Abstract
Bortezomib, a proteasome inhibitor capable of direct anti-tumor effects, has been shown to prevent acute graft-versus-host disease (aGVHD) when administered in a short course immediately after bone marrow transplantation (BMT) in mice. However, when given continuously, CD4+ T cell mediated gastrointestinal tract damages increase GVHD mortality. To investigate the protective effects of bortezomib on other organs, we have used a CD8 dependent aGVHD model of C3H.SW donor T cells engrafted into irradiated C57BL/6 recipients (minor MHC mismatch), which lack significant gut GVHD. Our data in this model show that bortezomib can be given continuously to prevent and treat aGVHD mediated by CD8+ T cells, but this effect is organ-specific such that only skin, but not liver, protection was observed. Despite the lack of hepatic protection, bortezomib still significantly improved survival primarily due to its skin protection. Reduced skin GVHD by bortezomib was correlated with reduced serum and skin IL-6 levels. Administration of a blocking IL-6 antibody in this model also resulted in similar cutaneous GVHD protection. These results indicate that bortezomib or blockade of IL-6 may prevent CD8+ T cell mediated cutaneous aGVHD.
Keywords: Bortezomib, anti-IL-6 therapy, Skin acute GVHD
Introduction
Graft-versus-host disease (GVHD) has severely limited the use of allogeneic hematopoietic stem cell transplantation (allo-HSCT) for the treatment of hematological malignancies [1]. Donor T cell mediated alloreactive responses and conditioning regimen induced tissue damage, with subsequent activation of the innate immune system and inflammatory cytokine release, are implicated in the pathogenesis of acute GVHD (aGVHD) following allo-HSCT [2, 3].
Cytokines, such as TNF-α, can augment aGVHD through direct cytotoxic impact on host tissues [4]. Other proinflammatory cytokines including IL-1, IL-17, IL-6 and IFN-γ can indirectly lead to priming, activation and differentiation of respective immune cells[5].
IL-6 is a pro-inflammatory cytokine with pleiotropic biological activities in immune regulation and inflammation [6]. Murine studies [7-9] have previously demonstrated the benefit of IL-6 inhibition in GVHD management. Recently, there have been anecdotal clinical trials suggesting the benefit of such an approach in a limited number of patients [10, 11]. However, with the heterogeneous presentations of aGVHD, target organs for such beneficial effects need to be better identified.
Bortezomib, a proteasome inhibitor, is routinely used for the treatment of hematological malignancies [12]. In addition to its direct anti-tumor effects, bortezomib specifically depletes alloreactive T cells, permits Treg cell survival, and attenuates pro-inflammatory cytokine responses [13-15]. Bortezomib also augments graft versus tumor (GVT) responses by sensitizing tumor cells to cytolytic effector mechanisms [16]. These effects make proteasome inhibition an attractive therapeutic approach for GVHD and GVT. Indeed, clinical trials of bortezomib as a prophylaxis in the management of acute GVHD have demonstrated encouraging results[17, 18]. However, we have also previously shown that bortezomib can have a time and dose dependent detrimental pro-inflammatory effect, by causing a marked increase in CD4+ T cell-mediated aGVHD and gut pathology induced lethality resulting in early death of animals. These outcomes have made assessment of bortezomib in other organs very difficult [19]. To investigate the protective effects of bortezomib on other organs, we have used a CD8 dependent aGVHD model of C3H.SW donor T cells engrafted into irradiated C57BL/6 recipients, creating a MHC-matched, minor histocompatibility antigen (miHAg) mismatched model which does not result in significant gut GVHD pathology.
Continuous administration of bortezomib post-HSCT resulted in tissue specific protection such that only cutaneous, but not hepatic, GVHD was protected and this protection was associated with decreased IL-6 levels. IL-6 blockade had similar beneficial effects on skin but not liver GVHD, suggesting the direct role of IL-6 in skin GVHD pathogenesis. Furthermore, bortezomib administration resulted in the specific down-regulation of CXCR3, a skin homing chemokine receptor, on donor CD8+ T cells. These data indicate that prophylactic bortezomib administration can induce organ-specific protection from cutaneous acute GVHD.
Materials and Methods
Animals
Female donor C3H.SW (H2b) and recipient C57BL/6 (H2b) mice were purchased from Jackson Laboratory (Bar Harbor, ME). Animals were kept in pathogen-free conditions and used at 8 to 10 weeks of age. All mice were maintained at the UC Davis Medical Center vivarium in accordance with Institutional Animal Care and Use Committee (IACUC) standards.
Allogeneic hematopoietic stem cell transplantation (HSCT)
Donor bone marrow cells (BM) were collected and T cell depleted bone marrow cells (TCD-BM) were prepared using the monoclonal anti-Thy 1.2 (38H12) antibody and rabbit complement as previously described [20]. CD8+ T cells were negatively selected from spleen cell suspensions using CD8 T cell isolation kit (Miltenyi Biotec). Briefly, C57BL/6 (H2b) recipient mice received 950 cGy myeloablative dose of total body irradiation (TBI) from a 137Cs source followed by the infusion of 1.0 × 107 donor C3H.SW (H2b) TCD-BM intravenously with or without 2-3 × 106 donor C3H.SW purified CD8+ T cells. Recipient C57BL/6 mice were randomly grouped and treated either with PBS or bortezomib in PBS at a dose of 0.125 mg/kg intraperitoneally (I.P.) on days 0 and 6 followed by every five days for a total of 10 injections post bone marrow transplantation (BMT). Anti-IL-6 antibody (Stock: 10.47 mg/ml; CNTO1322, Centocor, PA) or isotype control (rat IgG2a; Stock: 9.378 mg/ml; CNTO345, Centocor, PA) antibody was administered intraperitoneally (1 mg/mouse) every week starting on day 0 for a total of 10 injections post BMT. Skin clinical score was evaluated based on previous studies [21] as healthy appearance = 0; skin lesions with alopecia less than 1 cm2 = 1; skin lesions with alopecia 1 to 2 cm2 = 2; skin lesions with alopecia larger than 2 cm2 in area = 3. Mice with tail, ear and paw scaling were added for an additional 0.3-point for each lesion. Mice with clinical scores over 3.3 were euthanized based on IACUC regulations.
Reagents
Bortezomib was provided by Millennium Pharmaceuticals (Cambridge, MA). Stock bortezomib solution (1 mg/mL) was prepared in Dulbecco’s phosphate buffered saline (PBS) and stored at -80°C prior to use. Anti-IL-6 antibody (Stock: 10.47 mg/ml; CNTO1322) and isotype control (Stock: 9.378 mg/ml; CNTO345) were provided by Centocor Inc (PA).
Histology
Formalin fixed paraffin embedded tissue sections were stained with hematoxylin and eosin and evaluated blindly and independently by two pathologists. A semi-quantitative scale from 0 to 4 was used where histopathologic changes were identified as minimal = 1, mild = 2, moderate = 3, and severe = 4. Images were acquired with an Olympus BX45 clinical microscope, and DP72 digital camera.
Cytokine analysis
Serum cytokine levels (TNF-α, IFN-γ, IL-6 and IL-17) were determined using the BD™ Cytometric Bead Array (CBA) with cytokine-specific bead sets and standards according to the manufacturer’s protocol.
Quantitative Real-time RT-PCR (qRT-PCR)
Quantitative real-time RT-PCR was done by using Applied Biosystems Cycler (AB Step-ONE Plus) with SYBR Green SuperMix (Applied Biosystems). Tissue RNA was extracted from skin samples using a Qiagen kit according to the Manufacturer’s protocol. After obtaining cDNA, samples were evaluated for gene expression levels using primers purchased from Superarray Qiagen (SABiosciences). β-actin and GADPH were used as house keeping genes and relative fold change expression was calculated as below:
The data are presented as relative fold change expression of each gene in comparison to TCD-BM group.
Antibodies and flow cytometry
Protocols were conducted as previously described [19]. CD8α-AF700 (BioLegend), CXCR3-PE (BioLegend), CD4-PE-Cy7 (eBioscience), Foxp3-FITC (eBioscience) and CD45-PB (BioLegend) were used for these studies. Skin samples were prepared as previously described [22]. Briefly, single cell suspensions (1 million cells) were first incubated with Fc Block (BD Pharmingen. San Diego, CA) for 10 mins, then co-incubated with antibodies for 20 min at 4°C followed by washing with staining buffer (PBS + 1% FBS). Foxp3 intracellular staining was performed using an eBioscience kit (Cat#00-5523-00) according to the manufacturer’s protocol. Flow cytometry was performed on a LSR Fortessa and data were analyzed by FlowJo software (TreeStar).
Statistics
Survival data was plotted by the Kaplan-Meier method and analyzed by the log-rank test. One-way or two-way ANOVA or Student’s t tests were performed to determine if mean values were significantly different (P < 0.05) when appropriate.
Results
Skin aGVHD in MHC-matched, miHAg-mismatched model is associated with increased serum IL-6 levels
In this study, a CD8-dependent aGVHD murine model was used. This is a MHC-matched, multiple miHAg-mismatched murine model in which C3H.SW donor cells are infused into lethally irradiated C57BL/6 recipients, mimicking the majority of marrow grafts for allo-HSCT cases seen clinically [23]. As previously shown [8, 23], infusion of T cell depleted bone marrow (TCD-BM) plus CD8+ T cells isolated from spleen result in aGVHD in skin and/or liver with minimal gastrointestinal (GI) pathology when compared to mice infused with TCD-BM only (Fig 1A and 1B). Skin damage was particularly severe and the primary cause of mortality in this model. While the majority of the recipients showed combined skin and liver aGVHD, twenty percent of the recipients suffered from liver-only GVHD with variable levels of liver damage (Fig 1C). There were no recipients with skin-only GVHD. Serum levels of TNF-α, IFN-γ, IL-17 and IL-6 were measured and the results were compared among different GVHD pathologic groups. IL-6 serum levels correlated with skin but not liver GVHD occurrence demonstrating a tissue-specific association (Fig 1D; 72.66 ± 18.22 V.S. 21.61 ± 4.77 V.S. 9.88 ± 2.26). There was no significant difference in the level of serum IL-6 between liver-only GVHD and TCD-BM (control) groups. Similarly, increased IL-6 expression was detected by qRT-PCR in the skin samples when compared with the control group (Fig 1E; P < 0.05), but there was no change noted in liver IL-6 expression (Fig 1F). These findings demonstrate that both systemic and local tissue IL-6 levels are associated with skin but not liver GVHD pathology in this model.
Figure 1. Characteristics of murine GVHD in MHC-matched miHAg-mismatched model.

C57BL/6 recipient mice received 950 cGy myeloablative dose of total body irradiation (TBI) from a 137Cs source. A dose of 1.0 × 107 donor C3H.SW TCD-BM cells was infused with or without 2-3 × 106 donor C3H.SW purified CD8+ T cells after TBI. GVHD target samples (gut, skin and liver) were harvested and stained with hematoxylin and eosin. The grading of histopathological GVHD damage in transplanted recipients was assessed according to the scoring system described in the Methods section. Serum samples were taken by cardiac punctures and stored for analysis. (A) Representative histopathologic examination of H&E stained sections of paraffin-embedded skin, liver and gut tissues. Arrows indicate erosion of epidermis with inflammatory infiltrate in the skin and arrowheads indicate marked inflammatory infiltration over portal triads in the liver. (B) Semiquantitative histologic grading (grade 0-4) of different target tissues. (C) Liver pathology scores were graded from 1 to 4 and were shown as percentage between skin and liver GVHD and liver GVHD only groups. (D) Serum IL-6 level was examined in skin and liver GVHD compared to liver only or TCD-BM only groups. (E) Relative RNA expression of IL-6 levels in the skin. (F) Relative RNA expression of IL-6 levels in the liver. All the data were collected from 2-3 independent experiments with at least 8 mice (N=8) per group. Data were analyzed by one-way ANOVA or Student’s t tests to determine if mean values were significantly different. P < 0.05 were considered significant.
Protective effect of bortezomib is specific for cutaneous, but not hepatic GVHD and correlates with decreased IL-6 levels
Donor bone marrow cells plus CD8+ T cells from C3H.SW were adoptively transferred into C57BL/6 mice. Mice were randomized and received either bortezomib or vehicle. We observed a significant decrease in the occurrence of skin aGVHD with bortezomib compared to the vehicle group (Fig 2A and 2B) without any differential decline in body weight (Fig 2C). Pathological examination further documented a tissue specific protection effect of bortezomib in skin but not liver GVHD (Fig 2D-F). While the TCD-BM + CD8 T cell groups exhibited multifocal necrosis and T cell infiltration in the epidermal and dermal areas of the skin, treatment with bortezomib did not substantially decrease the T cell infiltrate in the liver. Additionally, liver enzyme levels concurred with histopathology scoring showing no effects on liver pathology with bortezomib treatment (Figure 2G). Serum IL-6 levels were significantly reduced by bortezomib treatment (Fig 2H), supporting that the skin specific protective effect of bortezomib correlated with a reduction in serum IL-6 levels.
Figure 2. Tissue specific protection by continuous application of bortezomib with decreased skin, but not liver, GVHD is associated with serum IL-6 level.
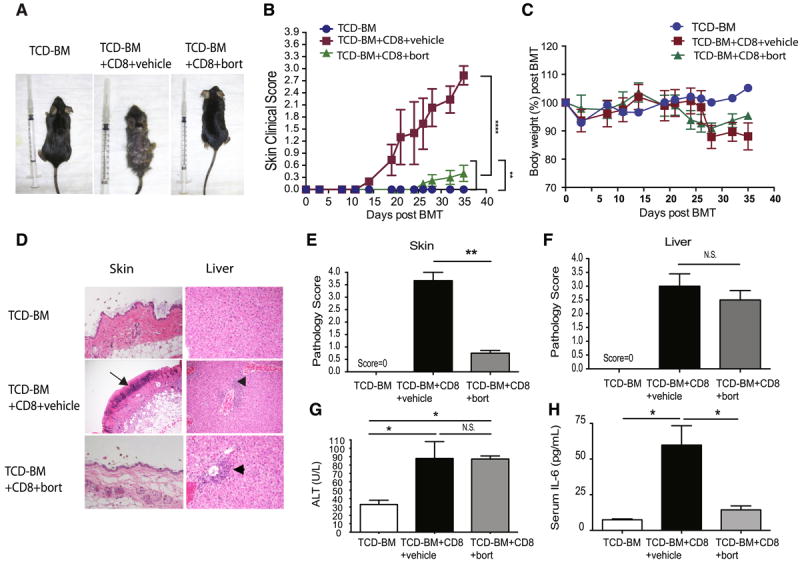
C57BL/6 recipient mice received 950 cGy myeloablative dose of total irradiation (TBI) from a 137Cs source. A dose of 1.0 × 107 donor C3H.SW BMCs was infused with or without 2-3 × 106 donor C3H.SW purified CD8+ T cells after TBI. Recipient C57BL/6 mice then received PBS or bortezomib at a dose of 0.125 mg/kg I.P. post BMT. (A) Representative clinical pictures of skin GVHD. (B) Skin clinical scores were evaluated twice a week. (C) Body weight changes among different groups. (D) Representative H&E stained sections of paraffin-embedded skin, liver from recipients. Arrows indicate erosion of epidermis with inflammation infiltrate. Arrowheads indicate inflammatory infiltration over portal triads in the liver. (E-F) Semiquantitative histological analysis (grade 0-4) of at least 6 examined recipients in each organ. (G) Liver enzymes from recipients with or without bortezomib therapy and TCD-BM control groups. (H) Serum IL-6 levels of recipients with or without bortezomib therapy were analyzed. All the data were collected from two independent experiments with at least 6 mice (N=6) per group. Data were analyzed by one-way ANOVA and post-hoc Tukey test to determine if mean values were significant among groups. P < 0.05 (*) and P < 0.01(**) were considered significant.
Anti-IL-6 therapy provides similar skin, but not liver, GVHD protection
To ascertain if IL-6 is indeed a target in cutaneous aGVHD pathogenesis or simply a surrogate of tissue inflammation and damage, similar experiments were done with weekly administration of anti-IL-6 monoclonal antibody post-HSCT. Anti-IL-6 treatment significantly decreased the severity of skin GVHD but had no protective effect on liver pathology (Fig 3A) or serum liver enzyme levels (Fig 3B) compared with the group that received an isotype control antibody. Both bortezomib and anti-IL-6 treatments led to a reduction in skin IL-6 gene expression (Fig 3C). It has been shown previously that IL-6 increases T cell trafficking to target tissues by augmenting chemokine expression levels in skin [24, 25]. Both bortezomib and anti-IL-6 therapy reduced donor derived CD8+T cell infiltration in the skin tissues (Fig 3D and 3E). CD8+CXCR3+ T cells from splenocytes were also reduced post bortezomib treatment compared to the GVHD control group (Fig 3F and 3G), indicating a potential mechanism for bortezomib on the tissue specific protection of skin GVHD. In addition, IL-6 blockade has been shown to induce regulatory T (Treg) cells following bone marrow transplantation [7]. Here, we also observed an increase in Treg cells with anti-IL-6 therapy compared to isotype controls (Fig 3H). The protective effects of both bortezomib and anti-IL-6 therapy further provided significant survival benefit compared with the GVHD group (Fig 3I) therefore supporting the role of IL-6 in mediating GVHD pathogenesis.
Figure 3. Anti-IL-6 therapy in skin GVHD model.
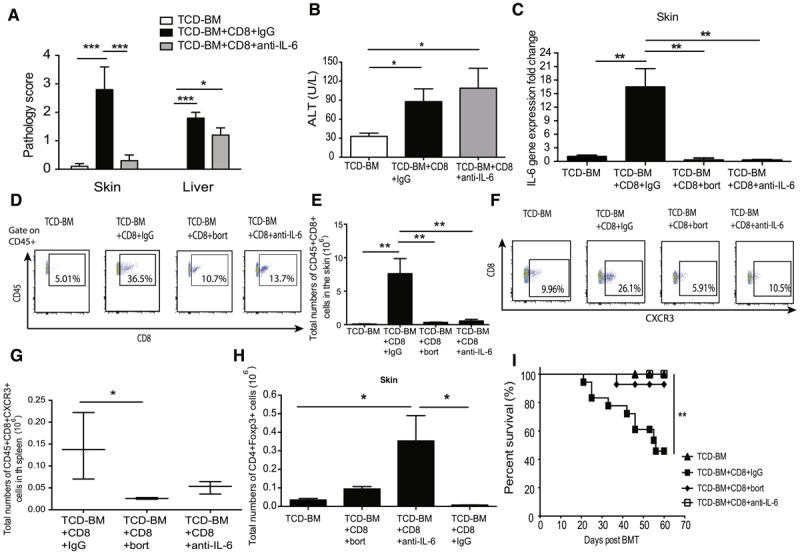
C57BL/6 recipient mice received 950 cGy TBI and were transplanted with TCD-BM with or without purified CD8+ T cells. Anti-IL-6 antibody or isotype control was given at a dose of 1 mg/mouse I.P. post BMT and every week there after. (A) Representative histopathologic scores were examined from paraffin-embedded skin, liver from recipients with or without anti-IL-6 therapy and TCD-BM control groups. (B) Liver enzymes from recipients with or without anti-IL-6 therapy and TCD-BM control groups. (C) Skin tissues were analyzed for IL-6 expression levels by qRT-PCR. (D) Skin samples were harvested and digested into single cell suspension for flow cytometry analysis. (E) Total numbers of CD45+CD8+ T cell in the skin tissues. (F) CD45+CD8+CXCR3+ T cells in spleen post BMT with different treatments. (G) Total numbers of CD45+CD8+CXCR3+ T cell in spleen (H) Total numbers of Treg cells were determined by flow cytometry in skin. (I) Survival curve among different treatment groups. All the data were collected from 2-3 independent experiments with at least 5 mice (N=5) per group. Data were analyzed by one-way ANOVA with post-hoc Tukey test. Survival curves were pooled from 2-3 independent experiments with 14-18 mice per group and plotted by the Kaplan-Meier method and analyzed by the log-rank test. P < 0.05 (*), P < 0.01 (**), P < 0.001 (***) were considered significant.
Discussion
We and others have shown protective effects of bortezomib administration in major MHC mismatched models when unselected T cell populations were used as a means to prevent aGVHD [26]. However, continuous treatment of bortezomib resulted in marked increases in GVHD mortality due to CD4+ T cell responses affecting the gut [27]. The severe toxicity and its associated early mortality prevented us from better understanding the effects of bortezomib on other GVHD target organs. The current study demonstrates that continuous bortezomib treatment can be safely administered in models where CD8+ T cells are the primary mediators of GVHD and this treatment results in the selective protection of skin GVHD. Furthermore, our study highlights the role of IL-6 in mediating skin aGVHD as well as it’s potential as a biomarker of response to bortezomib treatment of skin aGVHD. Our data on IL-6 inhibition further support that IL-6 may have a mechanistic effect in skin pathology as previously suggested [9] rather than solely being a biomarker for tissue injury and inflammation. It has also been demonstrated that macrophages are one of the main sources for IL-6 secretion and inhibiting macrophage activation can successfully abrogate skin GVHD in aGVHD models[28]. Therefore, it is likely that bortezomib, as an inhibitor of NF-kb, can directly affect macrophage activation and prevent cutaneous GVHD lesions. Binding of IL-6 to its receptors on endothelial cells can trigger trans-signaling and activate the signal transducer and activator of transcription-3 (STAT3) pathway [29], resulting in chemokine expression and T cell recruitment [24, 30]. In our experiments, decreased homing of CD8+ T cells to skin following anti-IL-6 therapy suggests a potential role of IL-6 in the homing of these T cells, although further experiments are required to prove this concept. In addition, we have also shown that the reduction of IL-6 can enhance regulatory T cells during donor T cell reconstitution, aiding in the further protection from the destructive inflammatory response to engraftment.
The concept of organ-specific pathological mechanisms in GVHD has recently been reevaluated. Chemokines and their receptors can be one of the explanations for the diversity of pathological changes in GVHD. CXCL10/CXCR3 axis has been shown to play a pivotal role in skin aGVHD responses post allo-HSCT [31]. In our experiments, bortezomib treatment resulted in a reduction of skin homing of CXCR3+CD8+ T cells, which likely contributes to its skin-specific protection effects. In addition, IL-6 has been shown to specifically decrease CXCL10, a chemokine ligand for CXCR3, suggesting that bortezomib’s effects on homing of T cells can be exerted through the IL-6-CXCL10-CXCR3 axis. Regarding T cell trafficking into the liver, it has been previously demonstrated that CCR2 is required for CD8 T cell migration into liver and the GI tract, but not into skin, in an acute GVHD model [32]. Furthermore, CCR2 has been shown to bind to CCL2, and CCL2 is induced by IFN-γ and TNF-α[33]. We did not see a difference of IFN-γ and TNF-α (data not shown) with or without bortezomib treatment in line with the lack of effects noted in liver pathology in our murine model. It is likely that bortezomib only affects certain chemokines that contribute to preferential target organ protection. It will be important for future clinical trials to investigate whether bortezomib has skin specific protective effects for select GVHD patients.
The pathology seen in GVHD in response to bortezomib also reveals a possible dose dependent characteristic of proteasome inhibition. While the higher dose of bortezomib treatment (0.625 mg/kg mouse) in this model led to early mortality (data not shown), lower doses (0.125 mg/kg) protected mice from GVHD pathogenesis. This suggests that the therapeutic index for bortezomib in allogeneic BMT given early post-BMT is narrow and appropriate caution should be exercised with regards to the benefits and risks of bortezomib treatment.
Recent clinical trials of bortezomib[17, 18] or anti-IL-6 receptor monoclonal antibody treatment (Tocilizumab)[11] have demonstrated promising results in the management of aGVHD, but such results require further dissection before larger clinical trials can be initiated. The animal model used in our current study can help improve future translational research in this area. The results of this study can increase our understanding of tissue specific GVHD pathology with an additive implication of appropriate GVHD patient population selection for bortezomib and/or anti-IL-6 therapy in clinical trials.
Acknowledgments
We thank Monja Metcalf and Weihong Ma for technical help and useful advice. This work has been supported by National Cancer Institute (NCI) grant 5R01CA102282-08.
Footnotes
Financial Disclosure: M.A. received research funding from Millennium Company.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7:340–52. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 2.Das R, Komorowski R, Hessner MJ, Subramanian H, Huettner CS, Cua D, et al. Blockade of interleukin- 23 signaling results in targeted protection of the colon and allows for separation of graft-versus-host and graft-versus-leukemia responses. Blood. 2010;115:5249–58. doi: 10.1182/blood-2009-11-255422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annual review of immunology. 2007;25:139–70. doi: 10.1146/annurev.immunol.25.022106.141606. [DOI] [PubMed] [Google Scholar]
- 4.Levine JE. Implications of TNF-alpha in the pathogenesis and management of GVHD. International journal of hematology. 2011;93:571–7. doi: 10.1007/s12185-011-0803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12:443–58. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kishimoto T. IL-6: from its discovery to clinical applications. Int Immunol. 2010;22:347–52. doi: 10.1093/intimm/dxq030. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Das R, Komorowski R, Beres A, Hessner MJ, Mihara M, et al. Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood. 2009;114:891–900. doi: 10.1182/blood-2009-01-197178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tawara I, Koyama M, Liu C, Toubai T, Thomas D, Evers R, et al. Interleukin-6 modulates graft-versushost responses after experimental allogeneic bone marrow transplantation. Clin Cancer Res. 2011;17:77–88. doi: 10.1158/1078-0432.CCR-10-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Huu D, Matsushita T, Jin G, Hamaguchi Y, Hasegawa M, Takehara K, et al. IL-6 blockade attenuates the development of murine sclerodermatous chronic graft-versus-host disease. The Journal of investigative dermatology. 2012;132:2752–61. doi: 10.1038/jid.2012.226. [DOI] [PubMed] [Google Scholar]
- 10.Gergis U, Arnason J, Yantiss R, Shore T, Wissa U, Feldman E, et al. Effectiveness and safety of tocilizumab, an anti-interleukin-6 receptor monoclonal antibody, in a patient with refractory GI graftversus-host disease. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:e602–4. doi: 10.1200/JCO.2010.29.1682. [DOI] [PubMed] [Google Scholar]
- 11.Drobyski WR, Pasquini M, Kovatovic K, Palmer J, Douglas Rizzo J, Saad A, et al. Tocilizumab for the treatment of steroid refractory graft-versus-host disease. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17:1862–8. doi: 10.1016/j.bbmt.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreau P, Richardson PG, Cavo M, Orlowski RZ, San Miguel JF, Palumbo A, et al. Proteasome inhibitors in multiple myeloma: 10 years later. Blood. 2012;120:947–59. doi: 10.1182/blood-2012-04-403733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tung D, Cheung PH, Kaur P, Foreman O, Kavirayani A, Hain HS, et al. Anti-Inflammatory and Immunomodulatory Effects of Bortezomib in Various in vivo Models. Pharmacology. 2011;88:100–13. doi: 10.1159/000330067. [DOI] [PubMed] [Google Scholar]
- 14.Cullen SJ, Ponnappan S, Ponnappan U. Proteasome inhibition up-regulates inflammatory gene transcription induced by an atypical pathway of NF-kappaB activation. Biochemical pharmacology. 2010;79:706–14. doi: 10.1016/j.bcp.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanco B, Perez-Simon JA, Sanchez-Abarca LI, Caballero-Velazquez T, Gutierrez-Cossio S, Hernandez-Campo P, et al. Treatment with bortezomib of human CD4+ T cells preserves natural regulatory T cells and allows the emergence of a distinct suppressor T-cell population. Haematologica. 2009;94:975–83. doi: 10.3324/haematol.2008.005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ames E, Hallett WH, Murphy WJ. Sensitization of human breast cancer cells to natural killer cellmediated cytotoxicity by proteasome inhibition. Clinical and experimental immunology. 2009;155:504– 13. doi: 10.1111/j.1365-2249.2008.03818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koreth J, Stevenson KE, Kim HT, McDonough SM, Bindra B, Armand P, et al. Bortezomib-based graftversus-host disease prophylaxis in HLA-mismatched unrelated donor transplantation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:3202–8. doi: 10.1200/JCO.2012.42.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koreth J, Stevenson KE, Kim HT, Garcia M, Ho VT, Armand P, et al. Bortezomib, tacrolimus, and methotrexate for prophylaxis of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation from HLA-mismatched unrelated donors. Blood. 2009;114:3956–9. doi: 10.1182/blood-2009-07-231092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun K, Wilkins DE, Anver MR, Sayers TJ, Panoskaltsis-Mortari A, Blazar BR, et al. Differential effects of proteasome inhibition by bortezomib on murine acute graft-versus-host disease (GVHD): delayed administration of bortezomib results in increased GVHD-dependent gastrointestinal toxicity. Blood. 2005;106:3293–9. doi: 10.1182/blood-2004-11-4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun K, Hsiao HH, Li M, Ames E, Bouchlaka M, Welniak LA, et al. IFN-gamma receptor-deficient donor T cells mediate protection from graft-versus-host disease and preserve graft-versus-tumor responses after allogeneic bone marrow transplantation. J Immunol. 2012;189:2033–42. doi: 10.4049/jimmunol.1102853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson BE, McNiff JM, Matte C, Athanasiadis I, Shlomchik WD, Shlomchik MJ. Recipient CD4+ T cells that survive irradiation regulate chronic graft-versus-host disease. Blood. 2004;104:1565–73. doi: 10.1182/blood-2004-01-0328. [DOI] [PubMed] [Google Scholar]
- 22.Ginhoux F, Collin MP, Bogunovic M, Abel M, Leboeuf M, Helft J, et al. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. The Journal of experimental medicine. 2007;204:3133–46. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shlomchik WD, Couzens MS, Tang CB, McNiff J, Robert ME, Liu J, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–5. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 24.Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol. 2005;175:3463–8. doi: 10.4049/jimmunol.175.6.3463. [DOI] [PubMed] [Google Scholar]
- 25.McLoughlin RM, Jenkins BJ, Grail D, Williams AS, Fielding CA, Parker CR, et al. IL-6 trans-signaling via STAT3 directs T cell infiltration in acute inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9589–94. doi: 10.1073/pnas.0501794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun K, Welniak LA, Panoskaltsis-Mortari A, O’Shaughnessy MJ, Liu H, Barao I, et al. Inhibition of acute graft-versus-host disease with retention of graft-versus-tumor effects by the proteasome inhibitor bortezomib. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8120–5. doi: 10.1073/pnas.0401563101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun K, Li M, Sayers TJ, Welniak LA, Murphy WJ. Differential effects of donor T-cell cytokines on outcome with continuous bortezomib administration after allogeneic bone marrow transplantation. Blood. 2008;112:1522–9. doi: 10.1182/blood-2008-03-143461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishiwaki S, Nakayama T, Murata M, Nishida T, Terakura S, Saito S, et al. Dexamethasone Palmitate Ameliorates Macrophages-Rich Graft-versus-Host Disease by Inhibiting Macrophage Functions. PloS one. 2014;9:e96252. doi: 10.1371/journal.pone.0096252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fielding CA, Jones GW, McLoughlin RM, McLeod L, Hammond VJ, Uceda J, et al. Interleukin-6 signaling drives fibrosis in unresolved inflammation. Immunity. 2014;40:40–50. doi: 10.1016/j.immuni.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hurst SM, Wilkinson TS, McLoughlin RM, Jones S, Horiuchi S, Yamamoto N, et al. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14:705–14. doi: 10.1016/s1074-7613(01)00151-0. [DOI] [PubMed] [Google Scholar]
- 31.Piper KP, Horlock C, Curnow SJ, Arrazi J, Nicholls S, Mahendra P, et al. CXCL10-CXCR3 interactions play an important role in the pathogenesis of acute graft-versus-host disease in the skin following allogeneic stem-cell transplantation. Blood. 2007;110:3827–32. doi: 10.1182/blood-2006-12-061408. [DOI] [PubMed] [Google Scholar]
- 32.Terwey TH, Kim TD, Kochman AA, Hubbard VM, Lu S, Zakrzewski JL, et al. CCR2 is required for CD8- induced graft-versus-host disease. Blood. 2005;106:3322–30. doi: 10.1182/blood-2005-05-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ichiba T, Teshima T, Kuick R, Misek DE, Liu C, Takada Y, et al. Early changes in gene expression profiles of hepatic GVHD uncovered by oligonucleotide microarrays. Blood. 2003;102:763–71. doi: 10.1182/blood-2002-09-2748. [DOI] [PubMed] [Google Scholar]


