Abstract
Asthma occurs as complex interactions of the environmental and genetics. Clinical studies and animal models of asthma indicate dietary factors such as vitamin E and vitamin D as protective for asthma risk. In this review, we discuss opposing regulatory functions of tocopherol isoforms of vitamin E and regulatory functions of vitamin D in asthma and how the variation in global prevalence of asthma may be explained, at least in part, by these dietary components.
Keywords: asthma, α-tocopherol, γ-tocopherol, vitamin D, human, animal models
Asthma is a heterogeneous disease resulting from complex interactions of environmental and genetic factors 1. The World Health Organization reported that the prevalence of asthma from 1950 to the present has increased in many countries including countries with high rates of asthma, intermediate rates of asthma or low rates of asthma 2–4. The marked rise in rates of asthma over a few decades and the differences in rates among countries and in migrating populations suggest an important role of the local environment, such as diet, in development of asthma. One environmental change over the past 40 years has been an increase in the γ-tocopherol isoform of vitamin E in the diet and in infant formulas 5, 6. We recently demonstrated that γ-tocopherol increases allergic lung inflammation in a mouse model of asthma and, we reported that, in humans, high plasma γ-tocopherol levels are associated with lower lung function 6–9. It is also suggested that a reduction over time in another vitamin, vitamin D, associates with the increase incidence in asthma. In this review, we discuss the regulation of asthma by vitamin D and the complex and potentially protective effects of specific isoforms of vitamin E on asthma in humans and in animal models of lung inflammation. We will also review how the variation in global prevalence of asthma may be explained, at least in part, by country-specific plasma γ-tocopherol concentrations.
VITAMIN E
Vitamin E consists of natural isoforms and synthetic racemic isoforms. The eight natural isomers are d-α-, d-β-, d-γ-, d-δ-tocopherol and d-α-, d-β-, d-γ-, d-δ-tocotrienol. Plants synthesize the natural isoforms from tyrosine and chlorophyll 10. Then, these tocols are consumed in the diet from plant lipids in vegetables. The most abundant dietary source is cooking oils from plants. Mammals do not synthesize or interconvert the tocopherol isoforms. The most abundant isoforms of vitamin E are α-tocopherol and γ-tocopherol which differ by one methyl group (Figure 1A). There are 10 fold higher tissue concentrations of α-tocopherol than γ-tocopherol because there is preferential loading of α-tocopherol on HDL/LDL particles in the liver by α-tocopherol transfer protein and because there is a higher rate of degradation of γ-tocopherol into its metabolites for excretion 11, 12. The tocopherol concentrations in plasma correlate with those in the lung tissue both in humans and mice 7, 8, 13. Other diet components may influence tocopherol absorption, including dietary L-carnitine which enhances absorption of α-tocopherol in rats 14. α-tocopherol concentrations are affected by genetic variants of the liver α-tocopherol transfer protein, resulting in human α-tocopherol deficiency 15. It is also reported that human plasma levels of α-tocopherol but not γ-tocopherol are increased in male adults and children by the apolipoprotein A5 1131T>C gene polymorphism 16, 17. In mice, apoE4 mice have lower plasma α-tocopherol than apoE3 mice 18.
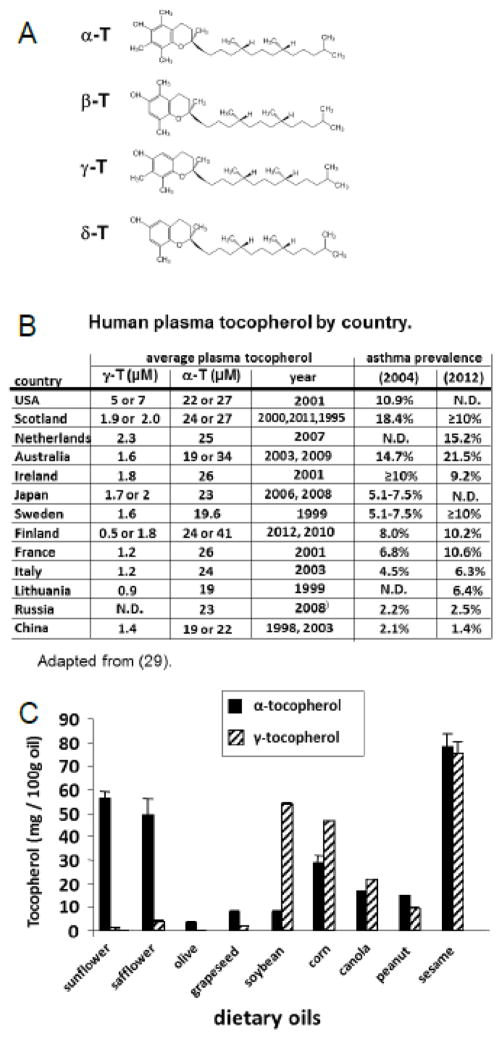
Figure 1. α-tocopherol and γ-tocopherol.
A) α-tocopherol differs from γ-tocopherol by one methyl group (arrows). B) Plasma γ-tocopherol (γ-T) and plasma α-tocopherol (α-T) in 1–2 reports/country and publication dates are indicated 29, 118–128. Global asthma prevalence in 2004 129 and 2012 130. C) Tocopherols were extracted from dietary oils (sunflower oil from Spectrum Organic Products, LLC; safflower oil from Spectrum; olive oil from Colavita; soybean oil from Crisco; corn oil from Mazola; grapeseed oil from Kusha, Inc; sesame oil from Lavita; peanut oil from Essentials by Supervalu; canola oil from Crisco; Sacha Inchi from Olivar). Extracted tocopherols were measured by HPLC with an electrochemical detector as previously described 8. ND, not determined.
The tocopherol isoforms are anti-oxidants. α-Tocopherol and γ-tocopherol, at equal molar concentrations, have a relatively similar capacity to scavenge reactive oxygen species (ROS) during lipid peroxidation in vitro and in cells 19, 20 and a relatively similar capacity to inhibit activation of protein kinase B (Akt) in cancer cells in vitro 21. Thus, because α-tocopherol is at a 10 fold higher concentration in tissues than γ-tocopherol, there is 10 fold more scavenging of ROS by α-tocopherol than by γ-tocopherol in vivo. Besides scavenging ROS, γ-tocopherol, in contrast to α-tocopherol, also reacts with reactive nitrogen species (RNS) such as peroxynitrite forming 5-nitro-γ-tocopherol 22. γ-Tocopherol scavenging of RNS may be beneficial for inflammation with increases in RNS such as neutrophilic inflammation that is induced by ozone or endotoxin in mice 23. Consistent with this, reports indicate that supplementation with a mixture of tocopherols enriched for γ-tocopherol blocks acute endotoxin-stimulated or ozone-stimulated neutrophil inflammation in the rat and human lung 24–26. In another study, γ-tocopherol supplementation reduced antigen induction of rat lung inflammation in which there was several fold more neutrophils than eosinophils 27. It is also reported that nebulized γ-tocopherol reduces neutrophilia in burn and smoke inhalation injury in sheep 28. Therefore, γ-tocopherol may be of benefit for acute neutrophilic inflammation. In contrast, it is reported that higher plasma γ-tocopherol associates with lower lung function in humans 9 and with higher lung eosinophilia and airway hyperresponsiveness in mouse models of allergic asthma 9.
There are studies reporting seemingly conflicting outcomes for vitamin E on allergy and other inflammatory diseases but these differences are consistent with the differences in levels of vitamin E isoforms present in the study supplements, vehicles and diets in the studies 6, 29, 30 and our mechanistic studies demonstrating opposing functions of vitamin E isoforms 7–9, 29, 31, 32.
We have demonstrated, in mouse models of allergic lung inflammation, that the isoform α-tocopherol is anti-inflammatory and blocks airway hyperreactivity, and that a 5-fold increase in the isoform γ-tocopherol is pro-inflammatory and increases airway hyperreactivity during eosinophilic allergic lung inflammation 6–8, 31, 32. In these studies, administration of α-tocopherol or γ-tocopherol subcutaneously to allergic adult mice during challenge with the antigen chicken egg ovalbumin (OVA) raised lung and plasma concentrations of the tocopherol isoform 4–5 fold 8. Subcutaneous administration of γ-tocopherol elevated lung eosinophil recruitment by 175%, and α-tocopherol reduced lung eosinophil recruitment by 65% when challenged with OVA 8. In these mice, α-tocopherol blocked and γ-tocopherol increased airway hyperresponsiveness 8. Whether tocopherols are administered subcutaneously or in the diet, the tocopherols enter the lymph, then the thoracic duct and then the liver where the tocopherols are loaded on lipoproteins that then enter circulation. In unpublished studies from our research group, lung inflammation in response to OVA was also reduced by administration of α-tocopherol through the diet and elevated by administration of γ-tocopherol through the diet, using a diets supplemented with 250 mg α- or γ-tocopherol /kg diet.
Interestingly, γ-tocopherol negates the anti-inflammatory benefit of α-tocopherol 8, 33. α-Tocopherol plus γ-tocopherol results in an airway response similar to that of the vehicle control-treated allergic mice, suggesting that these two tocopherols have competing opposing functions 8. This strong opposing function of γ-tocopherol occurs even though γ-tocopherol is about 5–10 times lower in concentration in vivo than α-tocopherol. The pro-inflammatory allergic effects of γ-tocopherol in mice are partially reversed by switching supplements from γ-tocopherol to α-tocopherol 7. Thus, we have demonstrated opposing and competing functions of α-tocopherol and γ-tocopherol in vivo. Okamoto et al. 34 found that feeding mice α-tocopherol starting 2 weeks before antigen sensitization did not affect IgE levels, but did reduce the number of eosinophils in the bronchoalveolar lavage, but, the form and purity of α-tocopherol were not indicated. Mabalirajan et al. 35 reported that oral administration of α-tocopherol in ethanol after antigen sensitization blocked OVA-induced lung inflammation and airway hyperresponsiveness. In a report by Suchankova et al. 36, purified α-tocopherol was administered in soy oil by gavage and they found no major effect of α-tocopherol on immune parameters, or lung airway responsiveness in mice challenged with OVA. However, the soy oil vehicle in this study contains an abundance of γ-tocopherol (Figure 1C) which can oppose the function of α-tocopherol. In their study, neither tissue tocopherol levels, nor vehicle tocopherol levels were reported. Therefore, differences among the reports for tocopherol regulation of eosinophilic lung inflammation reflect differences in the intake of tocopherol isoforms.
A mechanism for the opposing regulatory functions for α-tocopherol and γ-tocopherol on allergic inflammation in the mouse lung is, in part, a result of tocopherol regulation of signals for leukocyte transendothelial migration from the blood into the lung. We demonstrated in vitro that the migration of spleen leukocytes across endothelial cells is inhibited by pretreatment of the endothelial cells with α-tocopherol and elevated by pretreatment of the endothelial cells with γ-tocopherol 8. Pretreatment of endothelial cells with α-tocopherol plus γ-tocopherol results in spleen leukocyte transendothelial migration that is similar to the vehicle-treated control endothelial cells 8. Thus, α-tocopherol and γ-tocopherol have opposite regulatory functions during leukocyte recruitment and allergic lung inflammation in mice. A mechanism for this opposing function is through opposing regulatory effects on signals in the endothelial cells during leukocyte recruitment. During leukocyte recruitment, the endothelial cell adhesion molecules VCAM-1 and ICAM-1 activate signals in the endothelial cell through protein kinase C α (PKCα) that are required for spleen leukocyte migration between the endothelial cells 8, 31. We demonstrated that α-tocopherol inhibits VCAM-1 and ICAM-1 activation of PKCα in endothelial cells and that this is opposed by pretreatment of endothelial cells with γ-tocopherol 8, 31. It is also reported that α-tocopherol inhibits activation of PKCα in other cell systems or cell extracts but the mechanisms for inhibition were not know37. We demonstrated that α-tocopherol and γ-tocopherol directly bind to the C1a regulatory domain of PKCα 32 and that γ-tocopherol increases whereas α-tocopherol decreases recombinant PKCα activity 32. Thus, γ-tocopherol functions as an agonist of PKCα activity and α-tocopherol functions as an antagonist of PKCα activity 32, thereby regulating leukocyte recruitment during allergic inflammation.
The average human plasma γ-tocopherol levels are 2 to 5 times higher in the United States than those of many European and Asian countries (Figure 1B) whereas the average human plasma α-tocopherol levels are relatively similar among these countries 29. This 5-fold higher level of human plasma γ-tocopherol is similar to the 5-fold increase in plasma γ-tocopherol in mice that increased allergic lung inflammation with γ-tocopherol administration 8. The high human plasma γ-tocopherol levels in the United States are consistent with soybean oil, which is high in γ-tocopherol 38, 39 (Figure 1C), as the predominant food oil in the United States 40, 41. It is reported that dietary oils influence plasma tocopherol levels in humans. In studies with soybean oil administration, plasma γ-tocopherol is elevated 2–5 fold in humans and hamsters 42, 43. Also, in a study in which olive oil or soybean oil was administered to preterm human infants starting 24 hrs after birth, there was a significant 1.5 fold increase in plasma α-tocopherol after feeding with olive oil as compared to feeding with soybean oil, but unfortunately, γ-tocopherol was not reported 44. It is reported that as countries assume western lifestyles, diets change including increased consumption of soybean oil 45. In contrast to high levels of γ-tocopherol in soybean oil, γ-tocopherol is low in other oils such as sunflower oil, safflower oil and olive oil that are used in several European and Mediterranean countries (Figure 1C) 8. There are also differences in asthma prevalence among racial and ethnic groups 46. However, studies examining vitamin E association with clinical outcomes generally adjust for several known confounding factors such as gender, age, body mass index, race, and smoking. Although there may be other differences regarding the environment and genetics of people in different countries, the outcomes for tocopherol isoforms and asthma in clinical studies are consistent with the studies demonstrating opposing functions of the tocopherol isoforms on leukocyte recruitment and allergic inflammation in mice 8.
We reported that the findings of opposing regulatory functions of tocopherol isoforms in animal models can be translated to human lung function. We analyzed 4526 adults in the United States in the Coronary Artery Risk Development in Young Adults (CARDIA) multi-center cohort with available spirometry and tocopherol isoform data. In this cohort, there were equal numbers of blacks and whites and equal numbers of females and males by study design. Interestingly, increasing serum concentrations of γ-tocopherol were associated with lower FEV1 or FVC, whereas increasing serum concentrations of α-tocopherol were associated with higher FEV1 or FVC 9. Since these two tocopherols have opposing functions, we suggest that the analysis of opposing functions of tocopherol isoforms in clinical studies should include quartiles of plasma tocopherols with determination of whether there is an association of a tocopherol isoform with the clinical outcome when the concentration of the opposing tocopherol is low and causing the least competing opposing effects. Using this approach, in the analysis of the CARDIA cohort, we recently demonstrated that plasma γ-tocopherol is inversely associated with lung function (FEV1) and plasma α-tocopherol is positively associated with lung function (FEV1) in non-asthmatics and asthmatics 9 with adjustments for several known confounding factors such as gender, age, body mass index, race, and smoking. Thus, there were opposing outcomes for association of plasma α-tocopherol and γ-tocopherol with lung function. This is consistent with our mechanistic studies for these tocopherols in animal models.
Also consistent with the animal models 7, 8, a 5-fold magnitude of an increase in human plasma γ-tocopherol associated with a reduction in lung function in humans 9. Briefly, in mice challenged with OVA, a 5-fold increase in plasma γ-tocopherol increased lung hyperreponsiveness. This 5-fold difference in γ-tocopherol concentrations is consistent with 5-fold higher γ-tocopherol in Americans versus Western Europeans and Asians and higher prevalence of asthma in Americans (Figure 1B). In CARDIA, a 5-fold higher human plasma γ-tocopherol (>10 μM γ-tocopherol) was associated with reduced FEV1 and FVC in all participants (asthmatics and non-asthmatics) by age 21–27 years old. The γ-tocopherol-associated decreases in FEV1 and FVC before age 21 may occur during development and lung responses to environmental pollutants, allergens, or infections because tocopherols can directly regulate PKCα 6–8, 29. For the asthmatic group with plasma γ-tocopherol >10 μM, the participants had 350–570 mL lower FEV1 or FVC as compared to the low to moderate γ-tocopherol concentrations (<10 μM γ-tocopherol) at ages 21–279.
This 10 to 17% decrease in FEV1 with >10 μM plasma γ-tocopherol in asthmatics is similar to the 5–10% reduction in FEV1 reported for other environmental factors. For example, individuals with occupational allergen exposure have a 5–8% decrease in FEV1 compared to nonasthmatics and this decrease is associated with dyspnea, chest tightness, chronic bronchitis, and chronic cough 47. Responders to particulate matter have a 2 to 6% decrease in FEV1 48, responders to cold or exercise have a 5 to 11% decrease in FEV1 49 and responders to house dust mite or dog/cat dander have a 2–8% decrease in FEV1 50. Moreover, based on the 2% prevalence of serum γ-tocopherol >10μM in adults in CARDIA2 and the adult U.S. population in the 2011 census, we expect that the lower FEV1 and FVC at >10μM serum γ-tocopherol occur in up to 4.5 million adults in the U.S. population.
In other clinical studies, it is indicated that increased intake of α-tocopherol may confer a modest protective effect on adult-onset asthma, lung function (FEV1) or wheeze in studies in Finland and Italy, where plasma γ-tocopherol is low. In contrast, it is indicated that increased intake of α-tocopherol does not have a beneficial effect in the United States or the Netherlands, where plasma γ-tocopherol is up to 5 times higher 51–55. Moreover, the prevalence rate of asthma is higher in the United States, Netherlands, and Scotland than several European and Asian countries (Figure 1B). Interestingly, countries with the highest prevalence rate for asthma also tend to have high average human plasma levels of γ-tocopherol (Figure 1B). Administration of acetate-conjugated d-α-tocopherol oral supplementation at very high dose (1500 I.U. which is 1006 mg) to mild atopic asthmatics in the United States for 16 weeks resulted in increased plasma α-tocopherol, decreased plasma γ-tocopherol, and improved airway responsiveness to methacholine challenge 56. In a study in England, dietary supplementation with α-tocopherol in soy oil to asthmatics had no impact on FEV1, asthma symptom scores or bronchodilator use, but the γ-tocopherol in the soy oil may have opposed the benefit of the α-tocopherol 57. In a Scottish cohort, reduced maternal intake of vitamin E (likely referring to α-tocopherol) was associated with increased incidence of asthma and wheezing in children up to 5 years old 58, 59. In Devereaux’s review of this data and changes in the environment in Scotland 59 from 1967 to 2004, there was a significant increase in vegetable oil intake by Scottish and we suggest that this would at least resulted in an increase in γ-tocopherol since vegetable oil (soybean oil) is rich in γ-tocopherol (Figure 1C). In a meta-analysis 60, it was concluded that dietary vitamin E intake is not generally associated with asthma status. However, there were different vitamin E isoforms present in the diets, supplements and supplement vehichles in the meta-analysis. We interpret this meta-analysis as a combination of data across studies that included different vitamin E isoforms that have opposing functions. Most clinical studies on vitamin E include mixed forms of natural and synthetic tocopherols from supplementation or diet. Therefore, differences in outcomes from clinical reports on the associations of vitamin E and asthma may, in part, reflect the opposing regulatory effects of α-tocopherol and γ-tocopherol in the supplements, the vehicles for the supplements, and diets in the individuals.
It is reported that there are low plasma α-tocopherol levels in adults or children with asthma 52, 53, 61–64 and that plasma and tissue tocopherols correlate 7, 8, 13. Therefore, since α-tocopherol levels are low in asthmatics and since α-tocopherol can reduce inflammation, then an increase in α-tocopherol and importantly, a decrease in γ-tocopherol may be beneficial in combination with other regimens to either prevent or improve control of allergic disease/asthma. A decrease in γ-tocopherol consumption may be achieved by changing from diet cooking oils and changing supplements that are rich in γ-tocopherol to sources that are rich in α-tocopherol with little to no γ-tocopherol. Further intervention studies with analysis of the tocopherol isoforms in plasma are necessary to examine tocopherol isoform regulation of allergic lung inflammation and asthma. In summary, the opposing functions of α-tocopherol and γ-tocopherol in animal models 8 are consistent with the different outcomes for the clinical studies of tocopherol isoforms in people with asthma.
VITAMIN D
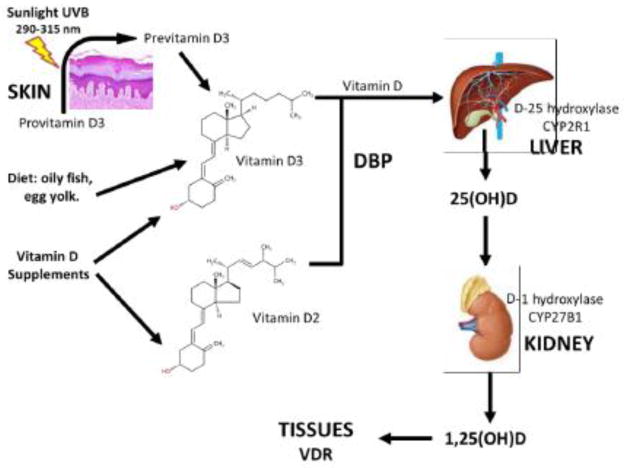
The experiments leading to the discovery of the chemical structure of vitamin D were initially based on ultraviolet light (UV) irradiation of mixtures of plant sterols. The first mistakenly identified vitamin D, named D1, is a term no longer used because it was later found to be a mixture of vitamin D2 (ergocalciferol) with tachysterol, a UV-derived isomer of ergosterol. The main forms of vitamin D are vitamin D2 and vitamin D3 (cholecalciferol) (Figure 2) 65. There are 3 sources of vitamin D: diet, supplements and sunlight. Dietary sources of vitamin D3 are oily fish (e.g. salmon, cod liver oil), egg yolk, and fortified (vitamin D3 added) foods 66. Vitamin D supplements contain either vitamin D3 or D2 from 400 IU to 50,000 IU per dose (1 IU = 25 ng), which are manufactured from UV-radiation of ergosterol from yeast or radiation of 7-dehydrocholesterolfrom lanolin, respectively, and both are effective for rickets. Ultraviolet B sunlight (wavelength 290–315 nm) causes photolysis of 7-dehydrocholesterol (provitamin D3), to previtamin D3 in the plasma membrane of skin cells, which is unstable and spontaneously converts to vitamin D3 67. 7-dehydrocholesterol originates from enzymatic conversion of cholesterol by the7-dehydrocholesterol reductase. Sunlight exposure does not cause vitamin D3 intoxication because sunlight destroys excess of previtamin D3 and vitamin D3 in the skin.
Figure 2. Vitamin D Metabolism.
Vitamins D2 or D3 circulate bound to vitamin D binding protein (DBP) which also transports 25-hydroxyvitamin D (25(OH)D) and 1-alpha-25- dihydroxyvitamin D (1,25(OH)D). Vitamin D hydroxylases include liver hydroxyvitamin D-25-hydroxylase (CYP2R1) and kidney 25-hydroxyvitamin D-1α-hydroxylase (CYP27B1). Both 25(OH)D and 1-alpha-25-hydroxyvitamin D (1,25(OH)D) bind to vitamin D receptor (VDR) to induce vitamin D effects on tissues.
Vitamins D2, D3 and their metabolites are lipophilic and circulate in the blood bound to vitamin D binding protein (DBP) 68. Once in the liver, vitamins D2 and D3 (from hereto called vitamin D) are metabolized by a microsomal cytochrome P450 mono-oxygenase called 25-vitamin D hydroxylase (gene symbol CYP2R1) into their 25-hydroxy vitamin D (25(OH)D) metabolites (specifically, 25(OH)D2, and 25(OH)D3 or calcidiol). 25(OH)D is 95%–99% bound to DPB and is the main vitamin D metabolite measured in the serum to diagnose vitamin D deficiency. Based mostly on bone health, vitamin D deficiency is defined as a serum level of 25(OH)D lower than 10 ng/ml (or 25 nmol/L as 1 ng/ml=2.5 nmol/L), insufficiency is between 10–30 ng/ml, sufficiency between 30–100 ng/ml with preferred range between 30–60 ng/ml, and intoxication >150 ng/ml 66, 69–71.
The final step of formation of vitamin D metabolites is 1-alpha-hydroxylation of 25(OH)D in the kidneys by another P450 mono-oxygenase located in the inner mitochondrial membrane. This 25-hydroxyvitamin D-1a-hydroxylase (CYP27B1 or 1a-hydroxylase) hydroxylates 25-hydroxyvitamin D3 at the 1-alpha position, forming the 1,25(OH)D metabolites (1,25(OH)D2 and 1,25(OH)D3 or calcitriol) 72. 1,25(OH)D binds to intracellular vitamin D receptor (VDR) to exert numerous functions is several tissues, including regulation of serum levels of parathyroid hormone, calcium, and phosphorus; intestinal absorption of calcium; synthesis of 1,25(OH)D; absorption of calcium in kidneys and intestine (bone and dental health); in addition to effects in the nervous system, immune system, cardiovascular system, skeletal muscles and others 73. Because of all these actions, vitamin D is considered more a hormone than a vitamin.
Regarding pharmacokinetics, vitamin D metabolites distribute into blood, muscle and adipose tissues. The circulating half-life for vitamins D2 and D3 is 2 days, for 25(OH)D3 is 2 weeks and for 1,25(OH)D3 is 12 hours. The functional half-lives for vitamins D2, D3 and 25(OH)D3 is 2–3 months and for 1,25(OH)D3 is 12 hours 74. As a result, high latitude regions with long winter seasons result in prolonged decreased sunlight exposure and increased prevalence of vitamin D deficiency during the winter without compensatory vitamin D ingestion by diet and/or supplementation. The Institute of Medicine of the United States recommends a daily intake of vitamin D3 of 400–600 IU per day, but up to 4000 IU/day can be tolerated without risk for intoxication 69.
Vitamin D influences several functions of the innate and adaptive immune systems. Its deficiency impairs innate immune response to Mycobacterium tuberculosis via toll-like receptor (TLR)-2 and nucleotide oligomerization domain (NOD)-2 which recognize triacylated lipoprotein and muramyl dipeptide from M. tuberculosis, respectively in monocytes and macrophages. Vitamin D increases expression of NOD-2 and its induction of human defensin beta 2, production of cathelicidin antimicrobial peptides (hCAP18, LL37) and autophagy in macrophages infected with mycobacteria 75. Vitamin D also enhances generation of reactive oxygen and nitrogen species in leukocytes 76, as well as enhances epithelial barrier function and chemokine secretion, 76, 77. Vitamin D affects corticosteroid responses, enhancing corticosteroid inhibition of RANTES secretion stimulated by TNF-alpha and suppressing corticosteroid induction of fractalkine in airway smooth muscle cells 78–80. The adaptive immune system is also affected by vitamin D. 1,25-OH(D) is produced by macrophages, dendritic cells, T and B cells, which all express vitamin D receptor (VDR). Vitamin D induces dendritic cells to promote tolerogenic T regulatory cells (Tregs) which suppress the development of both Th1 81 and Th2 82 cells in mice by decreasing dendritic cell expression of co-stimulatory molecules and IL-12, and increasing secretion of IL-10. VDR agonists inhibit development of pathogenic effector Th1 and Th17 cells and, may also promote deviation towards the Th2 pathway in certain conditions. In B cells, 1,25(OH)2D3 can induce apoptosis as well as inhibit proliferation, generation of memory B cells, plasma cell differentiation, and Ig production 83.
Epidemiological studies show conflicting results regarding vitamin D supplementation in asthma. In Europe, industrialization in the 20th century markedly changed the diet from fresh foods to processed foods, resulting in an increase in rickets. As vitamin D supplementation with cod liver oil and fortified milk were widely adopted, there was a decrease in rickets, but a parallel increase in prevalence of asthma in the latter half of the 20th century. This and other epidemiological studies 84, 85 led Wjst and Dold to hypothesize that vitamin D supplementation in early infancy may increase the risk of developing atopic diseases 86, 87. This hypothesis is corroborated by animal studies showing that in certain conditions vitamin D may skew immune response toward allergic (Th2) responses 88, 89.
In contrast, epidemiological observations from Camargo, Litonjua and Weiss showed a high prevalence of vitamin D insufficiency in the United States 90, and that lower maternal vitamin D intake during pregnancy increased risk of recurrent wheezing in children by 3 and 5 years of age 91, 92. Based on these clinical observations as well as laboratory evidence that vitamin D deficiency may skew immune responses towards Th2 responses, these researchers have raised the hypothesis that vitamin D supplementation during pregnancy or early infant life may be protective against development of asthma 93. As a result, interventional double-blinded, placebo-controlled, randomized clinical (DBPCRT) trials are now under way to examine this hypothesis 94. However, a recent study raised concerns about this strategy. Japanese researchers enrolled 164 breast-fed Japanese infants with facial eczema at 1 month of age and conducted a DBPCRT trial in which the mother took either placebo or 800 IU /day of vitamin D3 for 6 weeks. At 3 months of age, there were no differences in eczema frequency in the infants, but at 2 years of age food allergy was more common in children whose mother had taken vitamin D3 (25.7% vs. 7.5%, p=0.03). However, the high attrition rate of 50% reduces the confidence in these long term outcomes 95.
Vitamin D may not only be useful for primary prevention as discussed above, but it may also benefit those who already have asthma since serum vitamin D levels correlate with disease severity. In the third National Health and Nutrition Examination Survey (NHANES-III), low serum 25(OH)D levels were associated with worse lung function in a representative sample of 14,091 people of the United States population 96. In 616 children with asthma in Costa Rica, vitamin D insufficiency was prevalent (28%) and low serum 25(OH)D levels were related to both greater asthma severity (increased hospitalizations, use of anti-inflammatory asthma medication, and greater airway responsiveness) and atopy markers such as higher serum immunoglobulin E and blood eosinophilia 97. In 1,024 children aged 5–12 years with persistent asthma in the United States’ Childhood Asthma Management Program (CAMP) study, vitamin D insufficiency was present in 25% and deficiency in 10% of the children at baseline. After 12 months of inhaled corticosteroid therapy, improvement in lung function was directly related to baseline serum 25(OH)D levels 98.
Likewise, among 54 adults with asthma in Denver, lower serum 25(OH)D levels correlated with worse lung function, increased airway hyperreactivity, and reduced in vitro response to corticosteroids 99. Consistent with these findings, Xystrakis et al. showed that vitamin D3 reverses glucocorticoid resistance of CD4+T cells from steroid resistant severe asthmatic subjects, restoring secretion of IL-10 of their CD4+T cells upon dexamethasone stimulation both, after in vitro, or in vivo supplementation with D3 100. Although these results suggest that vitamin D3 supplementation enhances response to corticosteroid therapy in asthmatic patients, other studies have failed to show any significant differences in serum 25(OH)D levels between asthmatics and non-asthmatic adults, or correlations between serum 25(OH)D levels and measures of asthma severity in the United Kingdom 101 and Norway 102. Another study also showed inconsistencies between serum 25(OH)D levels and blood mononuclear responses to corticosteroids in vitro when comparing children versus adults with asthma 103. Finally, and most surprisingly, a recent large double-blinded, placebo-controlled, randomized clinical trial with 406 symptomatic adults with persistent asthma and serum 25(OH)D levels < 30 ng/ml failed to demonstrate an overt benefit in asthma outcomes after 100,000 IU of vitamin D3 bolus followed by 4,000 IU/day for 28 weeks 104. Vitamin D3 did provide some benefits in secondary outcomes, but they were small such as a 12% or 15 μg/day sparing effect on daily inhaled corticosteroids dose and an almost statistically significant 35% reduction in exacerbations. Additional trials are ongoing to further explore the effects of vitamin D supplementation in patients with asthma and other allergic diseases since this is currently a topic of considerable interest in the allergy field 105–107.
Another clinical effect of vitamin D3 supplementation being actively investigated is its ability to prevent or treat respiratory infections, a hypothesis that has arisen from its numerous effects in the innate and adaptive immune systems as discussed above. This is important because early life viral and bacterial respiratory infections increase risk of a child developing asthma and respiratory infections are a major cause of asthma exacerbations.
In a birth cohort in New Zealand, lower cord blood 25(OH)D levels were associated with higher risk of respiratory infection by 3 months of age, and wheezing in the first 5 years of life, although it was not associated with incidence of asthma diagnosis by 5 years of age 108. An analysis of 16,975 adults from the NHANES III showed that only 31% were vitamin D sufficient (serum 25(OH)D level ≥ 30 ng/ml) and 2.1% reported a community acquired pneumonia (CAP) in the previous year. Those with serum 25(OH)D levels <30 ng/ml were at 56% higher risk for reported CAP compared to those who were vitamin D sufficient109. Interestingly, Amrein et al. correlated serum 25(OH)D levels at admission with 90-day mortality in 24,094 adults patients hospitalized in 2 academic centers in Boston. Results suggested a U-shape relationship in that mortality was higher in those with levels below10 ng/ml or above 60 ng/ml 110. Together with the inconsistencies of results in clinical and laboratory studies described above, these results underscore the complex biological effects of vitamin D3 in humans.
Clinical trials of vitamin D3 supplementation to prevent respiratory infections have been undertaken. Vitamin D3 supplementation of 2000 IU (or 50 ug) per day for 3 months significantly increased serum 25(OH)D levels in a double-blinded, placebo-controlled, randomized clinical (DBPCRC) trial, but did not change serum levels of 10 cytokines in 162 healthy volunteers 111.
In a DBPCRC trial involving 247 Japanese school children aged 6–15 years, those who received 1200 IU of oral vitamin D3 per day for 4 months during the winter (Dec-Mar) experienced fewer influenza episodes as confirmed by rapid antigen detection in nasopharyngeal swab (18.6% to 10.8%, or a 42% reduction). They also experienced fewer asthma exacerbations, although there were few episodes for a robust analysis 112. A DBPCRC trial in 247 Mongolian school children showed that milk fortified with 300 IU of vitamin D3 taken during the winter (Jan-Mar) increased serum 25(OH)D levels three fold and reduced by 44% acute respiratory infections (chest infections and colds ascertained by questionnaire at the end of the trial) 113.
In the “Effect of Vitamin D3 Supplementation on Upper Respiratory Tract Infections in Healthy Adults” (VIDARIS) DBPCRC trial, 322 New Zealanders did not experience a reduction in the number or severity of upper respiratory tract infections after receiving two doses of 200,000 IU of oral vitamin D3 a month apart followed by 100,000 IU monthly doses for 18 months 114. Das et al. reviewed results of 3 large randomized controlled trials showing that oral vitamin D supplementation did not help children younger than 5 years of age presenting with acute pneumonia 115 and Bergman et al. published a meta-analysis of 11 randomized controlled trials indicating that vitamin D3 supplementation may prevent respiratory tract infections better when administered daily rather than in high doses intermittently, although heterogeneity of methodology among the trials prevented a firm conclusion 116.
Finally a recent DBPCRC trial of 600 students showed that 10,000 IU of oral vitamin D3 weekly for 2 months (Sep–Oct) reduced modestly the incidence of laboratory confirmed viral respiratory tract infections from 26.7% to 23.3% based on clinical symptoms and a positive nasal swab for virus PCR detection 117.
Taken together, the results of clinical trials are promising, but inconsistent to recommend a specific regimen of oral vitamin D3 supplementation to prevent acute respiratory tract infections.
SUMMARY
In summary, the anti-inflammatory function of α-tocopherol and pro-inflammatory function of γ-tocopherol in animal models of asthma 8 are consistent with the different outcomes for the clinical studies of tocopherol isoforms in asthma. The large variety of effects of vitamin D in many organ systems makes it difficult to predict its clinical effect. In addition, epidemiological and animal studies with vitamin D yielded conflicting results, but many suggest a potential therapeutic and preventive effect of vitamin D supplementation for patients with asthma (see Table 1). Few clinical trials of vitamin D supplementation thus far have shown an inconsistent benefit in reducing respiratory infections, and a single trial in asthma failed to show a clinically significant benefit in adults with vitamin D insufficiency.
Table 1.
Potential beneficial effects of vitamin D in asthma.
| Vitamin D effects on the Innate Immune System: |
| - Enhances epithelial barrier function and chemokine secretion. |
| - Increases production of antimicrobial peptides in macrophages and monocytes. |
| - Enhances generation of reactive oxygen and nitrogen species in leukocytes. |
| Vitamin D effects on the Adaptive Immune System: |
| - Promotes tolerogenic T regulatory cells (Tregs). |
| - Inhibits development of pathogenic effector Th1 and Th17 cells. |
| - Inhibits B cells, plasma cell differentiation, and Ig production. |
| Unproven potential clinical benefits: |
| - Protection against asthma development in early life. |
| - Protection against respiratory infections, a risk factor for asthma onset and exacerbations. |
| - Enhances anti-inflammatory response to corticosteroids. |
The marked differences in rates of asthma across the world, changes in disease prevalence over short periods, and changes with migrating populations means that environment influences the development and responses to triggers that worsen asthma and allergic inflammation. This means that changes in diet and/or lifestyle could modify disease. We suggest that we should rethink how we study and supplement specific isoforms of nutrients. We also suggest that in clinical studies, vitamin E isoforms and vitamin D should be measured in the supplements, vehicles for the supplements and the patient plasma.
HIGHLIGHTS.
Vitamin E tocopherol isoforms have opposing regulatory functions on cell signaling.
γ-tocopherol associates with lower lung function and increased allergic inflammation.
α-tocopherol associates with better lung function and reduced allergic inflammation.
Vitamin D function and epidemiology suggest beneficial roles in asthma and infections.
Vitamin D trials didn’t reduce asthma and inconsistently reduced respiratory infection.
Acknowledgments
Sources of Support: This study was supported by National Institutes of Health Grant R01 AT004837 (J.M.C-M), HL98096 (P.C.A) and Ernest S. Bazley Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martinez FD. Genes, environments, development and asthma: A reappraisal. Eur Respir J. 2007;29:179–184. doi: 10.1183/09031936.00087906. [DOI] [PubMed] [Google Scholar]
- 2.Bousquet J, Bousquet PJ, Godard P, Daures JP. The public health implications of asthma. Bull World Health Organ. 2005;83:548–554. [PMC free article] [PubMed] [Google Scholar]
- 3.Vollmer WM, Osborne ML, Buist AS. 20-year trends in the prevalence of asthma and chronic airflow obstruction in an hmo. Am J Respir Crit Care Med. 1998;157:1079–1084. doi: 10.1164/ajrccm.157.4.9704140. [DOI] [PubMed] [Google Scholar]
- 4.Friebele E. The attack of asthma. Environ Health Perspect. 1996;104:22–25. doi: 10.1289/ehp.9610422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uauy R, Hoffman DR, Birch EE, Birch DG, Jameson DM, Tyson J. Safety and efficacy of omega-3 fatty acids in the nutrition of very low birth weight infants: Soy oil and marine oil supplementation of formula. J Pediatr. 1994;124:612–620. doi: 10.1016/s0022-3476(05)83144-0. [DOI] [PubMed] [Google Scholar]
- 6.Cook-Mills JM, McCary CA. Isoforms of vitamin e differentially regulate inflammation. Endocr Metab Immune Disord Drug Targets. 2010;10:348–366. doi: 10.2174/1871530311006040348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCary CA, Abdala-Valencia H, Berdnikovs S, Cook-Mills JM. Supplemental and highly elevated tocopherol doses differentially regulate allergic inflammation: Reversibility of alpha-tocopherol and gamma-tocopherol’s effects. J Immunol. 2011;186:3674–3685. doi: 10.4049/jimmunol.1003037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berdnikovs S, Abdala-Valencia H, McCary C, Somand M, Cole R, Garcia A, Bryce P, Cook-Mills J. Isoforms of vitamin e have opposing immunoregulatory funcitons during inflammation by regulating leukocyte recruitment. J Immunol. 2009;182:4395–4405. doi: 10.4049/jimmunol.0803659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marchese ME, Kumar R, Colangelo LA, Avila PC, Jacobs DR, Jr, Gross M, Sood A, Liu K, Cook-Mills JM. The vitamin e isoforms alpha-tocopherol and gamma-tocopherol have opposite associations with spirometric parameters: The cardia study. Respir Res. 2014;15:31. doi: 10.1186/1465-9921-15-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter SC, Cahoon EB. Enhancing vitamin e in oilseeds: Unraveling tocopherol and tocotrienol biosynthesis. Lipids. 2007;42:97–108. doi: 10.1007/s11745-007-3028-6. [DOI] [PubMed] [Google Scholar]
- 11.Leonard SW, Paterson E, Atkinson JK, Ramakrishnan R, Cross CE, Traber MG. Studies in humans using deuterium-labeled alpha- and gamma-tocopherols demonstrate faster plasma gamma-tocopherol disappearance and greater gamma-metabolite production. Free Radic Biol Med. 2005;38:857–866. doi: 10.1016/j.freeradbiomed.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Bella DL, Schock BC, Lim Y, Leonard SW, Berry C, Cross CE, Traber MG. Regulation of the alpha-tocopherol transfer protein in mice: Lack of response to dietary vitamin e or oxidative stress. Lipids. 2006;41:105–112. doi: 10.1007/s11745-006-5077-7. [DOI] [PubMed] [Google Scholar]
- 13.Redlich CA, Grauer JN, Van Bennekum AM, Clever SL, Ponn RB, Blaner WS. Characterization of carotenoid, vitamin a, and alpha-tocopheral levels in human lung tissue and pulmonary macrophages. Am J Respir Crit Care Med. 1996;154:1436–1443. doi: 10.1164/ajrccm.154.5.8912761. [DOI] [PubMed] [Google Scholar]
- 14.Zou W, Noh SK, Owen KQ, Koo SI. Dietary l-carnitine enhances the lymphatic absorption of fat and alpha-tocopherol in ovariectomized rats. J Nutr. 2005;135:753–756. doi: 10.1093/jn/135.4.753. [DOI] [PubMed] [Google Scholar]
- 15.Cavalier L, Ouahchi K, Kayden HJ, Di Donato S, Reutenauer L, Mandel JL, Koenig M. Ataxia with isolated vitamin e deficiency: Heterogeneity of mutations and phenotypic variability in a large number of families. Am J Hum Genet. 1998;62:301–310. doi: 10.1086/301699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sundl I, Guardiola M, Khoschsorur G, Sola R, Vallve JC, Godas G, Masana L, Maritschnegg M, Meinitzer A, Cardinault N, Roob JM, Rock E, Winklhofer-Roob BM, Ribalta J. Increased concentrations of circulating vitamin e in carriers of the apolipoprotein a5 gene - 1131t>c variant and associations with plasma lipids and lipid peroxidation. J Lipid Res. 2007;48:2506–2513. doi: 10.1194/jlr.M700285-JLR200. Epub 2007 Aug 2510. [DOI] [PubMed] [Google Scholar]
- 17.Guardiola M, Ribalta J, Gomez-Coronado D, Lasuncion MA, de Oya M, Garces C. The apolipoprotein a5 (apoa5) gene predisposes caucasian children to elevated triglycerides and vitamin e (four provinces study) Atherosclerosis. 2010;212:543–547. doi: 10.1016/j.atherosclerosis.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Huebbe P, Jofre-Monseny L, Rimbach G. Alpha-tocopherol transport in the lung is affected by the apoe genotype--studies in transgenic apoe3 and apoe4 mice. IUBMB Life. 2009;61:453–456. doi: 10.1002/iub.177. doi:410.1002/iub.1177. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida Y, Saito Y, Jones LS, Shigeri Y. Chemical reactivities and physical effects in comparison between tocopherols and tocotrienols: Physiological significance and prospects as antioxidants. J Biosci Bioeng. 2007;104:439–445. doi: 10.1263/jbb.104.439. [DOI] [PubMed] [Google Scholar]
- 20.Keiko Nishio MH, Akazawa Yoko, Shichiri Mototada, Iwahashi Hitoshi, Yoshihisa Hagihara YY, Niki Etsuo. Attenuation of lipopolysaccharide (lps)-induced cytotoxicity by tocopherols and tocotrienols. Redox Biology. 2013;1:97–103. doi: 10.1016/j.redox.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang PH, Chuang HC, Chou CC, Wang H, Lee SL, Yang HC, Chiu HC, Kapuriya N, Wang D, Kulp SK, Chen CS. Vitamin e facilitates the inactivation of the kinase akt by the phosphatase phlpp1. Sci Signal. 2013;6:ra19. doi: 10.1126/scisignal.2003816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel A, Liebner F, Netscher T, Mereiter K, Rosenau T. Vitamin e chemistry. Nitration of non-alpha-tocopherols: Products and mechanistic considerations. J Org Chem. 2007;72:6504–6512. doi: 10.1021/jo0706832. [DOI] [PubMed] [Google Scholar]
- 23.Fakhrzadeh L, Laskin JD, Laskin DL. Ozone-induced production of nitric oxide and tnf-alpha and tissue injury are dependent on nf-kappab p50. Am J Physiol Lung Cell Mol Physiol. 2004;287:L279–285. doi: 10.1152/ajplung.00348.2003. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez ML, Wagner JG, Aline Kala R, Mills K, Wells HB, Alexis NE, Lay JC, Jiang Q, Zhang H, Zhou H, Peden DB. Vitamin E gamma-tocopherol reduces airway neutrophil recruitment after inhaled endotoxin challenge in rats and in healthy volunteers. Free Radic Biol Med. 2013;8:00054–00053. doi: 10.1016/j.freeradbiomed.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiser J, Alexis NE, Jiang Q, Wu W, Robinette C, Roubey R, Peden DB. In vivo gamma-tocopherol supplementation decreases systemic oxidative stress and cytokine responses of human monocytes in normal and asthmatic subjects. Free Radic Biol Med. 2008;45:40–49. doi: 10.1016/j.freeradbiomed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner JG, Harkema JR, Jiang Q, Illek B, Ames BN, Peden DB. Gamma-tocopherol attenuates ozone-induced exacerbation of allergic rhinosinusitis in rats. Toxicol Pathol. 2009;37:481–491. doi: 10.1177/0192623309335630. doi:410.1177/0192623309335630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner JG, Jiang Q, Harkema JR, Ames BN, Illek B, Roubey RA, Peden DB. Gamma-tocopherol prevents airway eosinophilia and mucous cell hyperplasia in experimentally induced allergic rhinitis and asthma. Clin Exp Allergy. 2008;38:501–511. doi: 10.1111/j.1365-2222.2007.02855.x. Epub 2007 Oct 2026. [DOI] [PubMed] [Google Scholar]
- 28.Hamahata A, Enkhbaatar P, Kraft ER, Lange M, Leonard SW, Traber MG, Cox RA, Schmalstieg FC, Hawkins HK, Whorton EB, Horvath EM, Szabo C, Traber LD, Herndon DN, Traber DL. Gamma-tocopherol nebulization by a lipid aerosolization device improves pulmonary function in sheep with burn and smoke inhalation injury. Free Radic Biol Med. 2008;45:425–433. doi: 10.1016/j.freeradbiomed.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cook-Mills JM, Marchese ME, Abdala-Valencia H. Vascular cell adhesion molecule-1 expression and signaling during disease: Regulation by reactive oxygen species and antioxidants. Antioxid Redox Signal. 2011;15:1607–1638. doi: 10.1089/ars.2010.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cook-Mills JM. Eosinophil-endothelial cell interactions. Eosinophils in health and disease. In: Lee JJaRHF., editor. Eosinophils in health and disease. Elsevier; 2012. pp. 139–153. [Google Scholar]
- 31.Abdala-Valencia H, Berdnikovs S, Cook-Mills JM. Vitamin e isoforms differentially regulate intercellular adhesion molecule-1 activation of pkcalpha in human microvascular endothelial cells. PLoS ONE. 2012;7:e41054. doi: 10.1371/journal.pone.0041054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCary CA, Yoon Y, Panagabko C, Cho W, Atkinson J, Cook-Mills JM. Vitamin e isoforms directly bind pkcalpha and differentially regulate activation of pkcalpha. Biochem J. 2012;441:189–198. doi: 10.1042/BJ20111318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cook-Mills JM, Marchese M, Abdala-Valencia H. Vascular cell adhesion molecule-1 expression and signaling during disease: Regulation by reactive oxygen species and antioxidants. Antioxid Redox Signal. 2011;15:1607–1638. doi: 10.1089/ars.2010.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okamoto N, Murata T, Tamai H, Tanaka H, Nagai H. Effects of alpha tocopherol and probucol supplements on allergen-induced airway inflammation and hyperresponsiveness in a mouse model of allergic asthma. Int Arch Allergy Immunol. 2006;141:172–180. doi: 10.1159/000094896. [DOI] [PubMed] [Google Scholar]
- 35.Mabalirajan U, Aich J, Leishangthem GD, Sharma SK, Dinda AK, Ghosh B. Effects of vitamin e on mitochondrial dysfunction and asthma features in an experimental allergic murine model. J Appl Physiol. 2009;107:1285–1292. doi: 10.1152/japplphysiol.00459.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suchankova J, Voprsalova M, Kottova M, Semecky V, Visnovsky P. Effects of oral alpha-tocopherol on lung response in rat model of allergic asthma. Respirology. 2006;11:414–421. doi: 10.1111/j.1440-1843.2006.00864.x. [DOI] [PubMed] [Google Scholar]
- 37.Mahoney CW, Azzi A. Vitamin e inhibits protein kinase c activity. Biochem Biophys Res Commun. 1988;154:694–697. doi: 10.1016/0006-291x(88)90195-7. [DOI] [PubMed] [Google Scholar]
- 38.Jiang Q, Christen S, Shigenaga MK, Ames BN. Gamma-tocopherol, the major form of vitamin e in the us diet, deserves more attention. Am J Clin Nutr. 2001;74:714–722. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 39.Talegawkar SA, Johnson EJ, Carithers T, Taylor HA, Jr, Bogle ML, Tucker KL. Total alpha-tocopherol intakes are associated with serum alpha-tocopherol concentrations in african american adults. J Nutr. 2007;137:2297–2303. doi: 10.1093/jn/137.10.2297. [DOI] [PubMed] [Google Scholar]
- 40.Bieri JG, Evarts RP. Tocopherols and fatty acids in american diets. The recommended allowance for vitamin e. J Am Diet Assoc. 1973;62:147–151. [PubMed] [Google Scholar]
- 41.Bieri JG, Evarts RP. Vitamin e adequacy of vegetable oils. J Am Diet Assoc. 1975;66:134–139. [PubMed] [Google Scholar]
- 42.Meydani M, Cohn JS, Macauley JB, McNamara JR, Blumberg JB, Schaefer EJ. Postprandial changes in the plasma concentration of alpha- and gamma-tocopherol in human subjects fed a fat-rich meal supplemented with fat-soluble vitamins. J Nutr. 1989;119:1252–1258. doi: 10.1093/jn/119.9.1252. [DOI] [PubMed] [Google Scholar]
- 43.Sanchez-Moreno C, Dorfman SE, Lichtenstein AH, Martin A. Dietary fat type affects vitamins c and e and biomarkers of oxidative status in peripheral and brain tissues of golden syrian hamsters. J Nutr. 2004;134:655–660. doi: 10.1093/jn/134.3.655. [DOI] [PubMed] [Google Scholar]
- 44.Gobel Y, Koletzko B, Bohles HJ, Engelsberger I, Forget D, Le Brun A, Peters J, Zimmermann A. Parenteral fat emulsions based on olive and soybean oils: A randomized clinical trial in preterm infants. J Pediatr Gastroenterol Nutr. 2003;37:161–167. doi: 10.1097/00005176-200308000-00015. [DOI] [PubMed] [Google Scholar]
- 45.Devereux G, Seaton A. Diet as a risk factor for atopy and asthma. J Allergy Clin Immunol. 2005;115:1109–1117. doi: 10.1016/j.jaci.2004.12.1139. quiz 1118. [DOI] [PubMed] [Google Scholar]
- 46.Zahran HS, Bailey C. Factors associated with asthma prevalence among racial and ethnic groups-united states, 2009–2010 behavioral risk factor surveillance system. J Asthma. 2013;11:11. doi: 10.3109/02770903.2013.794238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jacobs RR, Boehlecke B, van Hage-Hamsten M, Rylander R. Bronchial reactivity, atopy, and airway response to cotton dust. Am Rev Respir Dis. 1993;148:19–24. doi: 10.1164/ajrccm/148.1.19. [DOI] [PubMed] [Google Scholar]
- 48.Delfino RJ, Quintana PJ, Floro J, Gastanaga VM, Samimi BS, Kleinman MT, Liu LJ, Bufalino C, Wu CF, McLaren CE. Association of fev1 in asthmatic children with personal and microenvironmental exposure to airborne particulate matter. Environ Health Perspect. 2004;112:932–941. doi: 10.1289/ehp.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koskela H, Tukiainen H, Kononoff A, Pekkarinen H. Effect of whole-body exposure to cold and wind on lung function in asthmatic patients. Chest. 1994;105:1728–1731. doi: 10.1378/chest.105.6.1728. [DOI] [PubMed] [Google Scholar]
- 50.Blanc PD, Eisner MD, Katz PP, Yen IH, Archea C, Earnest G, Janson S, Masharani UB, Quinlan PJ, Hammond SK, Thorne PS, Balmes JR, Trupin L, Yelin EH. Impact of the home indoor environment on adult asthma and rhinitis. J Occup Environ Med. 2005;47:362–372. doi: 10.1097/01.jom.0000158708.32491.9d. [DOI] [PubMed] [Google Scholar]
- 51.Weiss ST. Diet as a risk factor for asthma. Ciba Fndn Symp. 1997;206:244–257. [PubMed] [Google Scholar]
- 52.Troisi RJ, Willett WC, Weiss ST, Trichopoulos D, Rosner B, Speizer FE. A prospective study of diet and adult-onset asthma. Am J Respir Crit Care Med. 1995;151:1401–1408. doi: 10.1164/ajrccm.151.5.7735592. [DOI] [PubMed] [Google Scholar]
- 53.Dow L, Tracey M, Villar A, Coggon D, Margetts BM, Campbell MJ, Holgate ST. Does dietary intake of vitamins c and e influence lung function in older people? Am J Respir Crit Care Med. 1996;154:1401–1404. doi: 10.1164/ajrccm.154.5.8912755. [DOI] [PubMed] [Google Scholar]
- 54.Smit HA, Grievink L, Tabak C. Dietary influences on chronic obstructive lung disease and asthma: A review of the epidemiological evidence. Proc Nutr Soc. 1999;58:309–319. doi: 10.1017/s0029665199000427. [DOI] [PubMed] [Google Scholar]
- 55.Tabak C, Smit HA, Rasanen L, Fidanza F, Menotti A, Nissinen A, Feskens EJ, Heederik D, Kromhout D. Dietary factors and pulmonary function: A cross sectional study in middle aged men from three european countries. Thorax. 1999;54:1021–1026. doi: 10.1136/thx.54.11.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoskins A, Roberts JL, 2nd, Milne G, Choi L, Dworski R. Natural-source d-alpha-tocopheryl acetate inhibits oxidant stress and modulates atopic asthma in humans in vivo. Allergy. 2012;67:676–682. doi: 10.1111/j.1398-9995.2012.02810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pearson PJ, Lewis SA, Britton J, Fogarty A. Vitamin e supplements in asthma: A parallel group randomised placebo controlled trial. Thorax. 2004;59:652–656. doi: 10.1136/thx.2004.022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martindale S, McNeill G, Devereux G, Campbell D, Russell G, Seaton A. Antioxidant intake in pregnancy in relation to wheeze and eczema in the first two years of life. Am J Respir Crit Care Med. 2005;171:121–128. doi: 10.1164/rccm.200402-220OC. [DOI] [PubMed] [Google Scholar]
- 59.Devereux G. Early life events in asthma--diet. Pediatr Pulmonol. 2007;42:663–673. doi: 10.1002/ppul.20640. [DOI] [PubMed] [Google Scholar]
- 60.Allen S, Britton JR, Leonardi-Bee JA. Association between antioxidant vitamins and asthma outcome measures: Systematic review and meta-analysis. Thorax. 2009;64:610–619. doi: 10.1136/thx.2008.101469. [DOI] [PubMed] [Google Scholar]
- 61.Kalayci O, Besler T, Kilinc K, Sekerel BE, Saraclar Y. Serum levels of antioxidant vitamins (alpha tocopherol, beta carotene, and ascorbic acid) in children with bronchial asthma. Turk J Peds. 2000;42:17–21. [PubMed] [Google Scholar]
- 62.Kelly FJ, Mudway I, Blomberg A, Frew A, Sandstrom T. Altered lung antioxidant status in patients with mild asthma. Lancet. 1999;354:482–483. doi: 10.1016/S0140-6736(99)01812-7. [DOI] [PubMed] [Google Scholar]
- 63.Schunemann HJ, Grant BJ, Freudenheim JL, Muti P, Browne RW, Drake JA, Klocke RA, Trevisan M. The relation of serum levels of antioxidant vitamins c and e, retinol and carotenoids with pulmonary function in the general population. Am J Respir Crit Care Med. 2001;163:1246–1255. doi: 10.1164/ajrccm.163.5.2007135. [DOI] [PubMed] [Google Scholar]
- 64.Al-Abdulla NO, Al Naama LM, Hassan MK. Antioxidant status in acute asthmatic attack in children. J Pak Med Assoc. 2010;60:1023–1027. [PubMed] [Google Scholar]
- 65.Deluca HF. Historical overview of vitamin d. In: Feldman D, Pike JW, Adams JS, editors. Vitamin d. London: Academic Press; 2011. pp. 3–12. [Google Scholar]
- 66.Holick MF. Vitamin d deficiency. New Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 67.Holick MF. Photobiology of vitamin d. In: Feldman D, Pike JW, Adams JS, editors. Vitamin D. London: Academic Press; 2011. pp. 13–22. [Google Scholar]
- 68.Bouillon R. The vitamin d binding protein dbp. In: Feldman D, Pike JW, Adams JS, editors. Vitamin d. London: Academic Press; 2011. pp. 13–22. [Google Scholar]
- 69.Institute of Medicine I. Dietary reference intakes for calcium and vitamin D. Committee to review dietary reference intakes for calcium and vitamin D; 2011. [Google Scholar]
- 70.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Guidelines for preventing and treating vitamin d deficiency and insufficiency revisited. J Clin Endocrin Metab. 2012;97:1153–1158. doi: 10.1210/jc.2011-2601. [DOI] [PubMed] [Google Scholar]
- 71.Rosen CJ, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Manson JE, Mayne ST, Ross AC, Shapses SA, Taylor CL. Iom committee members respond to endocrine society vitamin D guideline. J Clin Endocrin Metab. 2012;97:1146–1152. doi: 10.1210/jc.2011-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jones G, Prosser DE. The activating enzymes of vitamin d metabolism (25- and 1a-hydroxylases) In: Feldman D, Pike JW, Adams JS, editors. Vitamin D. London: Academic Press; 2011. pp. 23–42. [Google Scholar]
- 73.Feldman D, Pike JW, Adams JS. Vitamin d. London: Academic Press; 2011. [Google Scholar]
- 74.Vieth R. The pharmacology of vitamin d. In: Feldman D, Pike JW, Adams JS, editors. Vitamin d. London: Academic Press; 2011. pp. 1041–1056. [Google Scholar]
- 75.White JH. Vitamin D and innate immunity. In: Feldman D, Pike JW, Adams JS, editors. Vitamin D. London: Academic Press; 2011. pp. 1777–1787. [Google Scholar]
- 76.Liu PT. The role of vitamin D in innate immunity: Antimicrobial activity, oxidative stress and barrier function. In: Feldman D, Pike JW, Adams JS, editors. Vitamin D. London: Academic Press; 2011. pp. 1777–1787. [Google Scholar]
- 77.Brockman-Schneider RA, Pickles RJ, Gern JE. Effects of vitamin d on airway epithelial cell morphology and rhinovirus replication. PloS one. 2014;9:e86755. doi: 10.1371/journal.pone.0086755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Banerjee A, Damera G, Bhandare R, Gu S, Lopez-Boado Y, Panettieri R, Jr, Tliba O. Vitamin d and glucocorticoids differentially modulate chemokine expression in human airway smooth muscle cells. Brit J Pharmacol. 2008;155:84–92. doi: 10.1038/bjp.2008.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Damera G, Fogle HW, Lim P, Goncharova EA, Zhao H, Banerjee A, Tliba O, Krymskaya VP, Panettieri RA., Jr Vitamin D inhibits growth of human airway smooth muscle cells through growth factor-induced phosphorylation of retinoblastoma protein and checkpoint kinase 1. Brit J Pharmacol. 2009;158:1429–1441. doi: 10.1111/j.1476-5381.2009.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sambrook P. Glucocorticoids and vitamin d. In: Feldman D, Pike JW, Adams JS, editors. Vitamin D. London: Academic Press; 2011. pp. 1233–1244. [Google Scholar]
- 81.Adorini L. Tolerogenic dendritic cells induced by vitamin d receptor ligands enhance regulatory t cells inhibiting autoimmune diabetes. Annals of the New York Academy of Sciences. 2003;987:258–261. doi: 10.1111/j.1749-6632.2003.tb06057.x. [DOI] [PubMed] [Google Scholar]
- 82.Vasiliou JE, Lui S, Walker SA, Chohan V, Xystrakis E, Bush A, Hawrylowicz CM, Saglani S, Lloyd CM. Vitamin d deficiency induces th2 skewing and eosinophilia in neonatal allergic airways disease. Allergy. 2014 doi: 10.1111/all.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Adorini L. Control of adaptive immunity by vitamin d receptor agonists. In: Feldman D, Pike JW, Adams JS, editors. Vitamin d. London: Academic Press; 2011. pp. 1789–1809. [Google Scholar]
- 84.Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, Godfrey KM, Cooper C Princess Anne Hospital Study G. Maternal vitamin d status during pregnancy and child outcomes. Eur J Clin Nutri. 2008;62:68–77. doi: 10.1038/sj.ejcn.1602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hypponen E, Sovio U, Wjst M, Patel S, Pekkanen J, Hartikainen AL, Jarvelinb MR. Infant vitamin d supplementation and allergic conditions in adulthood: Northern finland birth cohort 1966. Anna New York Acad Sci. 2004;1037:84–95. doi: 10.1196/annals.1337.013. [DOI] [PubMed] [Google Scholar]
- 86.Wjst M. The vitamin d slant on allergy. Ped Allergy Immunol. 2006;17:477–483. doi: 10.1111/j.1399-3038.2006.00456.x. [DOI] [PubMed] [Google Scholar]
- 87.Wjst M, Dold S. Genes, factor x, and allergens: What causes allergic diseases? Allergy. 1999;54:757–759. doi: 10.1034/j.1398-9995.1999.00193.x. [DOI] [PubMed] [Google Scholar]
- 88.Jirapongsananuruk O, Melamed I, Leung DY. Additive immunosuppressive effects of 1,25-dihydroxyvitamin d3 and corticosteroids on Th1, but not Th2, responses. J Allergy Clin Immunol. 2000;106:981–985. doi: 10.1067/mai.2000.110101. [DOI] [PubMed] [Google Scholar]
- 89.Lemire JM, Archer DC, Beck L, Spiegelberg HL. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: Preferential inhibition of Th1 functions. J Nutrition. 1995;125:1704S–1708S. doi: 10.1093/jn/125.suppl_6.1704S. [DOI] [PubMed] [Google Scholar]
- 90.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin d insufficiency in the us population, 1988–2004. Arch Internal Med. 2009;169:626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, Kleinman K, Gillman MW. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutri. 2007;85:788–795. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Devereux G, Litonjua AA, Turner SW, Craig LC, McNeill G, Martindale S, Helms PJ, Seaton A, Weiss ST. Maternal vitamin d intake during pregnancy and early childhood wheezing. Am J Clin Nutri. 2007;85:853–859. doi: 10.1093/ajcn/85.3.853. [DOI] [PubMed] [Google Scholar]
- 93.Litonjua AA, Weiss ST. Is vitamin d deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120:1031–1035. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 94.Litonjua AA, Lange NE, Carey VJ, Brown S, Laranjo N, Harshfield BJ, O’Connor GT, Sandel M, Strunk RC, Bacharier LB, Zeiger RS, Schatz M, Hollis BW, Weiss ST. The vitamin D antenatal asthma reduction trial (vdaart): Rationale, design, and methods of a randomized, controlled trial of vitamin d supplementation in pregnancy for the primary prevention of asthma and allergies in children. Contemp Clin Trials. 2014;38:37–50. doi: 10.1016/j.cct.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Norizoe C, Akiyama N, Segawa T, Tachimoto H, Mezawa H, Ida H, Urashima M. Incre ased food allergy and vitamin d: Randomized, double-blind, placebo-controlled rial. Pediatrics Intl. 2014;56:6–12. doi: 10.1111/ped.12207. [DOI] [PubMed] [Google Scholar]
- 96.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128:3792–3798. doi: 10.1378/chest.128.6.3792. [DOI] [PubMed] [Google Scholar]
- 97.Brehm JM, Celedon JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, Laskey D, Sylvia JS, Hollis BW, Weiss ST, Litonjua AA. Serum vitamin D levels and markers of severity of childhood asthma in costa rica. Am J Respir Crit Care Med. 2009;179:765–771. doi: 10.1164/rccm.200808-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu AC, Tantisira K, Li L, Fuhlbrigge AL, Weiss ST, Litonjua A. Effect of vitamin d and inhaled corticosteroid treatment on lung function in children. Am J Respir Crit Care Med. 2012;186:508–513. doi: 10.1164/rccm.201202-0351OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sutherland ER, Goleva E, Jackson LP, Stevens AD, Leung DY. Vitamin d levels, lung function, and steroid response in adult asthma. Am J Respir Crit Care Med. 2010;181:699–704. doi: 10.1164/rccm.200911-1710OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xystrakis E, Kusumakar S, Boswell S, Peek E, Urry Z, Richards DF, Adikibi T, Pridgeon C, Dallman M, Loke TK, Robinson DS, Barrat FJ, O’Garra A, Lavender P, Lee TH, Corrigan C, Hawrylowicz CM. Reversing the defective induction of il-10-secreting regulatory t cells in glucocorticoid-resistant asthma patients. J Clin Investigation. 2006;116:146–155. doi: 10.1172/JCI21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Devereux G, Wilson A, Avenell A, McNeill G, Fraser WD. A case-control study of vitamin d status and asthma in adults. Allergy. 2010;65:666–667. doi: 10.1111/j.1398-9995.2009.02220.x. [DOI] [PubMed] [Google Scholar]
- 102.Mai XM, Langhammer A, Camargo CA, Jr, Chen Y. Serum 25-hydroxyvitamin d levels and incident asthma in adults: The hunt study. Am J Epidemiol. 2012;176:1169–1176. doi: 10.1093/aje/kws235. [DOI] [PubMed] [Google Scholar]
- 103.Goleva E, Searing DA, Jackson LP, Richers BN, Leung DY. Steroid requirements and immune associations with vitamin d are stronger in children than adults with asthma. J Allergy Clin Immunol. 2012;129:1243–1251. doi: 10.1016/j.jaci.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Castro M, King TS, Kunselman SJ, Cabana MD, Denlinger L, Holguin F, Kazani SD, Moore WC, Moy J, Sorkness CA, Avila P, Bacharier LB, Bleecker E, Boushey HA, Chmiel J, Fitzpatrick AM, Gentile D, Hundal M, Israel E, Kraft M, Krishnan JA, LaForce C, Lazarus SC, Lemanske R, Lugogo N, Martin RJ, Mauger DT, Naureckas E, Peters SP, Phipatanakul W, Que LG, Sheshadri A, Smith L, Solway J, Sullivan-Vedder L, Sumino K, Wechsler ME, Wenzel S, White SR, Sutherland ER. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: The vida randomized clinical trial. JAMA. 2014;311:2083–2091. doi: 10.1001/jama.2014.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Muehleisen B, Gallo RL. Vitamin D in allergic disease: Shedding light on a complex problem. J Allergy Clin Immunol. 2013;131:324–329. doi: 10.1016/j.jaci.2012.12.1562. [DOI] [PubMed] [Google Scholar]
- 106.Paul G, Brehm JM, Alcorn JF, Holguin F, Aujla SJ, Celedon JC. Vitamin d and asthma. Am J Respir Crit Care Med. 2012;185:124–132. doi: 10.1164/rccm.201108-1502CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hollams EM. Vitamin d and atopy and asthma phenotypes in children. Curr Opin Allergy Clin Immunol. 2012;12:228–234. doi: 10.1097/ACI.0b013e3283534a32. [DOI] [PubMed] [Google Scholar]
- 108.Camargo CA, Jr, Ingham T, Wickens K, Thadhani R, Silvers KM, Epton MJ, Town GI, Pattemore PK, Espinola JA, Crane J. Cord-blood 25-hydroxyvitamin d levels and risk of respiratory infection, wheezing, and asthma. Pediatrics. 2011;127:e180–187. doi: 10.1542/peds.2010-0442. [DOI] [PubMed] [Google Scholar]
- 109.Quraishi SA, Bittner EA, Christopher KB, Camargo CA., Jr Vitamin d status and community-acquired pneumonia: Results from the third national health and nutrition examination survey. PloS one. 2013;8:e81120. doi: 10.1371/journal.pone.0081120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Amrein K, Quraishi SA, Litonjua AA, Gibbons FK, Pieber TR, Camargo CA, Jr, Giovannucci E, Christopher KB. Evidence for a u-shaped relationship between prehospital vitamin d status and mortality: A cohort study. J Clin Endocrin Metab. 2014;99:1461–1469. doi: 10.1210/jc.2013-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yusupov E, Li-Ng M, Pollack S, Yeh JK, Mikhail M, Aloia JF. Vitamin d and serum cytokines in a randomized clinical trial. Intl J Endocrin. 2010;2010 doi: 10.1155/2010/305054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin d supplementation to prevent seasonal influenza a in schoolchildren. Am J Clin Nutri. 2010;91:1255–1260. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 113.Camargo CA, Jr, Ganmaa D, Frazier AL, Kirchberg FF, Stuart JJ, Kleinman K, Sumberzul N, Rich-Edwards JW. Randomized trial of vitamin d supplementation and risk of acute respiratory infection in mongolia. Pediatrics. 2012;130:e561–567. doi: 10.1542/peds.2011-3029. [DOI] [PubMed] [Google Scholar]
- 114.Murdoch DR, Slow S, Chambers ST, Jennings LC, Stewart AW, Priest PC, Florkowski CM, Livesey JH, Camargo CA, Scragg R. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: The vidaris randomized controlled trial. JAMA. 2012;308:1333–1339. doi: 10.1001/jama.2012.12505. [DOI] [PubMed] [Google Scholar]
- 115.Das RR, Singh M, Panigrahi I, Naik SS. Vitamin d supplementation for the treatment of acute childhood pneumonia: A systematic review. ISRN pediatrics. 2013;2013:459160. doi: 10.1155/2013/459160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bergman P, Lindh AU, Bjorkhem-Bergman L, Lindh JD. Vitamin d and respiratory tract infections: A systematic review and meta-analysis of randomized controlled trials. PloS one. 2013;8:e65835. doi: 10.1371/journal.pone.0065835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Goodall EC, Granados AC, Luinstra K, Pullenayegum E, Coleman BL, Loeb M, Smieja M. Vitamin d3 and gargling for the prevention of upper respiratory tract infections: A randomized controlled trial. BMC Infect Diseases. 2014;14:273. doi: 10.1186/1471-2334-14-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Boyle SP, Dobson VL, Duthie SJ, Hinselwood DC, Kyle JA, Collins AR. Bioavailability and efficiency of rutin as an antioxidant: A human supplementation study. Eur J Clin Nutr. 2000;54:774–782. doi: 10.1038/sj.ejcn.1601090. [DOI] [PubMed] [Google Scholar]
- 119.Johnstone AM, Lobley GE, Horgan GW, Bremner DM, Fyfe CL, Morrice PC, Duthie GG. Effects of a high-protein, low-carbohydrate v. High-protein, moderate-carbohydrate weight-loss diet on antioxidant status, endothelial markers and plasma indices of the cardiometabolic profile. Br J Nutr. 2011;106:282–291. doi: 10.1017/S0007114511000092. [DOI] [PubMed] [Google Scholar]
- 120.Hodge AM, Simpson JA, Fridman M, Rowley K, English DR, Giles GG, Su Q, O’Dea K. Evaluation of an ffq for assessment of antioxidant intake using plasma biomarkers in an ethnically diverse population. Public Health Nutr. 2009;12:2438–2447. doi: 10.1017/S1368980009005539. [DOI] [PubMed] [Google Scholar]
- 121.Samman S, Sivarajah G, Man JC, Ahmad ZI, Petocz P, Caterson ID. A mixed fruit and vegetable concentrate increases plasma antioxidant vitamins and folate and lowers plasma homocysteine in men. J Nutr. 2003;133:2188–2193. doi: 10.1093/jn/133.7.2188. [DOI] [PubMed] [Google Scholar]
- 122.Heinonen OP, Albanes D. The effect of vitamin e and beta carotene on the incidence of lung cancer and other cancers in male smokers. The alpha-tocopherol, beta carotene cancer prevention study group. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 123.Zieden B, Kaminskas A, Kristenson M, Kucinskiene Z, Vessby B, Olsson AG, Diczfalusy U. Increased plasma 7 beta-hydroxycholesterol concentrations in a population with a high risk for cardiovascular disease. Arterioscler Thromb Vasc Biol. 1999;19:967–971. doi: 10.1161/01.atv.19.4.967. [DOI] [PubMed] [Google Scholar]
- 124.Gylling H, Hallikainen M, Nissinen MJ, Miettinen TA. The effect of a very high daily plant stanol ester intake on serum lipids, carotenoids, and fat-soluble vitamins. Clin Nutr. 2010;29:112–118. doi: 10.1016/j.clnu.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 125.Valimaki IA, Vuorimaa T, Ahotupa M, Kekkonen R, Korpela R, Vasankari T. Decreased training volume and increased carbohydrate intake increases oxidized ldl levels. Int J Sports Med. 2012;33:291–296. doi: 10.1055/s-0031-1291223. [DOI] [PubMed] [Google Scholar]
- 126.Safronov ID, Trunov AN, Kokareva ED. Prooxidant-antioxidant factors in the blood of pregnant women with late gestosis of different severity. Bull Exp Biol Med. 2008;146:800–802. doi: 10.1007/s10517-009-0417-2. [DOI] [PubMed] [Google Scholar]
- 127.Ross MA, Crosley LK, Brown KM, Duthie SJ, Collins AC, Arthur JR, Duthie GG. Plasma concentrations of carotenoids and antioxidant vitamins in scottish males: Influences of smoking. Eur J Clin Nutr. 1995;49:861–865. [PubMed] [Google Scholar]
- 128.Wu LS, Sjakste T, Sakalauskas R, Sitkauskiene B, Paramonova N, Gasiuniene E, Jan RL, Wang JY. The burden of allergic asthma in children: A landscape comparison based on data from lithuanian, latvian, and taiwanese populations. Pediatr Neonatol. 2012;53:276–282. doi: 10.1016/j.pedneo.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 129.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: Executive summary of the gina dissemination committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 130.To T, Stanojevic S, Moores G, Gershon AS, Bateman ED, Cruz AA, Boulet LP. Global asthma prevalence in adults: Findings from the cross-sectional world health survey. BMC Public Health. 2012;12:204. doi: 10.1186/1471-2458-12-204. [DOI] [PMC free article] [PubMed] [Google Scholar]