Summary
Circulating branched-chain amino acid (BCAA) levels are elevated in obesity/diabetes and are a sensitive predictor for type 2 diabetes. Here we show in rats that insulin dose-dependently lowers plasma BCAA levels through induction of hepatic protein expression and activity of branched-chain α keto-acid dehydrogenase (BCKDH), the rate-limiting enzyme in the BCAA degradation pathway. Selective induction of hypothalamic insulin signaling in rats and genetic modulation of brain insulin receptors in mice demonstrate that brain insulin signaling is a major regulator of BCAA metabolism by inducing hepatic BCKDH. Short-term overfeeding impairs the ability of brain insulin to lower BCAAs in rats. High-fat feeding in non-human primates and obesity and/or diabetes in humans is associated with reduced BCKDH protein in liver. These findings support the concept that decreased hepatic BCKDH is a major cause of increased plasma BCAAs, and that hypothalamic insulin resistance may account for impaired BCAA metabolism in obesity and diabetes.
Introduction
The branched-chain amino acids (BCAAs) leucine, isoleucine and valine are essential amino acids that are elevated in obesity and Type II diabetes and have recently emerged as a predictor for the future risk of diabetes (Wang et al., 2011). In obese and/or diabetic humans and rodents BCAA levels and their incompletely oxidized degradation products, the short-chain acylcarnitines are elevated in plasma and/or tissues (Kim et al., 2010; Laferrere et al., 2011; Lanza et al., 2010; Mihalik et al., 2010; Newgard et al., 2009; She et al., 2007), suggesting that alterations in catabolism of BCAAs may play an important role in both insulin resistance and diabetes. Improvements in insulin sensitivity in response to a dietary/behavioral weight loss intervention and to bariatric surgery are associated with lower circulating BCAA levels7.
The mechanisms that are responsible for the elevation in plasma BCAAs in obese and/or diabetic individuals remain poorly understood and may be attributed to increased protein intake. However, even when protein intake was matched, diabetics had higher circulating BCAA levels compared to lean non-diabetic individuals (Tai et al., 2010). Increased proteolysis and/or reduced protein synthesis may account for decreased BCAA utilization leading to elevated circulating BCAA levels, but whole-body protein degradation and/or synthesis does not seem to differ between normal and diabetic individuals(Barazzoni et al., 2003; Halvatsiotis et al., 2002; Luzi et al., 1993; Tessari et al., 2005). Hence, reduced catabolism of BCAAs could be a major cause for the elevated BCAA levels seen in obese and/or diabetic individuals, which is supported by the finding that BCAA-catabolizing enzymes are decreased in fat and liver in genetically obese ob/ob mice and fa/fa rats4.
BCAA catabolism starts with the transamination of circulating BCAAs to α keto-acids by branched-chain aminotransferase (BCATm), expressed mainly in muscle, kidney, and heart (Harper et al., 1984; Hutson et al., 2005). The keto-acid products are then released back into the circulation, and upon entering the liver, they are oxidatively decarboxylated to acyl-CoA derivatives by the rate-limiting enzyme branched-chain α keto-acid dehydrogenase (BCKDH). While both adipose tissue and the liver perform oxidative decarboxylation of keto-acids, the liver is believed to be the organ with higher protein expression and activity of BCKDH (Suryawan et al., 1998), although as of yet BCKDH activity has not been directly compared in these two tissues.
While the nutritional and hormonal regulation of BCAA metabolism through a variety of hormones such as thyroid hormones and glucocorticoids has been studied (Shimomura et al., 2001), surprisingly the role of glucose and insulin in regulating BCAA catabolism is incompletely understood. Here we probe the role of insulin signaling in regulating BCAA catabolism and demonstrate that neuronal insulin signaling is an important pathway that controls hepatic BCAA catabolism which is evolutionarily conserved from C. elegans to mammals.
Results
Insulin lowers plasma BCAA levels and induces hepatic BCKDH expression and activity
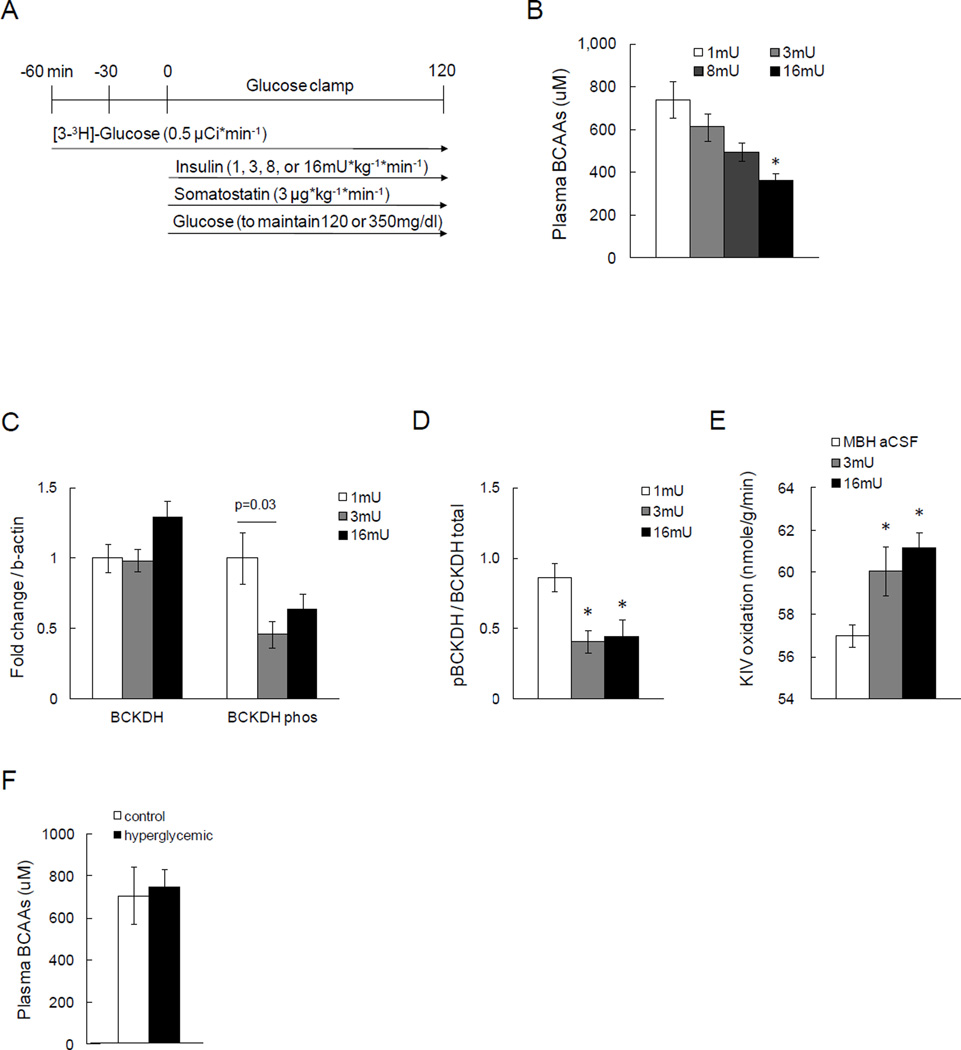
Obesity and pre-diabetes are characterized by insulin resistance and transient elevations in circulating glucose levels, both of which may affect BCAA metabolism (Palmer et al., 1985). Thus, we first set out to disentangle the role of insulin versus glucose in regulating BCAA metabolism in vivo. To delineate the physiological role of insulin in the regulation of circulating BCAA levels, we performed euglycemic clamps with plasma insulin at basal (1mU/kg/min), or induced moderate (3mU/kg/min) or marked hyperinsulinemic (8 or 16mU/kg/min) levels in 10 week-old male Sprague Dawley (SD) rats (experimental protocol depicted in Fig. 1A). This protocol allowed us to probe the role of insulin in regulating BCAAs independent of perturbations in glycemia. Somatostatin was infused during 1 and 3mU clamps to prevent endogenous insulin secretion and control counter-regulatory hormones such as glucagon. Plasma insulin levels increased dose-dependently, as did the glucose infusion rate (GIR) that was required to maintain euglycemia. Likewise, key parameters of insulin action such as the rate of glucose disposal (Rd) and the percent suppression of hepatic glucose production (hGP) increased with higher circulating insulin levels (Fig. S1). Total plasma BCAA levels were equally matched between all groups prior to the clamps as assessed through a spectrophotometric assay that measures NADH generated from BCAA oxidation by leucine dehydrogenase (Beckett, 2000). Insulin rapidly and dose-dependently lowered plasma BCAA levels (Fig. 1B), indicating that insulin is a major regulator of circulating BCAA levels. Since food had been removed at the start of the experiment, the animals transitioned into a fasting state during the clamp, and therefore, the lowering of plasma BCAA levels cannot be explained by reduced BCAA absorption in the gut, but instead could be due to alterations in BCAA metabolism.
Fig. 1. Insulin, but not glucose, regulates circulating BCAAs and hepatic BCAA metabolism.
A) Schematic representation of basal or hyperinsulinemic euglycemic clamp protocols in SD male rats. B) Plasma BCAA levels at the end of the clamp as measured by spectrophotometric assay (n ≥ 5 per group). C) Hepatic protein expressions of BCKDH and its phosphorylation state by Western blot (n ≥ 6 per group). D) The ratio of inactive BCKDH to total BCKDH protein in liver as an index of BCKDH activity (n ≥ 6 per group). E) Hepatic BCKDH activity as measured by α-keto isovalerate (KIV) oxidation assay using 1-14C isotope (n ≥ 4 per group). F) Circulating BCAAs at the end of hyperglycemic clamps (n ≥ 5 per group). * p<0.05 compared to basal condition. Values are mean±SEM.
The first step in BCAA catabolism is the conversion of BCAAs into branched-chain α keto-acids which is catalyzed by the enzyme BCATm in most peripheral tissues except the liver. The second step and rate-limiting step is the oxidative decarboxylation of branched chain keto-acids into acyl-CoAs catalyzed by BCKDH. BCKDH is expressed in both adipose tissue and liver and its activity is inhibited by phosphorylation. We therefore measured protein expression of BCATm in muscle, and in liver and adipose tissue we measured both protein levels and activation state of BCKDH, the latter through a phospho-specific antibody. BCATm levels were not altered by insulin in any of the groups. However, in the livers of the 16mU/kg/min clamped animals the expression of BCKDH protein was increased while levels of phospho-BCKDH were reduced (Fig. 1C, D). Since phosphorylation of BCKDH inactivates the enzyme, these data indicate that insulin enhances BCKDH activity both by increasing its expression and by decreasing its phosphorylation. To confirm that insulin indeed increases BCKDH activity, we determined the rate of oxidation of keto-isovalerate (KIV) in hepatic extracts. Hyperinsulinemia increased BCKDH activity, confirming that the insulin-induced expression and dephosphorylation of BCKDH protein resulted in an increase in activity (Fig. 1E).
BCKDH is also expressed in white adipose tissue (WAT) where BCKDH protein levels were higher in the 3mU/kg/min group but lower in the 16 mU/kg/min group compared to controls (Fig. S1), indicating that BCKDH in WAT is less or less consistently regulated by insulin than hepatic BCKDH. However, BCKDH protein levels may not adequately reflect its activity. To estimate the relative BCKDH activity in adipose tissue versus liver, we determined the enzymatic activity of BCKDH in WAT. BCKDH activity in perigonadal WAT was significantly lower compared to its activity in liver (Fig. 1 and Fig. S1). These data support the notion that insulin primarily regulates BCKDH activity in liver and that WAT BCKDH plays only a minor role in systemic BCAA metabolism in lean SD rats.
Hyperglycemia per se does not increase circulating BCAAs
In vitro evidence indicates that BCAA catabolism is inhibited by glucose, but to our knowledge there have been no in vivo studies which have investigated the role of glucose on BCAA catabolism independent of insulin (Palmer et al., 1985). We therefore studied the effects of increases in glycemia on BCAA levels and hepatic BCKDH expression. To this end, we performed hyperglycemic clamps during which we raised glucose levels to 330–360 mg/dl for 2h by infusing a variable rate of 45% glucose intravenously while maintaining basal insulin levels (Fig. S2). Transient hyperglycemia per se did not alter plasma BCAA levels (Fig. 1F), indicating that even marked changes in circulating glucose levels have little effect on BCAA metabolism in the absence of concomitant elevations of insulin. Taken together, these data demonstrate that insulin lowers circulating BCAA levels through induction of hepatic BCKDH protein expression and activity, independent of changes in glycemia.
Brain insulin signaling controls circulating BCAA levels by inducing hepatic BCKDH expression and activity
Insulin regulates glucose and lipid metabolism in target tissues such as the liver and adipose tissue both through direct effects mediated via the insulin receptors expressed in these tissues and indirectly by orchestrating organ cross talk where brain insulin signaling plays a critical role. For example, insulin suppresses hepatic glucose production and lipolysis in adipose tissue via cell autonomous effects and through signaling in the mediobasal hypothalamus (MBH) that alters parasympathetic and sympathetic outflow to these tissues, respectively (Pocai et al., 2005; Scherer et al., 2011). To initially test if BCAA metabolism is regulated through neuroendocrine mechanisms, we infused 2-deoxyglucose (2-DG) intracerebroventricularly (ICV) to induce glucopenia in the CNS, thereby triggering the counter-regulatory response. Plasma BCAA levels trended to be higher 60 and 90 min after the ICV 2-DG injection (Fig. S2). Since central 2-DG injection activates both the autonomic nervous system and the secretion of counter-regulatory hormones such as glucagon and corticosterone, these data suggest that BCAA metabolism is regulated by either counter-regulatory hormones or the autonomic nervous system.
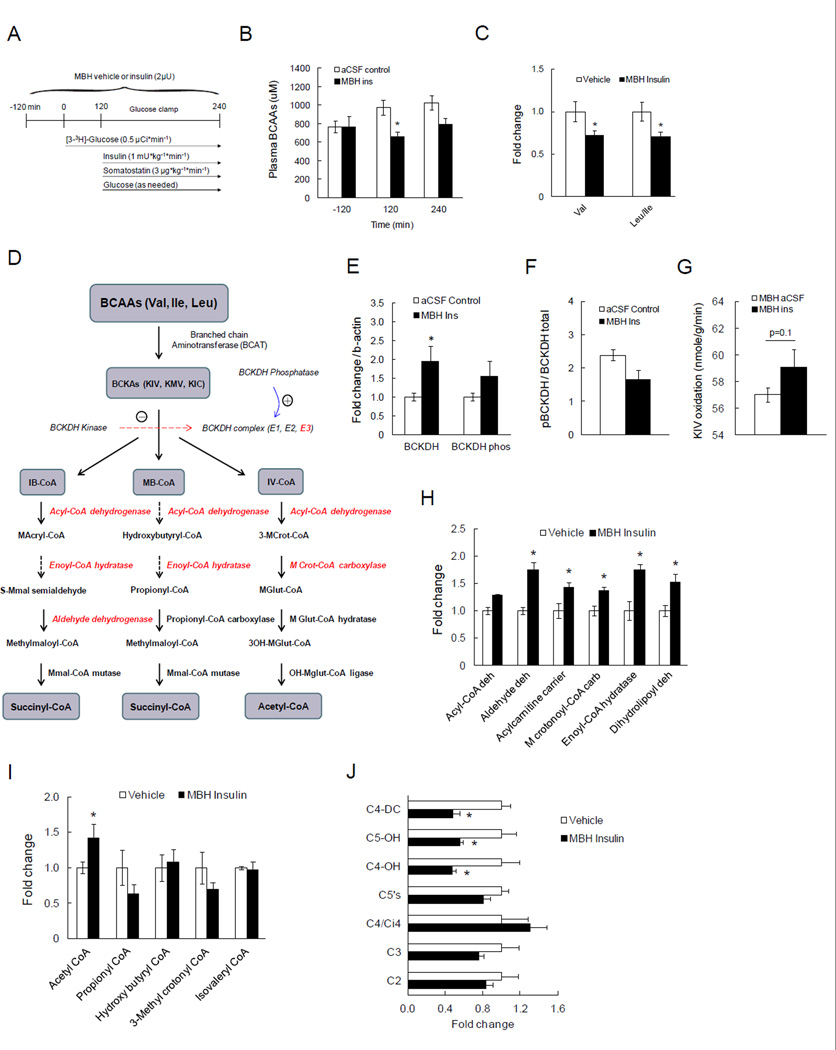
Hypothalamic insulin controls hepatic and adipose tissue metabolism via the autonomic nervous system (Buettner and Camacho, 2008; Scherer et al., 2011). To examine a potential role of hypothalamic insulin signaling in regulating BCAA metabolism that occurs independent of counter-regulatory hormones, we infused insulin (2µU) or vehicle (artificial cerebrospinal fluid; aCSF) into the MBH of SD rats over 6h (as described above). Since insulin infusion into the CNS can alter circulating insulin and glucose levels (Scherer et al., 2011), we infused insulin at 1mU/kg/min to maintain systemic insulin at basal levels and clamped glucose at euglycemic levels (Fig. 2A). Animals infused with MBH insulin required a higher glucose infusion rate (GIR) to maintain euglycemia. This increase in insulin sensitivity was due to a greater suppression of hepatic glucose production (Fig. S3) while peripheral glucose utilization as assessed by the Rd was unchanged, consistent with prior studies (Scherer et al., 2011). Plasma insulin levels during the clamps were not different between groups (Fig. S3). Since the animal transitioned to a fasting state during the clamp, BCAA levels gradually increased in MBH vehicle-infused animals. Importantly, MBH insulin was able to prevent this rise and even lowered plasma BCAAs (Fig. 2B, C; Fig. S3), demonstrating that hypothalamic insulin controls circulating BCAA levels. Since systemic hyperinsulinemia induced BCKDH expression in the liver, we next asked whether MBH insulin is sufficient to regulate hepatic BCKDH expression. Indeed, MBH insulin infusion induced hepatic BCKDH protein expression and activity (Fig. 2E–G). Further, we also observed a significant increase of BCKDH protein expression in white adipose tissue in the MBH infusion group compared to the controls (Fig. S3).
Fig. 2. Acute MBH insulin infusion lowers circulating BCAAs and induces hepatic BCKDH protein.
A) Schematic representation of euglycemic clamp protocol paired with MBH insulin infusion. B) Total plasma BCAA levels at baseline, at the start and the end of the clamps (n ≥ 4 per group). C) Individual plasma BCAA levels (n ≥ 4 per group). D) Schematic depiction of the simplified BCAA catabolic pathway with enzymes whose protein levels are regulated by MBH insulin highlighted in red color. E) Hepatic protein expressions of total and phosphorylated BCKDH by Western blot (n ≥ 6 per group). F) The ratio of inactive BCKDH to total BCKDH protein in liver as an index of BCKDH activity (n ≥ 6 per group). G) Hepatic BCKDH activity as measured by α-keto isovalerate (KIV) oxidation using 1-14C KIV (n ≥ 4 per group). H) Relative quantification of liver proteins that are regulated by MBH insulin as measured by global iTRAQ-based quantitative proteomics (n ≥ 4 per group). I, J) Circulating levels of short-chain acyl-CoAs and acylcarnitines that can be derived from BCAAs as measured by gas chromatography/mass spectrometry (GC/MS; (n ≥ 4 per group)). * p<0.05 compared to control group. Values are mean±SEM.
Of note, systemically administered leptin can also reduce circulating BCAAs comparable to systemically administered insulin in a mouse model of Type I diabetes (Wang et al., 2010). Both of these hormones regulate systemic metabolism in part through signaling in the MBH (Buettner et al., 2008; Scherer et al., 2011). Thus, we next tested whether central leptin signaling regulates circulating BCAA levels. Indeed MBH infused leptin also lowered BCAAs similar to MBH insulin (Fig. S3), suggesting that leptin and insulin exert similar effects on the regulation of BCAA metabolism via signaling in the CNS.
Brain insulin signaling induces several BCAA catabolic enzymes in addition to BCKDH and decreases incomplete degradation products of BCAAs
Apart from BCKDH, the second enzyme in the BCAA degradation pathway, several other enzymes downstream of BCKDH further catabolize BCAA to acetyl-CoA. Since antibodies to these proteins are not commercially available, we performed a global quantitative proteomic profiling by two-dimensional LC-MS/MS coupled with iTRAQ (isobaric tag for relative and absolute quantitation) labeling (Sigdel et al., 2014) to investigate whether MBH insulin induced downstream BCAA-catabolizing enzymes. This proteomic analysis led to the identification and quantification of 2418 proteins in these liver extracts, of which ~250 were significantly altered by MBH insulin infusion. Interestingly, five of these proteins, i.e. acyl-CoA dehydrogenase, aldehyde dehydrogenase, 3-Methylcrotonyl-CoA carboxylase, enoyl-CoA hydratase, and dihydrolipoyl dehydrogenase (E3 component of BCKDH complex), are enzymes involved in BCAA degradation and all of these proteins were significantly induced in livers of rats infused with MBH insulin (Fig. 2H). Incomplete degradation of BCAAs can lead to accumulation of partially oxidized intermediates in tissues and circulation which has been described in obesity and insulin-resistant states, giving rise to the notion that BCAA catabolism is reduced in these conditions (Laferrere et al., 2011; Newgard et al., 2009). If indeed BCAA degradation is enhanced by MBH insulin as suggested by the up-regulation of several catabolic enzymes and the reduction in circulating BCAAs, incompletely oxidized BCAA intermediates should also be reduced. To measure plasma short-chain acylcarnitines – C3, C4-DC, C5’s, and C5-OH – and liver acyl-CoAs such as 3-methylcrotonyl CoA and propionyl CoA, we utilized targeted metabolomics. This analysis revealed that incomplete degradation products such as propionyl-CoA and 3-methylcrotonyl CoA were lowered by MBH insulin (Fig. 2I, J) whereas the end product acetyl-CoA was increased in liver (Fig. 2I), indicating that brain insulin facilitates BCAA catabolism.
Some non-BCAA amino acids such as phenylalanine and asparagine were also reduced after insulin infusion in the MBH (Fig. S3). However, our proteomics analysis did not reveal changes in any of their catabolic enzymes such as phenylalanine hydroxylase, transaminase, or L-asparaginase, indicating that the hepatic expression of BCAA catabolizing enzymes is uniquely under the control of MBH insulin.
BCAA homeostasis is impaired in a mouse model of neuronal insulin receptor deficiency
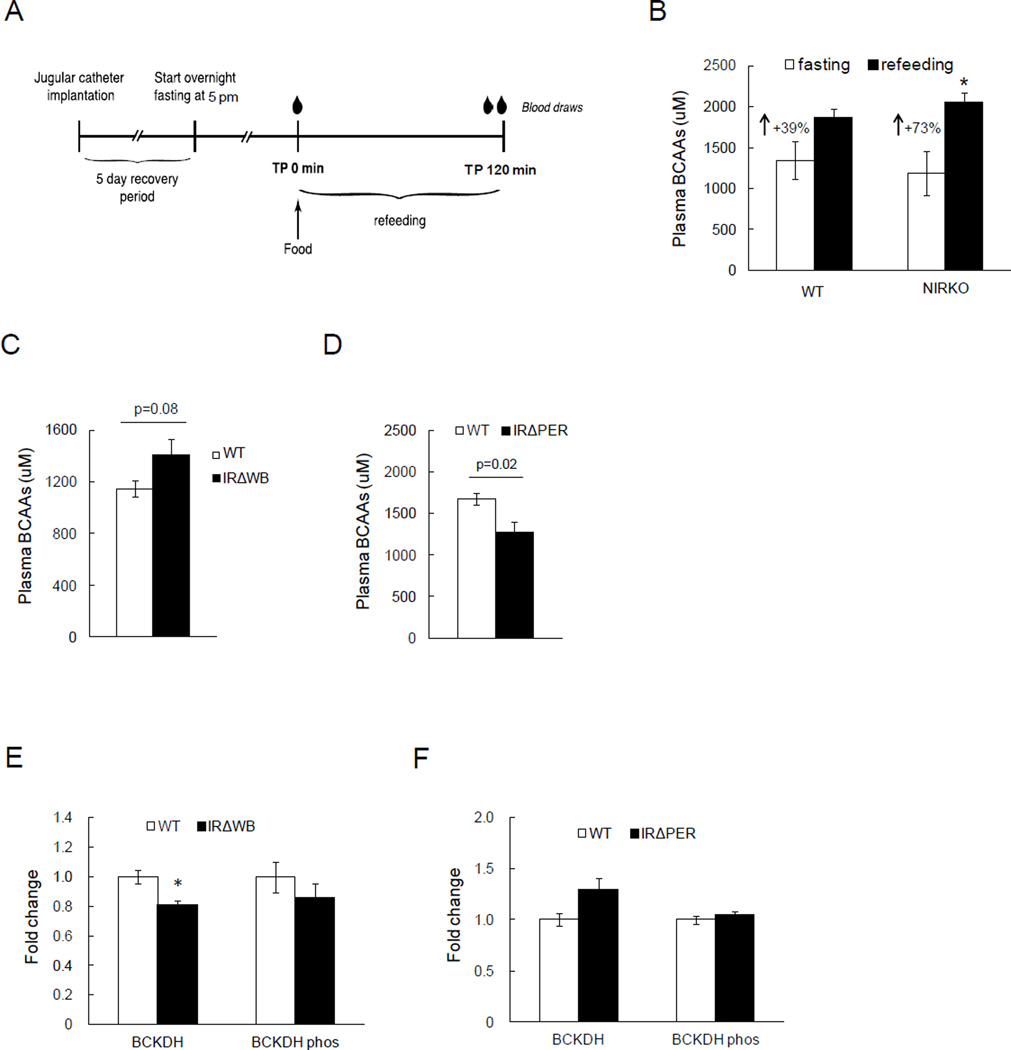
To test whether lifelong brain insulin resistance alters BCAA metabolism, we studied BCAA homeostasis in neuronal insulin receptor knockout mice (NIRKO) during the fast to fed transition as previously described (Scherer et al., 2011) (protocol depicted in Fig. 3A). Of note, NIRKO mice exhibit impaired regulation of lipid and glucose partitioning when studied with tracer dilution technique, although their fasting plasma lipid and glucose levels remain normal when fed a standard chow diet (Diggs-Andrews et al., 2010; Scherer et al., 2011). In the fasting state BCAA levels were not different between both strains. However after re-feeding, plasma BCAA levels were suppressed in controls likely due to higher insulin levels in the post-prandial state, and this suppression of plasma BCAAs was significantly diminished (~50%) in NIRKO mice (Fig. 3B), consistent with impaired BCAA homeostasis in the absence of brain insulin receptors.
Fig. 3. Lack of CNS insulin signaling leads to impaired regulation of BCAA metabolism.
A) Schematic representation of fasting and refeeding protocol in NIRKO mice. B) Plasma BCAA levels at fasting and refed state in NIRKO mice (n ≥ 5 per group). C) Plasma BCAA levels in IRΔWB mice after overnight fasting (n = 8 per group). D) Plasma BCAA levels in IRΔPER mice after overnight fasting (n ≥ 7 per group). E) Hepatic protein expressions of BCKDH and phosphorylated, inactive form of BCKDH by Western blot in IRΔWB mice after overnight fasting (n ≥ 4 per group). F) Hepatic protein expressions of BCKDH and phosphorylated, inactive form of BCKDH by western blot in IRΔPER mice after overnight fasting (n ≥ 4 per group). * p<0.05 compared to control group. Values are mean±SEM.
Inducible deletion of the IR in peripheral tissues including the liver does not increase circulating BCAA levels, but inducible whole-body IR deficiency does
Life-long knockout models such as the NIRKO mice have two main drawbacks in the study of physiology. First, a protein such as the insulin receptor (IR) may have an important role during development which may differ from its physiological role in adulthood. Second, because of developmental plasticity, metabolic defects may be “rescued” through the activation of compensatory pathways. These issues can largely be avoided through the use of inducible knockout mouse models as these animals undergo normal development and the insulin receptor is deleted only after development is complete. Therefore, we studied BCAA metabolism in two inducible knockout mouse models – first where the insulin receptor is knocked out in all peripheral tissues such as adipose tissue, liver and muscle but not the CNS (IRΔPER), and second where the insulin receptor is knocked out in the whole body including the CNS (IRΔWB) (Koch et al., 2008). Body weight was comparable between NIRKOs (Mean±SEM; 23.2g±0.6) and IRΔPER mice (23.2g±1.1), and slightly higher in the IRΔWB mice (25.8g±0.7). Both IRΔPER and IRΔWB mice developed comparable hyperglycemia and marked hyperinsulinemia (Fig. S2), the latter due to the absence of the hepatic insulin receptor that is required for insulin clearance. Since insulin receptor signaling in the brain is intact in the IRΔPER mice, we hypothesized that brain insulin signaling would be enhanced due to the marked hyperinsulinemia, resulting in increased hepatic BCAA catabolism and thus lower circulating BCAA levels. On the other hand, in the IRΔWB mice whose brain insulin receptor expression is markedly reduced, insulin should be unable to regulate BCAA metabolism and prevent the reduction of circulating BCAAs. As expected, IRΔWB mice had higher plasma BCAA levels compared to the controls after an overnight fast, while IRΔPER mice had lower plasma BCAA levels compared to control animals (Fig. 3C, D). Correspondingly, hepatic BCKDH protein expression was lower in IRΔWB and higher in IRΔPER mice after fasting (Fig. 3E, F). Taken together, these data confirm in genetic models that brain insulin signaling controls BCAA catabolism via regulation of hepatic BCKDH activity.
High-fat feeding impairs the ability of brain insulin to lower plasma BCAA levels in rats
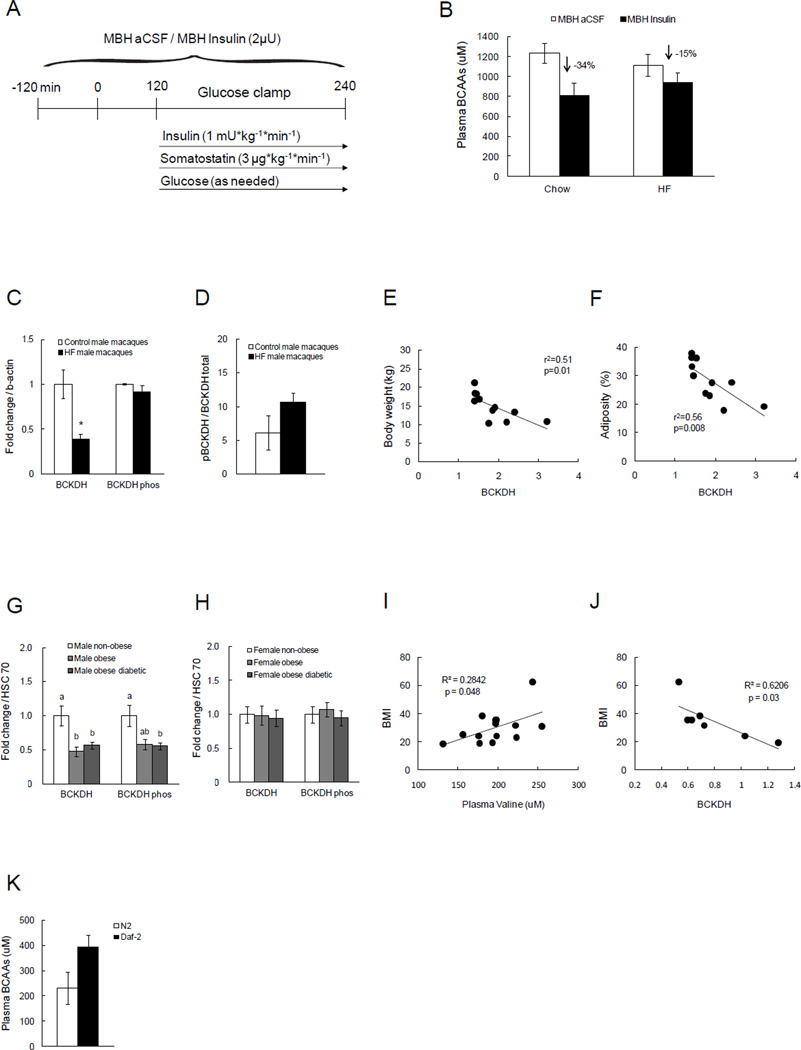
High-fat (HF) diet is well known to impair both peripheral and central insulin signaling, resulting in an insulin-resistant phenotype (Carvalheira et al., 2003; Clegg et al., 2011; Harris and Kor, 1992; Savage et al., 2007). We recently demonstrated that short-term voluntary overfeeding impairs hypothalamic insulin action in rats, defined as the ability of MBH insulin to suppress hepatic glucose production and adipose tissue lipolysis (Scherer et al., 2012). To test whether overfeeding-induced insulin resistance (a model for common forms of insulin resistance in humans) impairs the ability of MBH insulin to regulate BCAA metabolism, we fed rats a palatable 10% lard diet for 3 days, after which we performed clamp studies as described above, combining MBH insulin infusions with basal, euglycemic clamps (Protocol in Fig. 4A). Circulating BCAA levels did not differ between HF-fed and normal chow-fed rats at baseline likely due to the fact that insulin levels were higher after 3 days of overfeeding. While MBH insulin was able to effectively suppress circulating BCAA levels in rats fed regular chow diet (−34%; p=0.06), this suppression was attenuated by more than 50% (−15%; Fig. 4B) in rats on the HF diet, suggesting that excessive nutrient intake is sufficient to impair central insulin control of BCAA metabolism.
Fig. 4. Acute HF overfeeding leads to impaired suppression of circulating BCAAs by MBH insulin, and obesity and diabetes are associated with decreased BCKDH protein in liver.
A) Schematic representation of euglycemic clamp protocol paired with MBH insulin infusion in SD male rats following three days of either regular chow or HF (10% lard) feeding. B) Plasma BCAA levels in chow vs. HF-fed groups that were infused with either aCSF or insulin in the MBH (n ≥ 5 per group). C) Hepatic BCKDH and phosphorylation state of BCKDH in male Macaque monkeys after 1.5 years of HF diet (n ≥ 3 per group). D) Ratio of pBCKDH to total BCKDH protein expression as an index of BCKDH activity in liver (n ≥ 3 per group). E) Correlation between body weight and hepatic BCKDH protein expression in male Macaque monkeys (n = 10). F) Correlation between adiposity (%) and hepatic BCKDH protein expression in male Macaque monkeys (n = 11). G, H) Hepatic BCKDH and phosphorylated state of BCKDH in non-obese, obese, or obese and diabetic males or females (n = 4 per group). I, J) Correlation between BMI and plasma valine level or hepatic BCKDH protein of male subjects (n ≥ 7). K) BCAA levels in C. elegans deficient of insulin receptors (Daf-2; n ≥ 3 per group). * p<0.05 compared to control group. Groups with different letters are significantly different from each other. Values are mean±SEM.
Hepatic BCKDH expression is reduced in human obesity and diabetes
Emerging data suggest that human obesity and/or diabetes are also associated with central insulin resistance (Hallschmid et al., 2008; Tschritter et al., 2006). If human obesity indeed is associated with impaired hypothalamic insulin action, then the prediction would be that hepatic BCAA catabolism and BCKDH protein is reduced which could account for the increased plasma BCAA levels. To test this, we measured BCKDH protein in liver and circulating BCAA levels from obese or obese and diabetic humans, hypothesizing that hepatic BCKDH expression would be reduced while plasma BCAAs would be increased. Obese and obese-diabetic men showed decreased BCKDH protein expression in the liver compared to the non-obese control group (Fig. 4G), while in females there was no difference, suggestive of a sexual dimorphism in the regulation of BCAA metabolism (Fig. 4H).
High-fat feeding in non-human primates increases circulating BCAAs and lowers the expression of hepatic BCKDH
We next studied male non-human primates that had been fed a HF diet to test if HF diet-induced obesity increases plasma BCAA levels and reduces hepatic BCKDH expression. After 15 months of HF diet feeding, obese male macaques exhibited lower hepatic BCKDH protein expression without changes in its phosphorylation state, indicative of reduced BCKDH activity (Fig. 4C, D). Further, hepatic BCKDH protein expression was negatively and significantly associated with both body weight and adiposity in macaques (Fig. 4E, F). Plasma valine levels strongly correlated with the HOMA index, but not with trunk fat or hepatic BCKDH protein expression in the male macaques (Fig. S4). In men, BMI correlated with circulating valine levels (r=0.53, p=0.048; Fig. 4I), and inversely correlated with hepatic BCKDH protein level (r=−0.79, p=0.03; Fig. 4J), while HOMA index did not correlate with plasma valine or hepatic BCKDH protein level (Fig. S4). These results indicate that obesity and/or diabetes is associated with increased plasma BCAA levels and linked to lower hepatic BCAA metabolism, and that these effects are sex-specific.
Role of neuronal insulin signaling in regulating BCAAs is preserved in C. elegans
C. elegans is a commonly used model organism due to its genetic tractability. C. elegans expresses the insulin receptor homologue (Daf-2) mainly in neurons (Kenyon et al., 1993), therefore lends itself as a model of metabolic control through neuronal insulin signaling. Hence, we tested if BCAA levels are regulated through neuronal insulin signaling in this phylogenetically distant organism. Control and Daf-2 deficient worms were fasted for two hours after which the worms were homogenized and BCAA levels of whole-body lysates were analyzed. BCAA levels were increased in the Daf-2 deficient worms that lack insulin signaling (Fig. 4K), indicating that the role of brain insulin signaling is conserved from C. elegans to humans.
Discussion
Here we demonstrate that insulin action is a critical regulator of plasma BCAA level by inducing hepatic BCKDH protein and activity. Surprisingly, hyperglycemia per se did not alter BCAA levels in vivo, indicating that insulin resistance, but not hyperglycemia, is a major cause of the increase in circulating BCAAs in obesity and diabetes. Previous studies have indicated that infusion of glucose lowers plasma BCAAs in men and women (Felig et al., 1969). Our study demonstrates that this is due to glucose-induced insulin secretion and not due to a direct effect of glucose on BCAA metabolism per se. The ability of insulin to induce hepatic BCKDH activity and lower plasma BCAA levels depends on brain insulin signaling, specifically hypothalamic insulin signaling. We conclude this from a series of studies that utilize complementary approaches, where we inhibited insulin signaling through either pharmacological and/or genetic loss-of-function approaches. Further, high fat feeding impairs the ability of brain insulin to induce BCAA catabolism, suggesting that hypothalamic insulin resistance in part underlies the rise of BCAA plasma concentrations in obesity and diabetes. Since human obesity may be associated with brain insulin resistance (Tschritter et al., 2006), we speculated that obese humans and high fat-fed non-human primates would exhibit reduced expression of hepatic BCAA catabolic enzymes. We find that hepatic BCKDH protein is reduced in obese and diabetic humans as well as high fat-fed macaques, consistent with the concept of impaired brain insulin action in human obesity and diabetes.
The induction of hyperglycemia during basal insulinemia did not alter BCAA catabolism, indicating that hyperglycemia per se is not sufficient to increase BCAA levels and decrease BCAA catabolism, while circulating BCAA levels were dose-dependently lowered by insulin during maintained euglycemia. Although it is possible that the combined effect of hyperglycemia and hyperinsulinemia could reduce BCAA catabolism, the glucose infusion rate that would be needed to maintain hyperglycemia during hyperinsulinemia would be extremely high and likely unphysiological in an insulin-sensitive animal. Nonetheless, we believe that our studies effectively dissociated the effects of hyperglycemia and hyperinsulinemia on BCAA catabolism. While insulin dose-dependently lowered plasma BCAA levels, hepatic BCKDH activity was enhanced by physiological hyperinsulinemia, but was not further increased by marked hyperinsulinemia. The reason for this discrepancy is not clear, but supraphysiological hyperinsulinemia may further decrease BCAA levels possibly due to other mechanisms such as increased cellular BCAA uptake via an induction or activation of aromatic amino acid transporters and/or greater BCAA utilization by increased protein synthesis. An induction of aromatic amino acid transporters could also explain the observed reduction in aromatic amino acids.
We used genetic mouse models that lack insulin receptors either only in the brain, the periphery, or the whole-body including the CNS in addition to the clamp studies in rats where we infused insulin centrally. These complementary studies support the concept that circulating BCAAs and hepatic BCAA metabolism is regulated by brain insulin. Importantly, the degree of hyperglycemia and hyperinsulinemia in the two inducible insulin receptor knockout models were comparable at the time these animals were studied. We observed some minor differences in body composition in the genetic knockout models used herein which could have confounded some of our results. However, hepatic BCKDH protein expression was lower in the ΔWB mice despite lower adiposity, which would be predicted to increase BCKDH expression and activity. Thus, the moderate differences in body composition would be predicted to cause alterations in BCKDH expression opposite to what has been observed here, and therefore are unlikely to reduce the validity of our conclusions.
Both BCKDH protein and activity levels did not seem to be regulated by insulin in perigonadal WAT as opposed to liver. The average mass of liver is 12g and that of WAT is 3g in 12 week-old SD rats, and therefore the total BCKDH activity in liver induced by insulin would be estimated to be roughly over 500 fold higher than that in WAT, suggesting that WAT BCKDH activity is significantly less than that of liver in an insulin-sensitive rat. This conclusion rests on the assumption that the BCKDH activity of perigonadal WAT reflects that of other fat depots, which we did not assess in this study. Furthermore, animals markedly increase their fat mass as they become obese and/or diabetic, which may alter the relative contribution of hepatic vs. adipose BCKDH activity.
Circulating BCAAs are sensed by the hypothalamus which in turn can alter nutrient metabolism. The effects of central BCAAs such as leucine on hypothalamic control of systemic metabolism are somewhat controversial. Cota (Cota et al., 2006) and others (Blouet et al., 2009; Morrison et al., 2007) have shown that central administration of leucine increases activation of mTOR and S6K and reduces food intake, suggesting that hypothalamic BCAAs are important in limiting food intake. However, it is likely that the ability of BCAAs to exert this anorexigenic effect is lost in the obese and/or diabetic state. Further, BCAAs may trigger hypothalamic insulin resistance via hypothalamic mTOR over-activation to induce hepatic insulin resistance, that would in turn, impair systemic metabolism. In support of this, Ono (Ono et al., 2008) found that constitutive S6K activation in the hypothalamus can be a cause of hypothalamic insulin resistance. Based on our and others’ findings, we speculate that the increased circulating BCAA levels experienced by obese and/or diabetic individuals may lead to hyperactivation of the mTOR pathway within the MBH that impairs hypothalamic insulin action, worsening systemic insulin resistance and thus starting a vicious cycle. Conversely, the reduction of circulating BCAAs seen after bariatric surgery of obese individuals could be due to restored hypothalamic insulin signaling, a hypothesis we are currently examining. Whether a reduction of circulating BCAAs through the induction of BCAA catabolism is sufficient to improve glucose homeostasis remains to be tested.
We observed sexual dimorphism in the regulation of BCKDH levels in non-human primates. This finding mirrors that of a rodent study where sexual dimorphism in the diurnal variation in BCKDH activity in liver has been demonstrated (Kobayashi et al., 1997). Further, differences in the predictive values of BCAAs for insulin resistance were also reported to be sexually dimorphic in young human adults (Wurtz et al., 2012). The reason for the lack of association between obesity and diabetes in females and hepatic BCKDH protein expression is not clear, but the presence of sex difference may suggest that the male macaques are more sensitive to HFD-induced central insulin resistance, while females are more tolerant to HFD feeding and are protected from central insulin resistance. Human studies demonstrate that men are diagnosed with diabetes at a lower BMI compared to women across the age spectrum in a European cohort, suggesting that men are more susceptible to developing diabetes than women (Logue et al., 2011). The lack of HFD-induced changes in BKCDH expression in liver of females may be a reflection or even a mechanism for their improved metabolic state during chronic HFD feeding.
Collectively, these results suggest that brain insulin plays a critical role in the regulation of systemic BCAA metabolism, a metabolic pathway that is evolutionarily conserved from worm to man. Increased plasma BCAAs in obese and/or diabetic individuals may be due to impaired CNS insulin action. Thus, plasma BCAAs may represent a marker of hypothalamic insulin action.
Experimental Procedures
Animals
8–10 week-old male Sprague Dawley (SD) rats (Charles River Laboratories, Wilmington, MA) were provided with either standard laboratory chow (Rodent Diet 5001, LabDiet, St. Louis, MO) or a high-fat diet (5704C–K, purified modified rodent 10% lard, TestDiet, Richmond, IN). Animals were housed in separate cages at a constant ambient temperature of 21–23°C with a 12h light-dark cycle (lights on at 0700h, off at 1900h). Before pancreatic clamp studies, rats underwent stereotaxic implantation of indwelling cannulae targeting the MBH as previously described (Scherer et al., 2011). The coordinates for the MBH cannulae implantations were 3.3 mm posterior from bregma, 0.4 mm bilateral from midline, and 9.6 mm below the surface of the skull. After 7 days of recovery, rats were implanted with carotid and jugular catheters. Rats were allowed to recover for an additional 4–6 days and had to return to within 10% of their pre-surgical body weight. 0.003ul/min of insulin was infused in the MBH bilaterally for a total of 6h according to the clamp protocol, giving a total injection volume of 1.1ul for the duration of the clamp experiment. These coordinates had been previously validated where the spread of the infusate was assessed by infusion of radioactive tracers followed by sampling of several hypothalamic nuclei by micropunches such as the VMH, arcuate, and PVN. It was found that the infusate spread was contained to the arcuate and did not extend to the other nuclei (Lam et al., 2005). We routinely infused food dye immediately before removing the brain to confirm correct anatomical placement of the cannulae.
3–4 month-old SD rats were fed a high-fat diet for 3 days before the MBH infusion experiment. For the high-fat diet feeding study, rats were fed either standard chow which provided 59% calories from carbohydrates, 20% from protein, and 21% from fat or the same diet supplemented with 10% lard which provided 45% of calories from carbohydrates, 22% from protein, and 33% from fat. This led to an average increase of daily caloric intake from 61 to 83 kcal. Neuronal-specific insulin receptor knockout (NIRKO) mice were generated by crossing mice homozygous for the floxed insulin receptor allele with transgenic mice that express Cre recombinase under the control of nestin promoter (Bruning et al., 2000). IRΔWB mice were purchased from Taconic (Hudson, NY) and knockout of insulin receptors in the whole-body including the brain was induced by adding 2.5mg/kg doxycycline in 5% glucose drinking water throughout the study. Mice with insulin receptor deletion restricted in the periphery (IRΔPER) were generated by crossing IRflox/flox mice with RosaCre mice expressing Cre recombinase fused to the mutated ligand domain of estrogen receptor (ER) (Koch et al., 2008), and recombination was induced by an ip injection of tamoxifen (5mg) for five consecutive days. Blood glucose was carefully monitored daily. The mice were on a 12h light-dark cycle and fed a standard rodent diet (Mouse Diet 9F, PMI Nutrition International, St. Louis) unless otherwise noted. All protocols involved in the animal studies were approved by the Institutional Animal Care and Use Committee at the Icahn School of Medicine at Mount Sinai in accordance with guidelines established by the National Institutes of Health.
Worm Strains
The following strains were provided by Caenorhabditis Genetics Center (CGC): wild-type (N2), daf-2 (e1370). Caenorhabditis elegans wildtype worms were maintained on nematode growth media (NGM) at 20°C while daf-2 mutants were maintained at 15°C. Worms were fed Escherichia coli OP50.
Worm Extraction
Worms were maintained at 20°C and were washed off the plates with deionized water three days after axenization. Worms were allowed to sink to the bottom of micro-centrifuge tubes, and washed three times with water, after which worms were just maintained for another 2h in water to allow transitioning into the fasting state at which point they pelleted and sonicated in a lysis buffer made of 20 mM MOPS, 2 mM EGTA, 5 mM EDTA, 30 mM sodium fluoride, 40 mM β-glycerophosphate, 10 mM sodium pyrophosphate, 2 mM sodium orthovanadate, 0.5% NP-40, and complete protease inhibitor cocktail (Roche) and centrifuged at 13,000g for 15 min at 4°C. Protein concentration in the supernatant was measured with a BCA protein quantification kit (Thermo Scientific). The supernatant was then stored in an −80°C freezer and used for further analysis.
Mouse fasting and refeeding study
Three-month-old NIRKO and littermate control mice were deprived of food for 24h starting at 5 pm before the experiment, so the refeeding period coincided with the onset of the feeding cycle (protocol depicted in Fig. 3A). An initial blood sample was taken by tail bleeding at t=0 min, following which mice were given access to regular chow for two hours. The final blood sample (t=120 min) was obtained via tail bleed. Animals were anesthetized via isoflurane after which they were decapitated and organs including liver were harvested and clamp-frozen for western blot analysis.
Other analytic procedures
Blood glucose levels during the clamp were measured by hand-held glucometer (Onetouch Ultra Blood Glucose Monitor, LifeScan, Milpitas, CA, USA). Glucose flux and Ra glycerol were analyzed as described elsewhere (Obici et al., 2002; Scherer et al., 2011). Plasma free-glycerol and triglycerides were measured with a colorimetric assay from Sigma–Aldrich (St. Louis, MO) and plasma NEFA with a kit from Wako Chemicals (Richmond, VA). Plasma insulin was analyzed by ELISA kit from Mercodia (Uppsala, Sweden).
Western blots
At the end of clamp study, animals were sacrificed by decapitation, and liver, muscle, kidney, and adipose tissue were harvested and freeze-clamped in liquid nitrogen. The frozen samples were kept at −80°C until they were processed for western blots as previously described (Scherer et al., 2011). Briefly, ~100 mg of tissue was homogenized in lysis buffer and sonicated for 15 sec. The homogenates were centrifuged at 13,000 rpm at 4°C for 20 min and the supernatants were collected. Protein amount was quantified by BCA Protein Assay kit (Pierce, Rockford, IL) and 20ug of protein was loaded in 4–12% NuPAGE gels (Invitrogen, Carlsbad, CA). Primary antibodies against branched-chain α keto-acid dehydrogenase (BCKDH) total 32–50, 371–90, and phosphorylated form of BCKDH were generated and characterized as previously described (Lynch et al., 2003; Sweatt et al., 2004). Antibodies against branched-chain aminotransferase (BCATm) were purchased from AbCam (Cambridge, MA). Blots were scanned with the LI-COR Odyssey (LI-COR, Lincoln, NE) and quantified with Odyssey 3.0 software on the basis of direct fluorescence measurement.
BCAA assay
A spectrophotometric assay was employed to measure total BCAAs (leucine, isoleucine, and valine) from plasma samples as described previously (Beckett, 2000).
Serum acylcarnitines and amino acids
A panel of plasma acylcarnitines and amino acids were analyzed by MS/MS as described previously (Ferrara et al., 2008).
Circulating free fatty acids and organic acids in liver
Capillary gas chromatography/mass spectrometry (GS/MS) with TRACE DSQ instrument (Thermo Electron Corporation, Austin, TX) was used to analyze the derivatized fatty acids in the plasma and organic acids in the liver samples as described previously (Newgard et al., 2009).
Statistics
Plasma glucose levels during the clamp were analyzed by two-way repeated measures ANOVA, with treatment as a between-subject factor and time as a within-subject factor, followed by Bonferroni post-hoc multiple comparisons. Proteomics data, body weight, cumulative food intake, and plasma insulin were analyzed by student's t-test to compare the mean difference between vehicle vs. MBH insulin treatment. Clamp data, plasma BCAA levels, acylcarnitines, amino acids, derivatized CoAs, and western blot protein expression data were analyzed by one-way ANOVA followed by Bonferroni post-hoc test. Correlation analyses were conducted to determine the association between insulin sensitivity, BMI, hepatic BCKDH protein, and plasma valine. All data are expressed as mean ± SEM. Significant difference was set at p < 0.05.
Supplementary Material
Acknowledgements
We would like to thank James D. O’Hare, Tiffany Chi, Seta Degann, Brian Closs, Adam Spitz, and Lee Honig for their technical assistance and Dr. Charles V. Mobbs and Elizabeth Schwartz for help with the C. elegans study. This study was supported by NIH grants DK074873, DK083568, DK082724 (C.B), K01 DK099463 (A.C.S), P41 GM103493 (R.D.S), and by the Boehringer Ingelheim Foundation (M.F). Proteomic analysis was performed in the Environmental Molecular Sciences Laboratory (EMSL), a DOE national scientific user facility located at Pacific Northwest National Laboratory, which is operated by Battelle Memorial Institute under contract DE-AC05-76RLO 1830.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barazzoni R, Kiwanuka E, Zanetti M, Cristini M, Vettore M, Tessari P. Insulin acutely increases fibrinogen production in individuals with type 2 diabetes but not in individuals without diabetes. Diabetes. 2003;52:1851–1856. doi: 10.2337/diabetes.52.7.1851. [DOI] [PubMed] [Google Scholar]
- Beckett PR. Spectrophotometric assay for measuring branched-chain amino acids. Methods Enzymol. 2000;324:40–47. doi: 10.1016/s0076-6879(00)24217-1. [DOI] [PubMed] [Google Scholar]
- Blouet C, Jo YH, Li X, Schwartz GJ. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J Neurosci. 2009;29:8302–8311. doi: 10.1523/JNEUROSCI.1668-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- Buettner C, Camacho RC. Hypothalamic control of hepatic glucose production and its potential role in insulin resistance. Endocrinol Metab Clin North Am. 2008;37:825–840. doi: 10.1016/j.ecl.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Buettner C, Muse ED, Cheng A, Chen L, Scherer T, Pocai A, Su K, Cheng B, Li X, Harvey-White J, et al. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med. 2008;14:667–675. doi: 10.1038/nm1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalheira JB, Ribeiro EB, Araujo EP, Guimaraes RB, Telles MM, Torsoni M, Gontijo JA, Velloso LA, Saad MJ. Selective impairment of insulin signalling in the hypothalamus of obese Zucker rats. Diabetologia. 2003;46:1629–1640. doi: 10.1007/s00125-003-1246-x. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Gotoh K, Kemp C, Wortman MD, Benoit SC, Brown LM, D'Alessio D, Tso P, Seeley RJ, Woods SC. Consumption of a high-fat diet induces central insulin resistance independent of adiposity. Physiol Behav. 2011;103:10–16. doi: 10.1016/j.physbeh.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- Diggs-Andrews KA, Zhang X, Song Z, Daphna-Iken D, Routh VH, Fisher SJ. Brain insulin action regulates hypothalamic glucose sensing and the counterregulatory response to hypoglycemia. Diabetes. 2010;59:2271–2280. doi: 10.2337/db10-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P, Marliss E, Cahill GF., Jr Plasma amino acid levels and insulin secretion in obesity. N Engl J Med. 1969;281:811–816. doi: 10.1056/NEJM196910092811503. [DOI] [PubMed] [Google Scholar]
- Ferrara CT, Wang P, Neto EC, Stevens RD, Bain JR, Wenner BR, Ilkayeva OR, Keller MP, Blasiole DA, Kendziorski C, et al. Genetic networks of liver metabolism revealed by integration of metabolic and transcriptional profiling. PLoS Genet. 2008;4:e1000034. doi: 10.1371/journal.pgen.1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallschmid M, Benedict C, Schultes B, Born J, Kern W. Obese men respond to cognitive but not to catabolic brain insulin signaling. Int J Obes (Lond) 2008;32:275–282. doi: 10.1038/sj.ijo.0803722. [DOI] [PubMed] [Google Scholar]
- Halvatsiotis PG, Turk D, Alzaid A, Dinneen S, Rizza RA, Nair KS. Insulin effect on leucine kinetics in type 2 diabetes mellitus. Diabetes Nutr Metab. 2002;15:136–142. [PubMed] [Google Scholar]
- Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr. 1984;4:409–454. doi: 10.1146/annurev.nu.04.070184.002205. [DOI] [PubMed] [Google Scholar]
- Harris RB, Kor H. Insulin insensitivity is rapidly reversed in rats by reducing dietary fat from 40 to 30% of energy. J Nutr. 1992;122:1811–1822. doi: 10.1093/jn/122.9.1811. [DOI] [PubMed] [Google Scholar]
- Hutson SM, Sweatt AJ, Lanoue KF. Branched-chain [corrected] amino acid metabolism: implications for establishing safe intakes. J Nutr. 2005;135:1557S–1564S. doi: 10.1093/jn/135.6.1557S. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kim JY, Park JY, Kim OY, Ham BM, Kim HJ, Kwon DY, Jang Y, Lee JH. Metabolic profiling of plasma in overweight/obese and lean men using ultra performance liquid chromatography and Q-TOF mass spectrometry (UPLC-Q-TOF MS) J Proteome Res. 2010;9:4368–4375. doi: 10.1021/pr100101p. [DOI] [PubMed] [Google Scholar]
- Kobayashi R, Shimomura Y, Murakami T, Nakai N, Fujitsuka N, Otsuka M, Arakawa N, Popov KM, Harris RA. Gender difference in regulation of branched-chain amino acid catabolism. Biochem J. 1997;327(Pt 2):449–453. doi: 10.1042/bj3270449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch L, Wunderlich FT, Seibler J, Konner AC, Hampel B, Irlenbusch S, Brabant G, Kahn CR, Schwenk F, Bruning JC. Central insulin action regulates peripheral glucose and fat metabolism in mice. J Clin Invest. 2008;118:2132–2147. doi: 10.1172/JCI31073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laferrere B, Reilly D, Arias S, Swerdlow N, Gorroochurn P, Bawa B, Bose M, Teixeira J, Stevens RD, Wenner BR, et al. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci Transl Med. 2011;3:80re82. doi: 10.1126/scitranslmed.3002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam TK, Pocai A, Gutierrez-Juarez R, Obici S, Bryan J, Aguilar-Bryan L, Schwartz GJ, Rossetti L. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med. 2005;11:320–327. doi: 10.1038/nm1201. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Zhang S, Ward LE, Karakelides H, Raftery D, Nair KS. Quantitative metabolomics by H-NMR and LC-MS/MS confirms altered metabolic pathways in diabetes. PLoS One. 2010;5:e10538. doi: 10.1371/journal.pone.0010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue J, Walker JJ, Colhoun HM, Leese GP, Lindsay RS, McKnight JA, Morris AD, Pearson DW, Petrie JR, Philip S, et al. Do men develop type 2 diabetes at lower body mass indices than women? Diabetologia. 2011;54:3003–3006. doi: 10.1007/s00125-011-2313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzi L, Petrides AS, De Fronzo RA. Different sensitivity of glucose and amino acid metabolism to insulin in NIDDM. Diabetes. 1993;42:1868–1877. doi: 10.2337/diab.42.12.1868. [DOI] [PubMed] [Google Scholar]
- Lynch CJ, Halle B, Fujii H, Vary TC, Wallin R, Damuni Z, Hutson SM. Potential role of leucine metabolism in the leucine-signaling pathway involving mTOR. Am J Physiol Endocrinol Metab. 2003;285:E854–E863. doi: 10.1152/ajpendo.00153.2003. [DOI] [PubMed] [Google Scholar]
- Mihalik SJ, Goodpaster BH, Kelley DE, Chace DH, Vockley J, Toledo FG, DeLany JP. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity (Silver Spring) 2010;18:1695–1700. doi: 10.1038/oby.2009.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison CD, Xi X, White CL, Ye J, Martin RJ. Amino acids inhibit Agrp gene expression via an mTOR-dependent mechanism. Am J Physiol Endocrinol Metab. 2007;293:E165–E171. doi: 10.1152/ajpendo.00675.2006.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- Ono H, Pocai A, Wang Y, Sakoda H, Asano T, Backer JM, Schwartz GJ, Rossetti L. Activation of hypothalamic S6 kinase mediates diet-induced hepatic insulin resistance in rats. J Clin Invest. 2008;118:2959–2968. doi: 10.1172/JCI34277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TN, Caldecourt MA, Warner JP, Sugden MC. Modulation of branched-chain amino acid oxidation in rat hemidiaphragms in vitro by glucose and ketone bodies. Biochem Int. 1985;11:407–413. [PubMed] [Google Scholar]
- Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005;434:1026–1031. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507–520. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer T, Lindtner C, Zielinski E, O'Hare J, Filatova N, Buettner C. Short term voluntary overfeeding disrupts brain insulin control of adipose tissue lipolysis. J Biol Chem. 2012;287:33061–33069. doi: 10.1074/jbc.M111.307348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer T, O'Hare J, Diggs-Andrews K, Schweiger M, Cheng B, Lindtner C, Zielinski E, Vempati P, Su K, Dighe S, et al. Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab. 2011;13:183–194. doi: 10.1016/j.cmet.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She P, Van Horn C, Reid T, Hutson SM, Cooney RN, Lynch CJ. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am J Physiol Endocrinol Metab. 2007;293:E1552–E1563. doi: 10.1152/ajpendo.00134.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura Y, Obayashi M, Murakami T, Harris RA. Regulation of branched-chain amino acid catabolism: nutritional and hormonal regulation of activity and expression of the branched-chain alpha-keto acid dehydrogenase kinase. Curr Opin Clin Nutr Metab Care. 2001;4:419–423. doi: 10.1097/00075197-200109000-00013. [DOI] [PubMed] [Google Scholar]
- Sigdel TK, Salomonis N, Nicora CD, Ryu S, He J, Dinh V, Orton DJ, Moore RJ, Hsieh SC, Dai H, et al. The identification of novel potential injury mechanisms and candidate biomarkers in renal allograft rejection by quantitative proteomics. Mol Cell Proteomics. 2014;13:621–631. doi: 10.1074/mcp.M113.030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryawan A, Hawes JW, Harris RA, Shimomura Y, Jenkins AE, Hutson SM. A molecular model of human branched-chain amino acid metabolism. Am J Clin Nutr. 1998;68:72–81. doi: 10.1093/ajcn/68.1.72. [DOI] [PubMed] [Google Scholar]
- Sweatt AJ, Wood M, Suryawan A, Wallin R, Willingham MC, Hutson SM. Branchedchain amino acid catabolism: unique segregation of pathway enzymes in organ systems and peripheral nerves. Am J Physiol Endocrinol Metab. 2004;286:E64–E76. doi: 10.1152/ajpendo.00276.2003. [DOI] [PubMed] [Google Scholar]
- Tai ES, Tan ML, Stevens RD, Low YL, Muehlbauer MJ, Goh DL, Ilkayeva OR, Wenner BR, Bain JR, Lee JJ, et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia. 2010;53:757–767. doi: 10.1007/s00125-009-1637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessari P, Coracina A, Kiwanuka E, Vedovato M, Vettore M, Valerio A, Zaramella M, Garibotto G. Effects of insulin on methionine and homocysteine kinetics in type 2 diabetes with nephropathy. Diabetes. 2005;54:2968–2976. doi: 10.2337/diabetes.54.10.2968. [DOI] [PubMed] [Google Scholar]
- Tschritter O, Preissl H, Hennige AM, Stumvoll M, Porubska K, Frost R, Marx H, Klosel B, Lutzenberger W, Birbaumer N, et al. The cerebrocortical response to hyperinsulinemia is reduced in overweight humans: a magnetoencephalographic study. Proc Natl Acad Sci U S A. 2006;103:12103–12108. doi: 10.1073/pnas.0604404103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MY, Chen L, Clark GO, Lee Y, Stevens RD, Ilkayeva OR, Wenner BR, Bain JR, Charron MJ, Newgard CB, et al. Leptin therapy in insulin-deficient type I diabetes. Proc Natl Acad Sci U S A. 2010;107:4813–4819. doi: 10.1073/pnas.0909422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz P, Soininen P, Kangas AJ, Ronnemaa T, Lehtimaki T, Kahonen M, Viikari JS, Raitakari OT, Ala-Korpela M. Branched-Chain and Aromatic Amino Acids Are Predictors of Insulin Resistance in Young Adults. Diabetes Care. 2012 doi: 10.2337/dc12-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.