Summary
Allergy is an immune response to complex mixtures of multiple allergens yet current models use a single synthetic allergen. Multiple allergens were modeled using two well-defined tetravalent allergens each specific for a distinct IgE thus enabling a systematic approach to evaluate the effect of each allergen and percent of allergen specific IgE on mast cell degranulation. We found the overall degranulation response caused by two allergens is additive for low allergen concentrations or low percent specific IgE, does not change for moderate allergen concentrations with moderate to high percent specific IgE, and is reduced for high allergen concentrations with moderate to high percent specific IgE. These results provide further evidence that supra-optimal IgE cross-linking decreases the degranulation response and establishes the two allergen model as a relevant experimental system to elucidate mast cell degranulation mechanisms.
Introduction
Common allergen sources such as peanut contain complex mixtures of allergens. Recent efforts have been made to identify each allergen present in the mixtures to determine the major allergens that are capable of eliciting a degranulation response in the majority of patients that possess the specific allergy. For example, of the 11 different proteins present in peanuts that are capable of inducing IgE antibody production, 4 have been identified as major allergens (Zhuang and Dreskin, 2013). And of the four major peanut allergens, Ara h 2 and Ara h 6 are responsible for the majority of the degranulation response initiated by crude peanut extract (CPE) (Porterfield et al, 2009). Interestingly, removal of either Ara h 2 or Ara h 6 from CPE does not affect the activity of the crude extract (Chen et al, 2011). However, if both are removed, the potency of the CPE is significantly diminished indicating that the presence of the second major allergen has negligible effects on the degranulation response (Chen et al, 2011). Despite the presence of multiple major allergens capable of inducing a degranulation response in a natural system, current models use only a single synthetic allergen paired with a single monoclonal IgE to stimulate a degranulation response (Passante and Frankish, 2009). Even with their limitations, the current models have helped to discover critical aspects of mast cell signaling. Of particular interest, recent work has identified the Src homology 2-containing inositol polyphosphate 5′-phosphatase (SHIP1) as a critical regulator in the suppression of mast cell degranulation in response to supra-optimal allergen-IgE cross-linking (Huber, 2013). In this study, using a two allergen model, we sought to further investigate the conditions where supra-optimal allergen-IgE cross-linking results in reduced mast cell degranulation. This was accomplished by using two allergens, each specific for a different monoclonal IgE, at varying concentrations to assess the conditions where the effect of the second allergen resulted in increased degranulation, no change in degranulation, and decreased degranulation associated with supra-optimal IgE cross-linking. Additionally, we evaluated how the degranulation response changes with decreasing percent of allergen specific IgE.
Results
Design of the Two Allergen Model
In our previous work, we describe the design, synthesis, and characterization of synthetic tetravalent allergens (Handlogten et al, 2013; Handlogten et al, 2012; Handlogten et al, 2013). These well-defined tetravalent allergens have several advantages compared to the widely used haptenated proteins, such as DNP conjugated to BSA (DNP-BSA), as model allergens. The method used to synthesize these widely used allergens relies on the nonspecific conjugation of DNP to the ε-amine of lysine residues of BSA. This process results in poorly defined allergens with significant heterogeneity in both the number of haptens per protein, and the sites of hapten conjugation, resulting in variable potency from batch to batch. This complicates results as only the average number of haptens per carrier can be determined with many of the haptens likely unavailable to bind to surface bound IgE due to steric constraints (Hlavacek et al, 1999; Xu et al, 1998). As a result, a small subpopulation of the synthetic allergen, with properties distinct from the average, may be responsible for the majority of the degranulation response. In the design of the tetravalent allergens we ensured that each hapten is available to bind to a distinct IgE antibody without the capacity to bind bivalently to a single IgE. In addition, the tetravalency models the valency of several common allergens including Ara h 3 from peanuts, Tri a 14 from wheat, and Cuc m 2 from melon each of which have 4 immunodominant epitopes (Denery-Papini et al, 2011; Rabjohn et al, 1999; Tordesillas et al, 2010). Finally, the synthetic scheme used for the tetravalent allergens ensures that each allergen is identical thus allowing for direct analysis of the allergen properties on the stimulation of mast cell degranulation. In the present study, we used two homotetravalent allergens (HmTA), each specific for a different IgE, to model the multiple major allergens found in typical allergen sources. In addition, we used a third, non-allergen specific IgE to better represent the heterogeneity of IgE present on mast cell surfaces. Combined, the two allergen model provides a more physiologically relevant allergy system to evaluate the effect of multiple allergy inducing proteins present in typical allergen sources on mast cell degranulation.
Evaluation of the Tetravalent Allergens
The two allergen model required two hapten/IgE pairs and a third non-allergen specific IgE. The DNP/IgEDNP pair is the most commonly used system to study mast cell degranulation; consequently, it was selected as the first hapten/IgE pair (Andrews et al, 2009; Passante and Frankish, 2009). The second hapten/IgE pair selected was dansyl/IgEdansyl. This hapten/IgE pair was selected because dansyl, similar to DNP, is a small molecule that is easily incorporated into multivalent designs (Figure 1A). Finally, IgEcyclin A was selected as the third IgE to represent IgE of other specificity present on the surface of mast cells. The first step in characterizing the two allergen model was to determine the affinity of DNP and dansyl for their respective IgE antibodies using a fluorescence quenching assay as described previously (Handlogten et al, 2011). Using this technique, we determined that the Kd of DNP for IgEDNP was 22 ± 2 nM and the Kd of dansyl for IgEdansyl was 54 ± 4 nM (Figure 1B). With the same technique, we also determined there was no cross-reactivity between the hapten/IgE pairs (Figure S1).
Figure 1. Characterization and Structure of the Homotetravalent Allergens.
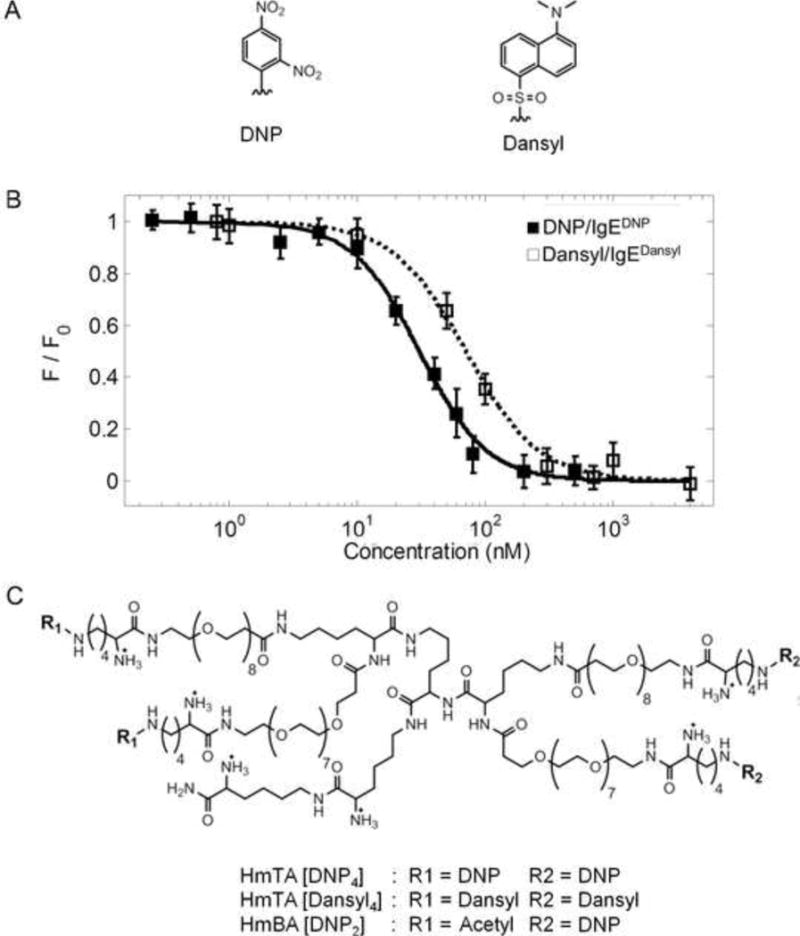
(A) Structures of the haptens DNP and dansyl used in the tetravalent allergens. (B) The affinity of each hapten was determined for its specific IgE using a fluorescence quenching technique (Supplemental Information). The Kd of DNP for IgEDNP was 22 ± 2 nM and the Kd of dansyl for IgEdansyl was 54 ± 4 nM. Data represents the means ± SD of triplicate experiments. (C) Structures of the synthetic allergens HmTA [DNP4], HmTA [dansyl4], and HmBA [DNP2].
Next, tetravalent versions of both DNP and dansyl were synthesized by conjugating each hapten to the tetravalent scaffold resulting in homotetravalent allergen DNP (HmTA [DNP4]) and homotetravalent allergen dansyl (HmTA [dansyl4], Figure 1C). In addition, a homobivalent allergen DNP (HmBA [DNP2]) with a structure identical to HmTA [DNP4], except with two acetylated arms, was also synthesized to evaluate the effect of allergen valency on mast cell degranulation (Figure 1C). To further establish that there was no cross-reactivity in the IgE-allergen pairs, mast cells were primed with IgEDNP, IgEdansyl, or IgEcyclin A and exposed to increasing concentrations of the synthetic allergens. As expected, the tetravalent allergens only stimulated a response when the mast cells were primed with the allergen specific IgE, IgEDNP for HmTA[DNP4] or IgEdansyl for HmTA [dansyl4] (Figure 2). Both HmTA [DNP4] and HmTA [dansyl4] stimulated a similar percent degranulation; however the HmTA [DNP4] allergen proved to be more potent, stimulating a maximum response at 10 nM compared to 100 nM for HmTA [dansyl4]. This is likely a reflection of the difference in affinities of DNP for IgEDNP (Kd of 22 nM) compared to dansyl for IgEdansyl (Kd of 54 nM). In line with previous work, the bivalent allergen, HmBA [DNP2] failed to stimulate a response under any condition due to insufficient valency (Figure 2) (Posner et al, 2007; Sil et al, 2007). Combined, these results validate that there is no cross-reactivity between the IgE-hapten pairs and demonstrates the suitability of the two allergen model to investigate the effect of multiple unique allergens on mast cell degranulation.
Figure 2. Specificity of the Tetravalent Allergens.
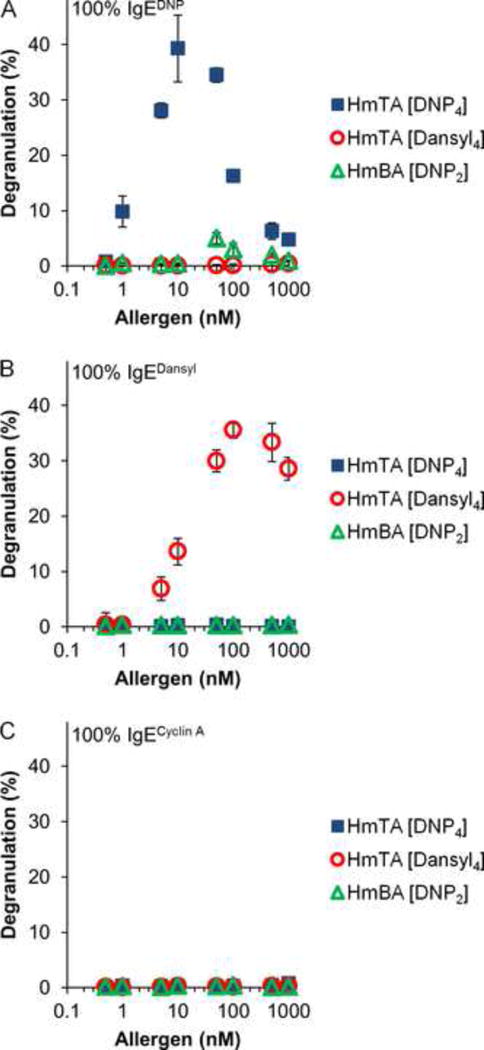
RBL cells were primed with (A) IgEDNP, (B) IgEdansyl, and (C) IgEcyclin A then exposed to increasing concentrations of the synthetic allergens HmTA [DNP4], HmTA [dansyl4], and HmBA [DNP2] to determine maximum degranulation response. HmTA [DNP4] and HmTA [dansyl4] only stimulated a response when the RBL cells were primed with IgEDNP and IgEdansyl respectively. HmBA [DNP2] did not stimulate a response under any condition. Data represents the means ± SD of triplicate experiments.
Evaluation of the Two Allergy Model System
Next, RBL cells were primed with an equimolar solution of IgEDNP and IgEdansyl. We previously established that the relative concentration of each IgE in solution is preserved on the surface of the mast cells (Handlogten et al, 2012). The mast cells were then exposed to mixtures of HmTA [DNP4] and HmTA [dansyl4] with concentrations ranging from 0.5 nM to 1000 nM (Figure 3, Figure S2). These results revealed a complex relationship between allergen concentration and the respective degranulation response. For low concentrations of HmTA [DNP4] and HmTA [dansyl4] the presence of both allergens resulted in increased degranulation, for moderate allergen concentrations the presence of a second allergen did not change the degranulation response, and for high allergen concentrations the presence of a second allergen decreased the degranulation response. Interestingly, at the highest allergen concentrations the overall degranulation response began to increase again as observed with HmTA [dansyl4] concentrations of 50 and 100 nM (Figure 3). The bell shaped dose-response has traditionally been attributed to the degree of IgE aggregation (Huber, 2013; Posner et al, 2007; Sil et al, 2007). As the allergen concentration increases, the degree of IgE cross-linking increases up to a point when monovalent allergen-IgE interactions begin to dominate due to excess allergen. According to this model, the presence of the second allergen could result in either increased degranulation when administered at moderate concentrations or no change in degranulation when administered at very high or very low concentrations. Instead, we observed that at HmTA [dansyl4] concentrations above 50 nM when the concentration of HmTA [DNP4] exceeded 10 nM the degranulation response decreased (Figure 3, Figure S2). Since these allergens each bind to a separate IgE, the decrease in the degranulation response cannot be attributed to competitive inhibition of IgE cross-linking caused by excess allergen. Instead, there is an optimal degree of IgE cross-linking on the surface of mast cells, above which, there are inhibitory pathways that become active to limit the degranulation response (Huber, 2013). Accordingly, the degranulation response continues to increase with allergen concentration until the optimal degree of IgE cross-linking is reached, as observed by the increased degranulation response with low concentrations of each HmTA [DNP4] and HmTA [dansyl4]. As the degree of cross-linking approaches the optimal amount, the additive effect of the second allergen decreases, as observed by the degranulation response with moderate concentrations of both HmTA [DNP4] and HmTA [dansyl4]. Finally, as the degree of cross-linking increases beyond the optimal level, the degranulation response decreases irrespective of which allergen is causing the increased IgE cross-linking as demonstrated by a decrease in degranulation observed at high concentrations of HmTA [DNP4] and HmTA [dansyl4].
Figure 3. Two Allergen Model Reveals Complex Relationship Between IgE Cross-linking and Degranulation.
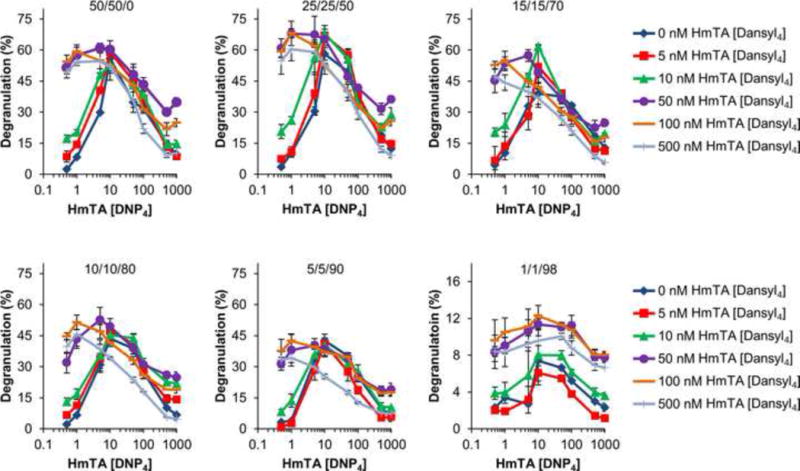
RBL cells were primed with mixtures of IgEs (50/50/0, 25/25/50, 15/15/70, 10/10/80, 5/5/90, and 1/1/98 IgEDNP/IgEdansyl/IgEcyclin A) at a saturating total IgE concentration of 1 μg/mL. The cells were washed and then exposed to mixtures of HmTA [DNP4] with HmTA [dansyl4] ranging from 0.5 to 1000 nM concentration of each allergen. Selected data shown here with complete results as Figure S2. Data represents the means ± SD of triplicate experiments.
Next, we investigated how the degranulation response changed with decreasing percent specific IgE by incorporating IgEcyclin A. As can be seen in Figure 3 and Figure S2, the trends observed with RBL cells primed with the 50/50 mix of IgEDNP and IgEdansyl are similar to those primed with 25/25/50 and 15/15/70 mix of IgEDNP/IgEdansyl/IgECyclin A. However, as the percent specific IgE is further decreased to 10/10/80 and 5/5/90: IgEDNP/IgEdansyl/IgEcyclin A the degranulation response exhibited a subtle change (Figure 3). Maximum degranulation still occurred at 10 nM HmTA [DNP4], however the decrease in degranulation previously observed with increasing HmTA [DNP4] concentration to 50 nM did not occur. Instead, the degranulation response reached a plateau at 10 nM HmTA [DNP4] and did not begin to decrease significantly until a concentration of 100 nM HmTA [DNP4]. This suggests that since there was a lower percent of allergen specific IgE on the surface of the mast cells, a higher concentration of the allergen was required to reach the same degree of IgE cross-linking. The binding curve of DNP to IgEDNP (Figure 1B) supports this theory. While the DNP-IgEDNP Kd is 22 nM (50% bound), 100% binding does not occur until ~200 nM demonstrating that there is increased binding of allergen to the surface bound IgE until a concentration of ~200 nM. Furthermore, as the percent of allergen specific IgE was decreased to 1/1/98: IgEDNP/IgEdansyl/IgEcyclin A, the degranulation response stimulated by the two allergens was approximately additive at all allergen concentrations. This result suggests that with only 1% specific IgE for each allergen there is not sufficient IgE to attain supra-optimal IgE cross-linking.
To further demonstrate that the changes in degranulation observed in Figure 3 were due to changes in IgE cross-linking and not due to some other property of DNP, a monovalent DNP ligand was used in place of HmTA [DNP4]. The RBL cells were primed with 25/25/50: IgEDNP/IgEdansyl/IgEcyclin A, which was the condition where maximum degranulation was observed (Figure 3). Then, the RBL cells were exposed to mixtures of HmTA [dansyl4] and monovalent DNP, with the concentration of each ranging from 0.5 to 1000 nM (Figure 4A). As expected, the monovalent DNP ligand did not cause any significant change in the degranulation response demonstrating that the differences in observed degranulation with increasing concentrations of HmTA [DNP4] in Figure 3 were due to changes in the cross-linking of the IgE antibodies on the RBL cell surface.
Figure 4. Effect of Valency on Degranulation in Two Allergen Model.
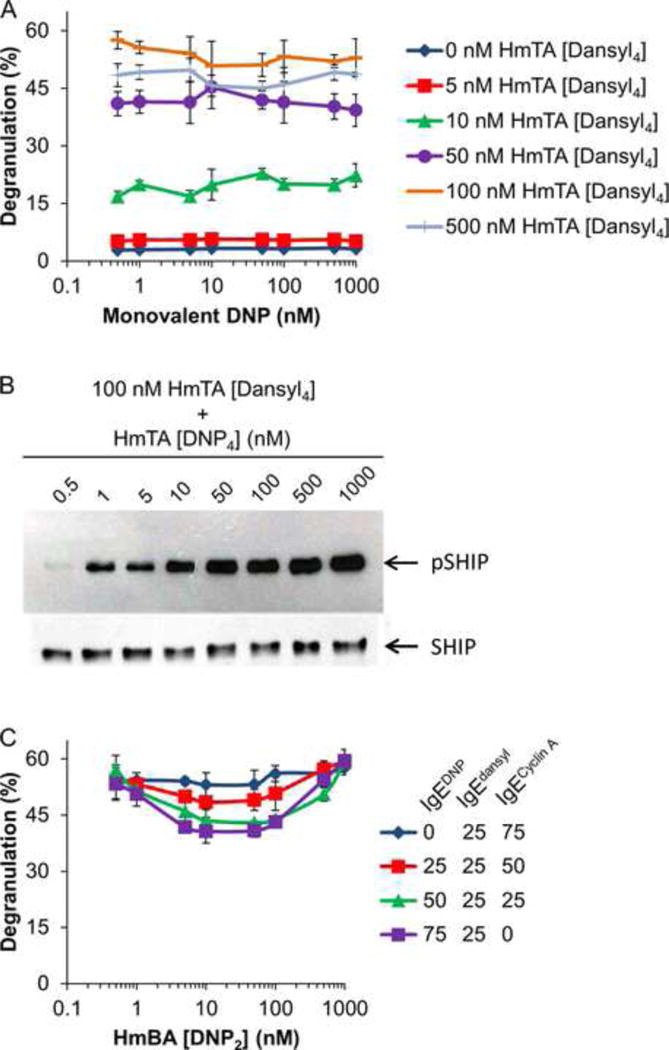
(A) RBL cells were primed with 25/25/50 IgEDNP/IgEdansyl/IgEcyclin A and then exposed to mixtures of HmTA [dansyl4] and monovalent DNP ranging from 0.5 to 1000 nM. (B) RBL cells were primed with an equimolar solution of IgEDNP and IgEdansyl then stimulated with 100 nM of HmTA [dansyl4] with 0.5–1000 nM of HmTA [DNP4]. The postnuclear supernatants were subjected to anti-SHIP immunoprecipitation then anti-phospho-tyrosine and anti-SHIP immunoblotting to demonstrate equal loading. (C) RBL cells were primed with mixtures of IgEs (0/25/75, 25/25/50, 50/25/25, and 75/25/0 IgEDNP/IgEdansyl/IgEcyclin A) at a total IgE concentration of 1 μg/mL followed by exposure to 100 nM HmTA [dansyl4] with HmBA [DNP2] ranging from 0.5 to 1000 nM. Data represents the means ± SD of triplicate experiments.
Several molecules have recently been identified as having a role in inhibitory pathways that limit degranulation in response to supra-optimal IgE cross-linking. Among these molecules, SHIP1 has been identified as a critical negative regulator of mast cell degranulation as demonstrated using SHIP1 deficient mast cells. SHIP1 deficient mast cells do not exhibit reduced degranulation in response to supra-optimal allergen concentrations. (Gimborn et al, 2005; Kraft and Kinet, 2007; Molfetta et al, 2007). Therefore, we investigated the phosphorylation of SHIP1 using the two allergen model. RBL cells were stimulated with 100 nM HmTA [Dansyl4], the concentration that elicited the highest response, (Figure 2B) with increasing HmTA [DNP4] from 0.5 to 1000 nM. Based on the results in Figure 3, we anticipated that there would be little phosphorylation of SHIP1 at the lowest concentrations of HmTA [DNP4] as these were the conditions where maximum degranulation occurred and that the phosphorylation of SHIP1 would increase with HmTA [DNP4] concentration to account for the decreased degranulation observed in Figure 3. Indeed, as observed in Figure 4B, the phosphorylation of SHIP1 increased with HmTA [DNP4] concentration confirming the role of SHIP1 as a negative regulator of mast cell degranulation that is activated in response to supra-optimal IgE cross-linking.
Bivalent allergens have been previously shown to form cyclic dimers with IgE and these clusters of cyclic dimers typically do not stimulate a degranulation response (Posner et al, 2007; Sil et al, 2007). We next investigated if the bivalent DNP allergen, HmBA [DNP2] which was unable to stimulate mast cell degranulation, (Figure 2) was able to decrease the degranulation response caused by HmTA [dansyl4]. For these experiments, the RBL cells were primed with four different IgE mixtures that had the same percent of IgEdansyl with increasing percent IgEDNP. The mixtures were 0/25/75, 25/25/50, 50/25/25, and 75/25/0: IgEDNP/IgEdansyl/IgEcyclin A. The RBL cells were then exposed to 100 nM HmTA [dansyl4] with increasing concentrations of HmBA [DNP2]. As observed in Figure 4B, in the absence of IgEDNP, the bivalent allergen HmBA [DNP2] had no effect on mast cell degranulation. However, as the percent of IgEDNP was increased there was a small reduction in the degranulation response observed from 5 to 100 nM HmBA [DNP2]. The reduction in degranulation response disappeared by 1000 nM HmBA [DNP2] presumably as IgEDNP cross-linking was inhibited due to excess HmBA [DNP2] in solution. The partial inhibition of degranulation observed with HmBA [DNP2] suggests that only large, signaling competent, clusters of IgE are capable of activating the pathways responsible for limiting the degranulation response.
Discussion
Mast cell degranulation experiences a bell-shaped dose response behavior with increasing allergen concentration. This response has traditionally been attributed to excess allergen competitively inhibiting IgE cross-linking on the mast cell surface such that at high allergen concentrations each IgE is bound monovalently to a different allergen preventing the formation of signaling competent clusters. Using the two allergen model we were able to demonstrate that the decrease in degranulation cannot be due to a decrease in IgE cross-linking, but to the contrary, is due to a further increase in IgE cross-linking. The specificity of the hapten-IgE pairs ensured that IgE cross-linking caused by the first allergen-IgE interactions on the RBL cells was not inhibited by the second allergen. Consequently, the decreased degranulation observed at high concentrations of both HmTA [DNP4] and HmTA [dansyl4] was due to increased IgE cross-linking. This confirms recent evidence suggesting that the decrease in degranulation at high allergen concentrations is caused by active inhibitory pathways (Huber, 2013). In particular, SHIP1 has been identified as a key regulator of mast cell degranulation which we confirmed using our two allergen model. However, the results in Figure 3 demonstrate that when mast cells are primed with high levels of allergen specific IgE and stimulated 50 or 100 nM HmTA [dansyl4], the degranulation response increased as the concentration of HmTA [DNP4] increased from 500 to 1000 nM despite increased SHIP1 phosphorylation (Figure 4B). The mechanisms through which SHIP1 and other regulatory molecules limit the degranulation response have yet to be fully elucidated. Further investigation and identification of the molecules involved in the suppression of degranulation will likely lead to the identification of novel molecular targets that could be exploited to treat patients with acute and chronic allergic diseases. The two allergen model is uniquely suited to analyze the effect of supra-optimal IgE cross-linking on mast cell degranulation and elucidate these critical aspects regulating mast cell degranulation.
Significance
Most allergen sources contain a complex mixture of allergens many of which are capable of stimulating a strong allergic response on their own and yet current allergen model systems use a single allergen specific for a single monoclonal IgE antibody. To investigate the influence of each allergen in these complex mixtures we used two well-defined tetravalent allergens each specific for a distinct IgE to model an allergen source containing multiple allergens. In addition, we used a third, non-allergen specific IgE to represent the heterogeneity of IgE present on mast cells. This system allowed us to investigate how the presence of two allergens effect the overall degranulation response and how the response stimulated by the two allergens changes as the percent IgE specific for each allergen is varied. Using this system, we demonstrated that at low allergen concentrations, the presence of a second allergen increases the degranulation response, at moderate allergen concentrations the second allergen does not change the degranulation response, and at high allergen concentrations the degranulation response decreases. Traditionally, the decreased degranulation observed at high allergen concentrations has been attributed to a decrease in IgE cross-linking caused by excess allergen in solution competitively inhibiting allergen-IgE interactions. However, each of the two allergens is specific for a different IgE so the decreased degranulation cannot be attributed to decreased IgE cross-linking. Therefore, our results provide further evidence of recently identified active inhibitory pathway that regulate mast cell degranulation. The mechanisms through which supra-optimal IgE cross-linking decreases the degranulation response remain to be fully elucidated and it is likely that further study of these pathways, using more physiologically relevant systems such as the two allergen model, will lead to novel molecular targets for treating allergy and asthma.
Experimental Procedures
Fluorescence Quenching Binding Assay
The binding constants of the monovalent haptens to the respective IgEs were determined using a previously described fluorescence quenching assay (Handlogten et al, 2011).
Synthesis of the tetravalent and bivalent synthetic allergens
All molecules were synthesized using standard Fmoc chemistry on a solid support as described in the supplemental materials.
Degranulation Assays
IgEdansyl (clone 27–74) and IgEcyclin A (clone BF683) were purchased from BD Biosciences. Degranulation assays were carried out as previously described (Handlogten et al, 2011).
Immunoprecipitation and Western Blotting
RBL cells were incubated over night with an equimolar solution of IgEDNP and IgEdansyl. Cells were washed twice and re-equilibrated to 37°C for 30 minutes prior to stimulation with the indicated allergen mixtures for 3 minutes. After stimulation, cells were scraped on ice, pelleted and solubilized with 0.5% NP-40 and 0.5% deoxycholate in 4°C phosphorylation solubilization buffer. Samples were normalized for protein content then subjected to immunoprecipitation using agaros conjugated monoclonal anti-SHIP antibody (P1C1) from Santa Cruz Biotechnology with three subsequent washing steps with phosphorylation buffer containing 0.5% NP-40. The precipitate was separated by SDS-PAGE and analyzed using Western blotting using an anti-p-Tyr antibody (PY99) from Santa Cruz Biotechnology or monoclonal anti-SHIP antibody (P1C1) from Santa Cruz Biotechnology as previously described (Gimborn et al, 2005).
Supplementary Material
Acknowledgments
We thank Dr. Bridget Wilson (University of New Mexico, Albuquerque, NM, U.S.A.) for generously providing us with IgEDNP and the RBL cell line and Dr. Bill Boggess at the Mass Spectrometry and Proteomics Facility in the University of Notre Dame for the use of MS instrumentation. This work was supported by the NIH-NIAID (National Institutes of Health National Institute of Allergy and Infectious Diseases) [grant numbers R03 AI085485 and R56 AI108884].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews NL, Pfeiffer JR, Martinez AM, Haaland DM, Davis RW, Kawakami T, Oliver JM, Wilson BS, Lidke DS. Small, mobile Fc epsilon R1 receptor aggregates are signaling competent. Immunity. 2009;31:469–479. doi: 10.1016/j.immuni.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhuang Y, Wang Q, Moutsoglou D, Ruiz G, Yen SE, Dreskin SC. Analysis of the effector activity of Ara h 2 and Ara h 6 by selective depletion from a crude peanut extract. J Immunol Methods. 2011;372:65–70. doi: 10.1016/j.jim.2011.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denery-Papini S, Bodinier M, Pineau F, Triballeau S, Tranquet O, Adel-Patient K, Moneret-Vautrin DA, Bakan B, Marion D, Mothes T, Mameri H, Kasarda D. Immunoglobulin-E-binding epitopes of wheat allergens in patients with food allergy to wheat and in mice experimentally sensitized to wheat proteins. Clin Exp Allergy. 2011;41:1478–1492. doi: 10.1111/j.1365-2222.2011.03808.x. [DOI] [PubMed] [Google Scholar]
- Gimborn K, Lessmann E, Kuppig S, Krystal G, Huber M. SHIP down-regulates Fc epsilon R1-induced degranulation at supraoptimal IgE or antigen levels. J Immunol. 2005;174:507–516. doi: 10.4049/jimmunol.174.1.507. [DOI] [PubMed] [Google Scholar]
- Handlogten MW, Kiziltepe T, Serezani AP, Kaplan MH, Bilgicer B. Inhibition of weak-affinity epitope-IgE interactions prevents mast cell degranulation. Nat Chem Biol. 2013;9:789–795. doi: 10.1038/nchembio.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handlogten MW, Kiziltepe T, Alves NJ, Bilgicer B. Synthetic allergen design reveals the significance of moderate affinity epitopes in mast cell degranulation. ACS Chem Biol. 2012;7:1796–1801. doi: 10.1021/cb300193f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handlogten MW, Kiziltepe T, Bilgicer B. Design of a heterotetravalent synthetic allergen that reflects epitope heterogeneity and IgE antibody variability to study mast cell degranulation. Biochem J. 2013;449:91–99. doi: 10.1042/BJ20121088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handlogten MW, Kiziltepe T, Moustakas DT, Bilgicer B. Design of a Heterobivalent Ligand to Inhibit IgE Clustering on Mast Cells. Chem Biol. 2011;18:1179–1188. doi: 10.1016/j.chembiol.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Hlavacek WS, Posner RG, Perelson AS. Steric effects on multivalent ligand-receptor binding: Exclusion of ligand sites by bound cell surface receptors. Biophys J. 1999;76:3031–3043. doi: 10.1016/S0006-3495(99)77456-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M. Activation/Inhibition of mast cells by supra-optimal antigen concentrations. Cell Commun Signal. 2013;11:7. doi: 10.1186/1478-811X-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft S, Kinet J. New developments in Fc epsilon RI regulation, function and inhibition. Nat Rev Immunol. 2007;7:365–378. doi: 10.1038/nri2072. [DOI] [PubMed] [Google Scholar]
- Molfetta R, Peruzzi G, Santoni A, Paolini R. Negative signals from Fc epsilon RI engagement attenuate mast cell functions. Arch Immunol Ther Exp. 2007;55:219–229. doi: 10.1007/s00005-007-0028-4. [DOI] [PubMed] [Google Scholar]
- Passante E, Frankish N. The RBL-2H3 cell line: its provenance and suitability as a model for the mast cell. Inflammation Res. 2009;58:737–745. doi: 10.1007/s00011-009-0074-y. [DOI] [PubMed] [Google Scholar]
- Porterfield HS, Murray KS, Schlichting DG, Chen X, Hansen KC, Duncan MW, Dreskin SC. Effector activity of peanut allergens: a critical role for Ara h 2, Ara h 6, and their variants. Clin Exp Allergy. 2009;39:1099–1108. doi: 10.1111/j.1365-2222.2009.03273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner RG, Geng D, Haymore S, Bogert J, Pecht I, Licht A, Savage PB. Trivalent antigens for degranulation of mast cells. Org Lett. 2007;9:3551–3554. doi: 10.1021/ol071175h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabjohn P, Helm E, Stanley J, West C, Sampson H, Burks A, Bannon G. Molecular cloning and epitope analysis of the peanut allergen Ara h 3. J Clin Invest. 1999;103:535–542. doi: 10.1172/JCI5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sil D, Lee JB, Luo D, Holowka D, Baird B. Trivalent ligands with rigid DNA spacers reveal structural requirements for IgE receptor signaling in RBL mast cells. ACS Chemical Biology. 2007;2:674–684. doi: 10.1021/cb7001472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordesillas L, Pacios LF, Palacin A, Cuesta-Herranz J, Madero M, Diaz-Perales A. Characterization of IgE epitopes of Cuc m 2, the major melon allergen, and their role in cross-reactivity with pollen profilins. Clin Exp Allergy. 2010;40:174–181. doi: 10.1111/j.1365-2222.2009.03401.x. [DOI] [PubMed] [Google Scholar]
- Xu KL, Goldstein B, Holowka D, Baird B. Kinetics of multivalent antigen DNP-BSA binding to IgE-Fc epsilon RI in relationship to the stimulated tyrosine phosphorylation of Fc epsilon RI. J Immunol. 1998;160:3225–3235. [PubMed] [Google Scholar]
- Zhuang Y, Dreskin SC. Redefining the major peanut allergens. Immunol Res. 2013;55:125–134. doi: 10.1007/s12026-012-8355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


