. Am J Psychiatry. 1995;152(6):862–868. doi: 10.1176/ajp.152.6.862. [DOI] [PubMed] [Google Scholar]
- 20.Leonhardt G, et al. Long-term follow-up of depression in seasonal affective disorder. Compr Psychiatry. 1994;35(6):457–464. doi: 10.1016/0010-440x(94)90229-1. [DOI] [PubMed] [Google Scholar]
- 21.Thompson C, Raheja SK, King EA. A follow-up study of seasonal affective disorder. Br J Psychiatry. 1995;167(3):380–384. doi: 10.1192/bjp.167.3.380. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz PJ, et al. Winter seasonal affective disorder: a follow-up study of the first 59 patients of the National Institute of Mental Health Seasonal Studies Program. Am J Psychiatry. 1996;153(8):1028–1036. doi: 10.1176/ajp.153.8.1028. [DOI] [PubMed] [Google Scholar]
- 23.Sato T, et al. Distinct seasonality of depressive episodes differentiates unipolar depressive patients with and without depressive mixed states. J Affect Disord. 2006;90(1):1–5. doi: 10.1016/j.jad.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Kasper S, Kamo T. Seasonality in major depressed inpatients. J Affect Disord. 1990;19(4):243–248. doi: 10.1016/0165-0327(90)90101-d. [DOI] [PubMed] [Google Scholar]
- 25.Garvey MJ, Wesner R, Godes M. Comparison of seasonal and nonseasonal affective disorders. Am J Psychiatry. 1988;145(1):100–102. doi: 10.1176/ajp.145.1.100. [DOI] [PubMed] [Google Scholar]
- 26.Suhail K, Cochrane R. Seasonal variations in hospital admissions for affective disorders by gender and ethnicity. Soc Psychiatry Psychiatr Epidemiol. 1998;33(5):211–217. doi: 10.1007/s001270050045. [DOI] [PubMed] [Google Scholar]
- 27.de Graaf R, et al. Seasonal variations in mental disorders in the general population of a country with a maritime climate: findings from the Netherlands mental health survey and incidence study. Am J Epidemiol. 2005;162(7):654–661. doi: 10.1093/aje/kwi264. [DOI] [PubMed] [Google Scholar]
- 28.McCarthy E, Tarrier N, Gregg L. The nature and timing of seasonal affective symptoms and the influence of self-esteem and social support: a longitudinal prospective study. Psychol Med. 2002;32(8):1425–1434. doi: 10.1017/s0033291702006621. [DOI] [PubMed] [Google Scholar]
- 29.Akhter A, et al. Seasonal variation of manic and depressive symptoms in bipolar disorder. Bipolar Disord. 2013 doi: 10.1111/bdi.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry. 1978;35(7):837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- 31.Endicott J, Spitzer RL. Use of the Research Diagnostic Criteria and the Schedule for Affective Disorders and Schizophrenia to study affective disorders. Am J Psychiatry. 1979;136(1):52–56. doi: 10.1176/ajp.136.1.52. [DOI] [PubMed] [Google Scholar]
- 32.Fiedorowicz JG, et al. Manic/hypomanic symptom burden and cardiovascular mortality in bipolar disorder. Psychosom Med. 2009;71(6):598–606. doi: 10.1097/PSY.0b013e3181acee26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiedorowicz JG, et al. Do risk factors for suicidal behavior differ by affective disorder polarity? Psychol Med. 2009;39(5):763–771. doi: 10.1017/S0033291708004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fiedorowicz JG, et al. Subthreshold hypomanic symptoms in progression from unipolar major depression to bipolar disorder. Am J Psychiatry. 2011;168(1):40–48. doi: 10.1176/appi.ajp.2010.10030328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fiedorowicz JG, et al. Course of illness following prospectively observed mania or hypomania in individuals presenting with unipolar depression. Bipolar Disord. 2012;14(6):664–671. doi: 10.1111/j.1399-5618.2012.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Association AP. Diagnostic and Statistical Manual of Mental Disorders. 5 ed. Washington DC: American Psychiatric Publishing; 2013. p. 991. [Google Scholar]
- 37.Keller MB, et al. The Longitudinal Interval Follow-up Evaluation. A comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44(6):540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- 38.Judd LL, et al. Long-term symptomatic status of bipolar I vs. bipolar II disorders. Int J Neuropsychopharmacol. 2003;6(2):127–137. doi: 10.1017/S1461145703003341. [DOI] [PubMed] [Google Scholar]
- 39.Fiedorowicz JG, et al. Chryptochrome 2 variants chronicity and seasonality of mood disorders. Psychiatr Genet. 2012;22(6):305–306. doi: 10.1097/YPG.0b013e3283539594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Persons JE, Coryell WH, Fiedorowicz JG. Cholesterol fractions symptom burden and suicide attempts in mood disorders. Psychiatry Res. 2012;200(2–3):1088–1089. doi: 10.1016/j.psychres.2012.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fiedorowicz JG, et al. Vasculopathy related to manic/hypomanic symptom burden and first-generation antipsychotics in a sub-sample from the collaborative depression study. Psychother Psychosom. 2012;81(4):235–243. doi: 10.1159/000334779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kasof J. Cultural variation in seasonal depression: cross-national differences in winter versus summer patterns of seasonal affective disorder. J Affect Disord. 2009;115(1–2):79–86. doi: 10.1016/j.jad.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 43.Wehr TA, et al. Contrasts between symptoms of summer depression and winter depression. J Affect Disord. 1991;23(4):173–183. doi: 10.1016/0165-0327(91)90098-d. [DOI] [PubMed] [Google Scholar]
- 44.Posternak MA, et al. The naturalistic course of unipolar major depression in the absence of somatic therapy. J Nerv Ment Dis. 2006;194(5):324–329. doi: 10.1097/01.nmd.0000217820.33841.53. [DOI] [PubMed] [Google Scholar]
- 45.Postolache TT, et al. Changes in allergy symptoms and depression scores are positively correlated in patients with recurrent mood disorders exposed to seasonal peaks in aeroallergens. ScientificWorldJournal. 2007;7:1968–1977. doi: 10.1100/tsw.2007.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coryell W, et al. Does major depressive disorder change with age? Psychol Med. 2009;39(10):1689–1695. doi: 10.1017/S0033291709005364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thalen BE, et al. Seasonal and non-seasonal depression. A comparison of clinical characteristics in Swedish patients. Eur Arch Psychiatry Clin Neurosci. 1995;245(2):101–108. doi: 10.1007/BF02190736. [DOI] [PubMed] [Google Scholar]
- 48.Levitt AJ, et al. Anxiety disorders and anxiety symptoms in a clinic sample of seasonal and non-seasonal depressives. J Affect Disord. 1993;28(1):51–56. doi: 10.1016/0165-0327(93)90076-v. [DOI] [PubMed] [Google Scholar]