Abstract
Heart failure represents a major cause of morbidity and mortality in the western society. Cardiac myocyte loss due to apoptosis plays a significant role in the progression of heart failure. The extracellular matrix (ECM) maintains the structural integrity of the heart and allows the transmission of electrical and mechanical signals during cardiac contraction and relaxation. Matricellular proteins, a class of non-structural ECM proteins, play a significant role in ECM homeostasis and intracellular signaling via their interactions with cell surface receptors, structural proteins, and/or soluble extracellular factors such as growth factors and cytokines. Osteopontin (OPN), also called cytokine Eta-1, is a member of matricellular protein family. Normal heart expresses low levels of OPN. However, OPN expression increases markedly under a variety of pathophysiological conditions of the heart. Many human and transgenic mice studies provide evidence that increased OPN expression, specifically in myocytes, associates with increased myocyte apoptosis and myocardial dysfunction. This review summarizes OPN expression in the heart, and its role in myocyte apoptosis and myocardial function.
Keywords: Osteopontin, Myocytes, Apoptosis, Heart Function
I. Introduction
Heart failure represents a major cause of morbidity and mortality in the western society, affecting nearly 5 million Americans. In United States, an estimated 400,000 to 700,000 new cases of heart failure are diagnosed each year. Heart failure, more common in people over the age of 65 years, is a progressive disease in which the heart loses the ability to pump enough blood to meet the metabolic demand of the body. Myocytes, a major cell-type of the heart, are responsible for the pumping ability of the heart. Adult cardiac myocytes are generally considered as terminally differentiated (Cheng and Force, 2010). Myocyte loss, either acute substantial loss or chronic low levels of apoptosis, is considered as a major contributing factor towards the development of heart failure.
Myocyte loss can occur due to autophagy, necrosis and/or apoptosis. All these types of cell death are observed in the heart during the progression of heart failure (Whelan et al., 2010). Autophagy, activated during nutrient deprivation, is a highly conserved process for the degradation of proteins and organelles. It involves the formation of double-membrane-bounded structures known as autophagosomes which fuse with lysosomes to form autophagolysosomes and their contents are then degraded by acidic lysosomal hydrolases (Mizushima et al., 2002). Autophagy is a critical process for maintenance of cellular and whole-body homeostasis. Increased autophagy is observed in a variety of pathologic conditions of the heart including cardiac hypertrophy, ischemia/reperfusion (I/R) injury and myocardial infarction (MI). Transgenic mice studies provide evidence that enhanced autophagic flux may contribute to cardiac dysfunction (Whelan et al., 2010). Necrosis occurs when cells are exposed to excessive stress or environmental conditions such as lack of oxygen and essential nutrients during an ischemic event (e.g. MI or stroke), elevated temperature and mechanical strain (e.g. trauma). It can also occur as a result of an incomplete execution of apoptosis (Formigli et al., 2000). Myocyte necrosis is a major contributor of heart failure associated with several cardiac pathologies (Whelan et al., 2010; Tavernarakis, 2007). Apoptosis is a highly regulated and energy-requiring process in which activation of signaling cascades induces cell death (Orogo et al., 2013). Myocyte loss due to apoptosis is recognized as an important determinant of structure and function of the heart, and is suggested to play a significant role in the progression of heart failure. Myocyte apoptosis occurs in the myocardium of patients during heart failure and in animal models of myocardial hypertrophy and failure (Whelan et al., 2010; Tavernarakis, 2007; Orogo and Gustafsson, 2013).
Extracellular matrix (ECM) modulates many cellular functions including cell adhesion, migration, differentiation, and survival (Bowers et al., 2010). The components of ECM include basic structural proteins such as collagen and elastin, and specialized proteins such as fibronectin, proteoglycans and matricellular proteins. Matricellular proteins are a class of non-structural ECM proteins exerting regulatory functions, most likely through their interactions with cell surface receptors, the structural proteins, and soluble extracellular factors such as growth factors and cytokines. Their expression is generally induced following an injury (Frangogiannis, 2012). Osteopontin (OPN) is a member of matricellular protein family. Heart expresses low basal levels of OPN. However, expression of OPN increases markedly in the heart under a variety of pathophysiological conditions (Singh et al., 2010a). Evidence has been provided that increased OPN expression, specifically in myocytes, associates with increased myocyte apoptosis and myocardial dysfunction (Subramanian et al., 2007; Renault et al., 2010; Dalal et al., 2014). While lack of OPN associates with reduced fibrosis in different models of myocardial hypertrophy and failure (Trueblood et al., 2001; Subramanian et al., 2007; Sam et al., 2004; Matsui et al., 2004), this review summarizes OPN expression in the heart, and its role in the induction of myocyte apoptosis and myocardial dysfunction.
II. OPN: a multifunctional protein
OPN (also called cytokine Eta-1) is a glycosylated phosphoprotein with a high acidic amino acid content (Singh et al., 2010a; Wolk, 2014). While first isolated from mineralized bone matrix in 1986 (Oldberg et al., 1986), OPN has since been shown to be synthesized by a variety of tissues and cell-types and secreted into body fluids (Wang and Denhardt, 2008; Singh et al., 2010a). OPN is a hydrophilic protein with isoelectric point of ~3.5. The predicted molecular weight of human full-length OPN is ~35kDa. It consists of 314 amino acid residues with 42 serine, 48 aspartic acid and 27 glutamic acid residues. 27 out of 42 serine residues can undergo phosphorylation. It has calcium and heparin binding domains, and undergoes O-linked and N-linked glycosylation. Due to the presence of acidic amino acid residues and extensive post-translational modifications, apparent molecular weight of OPN on SDS-PAGE can range from 45–75 kDa. Although, OPN is generally described as a cell-secreted protein, however, an alternative translation of a non-AUG site downstream of the canonical AUG sequence is suggested to generate an intracellular isoform (iOPN) (Wang and Denhardt, 2008). OPN has RGD (Arg-Gly-Asp) cell-binding sequence and interacts with αvβ1, αvβ3, αvβ5 and α8β1 integrins in an RGD-dependent manner (Kazanecki et al., 2007; Scatena et al., 2007). The SVVYGLR (Ser-Val-Val-Tyr-Gly-Leu-Arg) sequence, which becomes accessible upon cleavage of OPN by thrombin, interacts with α9β1 and α1β1 integrins. Variants of hyaluronan receptor, CD44, have also been identified as a receptor for OPN.
OPN is described as a protein with diverse biological functions including bone resorption and calcification, tumorigenesis, immune-modulation, wound healing, cell adhesion, chemotaxis, cell survival and apoptosis etc. (Wang and Denhardt, 2008; Singh et al., 2010a; Singh et al., 2010b; Kahles et al., 2014). Acting as a cytokine, OPN is shown to play a key role in immune cell recruitment and type-1 (Th1) cytokine expression at the sites of inflammation. With respect to cardiovascular disease, OPN is suggested to play a critical role in atherosclerosis, valvular stenosis, hypertrophy, myocardial infarction (MI) and heart failure (Singh et al., 2007; Scatena et al., 2007; Wolak et al., 2014). The diverse biological functions of OPN can be attributable due to its structural features, post-translational modifications, interaction with multiple receptors, and different isoforms. Matrix metalloproteinses (MMP) cleave OPN, thereby affecting migratory and adhesive properties of OPN (Scatena et al., 2007).
III. OPN: cell survival or death
In tumor cells, OPN is accepted as a pro-survival signal (Cao et al., 2012; Wai and Kuo, 2008). It is also shown to inhibit apoptosis in endothelial cells (Scatena et al., 1998) and melanocytes (Geissinger et al., 2002). In addition, mice lacking OPN exhibit significantly higher apoptosis in both tubular epithelium and interstitium during the injury phase of post-ischemic acute renal failure (Persy et al., 2003). Most of the anti-apoptotic actions of OPN are attributed to its interaction with αVβ3 integrins and activation of NF-kB. In IL-3-dependent cells, the anti-apoptotic actions of OPN are demonstrated via its interaction with CD44 receptor and activation of PI-3-kinase/Akt cascade (Lin and Yang-Yen, 2001). In contrast, chondrocyte apoptosis was lower in mice lacking OPN in an experimental model of rheumatoid arthritis (Yumoto et al., 2002). Mice lacking OPN also exhibited decreased apoptosis in their spleen and thymus in response to hindlimb-unloading by tail suspension (Wang et al., 2007). Cardiac fibroblasts isolated from the myocardium of mice lacking OPN exhibited enhanced necrosis, but decreased apoptosis, in response to H2O2 treatment when compared to their wild-type (WT) counterparts (Zohar et al., 2004). In vascular smooth muscle cells, treatment with OPN stimulated autophagy via the involvement of integrin and CD44 pathways (Zheng et al., 2011). In a recent study, knockdown of OPN inhibited breast cancer metastasis by regulating αvβ3 integrin expression and inducing autophagy (Zhang et al., 2014). Collectively these studies suggest involvement of OPN in all three types of cell death. However, the cell death or survival response appears to vary with different cell types and tissues.
IV. OPN expression and myocardial dysfunction
The normal adult heart expresses only low levels of OPN. Cell types of the heart, i.e. myocytes, fibroblasts and microvascular endothelial cells, express low basal levels of OPN. Stimuli, such as angiotensin II (Ang II), glucocorticoid hormone and cytokines (interleukin-1β+interferon-γ) increase OPN expression in different cell-types of the heart (Singh et al., 2010a; Singh et al., 2010b). Cultured neonatal cardiac myocytes express OPN where endothelin-1 and norepinephrine increased OPN expression (Graf et al., 1997). OPN expression increases markedly in the heart under a variety of pathophysiological conditions of the heart (Singh et al., 2010a; Singh et al., 2010b). OPN mRNA levels were readily detectable in the hypertrophied ventricles of rats subjected to the clipping of the renal artery (2K1C) for 6 weeks and aortic banding (AOB) for 4 weeks when compared to the normal adult heart or ventricles of sham-operated animals (Graf et al., 1997). Here, OPN expression correlated with the expression of ANP, a known marker of myocyte hypertrophy. In the above two models of left ventricular (LV) hypertrophy, myocytes were identified as a major source of OPN transcript and protein. Using spontaneously hypertensive rat (SHR) and AOB rats as models of myocardial hypertrophy and heart failure, it was demonstrated that increased OPN expression in the heart coincides with the development of heart failure. In AOB model, OPN mRNA levels increased by ~1.9-fold during myocardial hypertrophy phase. However, the increase in OPN mRNA levels was ~8-fold during heart failure (Singh et al., 1999). In this study, treatment of SHR with captopril, angiotensin converting enzyme inhibitor, before or after the onset of heart failure decreased OPN expression in the heart. In situ hybridization and immunohistochemical staining localized OPN primarily in nonmyocytes in the interstitial and perivascular space. In the MI model, increased OPN expression in the infarcted region peaked 3 days after MI, gradually decreasing over the next 28 days. In the remote LV, OPN expression was biphasic, with peaks at 3 and 28 days. In situ hybridization and immunohistochemical analyses showed increased OPN mRNA and protein primarily in the interstitium post-MI (Trueblood et al., 2001). Myocytes are identified as source of increased OPN expression in mice during streptozotocin-induced diabetic cardiomyopathy (Subramanian et al., 2007). Human myocardium with extensive fibrosis and myocyte hypertrophy from explanted hearts with either idiopathic or ischemic cardiomyopathy demonstrated substantial immunoreactivity for OPN in myocytes. In situ hybridization identified myocytes as the major source of OPN mRNA transcripts in the human hearts (Graf et al., 1997). Collectively, these data suggest that autocrine or paracrine neuroendocrine factors capable of inducing myocyte hypertrophy or apoptosis increase OPN expression in myocytes and the heart.
Several clinical reports provide evidence that increased OPN expression in the heart, specifically in myocytes, associates with myocardial dysfunction. In patients with severe heart failure due to dilated cardiomyopathy (DCM), increased OPN immunoreactivity within myocytes was observed in 13 out of 19 right ventricular biopsies. This increase in OPN expression in cardiac myocytes correlated negatively with left- and right-ventricular ejection fraction, and positively with LV end-systolic and end-diastolic volume index and LV end-diastolic pressure. Interestingly, OPN immunoreactivity in non-myocyte cells did not correlate with clinical data. Here, OPN expression also correlated with myocyte hypertrophy (Stawowy et al., 2002). Likewise, in situ hybridization identified myocytes as source of increased OPN expression in DCM patients and OPN mRNA levels correlated negatively with LV ejection fraction (Satoh et al., 2005). There was also a week positive correlation between OPN mRNA levels and LV end-diastolic diameter. In patients with MI, increased OPN plasma concentration correlated negatively with LV ejection fraction (Suezawa et al., 2005). Heart was identified as a source increased plasma OPN concentration since levels of plasma OPN were higher in the coronary sinus when compared to the aortic root (Tamura et al., 2003). A separate study quantified plasma OPN and OPN-expressing CD4+ T cells in 93 patients with heart disease (Soejima et al., 2007). Here, plasma OPN levels and the frequencies of OPN-expressing CD4+ T cells increased in proportion to the severity of the NYHA functional class. The frequencies of OPN-expressing CD4+ T cells were correlated inversely with LV ejection fraction. Rosenberg et al (Rosenberg et al., 2008) analyzed plasma levels in 420 patients with chronic heart failure. The patient cohort included 267 patients with DCM and 153 patients with ischemic heart failure. OPN plasma levels were found to be significantly elevated in patients with heart failure when compared to the healthy individual irrespective of heart failure origin (ischemic or DCM). OPN levels were higher in patients with moderate to severe heart failure as compared to patients with no or mild symptoms. Here, elevated plasma OPN levels correlated with the severity of heart failure and risk of subsequent death. In a recent study, increased OPN expression in the myocardium of heart failure patients correlated positively with lysol oxidase (an enzyme involved in cross-linking of ECM proteins to form insoluble fibrils), insoluble collagen and chamber stiffness, but inversely with LV ejection fraction (Lopez et al., 2013). Furthermore, OPN treatment increased expression and activity of lysol oxidase in human dermal and cardiac fibroblasts. This interesting observation suggests that the formation of insoluble collagen (i.e. stiff and resistant to degradation) and the subsequent alteration in LV mechanical properties and function in patients with heart failure might be facilitated by the OPN-lysol oxidase axis. OPN plasma levels are also found to be increased in patients with pulmonary hypertension (Rosenberg et al., 2012). In the setting of pulmonary hypertension, increased plasma OPN levels correlated with adverse right ventricular remodeling and dysfunction. Collectively, these studies provide strong evidence that increased OPN expression in the heart and/or plasma predicts adverse cardiac remodeling and dysfunction, and OPN may serve as a biomarker to identify patients with greater risk of decompensation and subsequent death.
V. OPN in myocyte apoptosis
Increased OPN expression associates with increased myocyte apoptosis in different models of heart disease (Subramanian, et al., 2007; Sam et al., 2004; Matsui et al., 2004). In streptozotocin-induced diabetic model, myocytes were identified as a source of OPN and myocyte apoptosis was significantly lower in mice lacking OPN (Subramanian et al., 2007). Likewise, cardiac myocyte-specific expression of OPN in the mouse heart during the first 11 weeks of life or in the adult heart led to increased myocyte apoptosis and myocardial dysfunction (Renault et al., 2010; Dalal et al., 2014). Myocardium of DCM patients associates with increased myocyte apoptosis (Di Napoli et al., 2003), where increased OPN expression in myocytes correlates negatively with heart function (Stawowy, et al., 2002). Recently, we provided evidence that treatment of adult rat cardiac myocytes with purified OPN protein or adenoviral-mediated expression of OPN in induces apoptosis (Dalal et al., 2014). However, the involvement of OPN in myocyte autophagy and necrosis requires further investigations.
In cells of non-cardiac origin, OPN is known to signal via its interaction with various integrins and CD44 receptor. Although, alternate translation of OPN generates intracellular isoform capable of distinct biological activities in dendritic cells, most of the effects of OPN are attributed via its interaction with the receptors on target cells. (Shinohara et al., 2008). Adult cardiac myocytes express α1, α3, α5, α6, α7, α7 and α10 integrin subunits (Ross, 2004). Myocytes predominantly express β1 subunit, although expression of β3 subunit has also been identified in feline myocytes (Daniel et al., 2014). Adult cardiac myocytes also express CD44 receptor. In adult rat cardiac myocytes, OPN co-immunoprecipitated with CD44 receptors, not with β1 or β3 integrins. Proximity ligation assay, which identifies interactions between two proteins in their native form, confirmed interaction of OPN with CD44 receptors. Furthermore, neutralizing anti-CD44 antibodies inhibited OPN-stimulated apoptosis (Dalal et al., 2014). Taken together, these data suggested that OPN, most likely acting via CD44 receptor, induces apoptosis in myocyte.
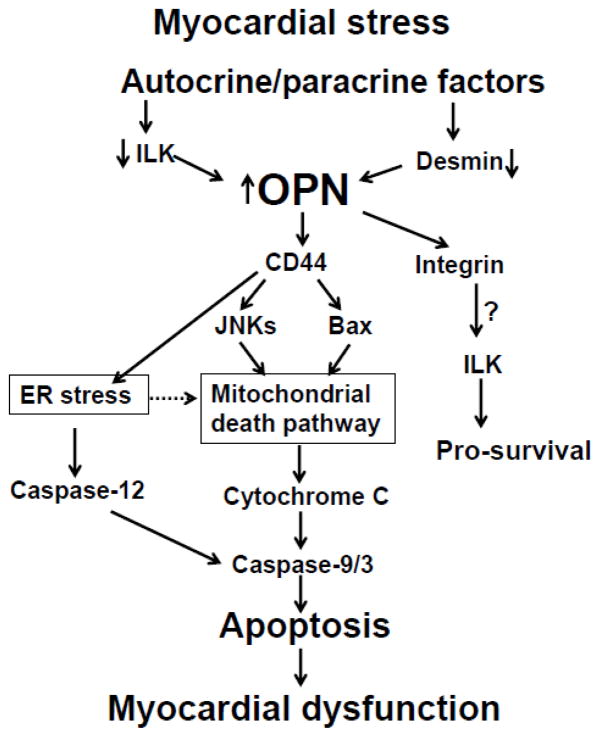
Significant strides have been made to understand the apoptotic process in myocytes and the heart. Organelles such as mitochondria and endoplasmic reticulum (ER; sarcoplasmic reticulum in myocytes) are suggested to play a crucial role in determining cell fate (Chen and Knowlton, 2010; Minamino and Kitakaze, 2010). Mitochondria play a central role in cellular metabolism, energy production and Ca++ handling. Mitochondria supply energy to support the high ATP consumption of the beating heart (Chen and Knowlton, 2010). Heart failure associates with diminished metabolism, calcium mishandling, oxidative stress and apoptosis, indicating mitochondrial dysfunction in the failing heart. Translocation of Bax to mitochondria and release of cytochrome c (i.e. increased levels of cytosolic cytochrome c) are considered as major events of mitochondrial dysfunction, leading to apoptosis. In isolated adult rat cardiac myocytes, activation of JNKs induces mitochondrial death pathway in response to β-adrenergic receptor stimulation (Remondino et al., 2003). The ER regulates protein synthesis, protein folding and trafficking, cellular responses to stress and intracellular Ca++ levels (Szegezdi et al., 2003; Rutkowski and Kaufman, 2004). Alteration in Ca++ homeostasis and accumulation of misfolded proteins initiate an adaptive response in the cell, termed the unfolded protein response (UPR, ER stress response). Prolonged ER stress triggers apoptosis in various cell types and is suggested to play a role in myocyte apoptosis and pathogenesis of heart failure (Szegezdi et al., 2003; Hamada et al., 2004; Okada et al., 2004). Increased Gadd-153 is considered as a hallmark of ER stress-induced apoptosis (Rao et al., 2004), while activation of caspase-12 plays a central role in ER-stress-induced apoptosis (Rutkowski and Kaufman, 2004). In adult rat cardiac myocytes, β-adrenergic receptor-stimulated induction of ER stress plays a pro-apoptotic role (Dalal et al., 2012). OPN-stimulated apoptosis in adult rat cardiac myocytes associates with activation of JNKs, increased Bax expression, decreased Bcl2/Bax ratio and increased levels of cytosolic cytochrome c (Dalal et al., 2014). It also associates with increased Gadd153 expression and activation of caspase-12. Furthermore, inhibition of JNKs, alleviation of ER stress or inhibition of caspase-12 inhibited OPN-stimulated apoptosis. These in vitro findings were recapitulated in vivo in heart where myocyte-specific expression of OPN in the adult heart led to the activation of mitochondrial death pathway and ER stress (Dalal et al., 2014). Thus, OPN-stimulated myocyte apoptosis involves both mitochondrial death pathway and ER stress (Figure). However, the molecular signals elicited by OPN-CD44 receptor interaction leading to the activation of mitochondrial death pathway and ER stress are not yet understood. It may involve members of the ezrin/radixin/moesin (ERM) family of proteins. Tumor suppressor protein merlin, a member of ERM family, regulates functions of the cell surface receptors, including CD44 (Stamenkovic et al., 2010).
Figure.
Schematic diagram illustrating signaling pathways involved in OPN-stimulated cardiac myocyte apoptosis. ILK, integrin linked kinase; JNKs, c-Jun-N-terminal kinase; ER, endoplasmic reticulum.
VI. Lessons learned from transgenic mice
Transgenic mice, i.e. mice lacking OPN and mice expressing OPN in a cardiac myocyte-specific manner, have been used to understand the role of OPN in heart disease (Table). In MI model, increased OPN expression mainly associated with interstitial and infiltrated cells. Here, mice lacking OPN exhibited reduced cardiac fibrosis and enhanced LV dilation 28 days post-MI. However, no difference in cardiac cell apoptosis was observed between WT and OPN−/− mice 28 days after MI (Trueblood et al., 2001). Of note, lack of CD44 also associates with reduced fibrosis and enhanced LV dilation in ischemia/reperfusion (I/R) model (Huebener et al., 2008). Ang II and aldosterone infusion led to decreased cardiac fibrosis, apoptosis and LV function in mice lacking OPN (Matsui et al., 2004; Sam et al., 2004). These two later studies did not identify if apoptosis was particularly occurring in the myocytes. However, increased OPN expression was associated with interstitial cells in response to Ang II (Matsui et al., 2004). Decreased fibrosis was also evident in mice lacking OPN in DOX-induced model of cardiac fibrosis (Schunke et al., 2013). In I/R model, OPN expression was found to be associated with cardiac myocytes. Mice lacking OPN exhibited reduced LV function following I/R (Duerr et al., 2014). Although this study did not measure apoptosis, however, mice lacking OPN exhibited reduced anterior wall thickness. No difference in myocardial apoptosis or fibrosis between WT and OPN−/− mice was observed in pressure overload model (Xie et al., 2004). However, compensatory hypertrophic response was lower in mice lacking OPN one month after the induction of pressure overload. In streptozotocin-induced diabetic cardiomyopathy model, myocytes were identified as source of OPN, and mice lacking OPN exhibited decreased cardiac fibrosis and myocyte apoptosis, and improved LV function 30 days after the induction of diabetes (Subramanian et al., 2007). Consistent with the diabetic model, expression of OPN in myocyte-specific manner during the first 11 weeks of mouse life and in the adult mouse (~4 months of age) associated with increased myocyte apoptosis, myocardial fibrosis and deterioration of heart function (Renault et al., 2010; Dalal et al., 2014). In general, these transgenic mice studies provide strong evidence that OPN plays pro-fibrotic and pro-apoptotic roles in the heart during myocardial remodeling, and establish a link between OPN expression and cardiomyopathies. However, the data with respect to heart function appears somewhat conflicting. Both lack of OPN and expression of OPN in myocyte-specific manner associated with LV dysfunction. ECM (fibrosis) affects heart function and the remodeling process of the heart. In normal heart, ECM provides a structural network for transmitting force generated by individual myocytes into organized systolic contraction of the heart. It also contributes to the passive stiffness in diastole and prevents overstretch, myocyte slippage, and tissue deformation during ventricular filling. Excessive fibrosis increases myocardial stiffness, thereby affecting mechanics of the heart and increasing the risk towards LV dysfunction (Creemers et al., 2011). On the other hand, a marked decrease in collagen 1 expression and fibrosis observed during OPN deficiency (Trueblood et al., 2001) may cause myocyte slippage, leading LV dilation and dysfunction. It should be noted, however, that increased OPN expression, specifically in myocytes, associates with LV dysfunction. This finding is consistent with the data obtained from patients with heart failure where increased OPN levels in the heart or plasma generally correlate negatively with heart function.
Table.
Observations from OPN transgenic mice
| Mouse model | Transgenic mice | Source of OPN | Apoptosis | Fibrosis | LV function | Ref |
|---|---|---|---|---|---|---|
| MI | OPN−/− | Interstitium/nonmyocytes | ↔ | ↓ | ↓ | (Trueblood et al., 2001) |
| Ang II infusion | OPN−/− | Interstitium | ↓ | ↓ | ↓ | (Matsui, et al., 2004) |
| Aldosteron e infusion | OPN−/− | ND | ↓ | ↓ | ↓ | (Sam, et al., 2004) |
| DOX treatment | OPN−/− | ND | ND | ↓ | ND | (Schunke et al., 2013) |
| Pressure Overload | OPN−/− | LV lysates | ↔ | ↔ | ↓ hypertrophic response | (Xie et al., 2004) |
| I/R (repetitive) | OPN−/− | Myocytes | ND (↓ wall thickness) | ↔ | ↓ | (Duerr, et al., 2014) |
| Diabetes | OPN−/− | Myocytes | ↓ (myocytes) | ↓ | ↑ | (Subramanian et al., 2007) |
| O/E | Myocyte- specific expression | Myocytes | ↑ (myocytes) | ↑ | ↓ | (Renault et al., 2010; Dalal, et al., 2014) |
MI, myocardial infarction; Ang II, angiotensin II, Dox, doxorubicin; I/R, ischemia/reperfusion; O/E, mice overexpressing OPN in the heart; ND, not determined
Other transgenic mice studies also provide evidence for the deleterious role of increased OPN expression in the heart. Integrin-linked kinase (ILK), a serine/threonine kinase, is recruited to the cytoplasmic domain of β1 and β3 integrin upon activation. ILK is implicated to play crucial roles in actin rearrangement, cell polarization, spreading, migration, proliferation and survival (Legate et al., 2006). Cardiac myocyte-specific deletion of ILK leads to spontaneous development of lethal dilated cardiomyopathy and heart failure with an early increase in apoptosis, fibrosis and cardiac inflammation (White et al., 2006). This Deletion of ILK associated with 47-fold increase in OPN expression in the heart. Interestingly, blocking antibodies against OPN partially rescued decline in heart functional in these mice (Dai et al., 2014). Desmin, an intermediate filament protein, is a target for cardiomyopathy and heart failure (Li et al., 1996). Desmin deficiency is characterized by increased expression of OPN in the heart (Mavroidis and Capetanaki, 2002). OPN appears to be a major regulator of myocardial remodeling in mice lacking desmin since mice lacking both desmin and OPN (des−/−OPN−/−) exhibit improved LV function and reduced myocardial fibrosis (Psarras et al., 2012).
VII. Conclusion and Future Directions
OPN expression increases in a variety of pathological conditions of the heart in human and experimental models. In animal models, lack of OPN associated with decreased myocyte apoptosis and improved heart function in a diabetic model. Expression of OPN in cardiac myocyte-specific manner led to increased myocyte apoptosis and LV dysfunction. In contrast, lack of OPN correlates with increased LV dilation and dysfunction in several experimental models, including MI, I/R, and Ang II- or aldosterone-infusion. In patients with heart failure, increased OPN expression in the heart or plasma levels generally correlated negatively with heart function. This shift in LV function in different experimental models may relate to the model system, cell-type/s involved in the expression of OPN, duration of OPN expression (transient vs chronic), time point at which heart function was analyzed in the experimental model etc. It is also possible that OPN synthesized by cardiac myocytes is different, with respect to post-translational modifications, from the OPN synthesized by other cell-types of the heart, e.g., fibroblasts, endothelial cells, macrophages etc? A better understanding of the stimuli involved in increased OPN expression, specifically in cardiac myocytes, may provide new opportunities to modulate OPN expression and myocyte apoptosis. OPN-mediated apoptosis in myocyte involves CD44 receptors, mitochondria and ER (Figure). However, future investigations aimed at identifying the intracellular signaling events leading to mitochondria death pathway and ER stress may provide new perspectives into the therapeutic strategies related to the role of OPN in modulating myocyte apoptosis and heart function.
Acknowledgments
This work was supported by a Merit Review award (numbers BX000640 and BX002332) from the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development, grants from The National Heart, Lung, and Blood Institute (Grant numbers R21HL-091405 and R21HL-092459), and funds from Institutional Research and Improvement account (to KS).
Footnotes
Conflict of Interest/Disclosure: The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Bowers SL, Banerjee I, Baudino TA. The extracellular matrix: at the center of it all. J Mol Cell Cardiol. 2010;48:474–482. doi: 10.1016/j.yjmcc.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Cao DX, Li ZJ, Jiang XO, Lum YL, Khin E, Lee NP, Wu GH, Luk JM. Osteopontin as potential biomarker and therapeutic target in gastric and liver cancers. World J Gastroenterol. 2012;18:3923–3930. doi: 10.3748/wjg.v18.i30.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Knowlton AA. Mitochondria and heart failure: new insights into an energetic problem. Minerva Cardioangiol. 2010;58:213–229. [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Force T. Molecular mechanisms of cardiovascular toxicity of targeted cancer therapeutics. Circ Res. 2010;106:21–34. doi: 10.1161/CIRCRESAHA.109.206920. [DOI] [PubMed] [Google Scholar]
- Creemers EE, Pinto YM. Molecular mechanisms that control interstitial fibrosis in the pressure-overloaded heart. Cardiovasc Res. 2011;89:265–272. doi: 10.1093/cvr/cvq308. [DOI] [PubMed] [Google Scholar]
- Dai J, Matsui T, Abel ED, Dedhar S, Gerszten RE, Seidman CE, Seidman JG, Rosenzweig A. Deep sequence analysis of gene expression identifies osteopontin as a downstream effector of integrin-linked kinase (ILK) in cardiac-specific ILK knockout mice. Circ Heart Fail. 2014;7:184–193. doi: 10.1161/CIRCHEARTFAILURE.113.000649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal S, Foster CR, Das BC, Singh M, Singh K. Beta-adrenergic receptor stimulation induces endoplasmic reticulum stress in adult cardiac myocytes: role in apoptosis. Mol Cell Biochem. 2012;364:59–70. doi: 10.1007/s11010-011-1205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal S, Zha Q, Daniels CR, Steagall RJ, Joyner WL, Gadeau AP, Singh M, Singh K. Osteopontin stimulates apoptosis in adult cardiac myocytes via the involvement of CD44 receptors, mitochondrial death pathway, and endoplasmic reticulum stress. Am J Physiol Heart Circ Physiol. 2014;306:H1182–H1191. doi: 10.1152/ajpheart.00954.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel LL, Joyner WL, Singh M, Singh K. Integrins: implications for aging in heart failure therapy. In: Jugdutt B, editor. Aging and Heart Failure: Mechanisms and management. New York, NY: Springer-Verlag; 2014. pp. 401–410. [Google Scholar]
- Di Napoli P, Taccardi AA, Grilli A, Felaco M, Balbone A, Angelucci D, Gallina S, Calafiore AM, De Caterina R, Barsotti A. Left ventricular wall stress as a direct correlate of cardiomyocyte apoptosis in patients with severe dilated cardiomyopathy. Am Heart J. 2003;146:1105–1111. doi: 10.1016/S0002-8703(03)00445-9. [DOI] [PubMed] [Google Scholar]
- Duerr GD, Mesenholl B, Heinemann JC, Zoerlein M, Huebener P, Schneider P, Feisst A, Ghanem A, Tiemann K, Dewald D, Welz A, Dewald O. Cardioprotective effects of osteopontin-1 during development of murine ischemic cardiomyopathy. Biomed Res Int. 2014;2014:124063. doi: 10.1155/2014/124063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formigli L, Papucci L, Tani A, Schiavone N, Tempestini A, Orlandini GE, Capaccioli S, Orlandini SZ. Aponecrosis: morphological and biochemical exploration of a syncretic process of cell death sharing apoptosis and necrosis. J Cell Physiol. 2000;182:41–49. doi: 10.1002/(SICI)1097-4652(200001)182:1<41::AID-JCP5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev. 2012;92:635–688. doi: 10.1152/physrev.00008.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissinger E, Weisser C, Fischer P, Schartl M, Wellbrock C. Autocrine stimulation by osteopontin contributes to antiapoptotic signalling of melanocytes in dermal collagen. Cancer Res. 2002;62:4820–4828. [PubMed] [Google Scholar]
- Graf K, Do YS, Ashizawa N, Meehan WP, Giachelli CM, Marboe CC, Fleck E, Hsueh WA. Myocardial osteopontin expression is associated with left ventricular hypertrophy. Circulation. 1997;96:3063–3071. doi: 10.1161/01.cir.96.9.3063. [DOI] [PubMed] [Google Scholar]
- Hamada H, Suzuki M, Yuasa S, Mimura N, Shinozuka N, Takada Y, Suzuki M, Nishino T, Nakaya H, Koseki H, Aoe T. Dilated cardiomyopathy caused by aberrant endoplasmic reticulum quality control in mutant KDEL receptor transgenic mice. Mol Cell Biol. 2004;24:8007–8017. doi: 10.1128/MCB.24.18.8007-8017.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebener P, Abou-Khamis T, Zymek P, Bujak M, Ying X, Chatila K, Haudek S, Thakker G, Frangogiannis NG. CD44 is critically involved in infarct healing by regulating the inflammatory and fibrotic response. J Immunol. 2008;180:2625–2633. doi: 10.4049/jimmunol.180.4.2625. [DOI] [PubMed] [Google Scholar]
- Kahles F, Findeisen HM, Bruemmer D. Osteopontin: A novel regulator at the cross roads of inflammation, obesity and diabetes. Mol Metab. 2014;3:384–393. doi: 10.1016/j.molmet.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanecki CC, Uzwiak DJ, Denhardt DT. Control of osteopontin signaling and function by post-translational phosphorylation and protein folding. J Cell Biochem. 2007;102:912–924. doi: 10.1002/jcb.21558. [DOI] [PubMed] [Google Scholar]
- Legate KR, Montanez E, Kudlacek O, Fassler R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol. 2006;7:20–31. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- Li Z, Colucci-Guyon E, Pincon-Raymond M, Mericskay M, Pournin S, Paulin D, Babinet C. Cardiovascular lesions and skeletal myopathy in mice lacking desmin. Dev Biol. 1996;175:362–366. doi: 10.1006/dbio.1996.0122. [DOI] [PubMed] [Google Scholar]
- Lin YH, Yang-Yen HF. The osteopontin-CD44 survival signal involves activation of the phosphatidylinositol 3-kinase/Akt signaling pathway. J Biol Chem. 2001;276:46024–46030. doi: 10.1074/jbc.M105132200. [DOI] [PubMed] [Google Scholar]
- Lopez B, Gonzalez A, Lindner D, Westermann D, Ravassa S, Beaumont J, Gallego I, Zudaire A, Brugnolaro C, Querejeta R, Larman M, Tschope C, Diez J. Osteopontin-mediated myocardial fibrosis in heart failure: a role for lysyl oxidase? Cardiovasc Res. 2013;99:111–120. doi: 10.1093/cvr/cvt100. [DOI] [PubMed] [Google Scholar]
- Matsui Y, Jia N, Okamoto H, Kon S, Onozuka H, Akino M, Liu L, Morimoto J, Rittling SR, Denhardt D, Kitabatake A, Uede T. Role of osteopontin in cardiac fibrosis and remodeling in angiotensin II-induced cardiac hypertrophy. Hypertension. 2004;43:1195–1201. doi: 10.1161/01.HYP.0000128621.68160.dd. [DOI] [PubMed] [Google Scholar]
- Mavroidis M, Capetanaki Y. Extensive induction of important mediators of fibrosis and dystrophic calcification in desmin-deficient cardiomyopathy. Am J Pathol. 2002;160:943–952. doi: 10.1016/S0002-9440(10)64916-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino T, Kitakaze M. ER stress in cardiovascular disease. J Mol Cell Cardiol. 2010;48:1105–1110. doi: 10.1016/j.yjmcc.2009.10.026. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Ohsumi Y, Yoshimori T. Autophagosome formation in mammalian cells. Cell Struct Funct. 2002;27:421–429. doi: 10.1247/csf.27.421. [DOI] [PubMed] [Google Scholar]
- Okada K, Minamino T, Tsukamoto Y, Liao Y, Tsukamoto O, Takashima S, Hirata A, Fujita M, Nagamachi Y, Nakatani T, Yutani C, Ozawa K, Ogawa S, Tomoike H, Hori M, Kitakaze M. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation. 2004;110:705–712. doi: 10.1161/01.CIR.0000137836.95625.D4. [DOI] [PubMed] [Google Scholar]
- Oldberg A, Franzen A, Heinegard D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci USA. 1986;83:8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orogo AM, Gustafsson AB. Cell death in the myocardium: my heart won’t go on. IUBMB Life. 2013;65:651–656. doi: 10.1002/iub.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persy VP, Verhulst A, Ysebaert DK, De Greef KE, De Broe ME. Reduced postischemic macrophage infiltration and interstitial fibrosis in osteopontin knockout mice. Kidney Int. 2003;63:543–553. doi: 10.1046/j.1523-1755.2003.00767.x. [DOI] [PubMed] [Google Scholar]
- Psarras S, Mavroidis M, Sanoudou D, Davos CH, Xanthou G, Varela AE, Panoutsakopoulou V, Capetanaki Y. Regulation of adverse remodelling by osteopontin in a genetic heart failure model. Eur Heart J. 2012;33:1954–1963. doi: 10.1093/eurheartj/ehr119. [DOI] [PubMed] [Google Scholar]
- Rao RV, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004;11:372–380. doi: 10.1038/sj.cdd.4401378. [DOI] [PubMed] [Google Scholar]
- Remondino A, Kwon SH, Communal C, Pimentel DR, Sawyer DB, Singh K, Colucci WS. Beta-adrenergic receptor-stimulated apoptosis in cardiac myocytes is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of the mitochondrial pathway. Circ Res. 2003;92:136–138. doi: 10.1161/01.res.0000054624.03539.b4. [DOI] [PubMed] [Google Scholar]
- Renault MA, Robbesyn F, Reant P, Douin V, Daret D, Allieres C, Belloc I, Couffinhal T, Arnal JF, Klingel K, Desgranges C, Dos SP, Charpentier F, Gadeau AP. Osteopontin expression in cardiomyocytes induces dilated cardiomyopathy. Circ Heart Fail. 2010;3:431–439. doi: 10.1161/CIRCHEARTFAILURE.109.898114. [DOI] [PubMed] [Google Scholar]
- Rosenberg M, Meyer FJ, Gruenig E, Lutz M, Lossnitzer D, Wipplinger R, Katus HA, Frey N. Osteopontin predicts adverse right ventricular remodelling and dysfunction in pulmonary hypertension. Eur J Clin Invest. 2012;42:933–942. doi: 10.1111/j.1365-2362.2012.02671.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg M, Zugck C, Nelles M, Juenger C, Frank D, Remppis A, Giannitsis E, Katus HA, Frey N. Osteopontin, a new prognostic biomarker in patients with chronic heart failure. Circ Heart Fail. 2008;1:43–49. doi: 10.1161/CIRCHEARTFAILURE.107.746172. [DOI] [PubMed] [Google Scholar]
- Ross RS. Cardiac remodeling: is 8 the heart’s lucky number? J Mol Cell Cardiol. 2004;36:323–326. doi: 10.1016/j.yjmcc.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Sam F, Xie Z, Ooi H, Kerstetter DL, Colucci WS, Singh M, Singh K. Mice lacking osteopontin exhibit increased left ventricular dilation and reduced fibrosis after aldosterone infusion. Am J Hypertens. 2004;17:188–193. doi: 10.1016/j.amjhyper.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Satoh M, Nakamura M, Akatsu T, Shimoda Y, Segawa I, Hiramori K. Myocardial osteopontin expression is associated with collagen fibrillogenesis in human dilated cardiomyopathy. Eur J Heart Fail. 2005;7:755–762. doi: 10.1016/j.ejheart.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Scatena M, Almeida M, Chaisson ML, Fausto N, Nicosia RF, Giachelli CM. NF-kappaB mediates alphavbeta3 integrin-induced endothelial cell survival. J Cell Biol. 1998;141:1083–1093. doi: 10.1083/jcb.141.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scatena M, Liaw L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol. 2007;27:2302–2309. doi: 10.1161/ATVBAHA.107.144824. [DOI] [PubMed] [Google Scholar]
- Schunke KJ, Coyle L, Merrill GF, Denhardt DT. Acetaminophen attenuates doxorubicin-induced cardiac fibrosis via osteopontin and GATA4 regulation: reduction of oxidant levels. J Cell Physiol. 2013;228:2006–2014. doi: 10.1002/jcp.24367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara ML, Kim HJ, Kim JH, Garcia VA, Cantor H. Alternative translation of osteopontin generates intracellular and secreted isoforms that mediate distinct biological activities in dendritic cells. Proc Natl Acad Sci USA. 2008;105:7235–7239. doi: 10.1073/pnas.0802301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Sirokman G, Communal C, Robinson KG, Conrad CH, Brooks WW, Bing OH, Colucci WS. Myocardial osteopontin expression coincides with the development of heart failure. Hypertension. 1999;33:663–670. doi: 10.1161/01.hyp.33.2.663. [DOI] [PubMed] [Google Scholar]
- Singh M, Ananthula S, Milhorn DM, Krishnaswamy G, Singh K. Osteopontin: a novel inflammatory mediator of cardiovascular disease. Front Biosci. 2007;12:214–221. doi: 10.2741/2059. [DOI] [PubMed] [Google Scholar]
- Singh M, Foster CR, Dalal S, Singh K. Osteopontin: role in extracellular matrix deposition and myocardial remodeling post-MI. J Mol Cell Cardiol. 2010a;48:538–543. doi: 10.1016/j.yjmcc.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Foster CR, Dalal S, Singh K. Role of osteopontin in heart failure associated with aging. Heart Fail Rev. 2010b;15:487–494. doi: 10.1007/s10741-010-9158-6. [DOI] [PubMed] [Google Scholar]
- Soejima H, Irie A, Fukunaga T, Oe Y, Kojima S, Kaikita K, Kawano H, Sugiyama S, Yoshimura M, Kishikawa H, Nishimura Y, Ogawa H. Osteopontin expression of circulating T cells and plasma osteopontin levels are increased in relation to severity of heart failure. Circ J. 2007;71:1879–1884. doi: 10.1253/circj.71.1879. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I, Yu Q. Merlin, a “magic” linker between extracellular cues and intracellular signaling pathways that regulate cell motility, proliferation, and survival. Curr Protein Pept Sci. 2010;11:471–484. doi: 10.2174/138920310791824011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawowy P, Blaschke F, Pfautsch P, Goetze S, Lippek F, Wollert-Wulf B, Fleck E, Graf K. Increased myocardial expression of osteopontin in patients with advanced heart failure. Eur J Heart Fail. 2002;4:139–146. doi: 10.1016/s1388-9842(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Subramanian V, Krishnamurthy P, Singh K, Singh M. Lack of osteopontin improves cardiac function in streptozotocin-induced diabetic mice. Am J Physiol Heart Circ Physiol. 2007;292:H673–H683. doi: 10.1152/ajpheart.00569.2006. [DOI] [PubMed] [Google Scholar]
- Suezawa C, Kusachi S, Murakami T, Toeda K, Hirohata S, Nakamura K, Yamamoto K, Koten K, Miyoshi T, Shiratori Y. Time-dependent changes in plasma osteopontin levels in patients with anterior-wall acute myocardial infarction after successful reperfusion: correlation with left-ventricular volume and function. J Lab Clin Med. 2005;145:33–40. doi: 10.1016/j.lab.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Szegezdi E, FitzGerald U, Samali A. Caspase-12 and ER-stress-mediated apoptosis: the story so far. Ann NY Acad Sci. 2003;1010:186–194. doi: 10.1196/annals.1299.032. [DOI] [PubMed] [Google Scholar]
- Tamura A, Shingai M, Aso N, Hazuku T, Nasu M. Osteopontin is released from the heart into the coronary circulation in patients with a previous anterior wall myocardial infarction. Circ J. 2003;67:742–744. doi: 10.1253/circj.67.742. [DOI] [PubMed] [Google Scholar]
- Tavernarakis N. Cardiomyocyte necrosis: alternative mechanisms, effective interventions. Biochim Biophys Acta. 2007;1773:480–482. doi: 10.1016/j.bbamcr.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Trueblood NA, Xie Z, Communal C, Sam F, Ngoy S, Liaw L, Jenkins AW, Wang J, Sawyer DB, Bing OH, Apstein CS, Colucci WS, Singh K. Exaggerated left ventricular dilation and reduced collagen deposition after myocardial infarction in mice lacking osteopontin. Circ Res. 2001;88:1080–1087. doi: 10.1161/hh1001.090842. [DOI] [PubMed] [Google Scholar]
- Wai PY, Kuo PC. Osteopontin: regulation in tumor metastasis. Cancer Metastasis Rev. 2008;27:103–118. doi: 10.1007/s10555-007-9104-9. [DOI] [PubMed] [Google Scholar]
- Wang KX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008;19:333–345. doi: 10.1016/j.cytogfr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Wang KX, Shi Y, Denhardt DT. Osteopontin regulates hindlimb-unloading-induced lymphoid organ atrophy and weight loss by modulating corticosteroid production. Proc Natl Acad Sci USA. 2007;104:14777–14782. doi: 10.1073/pnas.0703236104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol. 2010;72:19–44. doi: 10.1146/annurev.physiol.010908.163111. [DOI] [PubMed] [Google Scholar]
- White DE, Coutu P, Shi YF, Tardif JC, Nattel S, St Arnaud R, Dedhar S, Muller WJ. Targeted ablation of ILK from the murine heart results in dilated cardiomyopathy and spontaneous heart failure. Genes Dev. 2006;20:2355–2360. doi: 10.1101/gad.1458906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolak T. Osteopontin- a multi-modal marker and mediator in atherosclerotic vascular disease. Atherosclerosis. 2014;236:327–337. doi: 10.1016/j.atherosclerosis.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Xie Z, Singh M, Singh K. Osteopontin modulates myocardial hypertrophy in response to chronic pressure overload in mice. Hypertension. 2004;44:826–831. doi: 10.1161/01.HYP.0000148458.03202.48. [DOI] [PubMed] [Google Scholar]
- Yumoto K, Ishijima M, Rittling SR, Tsuji K, Tsuchiya Y, Kon S, Nifuji A, Uede T, Denhardt DT, Noda M. Osteopontin deficiency protects joints against destruction in antitype II collagen antibody-induced arthritis in mice. Proc Natl Acad Sci USA. 2002;99:4556–4561. doi: 10.1073/pnas.052523599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Guo M, Chen JH, Wang Z, Du XF, Liu PX, Li WH. Osteopontin knockdown inhibits alphav,beta3 integrin-induced cell migration and invasion and promotes apoptosis of breast cancer cells by inducing autophagy and inactivating the PI3K/Akt/mTOR pathway. Cell Physiol Biochem. 2014;33:991–1002. doi: 10.1159/000358670. [DOI] [PubMed] [Google Scholar]
- Zheng YH, Tian C, Meng Y, Qin YW, Du YH, Du J, Li HH. Osteopontin stimulates autophagy via integrin/CD44 and p38 MAPK signaling pathways in vascular smooth muscle cells. J Cell Physio. 2011;227:127–35. doi: 10.1002/jcp.22709. [DOI] [PubMed] [Google Scholar]
- Zohar R, Zhu B, Liu P, Sodek J, McCulloch CA. Increased cell death in osteopontin-deficient cardiac fibroblasts occurs by a caspase-3-independent pathway. Am J Physiol Heart Circ Physiol. 2004;287:H1730–H1739. doi: 10.1152/ajpheart.00098.2004. [DOI] [PubMed] [Google Scholar]