Abstract
In female rats sexual receptivity (lordosis) can be induced with either a single large dose of estradiol benzoate (EB), or a priming dose of EB that does not induce sexual receptivity followed by 17β-estradiol (E2). Estradiol priming initially inhibits lordosis through a multi-synaptic circuit originating in the arcuate nucleus of the hypothalamus (ARH) that activates and internalizes μ-opioid receptors (MOR) in medial preoptic nucleus (MPN) neurons. Lordosis is facilitated when MPN MOR are deactivated after the initial estradiol-induced activation. We tested the hypothesis that E2 given 47.5 hours post EB acts rapidly through G protein-coupled estrogen receptor 1 (GPER) in the ARH to deactivate MPN MOR and facilitate lordosis. Ovariectomized Long Evans rats implanted with a third ventricle cannula were primed with 2 μg EB. DMSO control, E2, or G1 (GPER selective agonist) was infused 47.5 hours later, and rats were tested for sexual receptivity. E2 and G1 infusions significantly increased levels of sexual receptivity compared to DMSO controls and pretreatment with G15 (GPER antagonist) blocked the facilitation of sexual receptivity. Brains were processed for MPN MOR immunohistochemistry to measure MPN MOR activation levels. E2 and G1 both significantly reduced MPN MOR activation compared to DMSO controls, while pretreatment with G15 blocked MPN MOR deactivation. In another group of EB treated ovariectomized rats, GPER immunofluorescence positive staining was observed throughout the ARH. Together these data indicate that in the 2 μg EB primed rat, E2 rapidly signals through GPER in the ARH to deactivate MPN MOR and facilitate lordosis.
Keywords: GPR30, GPER, Arcuate nucleus of the hypothalamus
INTRODUCTION
Sexual receptivity can be facilitated in ovariectomized (OVX) rats with estradiol-only treatments that coordinate classical nuclear, and non-classical extra-nuclear, estrogen receptor (ER) signaling mechanisms (reviewed in Micevych and Sinchak, 2013; Sinchak and Wagner, 2012). A single high dose of esterfied 17β-estradiol benzoate (5–50 μg; EB) or multiple sequential lower doses of estradiol can induce sexual receptivity (reviewed in Clemens and Weaver, 1985; Micevych and Sinchak, 2013). The single large EB dose facilitates sexual receptivity approximately 48 hours after the initial treatment (reviewed in Clemens and Weaver, 1985). Sequential estradiol dosing by priming with EB that does not facilitate sexual receptivity followed by nonesterfied 17β-estradiol (E2) 44 hours later induces sexual receptivity within four hours (Parsons et al., 1984). Esterfied estradiol (EB) maintains consistent blood levels of estradiol over a longer period of time, whereas nonesterfied estradiol produces faster elevation and faster clearance of estradiol from the circulation. A fundamental question is whether these steroid priming paradigms act on the same ER signaling pathways to facilitate sexual receptivity.
Initially, estradiol binds to ERα in the plasma membrane that complexes with and signals through metabotropic glutamate receptor 1a (mGluR1a) to rapidly activate β-endorphin neurons in the arcuate nucleus of the hypothalamus (ARH) that project to the medial preoptic nucleus (MPN; reviewed in Sinchak and Wagner, 2012). This activates MPN μ-opioid receptors (MOR) to inhibit sexual receptivity (Dewing et al., 2007; Eckersell et al., 1998; Sinchak and Micevych, 2001). Subsequent deactivation of MPN MOR facilitates sexual receptivity (Dewing et al., 2007; Sanathara et al., 2011; Sinchak et al., 2013; Sinchak and Micevych, 2001). Treating EB-primed (2 μg) nonreceptive rats with ERα/β antagonists (ICI 182,780 or tamoxifen) facilitates lordosis (Garcia et al., 2010). This could be through down regulation of ARH membrane ERα-mGluR1a complexes to reduce β-endorphin release and deactivate MPN MOR (Mahavongtrakul et al., 2013). However, these ER antagonists also activate G protein-coupled estrogen receptor-1 (GPER; aka GPR30 reviewed in Micevych and Sinchak, 2013; Prossnitz et al., 2008; Sinchak and Wagner, 2012). GPER mediates rapid extranuclear estradiol signaling events in many physiological systems and certain cancers of the reproductive system that are estrogen-responsive, but are exacerbated by anti-estrogen therapies, like tamoxifen (Prossnitz et al., 2008; Soltysik and Czekaj, 2013) reviewed in (Sinchak and Wagner, 2012). We tested the hypothesis that E2 acts rapidly through GPER in the ARH to deactivate MPN MOR and facilitate lordosis in 2 μg EB primed OVX rats.
MATERIALS AND METHODS
Animals
Adult Long Evans OVX rats (ovariectomized by supplier) and male rats (200 to 225 g; Charles River Laboratory Inc., Wilmington, MA) were housed in a light and climate controlled room (12/12 L/D cycle, lights on at 0600h), with food and water ad-libitum. Procedures were approved by California State University, Long Beach IACUC.
Steroid priming
For both experiments, animals received 2 μg EB (dissolved in 0.1ml safflower oil) subcutaneously in the nape of the neck once every four days for a total of four cycles (Sanathara et al., 2011; Sinchak et al., 2013; Sinchak and Micevych, 2001). In Long Evans rats, this EB priming paradigm produces estradiol blood levels observed at the peak of proestrus, and does not facilitate lordosis without other pharmacological treatments (Priest et al., 1995; Sinchak et al., 2013). The multiple EB treatment cycles reduce the variability in responsiveness to steroid that has been observed after ovariectomy (reviewed in Clemens and Weaver, 1985).
Experiment 1 – Rapid facilitation of lordosis by 17β-estradiol in 2 μg EB primed OVX rats
Experimental design
Each OVX rat was implanted with a cannula aimed at the third ventricle (3V) using standard stereotaxic surgical procedures (4 degree angle; coordinates from bregma; anterior −2.3mm, lateral 0.5mm, ventral −6.8mm from dura; tooth bar set at −3.3; for details see Sanathara et al., 2011; Sinchak et al., 2013). Animals received two sequential 3V infusions starting 47.25 hours after the third EB treatment. The first 3V infusion was either DMSO or G15 (GPER antagonist), followed by a second infusion of either DMSO, E2, or G1 (GPER agonist) fifteen minutes later (6 to 10 animals per drug treatment set). Animals were tested for sexual receptivity 30 minutes after the second 3V infusion (Sanathara et al., 2011; Sinchak et al., 2013; Sinchak and Micevych, 2001). Following the fourth EB treatment, animals received the same drug treatments. Thirty minutes after the second 3V infusion animals were deeply anesthetized, and brain tissue fixed via transcardial perfusion to measure MPN MOR activation by MOR-immunoreactivity (MORi) intensity levels (Dewing et al., 2007; Sanathara et al., 2011; Sinchak et al., 2013).
Drug infusions
All drugs infused were dissolved and delivered in 1 μL of DMSO: E2 (Steraloids, Newport, RI; 25 nmol), G1 (Sigma-Aldrich; 70 nmol), G15 (Sigma-Aldrich; 70 nmol). Infusions were performed by an infusion pump (Stoelting Co., Wood Dale, IL; 1.0 μl/minute) and infusion needle was left in for one minute after infusion for drug dispersion (for details see Sanathara et al., 2011; Sinchak et al., 2013).
Behavioral testing
Sexual receptivity testing began 30 minutes after the second 3V infusion. The female rat was placed in a Plexiglas testing arena containing an acclimated stimulus male rat. The male mounted the female 10 times, and the number of times the female displayed lordosis was recorded. Sexual receptivity was quantified as a lordosis quotient (LQ) by dividing the number of lordosis responses by the total number of mounts multiplied by 100 (Sanathara et al., 2011; Sinchak et al., 2013; Sinchak and Micevych, 2001). LQ data underwent square root transformation and analyzed by 1-way ANOVA with post hoc analysis by Student-Newman-Keuls (SNK; Sigma Stat V3.5) and effect size calculated by eta squared for ANOVA and Cohen’s d for pairwise effect size.
Brain sectioning and cannula guide placement confirmation
Brains were cryosectioned (20 μm thickness), collected in wells containing phosphate buffered saline (pH 7.5), and stored at 4°C (Sanathara et al., 2011; Sinchak et al., 2013). To confirm guide cannula placement, every fourth brain section was mounted onto Superfrost Plus slides (Fisher Scientific) and thionin stained using standard procedures followed by bright field microscopy (Sinchak and Micevych, 2001).
MPN MOR immunohistochemistry and analysis
MPN MOR immunohistochemistry was performed on free-floating MPN sections, as described previously (Dewing et al., 2007; Sanathara et al., 2011; Sinchak et al., 2013). Briefly, MOR primary antibody raised in rabbit (1:5000; Neuromics Antibodies Edina, MN) was visualized by Fluorescein detection using a Tyramide Signal Amplification kit according to the manufacturer’s protocol (TSA kit; Perkin Elmer/Life Science Products, Boston, MA). Sections were mounted out of 0.1 M Tris buffer (pH 7.5) onto Superfrost Plus slides, dried on a slide warmer for 15 minutes and coverslipped using Aqua-Poly/Mount mounting medium. Estimation of relative MPN MOR activation was performed by measuring positive MORi staining levels in the dorsal region of the MPN in epifluorescent photomicrographs (Dewing et al., 2007; Sanathara et al., 2011; Sinchak et al., 2013). Levels of MPN MORi intensity are correlated with increased MOR internalization into early endosomes (Eckersell et al., 1998), and used to measure MOR activation (Dewing et al., 2007; Sanathara et al., 2011; Sinchak et al., 2013). Grayscale MORi images were adjusted for brightness and contrast by Adobe Photoshop (version CS6; Adobe Systems Inc., San Jose, CA) and MPN MORi intensity levels were calculated in arbitrary units (AU) by ImageJ software (version 1.32j; National Institutes of Health, Bethesda, MD; for details see Dewing et al., 2007; Sanathara et al., 2011; Sinchak et al., 2013). MPN MORi intensity levels were analyzed by 1-way ANOVA with post hoc analysis by SNK (Sigma Stat V3.5) and effect size calculated by eta squared for ANOVA and Cohen’s d for pairwise effect size.
Experiment 2 – GPER is localized to the ARH in EB treated OVX rats
GPER Immunohistochemistry
ARH GPER expression was determined in OVX rats 48 hours after the last EB injection. Animals were perfused and brains prepared for immunohistochemistry as described previously followed by GPER immunohistochemistry on 20 μm thick free-floating brain sections. GPER primary antibody was raised in rabbit (1:3000; Novus Biologicals, Littleton, CO), and Hoesch 33258 nuclear stain (Life Technologies, Grand Island, NY) was added to the secondary Ab incubation step of the GPER immunohistochemistry at a dilution of 1:1000 for 1 hour. GPER immunostaining (GPERi) was visualized using Fluorescein TSA kit and mounted as described for the MOR immunohistochemistry.
Analysis of GPER in the ARH
Double-labeled images of GPERi and Hoesch nuclear staining were acquired using the Leica DM6000B epiluminescent microscope, Leica DFC 360FX monochrome digital camera, and Leica AF-LAS microscope software through FITC (480/40 nm excitation filter and a 527/30 nm bandpass filter) and DAPI (360/40 nm excitation filter and a 470/40 nm bandpass; Leica, Heidelberg, Germany) filters. Images were computer colorized: FITC – green; DAPI – red. Image brightness and contrast were adjusted in Adobe Photoshop (version CS6).
RESULTS/DISCUSSION
Experiment 1 – Activation of GPER rapidly facilitates lordosis through deactivation of MPN MOR
Behavior
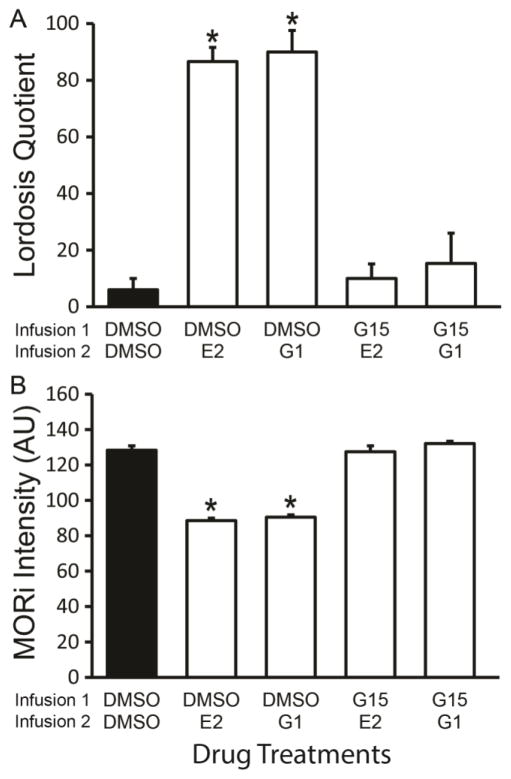
In EB-primed rats, infusions of E2 and the GPER agonist, G1, significantly increased LQ compared to DMSO controls (1-way ANOVA, df = 4,34 F = 28.68, P < 0.001; eta squared = 0.79; DMSO-E2, SNK, P < 0.001, Cohen’s d: d = 7.91, r = 0.97; DMSO-G1, SNK P < 0.001, Cohen’s d: d = 3.5, r = 0.87; Figure 1A). Infusion of the GPER antagonist, G15, prior to either E2 or G1 significantly reduced the LQ compared to E2 or G1 treated rats (SNK, P < 0.001; G15-E2 v DMSO-E2, Cohen’s d: d = 6.3, r = 0.95; G15-E2 v DMSO-G1, Cohen’s d: d = 3.2, r = 0.85; G15-G1 v DMSO-E2, Cohen’s d: d = 3.7, r = 0.88; G15-G1 v DMSO-G1, Cohen’s d: d = 2.4, r = 0.77) and were equivalent to DMSO controls (SNK, P > 0.05; Figure 1A). These data suggest that E2 and G1 facilitate lordosis in 30 minutes. Further, GPER antagonist pretreatment blocked E2 and G1 facilitation of lordosis, indicating that GPER is necessary for E2 induced lordosis.
Figure 1.
17β-estradiol (E2) rapidly facilitates lordosis through activation of GPER that deactivate MPN MOR. A) Animals primed with 2 μg EB received sequential 3V infusions at 47.25 (Infusion 1) and 47.5 hours (Infusion 2) post EB and 30 minutes later tested for sexual receptivity as measured by lordosis quotient (LQ). E2 and G1 (GPER agonist) rapidly facilitated lordosis indicated by higher LQ compared to DMSO controls (SNK, P < 0.001). Pretreatment with the GPER antagonist, G15, prior to E2 or G1 treatment blocked the facilitation of lordosis resulting in an LQ significantly lower from the E2 and the G1 groups, but not significantly different than the DMSO control. B) 3V infusions of either E2 or G1 significantly reduced MOR immunoreactive (MORi) intensity in arbitrary units (AU) as compared to DMSO. Pretreatment with G15 blocked the E2 induced reduction in MORi. In 2 μg EB primed OVX rats, a higher level of MORi indicates greater activation of the MPN MOR circuit and is associated with lordosis inhibition. Upon subsequent deactivation of the circuit the MORi is reduced and lordosis facilitated. These data indicate that in EB-primed rats E2 deactivates the MPN MOR circuit and facilitates lordosis via signaling through GPER. A & B)
 = significantly different from other treatment groups (SNK, P < 0.001).
= significantly different from other treatment groups (SNK, P < 0.001).
MOR immunohistochemistry
In EB primed rats, infusion of either E2 or G1 significantly reduced the MPN MORi intensity levels as compared to DMSO controls (1 way ANOVA df = 4,34 F = 121, P < 0.001; eta squared = 0.95; DMSO-E2, SNK, P < 0.001, Cohen’s d: d = 8.0, r = 0.97; DMSO-G1, SNK P < 0.001, Cohen’s d: d = 7.3, r = 0.96; Figure 1B). Pretreatment with G15 blocked the E2 and G1 reduction of MPN MORi intensity (SNK, P < 0.001; G15-E2 v DMSO-E2, Cohen’s d: d = 6.3, r = 0.95; G15-E2 v DMSO-G1, Cohen’s d: d = 5.8, r = 0..94; G15-G1 v DMSO-E2, Cohen’s d: d = 14.1, r = 0.99; G15-G1 v DMSO-G1, Cohen’s d: d = 12.4, r = 0.99), which were equivalent to DMSO (SNK P > 0.05). These data indicate that E2 rapidly signals through GPER to reduce activation of MPN MOR, presumably through the deactivation of ARH β-endorphin neurons that project to the MPN as part of the mechanism to facilitate lordosis (Sanathara et al., 2011; Sinchak and Micevych, 2001).
Experiment 2 – GPER expression in ARH
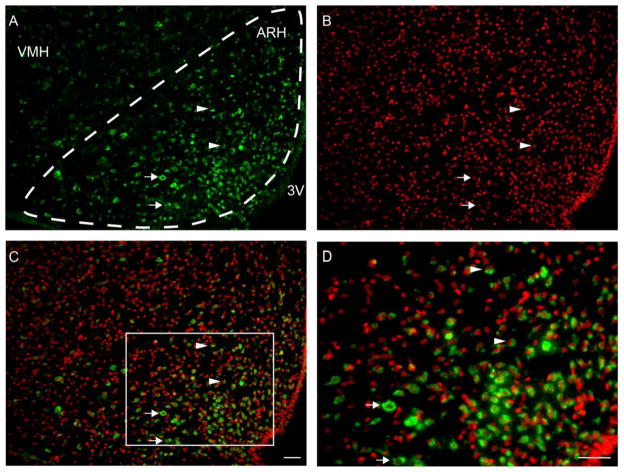
GPERi staining was observed throughout the ARH, especially at the level of the ventromedial nucleus of the hypothalamus (VMH; Figure 2). Although GPERi was observed throughout the ARH, the density of GPERi cells appeared greatest in the central region compared to the dorsomedial and ventrolateral regions of the ARH (Figure 2). In the dorsomedial and central ARH regions GPERi appeared more constrained to the perinuclear region of the cells. In contrast, GPERi appeared to fill the cytoplasm of cells located in the ventrolateral ARH region. In relationship to the nuclear Hoesch staining, GPERi was observed mainly in the cytoplasm and closely associated with or partially surrounding the Hoesch nuclear staining, but not observed in the nucleus. Although found in the plasma membrane in peripheral tissue, GPERi did not appear to be associated with the plasma membrane, which has been reported by others for CNS neurons and astrocytes (Gorosito et al., 2008; Kuo et al., 2010). Much of the GPER cytoplasmic staining appears closely associated with the nucleus. Therefore, E2 may act on intracellular GPER within the ARH to facilitate lordosis through reducing β-endorphin activity and MPN MOR activation as observed with other steroid paradigms (Sanathara et al., 2011; Sinchak and Micevych, 2001).
Figure 2.
GPER immunoreactive staining (GPERi; FITC, green) in the ARH at the level of the VMH in 2 μg EB treated rats. A) GPERi cells were observed throughout the area of the ARH as well as outside of the ARH in the VMH and shell regions. The distribution of GPERi cells appear to most dense in the central and medioventral ARH regions compared to the dorsomedial and ventrolateral ARH regions. B) Hoesch staining of the nucleus of the cells within the ARH (red). C) Merged image of GPERi and Hoesch staining. In the more dorsal region of the ARH, GPERi staining tends to be compact within the cytoplasm and closely associated with the Hoesch nuclear staining (arrowheads). In the more ventral regions GPERi appears to fill more of the cytoplasm of cells (arrows). D) Photomicrograph of higher power image of merged GPERi and Hoesch staining of boxed region in panel C. In the dorsal region GPERi staining is frequently observed only on a single side of the Hoesch stained nucleus (arrowheads). Although this pattern of staining was also observed in the ventral region of the ARH, GPERi appears to fill the cytoplasm of more cells in the ventral ARH region (arrows). GPERi appears to be mainly extranuclear and cytoplasmic, and not localized to the plasma membrane. The GPERi that is in apposition to the Hoesch nuclear staining may be asscociated with the endoplasmic reticulum (Revankar et al., 2005). Scale bars = 25 μm, 3V – third ventricle; ARH – arcuate nucleus of the hypothalamus; VMH – ventromedial nucleus of the hypothalamus.
CONCLUSIONS
Our data demonstrate that 48 hours after 2 μg EB priming, E2 rapidly facilitates lordosis within 30 minutes via activation of GPER that deactivate MPN MOR. The subcellular localization of ARH GPERi suggests that E2 acts on an intracellular GPER that mediates an extranuclear-signaling pathway, which is supported by our preliminary data that membrane impermeable estradiol conjugated to biotin did not facilitate lordosis in EB primed rats (Long et al., 2012). This study provides in vivo evidence and supports our hypothesis of an estradiol-GPER extranuclear signaling cascade in the hypothalamus that rapidly facilitates lordosis via deactivation of MPN MOR. It is unclear whether EB-only and E2 facilitation of lordosis use the same ER signaling pathways. However, the ARH distribution of GPER and the opioid peptide, orphanin FQ, (OFQ) are very similar (Sanathara et al., 2014), and both estradiol paradigms appear to require activation of the OFQ-opioid receptor-like receptor-1 (ORL-1) system in the ARH to reduce β-endorphin signaling and facilitate lordosis (Long et al., 2013; Sanathara et al., 2011). Thus, E2 GPER and EB only signaling appears to converge on the OFQ-ORL-1 system to reduce ARH β-endorphin output to MPN MOR as part of the mechanism for facilitation sexual receptivity.
Highlights.
G protein-coupled estrogen receptor 1 (GPER) is expressed in arcuate nucleus (ARH)
GPER immunoreactivity appears localized to cytoplasm
Activation of GPER in ARH of estradiol primed rats rapidly facilitates lordosis
Activation of GPER in ARH deactivates MPN μ-opioid receptors
Rapid extranuclear estradiol GPER signaling in the ARH facilitates lordosis
Acknowledgments
Authors thank Amelia Welborn, Dream Le, Lam Nguyen, Lauren Jewel, Mari Maciel, Caitlin Hengeveld, Priscilla Tea, Heang Seng, Veronica Thach and Nayna Sanathara for their technical assistance. Research was supported by NIH grant HD058638 (KS) and 5R25GM071638 (NIH RISE – CSULB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Clemens LG, Weaver DR. The role of gonadal hormone in the activation of feminine sexual behavior. In: Adler N, Pfaff D, Goy RW, editors. Handbook of Behavioral Neurobiology. Plenum Press; New York: 1985. pp. 183–227. [Google Scholar]
- Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of mu-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J Neurosci. 1998;18:3967–3976. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia BL, Mana A, Kim A, Sinchak K. Antagonism of estrogen receptors facilitates sexual receptivity through opioid circuits in the arcuate nucleus of the hypothalamus and the medial preoptic nucleus in estradiol primed non-receptive female rats, Society for Neuroscience. Abstract Viewer/Itinerary Planner; San Diego. Washington, DC: Society for Neuroscience; 2010. [Google Scholar]
- Gorosito SV, Lorenzo AG, Cambiasso MJ. Estrogen receptor alpha is expressed on the cell-surface of embryonic hypothalamic neurons. Neuroscience. 2008;154:1173–1177. doi: 10.1016/j.neuroscience.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych P. Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. J Neurosci. 2010;30:12950–12957. doi: 10.1523/JNEUROSCI.1158-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long N, Serey C, Welborn A, Sinchak K. Free estradiol signals through G-protein coupled receptor 30 (GPER) to rapidly facilitate lordosis via deactivation of medial preoptic nucleus-μ-opioid receptors in estradiol primed rats, Society for Neuroscience. Abstract Viewer/Itinerary Planner; San Diego, CA. USA; Washington, DC: Society for Neuroscience; 2013. p. 278.202. [Google Scholar]
- Long NP, Chhorvann S, Sinchak K. In estradiol primed rats subsequent free estradiol rapidly facilitates lordosis through G-protein coupled receptor 30 (GPR30), Society for Neuroscience. 2012 Neuroscience Meeting Planner; New Orleans. Washington, DC: Society for Neuroscience; 2012. [Google Scholar]
- Mahavongtrakul M, Kanjiya MP, Maciel M, Kanjiya S, Sinchak K. Estradiol dose-dependent regulation of membrane estrogen receptor-alpha, metabotropic glutamate receptor-1a, and their complexes in the arcuate nucleus of the hypothalamus in female rats. Endocrinol. 2013;154:3251–3260. doi: 10.1210/en.2013-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych P, Sinchak K. Temporal and concentration-dependent effects of oestradiol on neural pathways mediating sexual receptivity. J Neuroendocrinol. 2013;25:1012–1023. doi: 10.1111/jne.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons B, Rainbow TC, Snyder L, McEwen BS. Progesterone-like effects of estradiol on reproductive behavior and hypothalamic progestin receptors in the female rat. Neuroendocrinology. 1984;39:25–30. doi: 10.1159/000123950. [DOI] [PubMed] [Google Scholar]
- Priest CA, Vink KL, Micevych PE. Temporal regulation by estrogen of beta-preprotachykinin mRNA expression in the rat ventromedial nucleus of the hypothalamus. Brain Res Mol Brain Res. 1995;28:61–71. doi: 10.1016/0169-328x(94)00184-g. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol. 2008;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Sanathara NM, Moraes J, Kanjiya S, Sinchak K. Orphanin FQ in the mediobasal hypothalamus facilitates sexual receptivity through the deactivation of medial preoptic nucleus mu-opioid receptors. Horm Behav. 2011;60:540–548. doi: 10.1016/j.yhbeh.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanathara NM, Moreas J, Mahavongtrakul M, Sinchak K. Estradiol Upregulates Progesterone Receptor and Orphanin FQ Colocalization in Arcuate Nucleus Neurons and Opioid Receptor-Like Receptor-1 Expression in Proopiomelanocortin Neurons That Project to the Medial Preoptic Nucleus in the Female Rat. Neuroendocrinology. 2014 doi: 10.1159/000363324. Epub, May 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinchak K, Dewing P, Ponce L, Gomez L, Christensen A, Berger M, Micevych P. Modulation of the arcuate nucleus-medial preoptic nucleus lordosis regulating circuit: A role for GABAB receptors. Horm Behav. 2013;64:136–143. doi: 10.1016/j.yhbeh.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinchak K, Micevych PE. Progesterone blockade of estrogen activation of m-opioid receptors regulates reproductive behavior. J Neurosci. 2001;21:5723–5729. doi: 10.1523/JNEUROSCI.21-15-05723.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinchak K, Wagner EJ. Estradiol signaling in the regulation of reproduction and energy balance. Front Neuroendocrinol. 2012;33:342–363. doi: 10.1016/j.yfrne.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltysik K, Czekaj P. Membrane estrogen receptors - is it an alternative way of estrogen action? J Physiol Pharmacol. 2013;64:129–142. [PubMed] [Google Scholar]