Abstract
Objective
We assessed the relationships between supportive and obstructive family behaviors and patients’ diabetes self-care activities and HbA1C, and potential interaction effects and differences by demographic characteristics.
Methods
In a cross-sectional study, 192 adults with type 2 diabetes completed the Diabetes Family Behavior Checklist-II, the Summary of Diabetes Self-Care Activities, and a glycemic control (HbA1C) test.
Results
Participants reported similar rates of supportive and obstructive behaviors that were positively correlated (rho=0.61, p<.001). In adjusted analyses, supportive family behaviors were associated with adherence to different self-care behaviors (β=0.20–0.50, p<.05), whereas obstructive family behaviors were associated with less adherence to self-care behaviors (β=−0.28–−0.39, p<.01) and worse HbA1C (β=0.18, p<.05). Supportive behaviors protected against the detrimental effect of obstructive behaviors on HbA1C (interaction β=−0.22, p<.001). Non-Whites reported more supportive and obstructive behaviors than Whites, but race did not affect the relationships between family behaviors and self-care or HbA1C.
Conclusion
Involving family members in patients’ diabetes management may compromise patients’ self-care and glycemic control unless family members are taught to avoid obstructive behaviors.
Practice Implications
Our findings endorse interventions that help family members develop actionable plans to support patients’ self-care and train them to communicate productively about diabetes management.
Keywords: family, social support, type 2 diabetes, self-care, glycemic control, HbA1C
1. Introduction
For adults with type 2 diabetes mellitus (T2DM), performing recommended self-care is essential for avoiding complications, yet patient adherence remains challenging [1, 2]. Self-care interventions have largely focused on ‘the individual patient’, giving less attention to the socioecological conditions (e.g., families, communities) in which patients perform self-care [3]. Across chronic disease contexts, including diabetes, disease-specific instrumental support from significant others (i.e., family members’ practical actions that make self-care easier/possible) has been more strongly associated with patients’ adherence than other types of support (i.e., emotional, informational, or appraisal)[4, 5]. Family members provide instrumental support by attending medical appointments [6], reminding/helping patients to perform a behavior [7, 8], and creating an environment to reinforce adherence (e.g., preparing healthy meals)[9]. Furthermore, such instrumental support has been associated with adults’ adherence to diet [10], exercise [7, 11], blood glucose testing [11, 12], diabetes medications [10], and general self-care [13, 14].
Family members’ involvement in diabetes care can also be harmful [11, 15-18]. Family members may sabotage or undermine patients’ self-care efforts by planning unhealthy meals, tempting patients to eat unhealthy foods, or questioning the need for medications [15-17]. Family members may also nag or argue with patients in an attempt to ‘support’ adherence [16] only to undermine patients’ self-efficacy and create family conflict [19]. Among adults with T2DM, family members’ obstructive behaviors have been associated with patients having less adherence-related motivation and self-efficacy [13], and less adherence to diet recommendations [8, 11] and medications [7, 16].
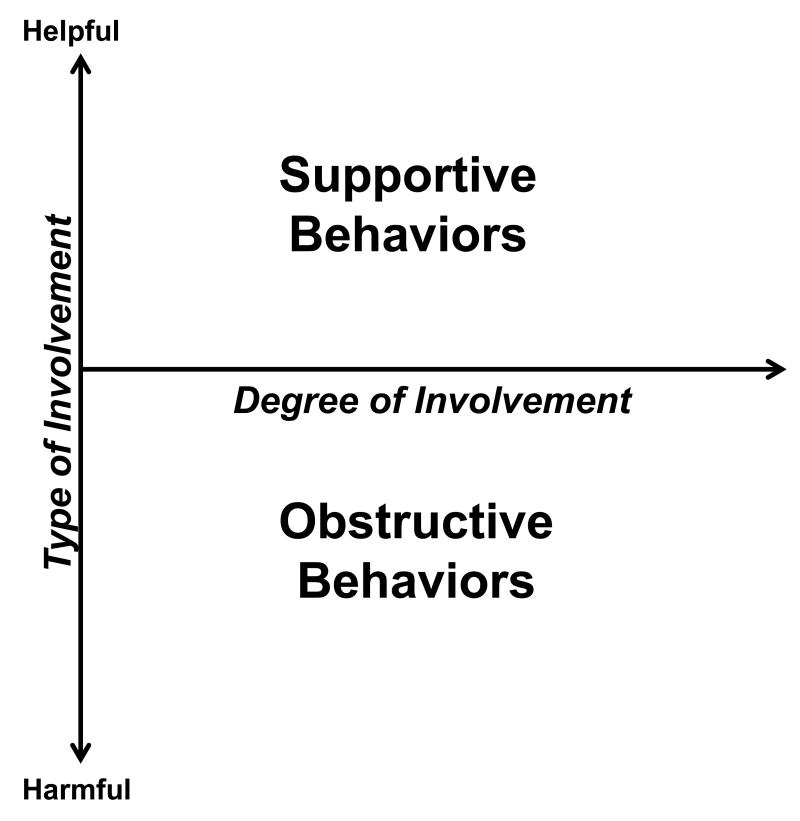
Supportive and obstructive family behaviors have been studied separately, but examining one form of family involvement without the other may misrepresent the lived experiences of patients and their families. Often supportive and obstructive family behaviors co-occur and are positively correlated [11, 15, 16, 18, 20] and providing no support (i.e., being inactive) is different from actively sabotaging or nagging a patient [15]. Typically, family involvement is conceptualized as a unidimensional construct (e.g., more is better). However, the literature suggests that a complete conceptualization of family involvement in diabetes care is two-dimensional, consisting of the degree of involvement in the patient’s care and the type of involvement (i.e., helpful vs. harmful; Figure 1). To date, quantitative studies often operationalize “family support” as a unidimensional construct, either by assessing only the helpful aspects of family involvement [14, 21] or by subtracting harmful family involvement from helpful family involvement and treating what’s left as a single variable [8, 12, 22]. Both approaches preclude examination of the independent and co-occurring role of supportive and obstructive family involvement. For example, supportive involvement may protect patients from the detrimental effects of obstructive involvement. However, to our knowledge, studies have not yet explored how these factors may interact to affect adults’ diabetes outcomes.
Figure1.
Family involvement in adults’ diabetes management is a two-dimensional construct. Family involvement is a function of degree (uninvolved to involved) and type (helpful to harmful).
Studies of family involvement in adults’ diabetes management have largely used racially homogenous samples [7, 8, 11-13, 16, 22], limiting our knowledge of racial/ethnic variation in the amount of helpful and harmful family behaviors patients experience and their consequences. This is worthy of exploration, given what we do know about racial/ethnic variation in both family household composition and family dynamics. For instance, older African Americans (AA)/Blacks are more likely to live with children/grandchildren and have stronger expectations for intergenerational co-residence than Whites [23], but report receiving less help with self-care[24]. Moreover, Hispanics report more assistance from adult children than AA/Blacks or Whites, and may view diabetes as the family’s responsibility rather than the individual’s responsibility[25]. Thus, studies focusing only on spouse/partner involvement [15, 18, 26] may be less relevant for patients living in intergenerational households. While the research on racial/ethnic variation has been sparse, gender differences in family involvement have been identified, with men experiencing and benefiting more from helpful family behaviors than women [12, 13, 26].
In an effort to fill some of the aforementioned gaps in the literature, we sampled from a racially/ethnically diverse patient population of adults with T2DM and low socioeconomic statuts (SES) to: (1) explore whether both supportive and obstructive family behaviors predict patients’ diabetes self-care activities and glycemic control; (2) assess whether the type (supportive versus obstructive) of family involvement matters more than simply having family members who are involved in the patients’ self-care, regardless of the type of their involvement; (3) test whether supportive family behaviors buffer the effects of obstructive family behaviors; (4) and examine whether there are racial/ethnic, gender, and living alone versus living with others differences in the amount of supportive and obstructive behaviors experienced and subsequent effects on patients’ self-care and glycemic control.
2. Methods
After identifying the importance of diabetes-specific family behaviors in our previous mixed-methods study [16], we added a measure assessing these family behaviors to a crosssectional study examining modifiable determinants of diabetes medication adherence. The parent study consecutively recruited patients arriving for medical appointments at a Federally Qualified Health Center in Nashville, TN from June 2010 to November 2012, and this measure was added in June 2011. English- or Spanish-speaking adults (age≥18 years) diagnosed with T2DM and prescribed diabetes medications were eligible. Exclusion criteria included not having a social security number required for compensation, unintelligible speech, delirium/dementia or other cognitive impairment, severe hearing impairment, and administration of all medications by a caregiver as determined by RAs in collaboration with clinic personnel. For the larger study, 588 patients with T2DM arrived for a clinic appointment and 83.3% of the eligible patients (314 out of 377) were enrolled [27]. Of these 314 participants, 192 were enrolled after the measure of family behaviors was added to the study protocol and therefore were included in these analyses.
Interested and eligible participants were taken to a private room at the clinic before/after their clinic appointment to provide informed consent and complete an research assistant (RA)-administered survey. RAs read all items and response options in participants’ preferred language, and provided a copy of each set of response options printed in large font for participants’ reference. Materials were translated using the forward-backward technique [28] by licensed translators. Clinic nurses administered a point-of-care HbA1C test, and RAs collected information from the medical record. Participation took approximately 1 hour and participants were compensated $20. The Vanderbilt University Institutional Review Board approved all study procedures.
2.1. Measures
We collected self-reported age, gender, race, ethnicity, income, education, insurance status, living alone versus living with others, and diabetes duration (time since diabetes diagnosis in years and months). RAs collected the number and type of diabetes medication(s) from participants’ medical record.
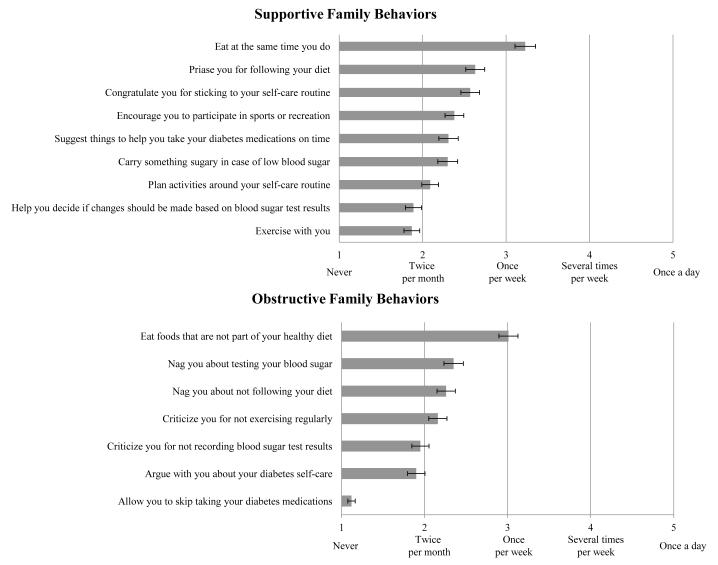
Participants’ perceptions of family members’ supportive and obstructive behaviors were assessed with the supportive and nonsupportive subscales from the Diabetes Family Behavior Checklist-II (DFBC-II) [11]. The 16-item DFBC-II asks respondents how often their family members have performed specific behaviors in the past month on a scale from 1=never to 5=at least once a day. The instruments’ developers characterize certain items as supportive or nonsupportive (i.e., obstructive). Each item asks “How often do your family members…” perform a certain behavior with response options from 1=never to 5=once a day. Items for each subscale are shown in Figure 2. We averaged the 9 supportive items and 7 nonsupportive items to create two subscales ranging from 1–5, with higher scores indicating more supportive or obstructive behaviors, respectively [11]. Schafer et al.[20] reported test-retest reliability and good convergent validity with family member-reported scores. In our sample, the supportive and nonsupportive subscales had internal consistency reliability (Cronbach’s α) of 0.85 and 0.78, respectively.
Figure 2.
Average frequency and standard error for each family behavior on the Diabetes Family Behavior Checklist-II.
We used the Summary of Diabetes Self-Care Activities (SDSCA) subscales to assess participants’ adherence to different self-care behaviors over the last 7 days [29]. Each SDSCA subscale ranges from 0-7, with higher scores indicating greater adherence. Glycemic control was assessed with a valid and reliable point-of-care HbA1C (%) test [30] administered by a clinic nurse on the day of participation.1
2.2. Analyses
Using Stata 12, we conducted a series of regression models to test the relationships between family members’ supportive and, separately, obstructive behaviors and participants’ diabetes self-care and HbA1C. First, we examined unadjusted associations between supportive and obstructive behaviors and self-care and glycemic control. Next, to answer the question “Is it simply family involvement that matters, or does the type of involvement matter?” we conducted partially adjusted regression models with both supportive and obstructive family behaviors as predictors in each model. Including both variables adjusted for the overlap between supportive and obstructive behaviors, representing the degree of family involvement, to allow for an understanding of how supportive and obstructive family behaviors were associated with self-care and glycemic control, over and beyond that involvement. Collinearity was not problematic (tolerance=0.64). Fully adjusted models included apriori covariates – participants’ age, gender, race (White, Black, or other), education, insurance status (uninsured, public, or private), diabetes duration, and insulin status. To assess if the effects of obstructive behaviors were weaker at high levels of supportive behaviors, we conducted unadjusted and adjusted regression models with an interaction term.
We used analysis of variance/covariance models with a Bonferroni correction for multiple comparisons to assess if racial/ethnic minorities reported more supportive and/or obstructive family behaviors than Whites, and to explore differences by gender and living alone versus living with others. Adjusted models included both supportive and obstructive behaviors and the aforementioned apriori covariates. Finally, to assess if relationships between supportive and obstructive behaviors and self-care and HbA1C were consistent across race and gender, we assessed interactions between supportive and obstructive behaviors and race (White versus non-White due to insufficient number of “other” race participants) and, separately, gender in regression models. Because of the small number of participants living alone, we could not assess effect modification with this variable.
3. Results
3.1. Participant Characteristics
Most participants (70%) were women; 56% were AA/Black, 34% were White, and 10% reported another race. Of the 20 other race participants, 80% reported Hispanic ethnicity and 11 interviews were conducted in Spanish. Most (71%) reported incomes <$15,000, 30% had <a high school degree and 47% were uninsured. Only 28% were married/partnered, but 74% did not live alone, suggesting at least half (48%) lived with someone other than a spouse/partner. Participants had an average age of 51.6±10.9 and had been diagnosed with diabetes for an average of 7.7±7.2 years. Given that the majority of the sample had low SES and 66% were members of a racial/ethnic group, the young age of our sample is consistent with the younger age of racial/ethnic minorities diagnosed with diabetes in the U.S. [31]. On average, participants reported experiencing each supportive and obstructive family behavior at least twice per month (Table 1). Average frequencies and standard errors for each family behavior are depicted in Figure 2. Listwise deletion was used to handle missing data on diabetes duration (n=3) and the DFBC-II (n=2).
Table 1. Participant characteristics.
| N = 192 | M ± SD or n (%) |
|---|---|
| DEMOGRAPHIC CHARACTERISTICS | |
| Age, years | 51.6 ± 10.9 |
| Gender | |
| Men | 57 (29.7) |
| Women | 135 (70.3) |
| Race | |
| White | 65 (33.9) |
| African American/Black | 107 (55.7) |
| Other race | 20 (10.4) |
| Hispanic ethnicity | 19 (9.9) |
| Education, years | 12.0 ± 3.0 |
| Incomea | |
| <$10,000 | 78 (43.6) |
| $10,000 – $14,999 | 49 (27.4) |
| $15,000 – $19,999 | 27 (15.1) |
| ≥$20,000 | 25 (14.0) |
| Insurance Status | |
| Uninsured | 90 (46.9) |
| Public insurance | 87 (45.3) |
| Private insurance | 15 (7.8) |
| DIABETES CHARACTERISTICS | |
| Diabetes duration, years | 7.7 ± 7.2 |
| Type of diabetes medications | |
| Oral agents only | 102 (53.1) |
| Insulin only | 42 (21.9) |
| Both | 48 (25.0) |
| FAMILY BEHAVIORS (DFBC-II) | |
| Supportive behaviors | 2.4 ± 1.0 |
DFBC-II = Diabetes Family Behavior Checklist-II, HbA1c = point-of-care hemoglobin A1C, M = mean, SD = standard deviation, SDSCA = Summary of Diabetes Self-Care Activities.
13 participants did not report their income
3.2. Family Behaviors and Self-care
Family members’ supportive and obstructive behaviors were more strongly related to participants’ self-care and explained more variation (increase in incremental R2) in the outcomes when both were included in regression models (Table 2). Adjusting for covariates typically decreases the coefficient of the predictor, so reciprocal suppression is indicated if both predictors have a stronger association when included in a single model [32]. By “suppressing” the effects of family involvement (represented by Spearman’s rho=0.61, p<.001 between supportive and obstructive behaviors), we can examine the unique contributions of supportive and obstructive family behaviors on the outcomes of interest.
Table 2. Effects of supportive and obstructive family behaviors on participants’ adherence to self-care behaviors and glycemic control.
| Unadjusted | Partially Adjusteda | Fully Adjustedb | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| β | P | R2(%) | β | P | Incremental R2 (%) | β | P | Incremental R2 (%) | |
| General diet | |||||||||
| Supportive | .19 | .007 | 3.6 | .44 | <.001 | 12.3*** | .43 | <.001 | 11.5*** |
| Obstructive | −.15 | .033 | 2.4 | −.42 | <.001 | 11.2*** | −.39 | <.001 | 9.7*** |
| Specific diet | |||||||||
| Supportive | .03 | .718 | 0.0 | .22 | .015 | 3.1* | .22 | .019 | 3.1* |
| Obstructive | −.19 | .015 | 3.7 | −.32 | <.001 | 6.7*** | −.31 | .003 | 5.5** |
| Exercise | |||||||||
| Supportive | .26 | <.001 | 6.8 | .49 | <.001 | 15.7*** | .50 | <.001 | 15.3*** |
| Obstructive | −.09 | .149 | 0.9 | −.39 | <.001 | 9.7*** | −.31 | <.001 | 7.6*** |
| Blood glucose testing | |||||||||
| Supportive | .07 | .378 | 0.4 | .15 | .097 | 1.4 | .20 | .045 | 2.5* |
| Obstructive | −.05 | .451 | 0.3 | −.14 | .092 | 1.3 | −.08 | .373 | 0.8 |
| Medications | |||||||||
| Supportive | .11 | .151 | 1.2 | .27 | .001 | 4.9** | .30 | .001 | 5.6** |
| Obstructive | −.11 | .125 | 1.3 | −.28 | .001 | 5.0** | −.28 | .003 | 4.1** |
|
| |||||||||
| Glycemic control (HbA1C) | |||||||||
| Interaction | .27 | <.001 | 7.4 | −.22 | .017 | 4.5** | −.22 | <.001 | 4.5** |
| Main Effects | |||||||||
| Supportive | .10 | .125 | 1.1 | −.09 | .309 | 0.5 | −.10 | .198 | 0.7 |
| Obstructive | .27 | <.001 | 7.4 | .40 | <.001 | 9.6*** | .25 | .007 | 4.5** |
p < .05
p < .01
p < .001.
β = standardized regression coefficients; p = probability value; R2 = percent of variance in outcome variable explained by the predictor variable; Incremental R2 = percent of variance in outcome variable uniquely explained by the predictor variable, controlling for the other predictor variables in the model.
Partially adjusted models include both predictors (i.e., supportive and obstructive behaviors).
Fully adjusted models include both predictors (i.e., supportive and obstructive behaviors), age, gender, race, education, diabetes duration, insulin status, and insurance status; n = 187 due to missing data.
Family members’ supportive and obstructive behaviors were positively and negatively associated, respectively, with participants’ adherence to general diet, specific diet, exercise, and medications. In fully adjusted models, these associations were maintained. As shown in Table 2, in fully adjusted models supportive and obstructive behaviors demonstrated moderate associations with self-care behaviors. Combined, supportive and obstructive behaviors explained a substantial and significant percent of variance in self-care behaviors over and above the variance explained by apriori covariates: 22.9% in adherence to exercise, 21.2% in adherence to general diet, 9.7% in adherence to medications, and 8.6% in adherence to specific diet.
Supportive behaviors were associated with blood glucose self-monitoring in fully adjusted models, but explained a negligible percent of variance in this outcome. There was a significant interaction between supportive and obstructive behaviors on adherence to general diet (β=0.17, p<.001), but this interaction was nonsignificant when adjusted for covariates (β=0.13, p=.06).
3.3. Family Behaviors and HbA1C
When family members’ supportive and obstructive behaviors were included in a single model predicting HbA1C, supportive behaviors acted as a suppressor variable for obstructive behaviors, which had a stronger association with the outcome when the shared error variance was suppressed (i.e., classical suppression [33]). Obstructive behaviors were associated with worse HbA1C in the unadjusted model (β=0.27, p<.001), after adjusting for supportive behaviors (β=0.33, p<.001) and in the fully adjusted model (β=0.18, p<.05). Supportive behaviors were not associated with HbA1C, but moderated the effect of obstructive behaviors on HbA1C(partially adjusted interaction β=−0.22, p<.05; fully adjusted interaction β=−0.22, p<.001). As shown in Figure 3, for participants reporting low supportive behaviors, obstructive behaviors were significantly associated with worse HbA1C (simple slope β=0.47, p=.001), whereas obstructive behaviors were not associated with HbA1C for participants reporting high supportive behaviors. When the interaction effect was included (Table 2), family behaviors explained 9.7% of the variance in HbA1C over and above the variance explained by apriori covariates.
Figure 3.
Estimated values and simple slopes for the effects of obstructive family behaviors on glycemic control (HbA1C, %) given different degrees of supportive family behaviors. Obstructive family behaviors have a detrimental effect on glycemic control (i.e., HbA1C values are higher) for participants reporting low supportive family behaviors, but not for participants reporting high supportive family behaviors. Low and high values represent ± 1 standard deviation from the mean. Models are adjusted for age, gender, race, education, insurance status, diabetes duration, and insulin status. β = standardized regression coefficients.
3.4. Race, Gender, and Living Alone versus Living with Others
Supportive family behaviors differed by participants’ race/ethnicity in unadjusted (F(2, 187)=13.46, p<.001) and adjusted (F(2, 176)=11.38, p<.001) analyses. Other race participants reported more supportive behaviors (3.2±1.1) than AA/Blacks (2.5±1.0, p<.001) or Whites(1.9±0.9, p<.01), and AA/Blacks reported more supportive behaviors than Whites (p<.01). Obstructive family behaviors also differed by race/ethnicity with the same pattern in unadjusted analyses (F(2, 187)=37.63, p<.001), but these differences were nonsignificant in adjusted analyses (F(2, 176)=2.66, p=.07). Neither supportive nor obstructive family behaviors differed by gender. Neither race (White versus non-White) nor gender moderated the relationships between supportive or obstructive family behaviors and participants’ self-care or HbA1C. Participants who lived alone reported less supportive behaviors than those who lived with others(2.0±1.1 versus 2.5±1.0) in unadjusted (F(1, 188)=9.28, p<.01) and adjusted (F(1, 177)=4.62, p<.05) analyses, but reported the same amount of obstructive behaviors as those who lived with others.
4. Discussion and Conclusion
4.1. Discussion
In a cross-sectional study of adults with T2DM and low SES, participants reported that their family members performed actions that impeded their diabetes self-care nearly as often as their family members performed helpful actions. Both supportive and obstructive family behaviors were associated with patients’ adherence to different self-care behaviors, and in the expected directions. These effect sizes were moderate and supportive and obstructive family behaviors combined explained a substantial and significant percent of the variance in adherence to each self-care behavior (with the exception of blood glucose testing). However, only obstructive behaviors were associated with worse glycemic control. Although supportive behaviors were not associated with glycemic control, they protected against the detrimental effect of obstructive behaviors on glycemic control. Because non-Whites reported significantly more supportive family behaviors, they may benefit most from this buffering effect. However, they also reported more obstructive behaviors than Whites in unadjusted analyses, and the relationships between supportive and obstructive family behaviors and diabetes self-care or glycemic control were consistent regardless of race/ethnicity or gender. We also found that participants living alone reported the same rates of obstructive behaviors as those living with others, but less supportive behaviors. Family members may find it easier to nag/argue about nonadherence from afar than to support daily self-care (e.g., prepare healthy meals).
This study contributes most to our understanding of how family members’ supportive and obstructive behaviors may jointly affect adults’ diabetes self-care and glycemic control. Analyzing the effects of both types of behaviors simultaneously isolated the contribution of each over and above the effect of family involvement in patients’ self-care [32, 33]. These relationships were substantially stronger, suggesting that the type of interactions family members have with patients was more important than the degree of family involvement in diabetes self-care. As a result of not acknowledging and accommodating suppressor effects, prior studies have reported inconsistent findings when using the DFBC subscales, and resorted to conceptualizing family involvement as a single variable [11]. Consequently, the relationship between obstructive family behaviors and patients’ glycemic control and the moderating role of supportive behaviors in this relationship, have been previously overlooked.
4.2. Limitations and Future Research
There are limitations to acknowledge. Our cross-sectional design limits conclusions about causality. Stephens et al.[18] reported spouses’ diet-specific supportive behaviors affected patients’ diet adherence the next day, and a longitudinal study [34] reported patients in low conflict families had better HbA1C values six months later. These studies suggest family behaviors may exert a causal effect on self-care and glycemic control, but reciprocal causality is more plausible [35]. For instance, poorly controlled patients may elicit more nagging/arguing from their family members, which, in turn, may be detrimental to patients’ adherence and glycemic control. Thus, in our opinion, intervention studies and studies that seek to understand the moderators and mediators of the relationships between family behaviors and patients’ diabetes-management are more informative than those seeking to establish cause-effect relationships.
Our reliance on self-report measures may have introduced recall and social-desirability bias. Future studies should consider objective measures of patients’ self-care activities. We also sampled from a single clinic, limiting generalizability. There was little variability with regard to gender (70% female) and SES. The type of family behaviors that matter may vary by gender in families with traditional gender roles around meal preparation. Future research should test these relationships among larger samples of male and female participants. Patients with low SES may be more vulnerable to family behaviors than patients with higher SES. Others have found that gender [12, 26] or race [9] moderated the effects of family constructs on self-care or glycemic control. Such modifying effects may exist in samples with more SES and gender heterogeneity. Although our results are likely robust with respect to Black/White differences, conclusions about Hispanic participants cannot be drawn due to a small number of Hispanic participants.
We also did not have information on participants’ family composition beyond marital status and living alone versus living with others. Future studies should explore what these families look like – how many people live with the patient, how much contact does the patient have with his/her family members, and is the patient a primary family caregiver? Now that we are aware of the importance of family behaviors for this patient population, a more thorough understanding of adult patients’ family context is critical. Constructs such as diabetes distress and self-efficacy for diabetes self-care should be included in future studies to clarify the mechanisms underlying the associations between family behaviors and patients’ self-care and glycemic control. For instance, Rosland et al.[13] found that obstructive family behaviors were associated with decreased self-efficacy for self-care but it remains unclear if self-efficacy mediates the associations identified here. Additional research should also explore how family dynamics previously identified as important to diabetes self-management (e.g., family relationship quality and conflict resolution) [9, 35] interact with supportive and obstructive family behaviors to influence patients’ self-care and glycemic control.
4.3. Conclusions
Our findings extend the current understanding of how families are involved in adults’ diabetes self-care and the ramifications of harmful family involvement for diabetes outcomes. Research efforts should assess both positive and negative aspects of family involvement, and not make the assumption that more family involvement is beneficial. The positive association between supportive and obstructive family behaviors suggests family members are involved in patients’ self-care, but may not know how to best help and not hinder patients’ efforts. In the recent international Diabetes Attitudes Wishes and Needs 2 study, nearly 40% of family members of an adult with diabetes reported wanting to be more involved in the patients’ diabetes management, but did not know how to help [36]. However, educating family members about diabetes and/or involving families in patients’ care without working to reduce obstructive family behaviors may actually evoke less patient adherence. In one study [16], participants reported that family members’ knowledge about diabetes management was associated with more supportive but not with less obstructive family behaviors. Future work should determine what intervention content effectively reduces obstructive family behaviors while increasing supportive behaviors.
4.4. Practice Implications
This study has implications for behavioral interventions and not directly for clinical practice; future work is necessary to determine recommendations for patient-provider interactions. Family-based interventions for adults with diabetes are relatively new, have tried various approaches, and have not been effective at reducing HbA1C [37]. While complex family characteristics affect adults’ diabetes management (e.g., family relationship quality and conflict resolution) [9, 35], these constructs are relatively stable and resistant to health behavior interventions. Interventions may be able to redirect family members’ efforts to “help” from nagging/arguing to supportive behaviors, without addressing underlying relationship issues.
Recent interventions involving families in adults’ disease management have focused on either (1) guiding family members to set specific goals to support patients’ self-care, (2) training family members in supportive communication techniques around disease management, or (3) giving family members helpful roles in the clinical care process (e.g., tracking clinical data, communicating with providers) [6]. Applied individually, these approaches have had mixed success in improving patient outcomes [6]. Interventions incorporating all of these approaches may address both the helpful and harmful aspects of family involvement. Our findings endorse interventions that help family members develop actionable plans to support patients’ self-care goals and train them to communicate productively about diabetes management. In the event obstructive behaviors cannot be successfully reduced due to the complexities inherent to families (e.g., personalities, contentious family environments), increasing the amount of diabetes-specific family support may offset the detrimental effect of obstructive family behaviors on patients’ glycemic control.
We confirm all patient/personal identifiers have been removed or disguised so the patient/person(s) described are not identifiable and cannot be identified through the details of the story.
Highlights.
Family involvement in adult’ diabetes management can be both helpful and harmful.
Both supportive and obstructive behaviors were associated with patients’ self-care.
Type mattered more than degree of family involvement for patients’ self-care.
Obstructive behaviors were associated with worse HbA1C.
Supportive behaviors buffered this deleterious effect on HbA1C.
Acknowledgements
The manuscript will not be submitted to another journal while under review for Patient Education and Counseling. This research was funded with support from the Vanderbilt CTSA award (UL1TR000445) from the National Center for Advancing Translational Sciences. Dr. Mayberry was supported by a National Research Service Award (NIDDK F32DK097880) and Dr. Osborn was supported by a career development award (NIDDK K01DK087894) from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We also collected the most recent lab HbA1C value and its associated collection date from participants’ medical charts. A substantial proportion of participants either had an out of date (i.e., >90 days from study participation) lab HbA1C value in their medical chart (n=29) or did not have an HbA1C value in their medical chart (n=4). The point-of-care HbA1C values demonstrated good convergent validity with lab HbA1C values among participants with <90 days between the tests (Spearman’s rho = .87, p<.001). Time between test dates explained why six participants had a >1.0% discrepancy between the two HbA1C values (all six had >90 days between values and three had >1 year between values).
Conflict of interest statement: The authors have no conflict of interest to disclose.
Both authors contributed to the development of the analyses and manuscript.
References
- [1].DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42:200–9. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- [2].Bowen DJ, Helmes A, Lease E. Predicting compliance: how are we doing? In: Burke L, Ockene I, editors. Compliance in healthcare and research. Futura; Armonk: 2001. pp. 25–41. [Google Scholar]
- [3].Glasgow RE, Wagner EH, Kaplan RM, Vinicor F, Smith L, Norman J. If diabetes is a public health problem, why not treat it as one? A population-based approach to chronic illness. Ann Behav Med. 1999;21:159–70. doi: 10.1007/BF02908297. [DOI] [PubMed] [Google Scholar]
- [4].DiMatteo MR. Social support and patient adherence to medical treatment: A meta-analysis. Health Psychol. 2004;23:207–18. doi: 10.1037/0278-6133.23.2.207. [DOI] [PubMed] [Google Scholar]
- [5].Rosland AM, Heisler M, Piette JD. The impact of family behaviors and communication patterns on chronic illness outcomes: a systematic review. J Behav Med. 2012;35:221–39. doi: 10.1007/s10865-011-9354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rosland AM, Piette JD. Emerging models for mobilizing family support for chronic disease management: a structured review. Chronic Illn. 2010;6:7–21. doi: 10.1177/1742395309352254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tang TS, Brown MB, Funnell MM, Anderson RM. Social support, quality of life, and self-care behaviors among African Americans with type 2 diabetes. Diabetes Educ. 2008;34:266–76. doi: 10.1177/0145721708315680. [DOI] [PubMed] [Google Scholar]
- [8].Wen LK, Parchman ML, Shepherd MD. Family support and diet barriers among older Hispanic adults with type 2 diabetes. Fam Med. 2004;36:423–30. [PubMed] [Google Scholar]
- [9].Fisher L, Chesla CA, Skaff MM, Gilliss C, Mullan JT, Bartz RJ, et al. The family and disease management in Hispanic and European-American patients with type 2 diabetes. Diabetes Care. 2000;23:267–72. doi: 10.2337/diacare.23.3.267. [DOI] [PubMed] [Google Scholar]
- [10].Garay-Sevilla ME, Nava LE, Malacara JM, Huerta R, Diaz de Leon J, Mena A, et al. Adherence to treatment and social support in patients with non-insulin dependent diabetes mellitus. J Diabetes Complications. 1995;9:81–6. doi: 10.1016/1056-8727(94)00021-f. [DOI] [PubMed] [Google Scholar]
- [11].Glasgow RE, Toobert DJ. Social environment and regimen adherence among type II diabetic patients. Diabetes Care. 1988;11:377–86. doi: 10.2337/diacare.11.5.377. [DOI] [PubMed] [Google Scholar]
- [12].Choi SE. Diet-specific family support and glucose control among Korean immigrants with type 2 diabetes. Diabetes Educator. 2009;35:978–85. doi: 10.1177/0145721709349220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rosland AM, Heisler M, Choi HJ, Silveira MJ, Piette JD. Family influences on self-management among functionally independent adults with diabetes or heart failure: do family members hinder as much as they help? Chronic Illn. 2010;6:22–33. doi: 10.1177/1742395309354608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Xu Y, Toobert D, Savage C, Pan W, Whitmer K. Factors influencing diabetes self-management in Chinese people with type 2 diabetes. Res Nurs Health. 2008;31:613–25. doi: 10.1002/nur.20293. [DOI] [PubMed] [Google Scholar]
- [15].Henry SL, Rook KS, Stephens MA, Franks MM. Spousal undermining of older diabetic patients’ disease management. J Health Psychol. 2013 doi: 10.1177/1359105312465913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mayberry LS, Osborn CY. Family support, medication adherence, and glycemic control among adults with type 2 diabetes. Diabetes Care. 2012;35:1239–45. doi: 10.2337/dc11-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Carter-Edwards L, Skelly AH, Cagle CS, Appel SJ. “They care but don’t understand”’: Family support of African American women with type 2 diabetes. Diabetes Educator. 2004;30:493–501. doi: 10.1177/014572170403000321. [DOI] [PubMed] [Google Scholar]
- [18].Stephens MAP, Franks MM, Rook KS, Iida M, Hemphill RC, Salem JK. Spouses’ attempts to regulate day-to-day dietary adherence among patients with type 2 diabetes. Health Psychol. 2013;32:1029–37. doi: 10.1037/a0030018. [DOI] [PubMed] [Google Scholar]
- [19].Harris MA. The family’s involvement in diabetes care and the problem of miscarried helping. Business Briefing: European Endocrine Review. 2006:1–3. Reference Section. [Google Scholar]
- [20].Schafer LC, Mccaul KD, Glasgow RE. Supportive and nonsupportive family behaviors - relationships to adherence and metabolic control in persons with type-I diabetes. Diabetes Care. 1986;9:179–85. doi: 10.2337/diacare.9.2.179. [DOI] [PubMed] [Google Scholar]
- [21].Latham CL, Calvillo E. Predictors of successful diabetes management in low-income Hispanic people. West J Nurs Res. 2009;31:364–88. doi: 10.1177/0193945908328263. [DOI] [PubMed] [Google Scholar]
- [22].Glasgow RE, Toobert DJ, Riddle M, Donnelly J, Mitchell DL, Calder D. Diabetes-specific social learning variables and self-care behaviors among persons with type II diabetes. Health Psychol. 1989;8:285–303. doi: 10.1037//0278-6133.8.3.285. [DOI] [PubMed] [Google Scholar]
- [23].Cohen PN, Casper LM. In whose home? Multigenerational families in the United States, 1998-2000. Sociol Perspect. 2002;45:1–20. [Google Scholar]
- [24].Silverman M, Musa D, Kirsch B, Siminoff LA. Self care for chronic illness: older African Americans and whites. J Cross Cult Gerontol. 1999;14:169–89. doi: 10.1023/a:1006676416452. [DOI] [PubMed] [Google Scholar]
- [25].Gallant MP, Spitze G, Grove JG. Chronic illness self-care and the family lives of older adults: a synthetic review across four ethnic groups. J Cross Cult Gerontol. 2010;25:21–43. doi: 10.1007/s10823-010-9112-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hagedoorn M, Keers J, Links T, Bouma J, Ter Maaten J, Sanderman R. Improving selfself-management in insulin-treated adults participating in diabetes education. The role of overprotection by the partner. Diab Med. 2006;23:271–7. doi: 10.1111/j.1464-5491.2006.01794.x. [DOI] [PubMed] [Google Scholar]
- [27].Mayberry LS, Gonzalez JS, Wallston KA, Kripalani S, Osborn CY. The ARMS-D out performs the SDSCA, but both are reliable, valid, and predict glycemic control. Diabetes Res Clin Pract. 2013 doi: 10.1016/j.diabres.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Behling O, Law KS. Translating questionnaires and other research instruments: Problems and solutions. In: Lewis-Beck M, editor. Quantitative Applications in the Social Sciences. Sage; Thousand Oaks: 2000. p. 1.p. 70. [Google Scholar]
- [29].Toobert DJ, Hampson SE, Glasgow RE. The Summary of Diabetes Self-Care Activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23:943–50. doi: 10.2337/diacare.23.7.943. [DOI] [PubMed] [Google Scholar]
- [30].Kennedy L, Herman WH. Glycated hemoglobin assessment in clinical practice: comparison of the A1cNow point-of-care devise with central laboratory testing (GOAL A1C Study) Diabetes Technol Ther. 2005;7:907–12. doi: 10.1089/dia.2005.7.907. [DOI] [PubMed] [Google Scholar]
- [31].Centers for Disease Control and Prevention (CDC) National Center for Health Statistics [Accessed 24 July 2014];Division of Health Interview Statistics, data from the National Health Interview Survey. Data computed by personnel in CDC’s Division of Diabetes Translation, National Center for Chronic Disease Prevention and Health Promotion. from: http://www.cdc.gov/diabetes/statistics/age/fig4.htm.
- [32].Velicer WF. Suppressor variables and the semipartial correlation coefficient. Educ Psychol Meas. 1978;38:953–8. [Google Scholar]
- [33].Conger AJ. A revised definition for suppressor variables: A guide to their identification and interpretation. Educ Psychol Meas. 1974;34:35–46. [Google Scholar]
- [34].Edelstein J, Linn MW. The influence of the family on control of diabetes. Soc Sci Med. 1985;21:541–4. doi: 10.1016/0277-9536(85)90038-3. [DOI] [PubMed] [Google Scholar]
- [35].Fisher L, Chesla CA, Bartz RJ, Gilliss C, Skaff MA, Sabogal F, et al. The family and type 2 diabetes: a framework for intervention. Diabetes Educ. 1998;24:599–607. doi: 10.1177/014572179802400504. [DOI] [PubMed] [Google Scholar]
- [36].Burns KK, Nicolucci A, Holt RIG, Willaing I, Hermanns N, Kalra S, et al. Diabetes Attitudes, Wishes and Needs second study (DAWN2 (TM)): Cross-national benchmarking indicators for family members living with people with diabetes. Diabetic Med. 2013;30:778–88. doi: 10.1111/dme.12239. [DOI] [PubMed] [Google Scholar]
- [37].Torenholt R, Schwennesen N, Willaing I. Lost in translation—the role of family in interventions among adults with diabetes: a systematic review. Diabetic Med. 2014;31:15–23. doi: 10.1111/dme.12290. [DOI] [PubMed] [Google Scholar]