Abstract
Synaptic inhibition, brought about by a rich variety of interneuron types that target different domains of principal cells and other interneurons, counters excitation, modulates the gain, timing, tuning, bursting properties of principal cell firing, and exerts selective filtering of synaptic excitation. At the network level, it allows for coordinating transient interactions among the principal cells to form cooperative assemblies for efficient transmission of information and routing of excitatory activity across networks, typically in the form of brain oscillations. Targeted expression of neuronal activity modulators, such as optogenetics, allow physiological identification and perturbation of specific interneuron subtypes. Combined with large-scale recordings or imaging techniques, these approaches facilitate our understanding of the multiple roles of inhibitory interneurons in shaping circuit functions.
Keywords: inhibition, interneurons, circuits, oscillations, optogenetics, pharmacogenetics
Introduction
Inhibition is a unique operational mechanism in the brain. While in its simple form, inhibition can be conceived as a break or pull back mechanism, it rather functions as a multi-faceted mechanism that coordinates the action of the numerous principal cells. Depending on when and where on the principal cell soma-dendritic domain it exerts its action, inhibition counters excitation, modulates the gain, timing, tuning, bursting properties of pyramidal cell firing, and exerts selective filtering of synaptic excitation (Buzsaki et al., 1996; Miles et al., 1996; Wehr & Zador, 2003; Markram et al., 2004; Pouille & Scanziani, 2004; Pouille et al., 2009; Isaacson & Scanziani, 2011; Atallah et al., 2012; Gentet et al., 2012; Lee et al., 2012; Lovett-Barron et al., 2012; Royer et al., 2012; Wilson et al., 2012). At the network level, inhibition allows for transient autonomy of principal cell groups both in time and space, though the maintenance of brain oscillations (Buzsaki & Chrobak, 1995; Freund & Buzsaki, 1996; Csicsvari et al., 1999; Klausberger & Somogyi, 2008; Stark et al., 2013). The inhibition-based time windows of brain rhythms, in turn, allow for short-time interactions among the principal cells to form cooperative assemblies for efficient transmission of information. A rich variety of GABAergic inhibitory interneuronal classes, reflecting a considerable division of labor, have been identified to support these functions (Freund & Buzsaki, 1996; Cauli et al., 1997; Kawaguchi & Kubota, 1998; Markram et al., 2004; Monyer & Markram, 2004; Ascoli et al., 2008; Klausberger & Somogyi, 2008; Moore et al., 2010; Fishell & Rudy, 2011; DeFelipe et al., 2013). With the advent of optogenetics and pharmacogenetics, which allow reversible modulation of neuronal activity, such assumed functions can now be challenged by targeted perturbations of interneurons in the intact brain (Kepecs & Fishell, 2014; Lovett-Barron & Losonczy, 2014; Roux et al., 2014). Our review briefly overviews some of these functions, and focuses on recent cell-type specific manipulations that allow for testing causal mechanisms brought about by molecularly defined interneuron subtypes.
Inhibitory control in circuits
Inhibitory interneurons can provide stability to the principal cell populations by at least two different ways: feedforward and feedback and inhibition (Figure 1A, 1B). All known excitatory afferents to the various dendritic domains of the principal cells have their ‘own’ classes of dedicated interneurons. These interneurons target the same domains as the excitatory afferents they receive inputs from, providing a template for feedforward inhibition (Buzsaki, 1984). In addition to dendritic inhibition, interneurons with somatic targets (basket cells) or axon initial segment targets (chandelier or axo-axonic cells) can also form feed-forward circuits. Feed-forward inhibition thus can reduce the spike responses of principal neurons, by competing with dendritic excitation or reducing output spiking. A recent study suggested that cholecystokinin (CCK) positive basket cells are primarily involved in the feed-forward inhibition of hippocampal CA1 pyramidal cells (Basu et al., 2013). Whether feed-forward inhibition brings about a subtractive or divisive action in the visual cortex is still debated (Atallah et al., 2012; Lee et al., 2012; Wilson et al., 2012; Lee et al., 2014a) and may depend whether the inhibitory target is dendrites or the perisomatic region. In a feedforward circuit, even though the inhibitory action is disynaptic, several mechanisms are in place to accelerate the inhibitory action. These include lower firing threshold and larger and more efficient excitatory synapses on interneurons (Gulyas et al., 1993; Gabernet et al., 2005; Cruikshank et al., 2007) (Glickfeld & Scanziani, 2006; Helmstaedter et al., 2009; Hull et al., 2009; Stokes & Isaacson, 2010). As a result, feed-forward inhibition can arrive in time before the principal cell’s membrane can be charged to threshold and prevent the occurrence of the spike, or at least the occurrence of multiple spikes (Buzsaki & Eidelberg, 1981; 1982). This mechanism can effectively shorten the time window within which the principal cell responds giving rise to very high temporal precision of evoked spiking (Pouille & Scanziani, 2001). Similarly, inhibition has been shown to increase temporal precision in other circuits such as the auditory cortex (Wehr & Zador, 2003) or in the CA1 region of the hippocampus by reducing the signal-to-noise ratio (Owen et al., 2013). Alternatively or in addition, feed-forward inhibition may veto the occurrence of the spike responses from a given afferent input, and can serve to reduce synchrony of parallel activated nearby neurons (Renart et al., 2010). Long-term changes in feed-forward inhibition can also be involved in a heterosynaptic form of plasticity (Basu et al., 2013) and modulate temporal association memory (Kitamura et al., 2014).
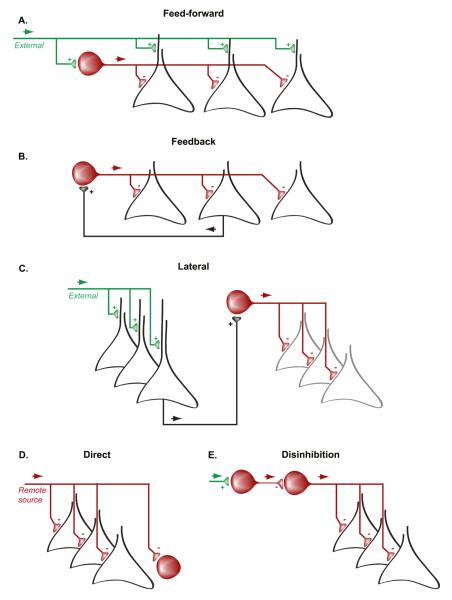
Figure 1.
Main forms of inhibitory microcircuits. A. In a feed-forward inhibitory circuit, interneurons (red) receive excitatory inputs from an external source (green) and in turn inhibit the local principal neurons (black). These latter neurons are often also targeted by the external excitatory input and the relative strength of excitation on the principal cells and interneurons as well as the interneuron-induced inhibition determine the firing discharge of principal cells. B. In a feed-back inhibitory circuit, interneurons receive excitation from principal cells and, in turn they inhibit the principal cells. Thus, local excitation is a condition for inhibition. C. Lateral inhibition allows a first assembly of principal cells (black) to suppress the activity of another assembly of principal cells (grey) through the excitation of inhibitory interneurons. In real networks, lateral inhibition is usually reciprocal and such connectivity allows for assembly competition (an exclusive OR operation in Boolean terms). D. Direct inhibition involves the suppression of local principal cell or interneuron activity by long-range interneurons from remote brain regions. E. Disinhibition of the principal cells occurs when their direct inhibitory inputs are suppressed by another population of inhibitory interneurons.
Feed-back or recurrent inhibition, on the other hand, requires that principal neurons in a given circuit discharge first and recruit a postsynaptic inhibitory neuron, whose feed-back action can prevent further discharges of the principal cells (Miles, 1990) (Figure 1B). Strictly speaking, recurrent inhibition involves a loop in which the excited inhibitory neuron feeds back inhibition to the very neuron(s) that brought about its excitation. Indeed, reciprocal excitatory-inhibitory connections have been identified in multiple circuits (Csicsvari et al., 1998; Thomson et al., 2002; Urban & Sakmann, 2002; McCormick et al., 2003; Bartho et al., 2004; Markram et al., 2004; Maurer et al., 2006; Huguenard & McCormick, 2007; Ko et al., 2011; Pfeffer et al., 2013; Zaitsev & Lewis, 2013; Lee et al., 2014b). A related form of inhibition is lateral inhibition, in which the excited interneuron suppresses the activity of similar type of principal cells, which are different than those that gave rise to the interneuron excitation (Hartline et al., 1956; Isaacson & Scanziani, 2011; Adesnik et al., 2012; Lee et al., 2014b) (Figure 1C). Lateral inhibition is the fundamental mechanism of neuronal group selection and assembly competition.
It is important to emphasize that feedforward and feedback inhibition are simply principles and do not correspond to specific interneuron types. First, both types of inhibition can affect either the perisomatic region or the dendrites of the principal cell. Second, the feedback and feedforward inhibitory functions are not mutually exclusive for a given interneuron. For example, ‘feedback interneurons’ are rare since interneurons taking part the feedback loops also often receives afferents from distant sources, thus being part of a feedforward inhibition circuit as well.
Yet another form of inhibition, called direct inhibition, takes place when GABAergic afferents from a distant source can bring about a local inhibitory action (Figure 1D). For example, GABAergic neurons of the pars reticulata of the substantia nigra provides direct inhibitory output to a variety of basal ganglia structures (Bolam et al., 2000), medium spiny neurons of the striatum directly inhibit their distant targets (Tepper et al., 2004), Purkinje cells provide the sole output from the cerebellar cortex and inhibit the deep cerebellar nuclei (Eccles et al., 1967) and long-range inhibitory neurons in the hippocampus can exert direct inhibition of neurons in the medial septum or entorhinal cortex (Alonso & Kohler, 1982; 1984; Kohler et al., 1984; Freund & Antal, 1988; Toth et al., 1993; Jinno et al., 2007; Melzer et al., 2012; Caputi et al., 2013). Similarly, axons of neocortical interneurons can cross the corpus callosum and directly innervate their contralateral hemispheric targets (Higo et al., 2007; Tomioka & Rockland, 2007).
A further ‘complication’ is that, in addition to principal cells, GABAergic interneurons also make inhibitory, often reciprocal, contacts onto each other (Freund & Buzsaki, 1996; Tamas et al., 1998; Gibson et al., 1999; Galarreta & Hestrin, 2002; Chamberland et al., 2010; Letzkus et al., 2011; Lovett-Barron et al., 2012; Pfeffer et al., 2013; Xu et al., 2013) and some interneurons target only other interneurons (“interneuron specific interneurons”; Acsady 1996; Gulyas et al., 1996; Hajos 1996, David 2007; Pi et al., 2013) (Figure 1E). Such interactions can induce network synchrony (Van Vreeswijk et al., 1994; Hu et al., 2011) or can mediate dis-inhibition of the principal cells (Letzkus et al., 2011; Lovett-Barron et al., 2012; Xu et al., 2013). A recent study demonstrated the specific role of some vasoactive intestinal polypeptide positive (VIP+) interneurons in suppressing firing in other interneuron classes in vivo (Pi et al., 2013). Similar to the complexity of the interneuron-principal cell circuits, the connectivity within and across different classes of interneurons is also not well understood. The dynamic consequences of such complex circuits must be enormous (Freund & Buzsaki, 1996; Markram et al., 2004; Somogyi & Klausberger, 2005), the disentangling of which will require identification of the individual components, their targeted perturbation, computational modeling and application of control theory in future experiments.
Balanced inhibition and excitation versus communication
It follows from the principles of inhibition and the strong mutual connectivity between principal cells and inhibitory interneurons, that their interactions will effectively shape spatial and temporal features of their firing patterns. Proper dynamics in neuronal networks can only be maintained if the excitatory forces are counteracted by effective inhibitory forces (van Vreeswijk & Sompolinsky, 1996). These interactions are often referred to by the term ‘balanced’ inhibition-excitation (Anderson et al., 2000; Wehr & Zador, 2003; Zhang et al., 2003; Wilent & Contreras, 2004; Poo & Isaacson, 2009; Isaacson & Scanziani, 2011).
Nonetheless, it should be emphasized that the concept of balance is always a function of a given time frame. What appears balanced at a time scale of seconds can often be characterized by large swings in excitability at the sub-second scales. For example, slow oscillations (Steriade et al., 1993) shift back and forth between UP and DOWN states approximately every second. However, the UP state does not correspond to a steady state either. The transition to the UP state can trigger oscillatory sleep spindles and induce gamma oscillations, characterized by principal cell and interneuron spiking at distinct phases of these faster rhythms (Hasenstaub et al., 2005). Importantly, if cortical excitation and inhibition were at balance at all time scales, neuronal communication would not be possible. Sending spikes across structures is in fact possible only because of the transient imbalance between excitation and inhibition. Such imbalance-balance is most often achieved in the brain by network oscillations (Buzsaki & Draguhn, 2004). For instance, during hippocampal sharp wave ripple events, the excitatory gain can increase as much as 300% transiently for approximately 50 msec (Csicsvari et al., 1999), allowing transfer of hippocampal information to neocortical targets (Chrobak & Buzsaki, 1994). In the case of gamma oscillations, the rivalry between excitatory and inhibitory neurons ensures the stability of global neuronal firing rates over extended territories of the cortex, and yet also allows for dramatic increases of local excitability in short time windows, ä condition necessary for sending messages and modifying network connections (Buzsaki & Wang, 2012).
Self-organized circuits with delicate excitatory-inhibitory balance appear to be maximally sensitized to external perturbations, yet they are capable of absorbing large external effects without undergoing functional breakdown. However, alterations of these interactions may result in abnormal physiological patterns (Cossart et al., 2001; Yizhar et al., 2011), as it has been well-documented in epilepsy and various forms of psychiatric diseases (Lewis, 2009).
Grouping and routing by inhibition-based oscillations
The inhibitory neuronal network, when coupled to the principal cells, provides the flexibility needed for the complex operations of the brain. Competition between opposing forces and feedback control by inhibition are often reflected by population oscillations. Interneuron networks are the backbone of many brain oscillators as they provide rhythm-based timing to principal cells (Buzsaki & Chrobak, 1995; Engel et al., 2001; Salinas & Sejnowski, 2001). Once a collective oscillatory pattern arises, the rhythmic inhibitory volleys provide alternating windows of reduced and enhanced excitability to the principal cells, in a temporally coordinated manner (Whittington et al., 2000; Buzsaki, 2006). This framework constrains the windows of opportunity for the principal cells to discharge, which leads to their synchronization. Synchronization by oscillation occurs at multiple time scales, covering time epochs from milliseconds to seconds. The duration of the oscillation, in turn, regulates the length of messages that can be transmitted, as well as the spatial extent of the involved neuronal pools. Thus, by way of oscillations, inhibition can create multiple temporal and spatial organizations of principal cells in the cerebral cortex (Buzsaki, 2010).
Most oscillations observed in the brain are called ‘relaxation’, or pulse type (Strogatz, 1994; Buzsaki, 2006). This implies that spikes of principal cells are typically concentrated in a limited phase range (called the output or duty cycle) and separated from the phase range in which inhibitory neurons are active, and in which the cycle length and amplitude can be affected by external inputs (so called ‘perturbation phase’; Buzsaki, 2006). Despite the wide range of phase preferences observed for the multiple classes of interneurons and the different mechanisms of network oscillations, the separation of duty phase and perturbation phase applies to all rhythms and forms the basis of effective synchronization of neuronal groups (Ermentrout & Kopell, 1998).
Brain rhythms in mammals, as measured with the mesoscopic local field potential (LFP), form a system and span from approximately 0.05 Hz to 500 Hz. The mean frequencies of their bands can be fit to a natural logarithmic scale and provide constant ratios between any given pair of neighboring frequencies (Penttonen & Buzsáki, 2003). The system of cortical rhythms is hierarchical both in space and time, largely due to anatomical wiring of networks and the limited speed of axon conduction speeds. In the case of higher frequency oscillations, the participating neurons are confined to a small volume of nervous tissue, as the available time for neurons recruitment at each cycle is short. In contrast, during slow oscillations many neurons in a large volume of tissue can be recruited to the rhythm. As a result, when multiple rhythms are present simultaneously, the slow oscillation can unidirectionally affect the faster oscillations. The nature of this interaction is reflected by cross-frequency coupling, so that the phase of the slow rhythm(s) modulates the power of the faster one(s). This ‘cross-frequency phase coupling’, first demonstrated between theta (4-9 Hz) and gamma (30-90 Hz) oscillations (Soltesz & Deschenes, 1993; Bragin et al., 1995; Buzsaki & Wang, 2012), is a general mechanism for all known rhythms (Chrobak & Buzsaki, 1998; Sirota et al., 2008; Schroeder & Lakatos, 2009) and it undergirds the temporal hierarchical organization of brain rhythms (Buzsaki, 2006). Inhibition plays an important role in theta-gamma coupling since the same perisomatic interneurons are involved in both rhythms. In the olfactory bulb however, two anatomically segregated populations of interneurons are suggested to modulate the respiration-coupled theta activity on the one hand, and the gamma rhythm on the other hand (Fukunaga et al., 2014).
Dynamic congregation and segregation of excitatory principal cells into functional groups, often referred to as cell assemblies and assembly sequences (Hebb & Konzett, 1949), is perhaps the most important function performed by the large family of inhibitory neuron classes in the cortex (Freund & Buzsaki, 1996; Klausberger & Somogyi, 2008). Inhibition-based oscillations may do so by ‘chunking’ streams of neuronal information flow into shorter time frames, by transiently silencing the principal cells. Indeed, oscillations have well-defined onsets and offsets, with characteristic maximum and minimum spiking activity in the information-transmitting principal cells (Masquelier et al., 2009). This stop-start parsing function of neuronal oscillators, coordinated across networks, is somewhat analogous to written text where words and expressions are separated by spaces, commas, periods, etc. Parsing of information by inhibition can make communication more straightforward as compared to a situation where downstream “readers” would have to “interpret” long uninterrupted messages (Wickelgren, 1999) or stochastic patterns of spikes (MacLeod et al., 1998; Buzsaki, 2010). Of course, neuronal transmission can also be coordinated without oscillations as long as some other mechanisms, e.g., saccadic eye movements or external signals can provide the necessary timing.
Some basic functions accomplished by neuronal networks are pattern completion and pattern separation, two functions that are related to integration/congregation and differentiation. Separation of inputs is difficult in a network with only excitatory connections. However, with inhibitory connections, the competing cell assemblies (and even neighboring excitatory neurons) can be functionally isolated, such that excitatory paths can be re-routed by the traffic-controlling ability of coordinated interneuron groups (Freund & Buzsaki, 1996). The specific firing patterns of principal cells in a network will depend largely on the temporal and spatial distribution of inhibition (Pouille & Scanziani, 2004). As a result, in response to the same input, the same network can potentially produce several different output patterns at different times, depending on the distribution of inhibition. Coordinated inhibition can ensure (1) that excitatory activity recruits the appropriate number of neurons in the appropriate temporal window and (2) that excitation spreads in the right direction.
Interneuron diversity enhances computational ability of cortical circuits
Cortical structures have evolved several types of principal cells and numerous classes of GABAergic inhibitory interneurons (Freund & Buzsaki, 1996; Cauli et al., 1997; Markram et al., 2004; Somogyi & Klausberger, 2005; Ascoli et al., 2008; Klausberger & Somogyi, 2008; DeFelipe et al., 2013). The addition of functionally different interneuron types to a network, even in small numbers, offers a dramatic expansion of computational possibilities for the neuronal circuit. Virtually every segment of the somato-dendritic surface of cortical principal cells is under the control of a unique set of interneuron subtypes. Each interneuron class targets specific somato-dendritic compartments, but multiple classes of interneurons can target the same domain (such as the soma). This domain-specific innervation can enhance the functional repertoire of principal cells. For instance, interneuron-mediated inhibition can functionally “eliminate” a dendritic segment or a whole dendrite, selectively deactivate Ca2+ channels, or segregate dendrites from the soma or the soma from the axon (Buzsaki et al., 1996; Miles et al., 1996; Tsubokawa & Ross, 1996; Chiu et al., 2013). Such actions of interneurons are functionally equivalent to replacing a principal cell by a morphologically different type. Appropriately timed inhibition targeted to specific somato-dendritic domains of principal cells can selectively filter synaptic excitation and control bursting properties of pyramidal cell firing (Figure 2) (Isaacson & Scanziani, 2011). In summary, interneurons can divide the full computational power of principal cells into numerous subroutines, which can be flexibly used according to momentary needs.
Figure 2.
Dendrite-targeting interneurons control principal cell bursting. A-B. Dendritic recording from a CA1 pyramidal cell performed in vivo with a sharp electrode showed that inhibition modulates dendritic spike invasion. The recording configuration is shown in B. A. (a) Intradendritic depolarization (0.4 nA) evoked fast sodium spikes and a slow spike, likely a calcium spike. (b-d) Dendritic depolarization was paired with commissural stimulation (short arrows) in order to activate inhibitory interneurons. Bottom traces: extracellular recordings from the pyramidal layer. Weak commissural stimulation delayed (b, 30 μA), abolished (c, 50 μA) or aborted the slow spike (d, 50μA). (c) [triangleup], inhibitory postsynaptic potential evoked by the commissural stimulus. B. After the recordings, the cell was injected with biocytin for morphological reconstruction with a drawing tube. Adapted from Buzsaki et al., 1996. Copyright (1996) National Academy of Sciences, U.S.A. C-D. Current-clamp recordings from the distal apical dendrites of CA1 pyramidal cells performed in vitro, combined with pharmacogenetic manipulations (via the expression of the ligand-gated Cl− channel, PSAML141F-GlyR in the SOM+ cells, and application of its ligand PSEM308), showed that silencing the dendrite-targeting SOM+ cells increases dendritic spike generation during Schaffer Collateral photostimulation, whereas silencing of the soma-targeting PV+ cells does not. Adapted by permission from Macmillan Publishers Ltd: Nature Neuroscience (Lovett-Barron et al. 2012), copyright (2012). E. Left: Example whole-cell recording from an excitatory neuron during optogenetic inhibition of nearby dendrite-targeting SOM+ neurons in the somatosensory cortex of anesthetized mice. Right: Optogenetic silencing (Light ON) of the SOM+ cells increased burst firing in the nearby principal cells. Each thin line represents an individual neuron and filled circles with error bars connected by thick lines represent mean ± s.e.m. Adapted by permission from Macmillan Publishers Ltd: Nature Neuroscience (Gentet et al. 2012), copyright (2012). F. In vivo extracellular recordings combined with optogenetics in freely moving mice showed that SOM+ silencing (but not PV+ cell silencing) increases burst firing in the putative pyramidal cells, as shown by the relative increase (mean ± s.e.m.) of occurrence for different burst lengths (comparison between PV+ and SOM+ cell silencing, *P < 0.05, #P < 0.0005). Adapted from Royer et al. 2012.
Types of interneurons
Although researchers agree on the rich diversity of inhibitory interneurons, to date, no widely acceptable taxonomy exists. Novel interneuron types are being discovered with accelerated speed and currently >20 different interneuron types are recognized in the cortex (Klausberger & Somogyi, 2008). The existing classification schemes depend largely on how the first division is implemented. Typically, it is based on genetic, morphological, developmental origin, chemical content, in vitro or in vivo firing patterns and embeddedness in circuits (Freund & Buzsaki, 1996; Cauli et al., 1997; Kawaguchi & Kubota, 1998; Parra et al., 1998; McBain & Fisahn, 2001; Markram et al., 2004; Masland, 2004; Monyer & Markram, 2004; Ascoli et al., 2008; Klausberger & Somogyi, 2008; Moore et al., 2010; Fishell & Rudy, 2011; Isaacson & Scanziani, 2011; Rudy et al., 2011; Tricoire et al., 2011; DeFelipe et al., 2013; Kepecs & Fishell, 2014). Because the main function of interneurons is to control the activity of principal cells, one classification scheme divides them according their axonal targets (Freund & Buzsaki, 1996; Klausberger & Somogyi, 2008). In such a scheme, three or four first major divisions can be made. (1) The perisomatic group. It constitutes the largest family of interneurons and comprises basket cells and axo-axonic (or chandelier) cells. By providing perisomatic inhibition, it controls the spiking output of principal cells. Consequently, these interneurons are likely most critical for the precise timing of pyramidal cells spikes, coordinating their synchrony through gamma and other oscillations (Buzsaki & Wang, 2012). (2) The dendrite-targeting group. Interneurons in this family target specific dendritic domains of principal cells. Every known excitatory pathway in the cortex has a matching family of interneurons, which innervates the same dendritic domain (Buzsaki, 1984). Several additional subclasses in this group seek out two or more (overlapping or non-overlapping) dendritic regions, and yet other subclasses innervate the somata and nearby dendrites with similar probability. The different domains of principal cells have different functional dynamics, and interneurons innervating these specific domains appear to effectively and specifically control the kinetic properties of their target domains (e.g. by suppressing the activity of Ca2+ channels; Miles et al., 1996; Figure 2). Members of the dendrite-targeting interneuron family display large variability. (3) The interneuron-specific group (Gulyas et al., 1996; Pi et al., 2013). These interneurons have the distinguishing characteristic that their axons preferentially contact other interneurons but avoid principal cells, and therefore can exert an indirect large effects on the principal cells (Gulyas et al., 1996; Pi et al., 2013) (Figure 1E). (4) The long-range group. Members of this morphologically diverse group have axon trees that span two or more anatomical brain regions (Alonso & Kohler, 1982; Kohler et al., 1984; Freund & Antal, 1988; Germroth et al., 1989; Toth et al., 1993; Sik et al., 1994; Gulyas et al., 2003; Buzsaki et al., 2004; Tomioka et al., 2005; Jinno et al., 2007; Tomioka & Rockland, 2007; Melzer et al., 2012; Caputi et al., 2013) (Figure 1D). Some axon collaterals of long-range interneurons even cross the hemispheric midline and/or innervate sub-cortical structures. Their large-caliber axons provide fast communication between the innervated areas (Jinno et al., 2007). Since this group of inhibitory cells projects over large distances, the term ‘interneuron’ is not strictly accurate. Nevertheless, for historical reasons all GABAergic cells in the cerebral cortex are referred to as “inhibitory interneurons”. An important, yet perhaps least understood aspect, of inhibitory control is its scalability across species. A recipe of proportional change with increased brain size is unlikely due to the increasing volume and energy demands associated with increased wiring. Instead, a small world-like or similar scaling rule may provide a better explanation. Long-range interneurons are potentially key players in such growing networks (Buzsaki et al., 2004), but more research is needed to understand how wiring of interneurons change by certain rules in growing brains.
With so many interneuron types, one wonders about a “recipe” that describes how principal cells are innervated by their inhibitory peers. Even in the single layer hippocampus, it is unlikely that each and every pyramidal cell is targeted by all the different types of interneurons. It appears that the axon initial segment and cell body of all principal cells is contacted by inhibitory boutons. However, it is less clear whether similar or largely different fractions of perisomatic basket parvalbumin (PV) and CCK neurons innervate the somata of neighboring neurons. Even less is known about the relative contributions of dendrite-targeting interneurons to pyramidal cell inhibition. Is each domain of the dendrite innervated equally? Are different principal cells under the control of a different set of interneurons? In the multi-layer neocortex, it is also not clear whether a first set of dendrite-bound Martinotti neurons innervate principal cells of layers II, III and V in layer one, and another set of Martinotti cells contact distal dendrites of layer VI and layer IV neurons, or whether each set of pyramidal cells have their ‘own’ Martinottis. Does each layer have a similar principal cell-interneuron connection pattern or, alternatively, interneurons are posed to coordinate the activity accross multiple layers? Recent works indicate that such ‘plans’ are far from random but, instead, highly specific sub-circuits exist (but see (Fino & Yuste, 2011). For example, in the somatosensory cortex, somatostatin positive (SOM+) interneurons are involved in different circuits/functions depending on their location. In the layer IV, non-Martinotti SOM+ interneurons dis-inhibit the principal cells via the inhibition of fast spiking interneurons, while in layer II/III, SOM+ cells (mostly Martinotti cells) have an direct inhibitory effect on the principal cells (Xu et al., 2013). In the entorhinal cortex, cannabinoid type 1 receptor–expressing GABAergic basket cells selectively innervate principal cells in layer II that project outside the hippocampus but avoid neighboring cells that give rise to the perforant pathway (Varga et al., 2010). Heterogeneous inhibitory circuits have recently been described in the CA1 region of the hippocampus where PV positive (PV+) basket interneurons evoke stronger inhibition in deep as compared to superficial pyramidal cells (Lee et al., 2014b). Furthermore, deep pyramidal cells that project to the amygdala also receive stronger inhibition from PV+ cells as compared to the prefrontal cortex-projecting ones, presumably allowing for appropriate trafficking of contextual and emotional signals (Lee et al., 2014b). Altogether these results show that the organization of GABAergic microcircuits can specifically modulate the long-distance efferent effects of principal neurons. Heterogeneity of inhibition has also recently been observed a the single cell level: in the somatosensory cortex, a single PV+ (but not SOM+) interneuron provides different inputs to its postsynaptic pyramidal cells depending on their respective level of activity, resulting in an equalization of the excitation-inhibition ratios (Xue et al., 2014). Another level of complexity is illustrated by the different subunit composition of the target GABAergic receptors even in the same somato-dendritic subdomains (Freund, 2003).
Optogenetics and physiological characterization of interneurons
The recent advent of optogenetics (Zemelman et al., 2002; Boyden et al., 2005; Deisseroth, 2011) provides a solution for identifying genetically-defined interneuronal subtypes in blind extracellular recordings, by expressing light-sensitive opsins in a given neuronal population (Lima et al., 2009). When used with the right combination of large-scale recording and light delivery methods, optogenetics provide both high temporal and spatial resolution, thus becoming adequate for addressing many outstanding and new questions (Cardin et al., 2010; Royer et al., 2010; Stark et al., 2012; Roux et al., 2014). Integrated recording-optogenetic methods can be used to accomplish at least two goals: (a) identification of genetically labeled neurons (optogenetic-assisted ‘tagging’ or Photostimulation-assisted Identification of Neuronal Populations) (Lima et al., 2009), for physiological characterization and classification of neuron types and (b) testing the causal roles of the identified neurons on the performance of local circuits.
Knowledge about the molecular identity of the different components part of a circuit can considerably improve the interpretation of correlational observations provided by extracellular recordings. Numerous classification schemes have been developed to assign extracellular spikes to putative interneurons, pyramidal cells and their putative subtypes, on the basis of a variety of physiological criteria. These include waveform features, firing rate statistics in different brain states, embeddedness in various population activities, firing patterns characterized by their autocorrelograms, and putative monosynaptic connections to other neurons (Csicsvari et al., 1999; Bartho et al., 2004; Fujisawa et al., 2008; Sirota et al., 2008). However, the ‘ground truth’ of these physiological classifying methods is largely missing. An important application of the optogenetic approach is to assist the identification of distinct subtypes of neurons, within individual molecularly identified classes. An iterative refinement of a library of physiological parameters is needed so that subsequently, the various neurons can be recognized reliably by using purely physiological criteria without the need for optogenetics. Although there are many technical hurdles that need to be addressed for unequivocal identification of genetically labeled neurons, these methods are improving rapidly (Roux et al., 2014). Notably, methods traditionally used for physiological characterization of cell types can help refining classification of optogenetically identified neurons. For instance, an hitherto unexploited approach to characterize the physiological properties of the optically tagged neurons is their input-output analysis. By analogy to intracellular current injections (Ascoli et al., 2008), localized optical stimulation can be used to activate neurons and observe their characteristic response properties to pulses, sinusoids, white noise stimuli or more complex patterns. Comparison of pyramidal cells and interneurons has already demonstrated that channelrhodopsin 2 expressing PV+ interneurons follow optical responses much more efficiently than neighboring pyramidal neurons (Stark et al., 2013).
Optogenetic identification of many individual interneurons, in combination with simultaneous recording from a large number of their neighbors, allows to subsequently investigate the physiological activity of different interneuron subtypes during sensory stimuli, specific network oscillation patterns and/or specific behavioral periods, without light stimulation. So far, these studies only focused on three main interneuron populations, characterized by the expression of the PV, SOM and VIP markers. Although these three populations are not homogeneous (see below), they represent distinct non-overlapping groups of cells and together account for the majority of the interneuron population in the somatosensory cortex (Rudy et al., 2011; but see Tricoire et al., 2011 for hippocampal data).
A pioneering work introduced the method of optogenetic tagging by comparing the responses of ‘tagged’ PV+ cells and nearby untagged neurons in the auditory cortex, for a white noise stimulus (Lima et al., 2009). Sound-evoked firing rates were similar between these two populations, showing in both cases an increase compared to baseline. A more detailed description of PV+ interneuron receptive fields in the auditory cortex indicated that these cells are well tuned for frequency but show shallower response gain and are less intensity-tuned than PV negative neurons (Moore & Wehr, 2013). In the primary visual cortex however, tagged PV+ neurons showed a broad tuning to sensory stimuli (Lee et al., 2012), consistent with other findings in V1 (Kerlin et al., 2010; Ma et al., 2010; Atallah et al., 2012; but see Runyan et al., 2010), and in the olfactory bulb (Kato et al., 2013).
Optogenetically identified PV+ and SOM positive (SOM+) neurons have also been studied in the hippocampal formation in freely moving rodents (Royer et al., 2012; Stark et al., 2013; Buetfering et al., 2014). These studies provided information about the spiking relationship of PV+ and SOM+ cells in relation to theta and ripple oscillations (Royer et al., 2012; Stark et al., 2013; Stark et al., 2014), complementing previous juxtacellular studies, mostly performed under anesthesia (reviewed in Klausberger & Somogyi, 2008; but see Varga et al., 2010; Lapray et al., 2012; Viney et al., 2013; Katona et al., 2014 for waking data). Recent data collected in the medial entorhinal cortex indicated that PV+ cells in this region share many properties with PV+ cells identified in the hippocampus (Royer et al., 2012), such as the shape of their spike waveforms, their high firing rate and the low spatial selectivity of their firing fields (Buetfering et al., 2014).
Optical tagging was also used in the amygdala to correlate the activity of PV+ and SOM+ cells with specific aspects of behavior during auditory fear conditioning. While both cell types show a decreased firing during the US, only PV+ cells increase their activity during the CS, presumably inhibiting the SOM+ cells, and favoring dendritic dis-inhibition of the pyramidal cells (Wolff et al., 2014). In the prefrontal cortex, identified PV+ cells were recruited during freezing in an auditory fear conditioning task (Courtin et al., 2014). In a different study, also performed in the prefrontal cortex but in a different behavioral context, identified PV+ neurons showed a uniform increase in activity when the animal was leaving the reward location. Conversely, a subset of SOM+ cells fired at reward location approach (Kvitsiani et al., 2013). This latter study also investigated the impact of PV+ and SOM+ positive cell spiking on their targets, the putative pyramidal cells, during light-free epochs: PV+ cells exert a brief and uniform inhibitory effect whereas SOM+ cells had a longer and more variable impact. Similarly, VIP+ neurons were identified in extracellular recordings via optogenetics in the auditory and medial prefrontal cortices of freely moving mice (Pi et al., 2013). This study shows that reinforcement signals strongly and uniformly activated VIP+ neurons in auditory cortex during the performance of an auditory discrimination task. Another study used an auditory trace conditioning procedure and found that optogenetically identified GABAergic interneurons in the ventral midbrain responded to auditory stimuli before the onset of the phasic response of the dopaminergic neurons. The fact that these neurons show an enhanced response during extinction and provide monosynaptic inputs to the dopaminergic neurons suggests that they play a key role in extinction by attenuating dopaminergic neuron responses (Pan et al., 2013). The activity of optogenetically ‘tagged’ GABAergic neurons in the VTA was also monitored, while mice associated different odor cues with appetitive and aversive outcomes (Cohen et al., 2012). They responded with persistent activity during the delay between odor and outcome when a reward was expected, suggesting their role in reward prediction error.
Although optogenetic-assisted tagging of cell populations brings an important contribution to the understanding of brain function, one important limitation is that molecularly-defined classes of interneurons often contain multiple sub-populations, with heterogeneous morphological and physiological features (Freund & Buzsaki, 1996; Kawaguchi & Kubota, 1998; Parra et al., 1998; McBain & Fisahn, 2001; Markram et al., 2004; Masland, 2004; Monyer & Markram, 2004; Ascoli et al., 2008; Klausberger & Somogyi, 2008; Fishell & Rudy, 2011; Isaacson & Scanziani, 2011; Rudy et al., 2011; Tricoire et al., 2011; DeFelipe et al., 2013; Kepecs & Fishell, 2014), preventing unambiguous identification of cell types. Studies using single-cell recordings and labeling in freely behaving animals represent complementary approaches towards an exhaustive knowledge of interneuron physiology in the freely behaving animal because they can provide information about the morphology, expression of multiple markers as well as intracellular properties (Isomura et al., 2009; Lapray et al., 2012; Varga et al., 2012; Viney et al., 2013; Katona et al., 2014). Alternatively, refinement of genetic approaches employed to target distinct functional sub-populations of interneurons (Fenno et al., 2014) will allow a more detailed dissection of the circuit elements involved in specific behaviors.
Roles for interneurons in circuit function
Permanent manipulations of specific interneuron sub-populations, such a genetic ablation of AMPA or NMDA receptors (Fuchs et al., 2007; Korotkova et al., 2010; Carlen et al., 2012) or expression of tetanus toxin light chain (Murray et al., 2011), have provided important insight about the roles of PV+ cells in different behavioral tasks. In vivo manipulations of single interneuron activity via whole-cell recordings, while monitoring layer 5 pyramidal cells, also highlighted functional differences between two classes of layer 1 interneurons in the neocortex, regarding the initiation of complex spikes (Jiang et al., 2013). Although these experiments employed useful approaches to investigate interneuron function, we will focus our review on some of the recent studies that took advantage of optogenetic and pharmacogenetic tools to manipulate the activity of molecularly defined classes of interneurons in vivo. The spatial and temporal precisions offered by these perturbation strategies, combined with electrophysiological recordings or imaging techniques, allow probing the involvement of specific interneuron classes in virtually any defined computation.
Visual processing
How the different interneuron subtypes influence the input-output transformation in the pyramidal cells of the primary visual cortex has recently been addressed in numerous studies. However, so far, no consensus has emerged whether dendritic-targeting SOM+ and soma-targeting PV+ cells exert divisive or substractive gain modulation (but see Lee et al., 2014a). Studies performed in anesthetized animals with optogenetic tools indicated that PV+ cells linearly transform the response properties of the pyramidal cells, with a minor contribution to the width of their tuning curves (mostly a divisive gain modulation) (Atallah et al., 2012; Wilson et al., 2012). In contrast, SOM+ cell optogenetic-activation was shown to induce a sharpening of orientation tuning (substractive transformation) in pyramidal neurons (Wilson et al., 2012). In awake mice however, PV+ cell photo-activation (but not SOM+ or VIP+ cell activation) was shown to sharpen the orientation tuning and enhance the direction selectivity of their target pyramidal cells, possibly due to a substractive gain modulation (Lee et al., 2012). This manipulation had an impact at the behavioral level as it was shown to increase the performance in a go/no-go discrimination task. In contrast, SOM+ cells exerted a divisive gain modulation, but did not impact the orientation tuning.
Besides these works studying the impact of interneurons on orientation selectivity and gain modulation of the principal cells, the mechanisms involved in surround suppression (or lateral inhibition) also attracted considerable interest. The role of SOM+ cells on the responses of pyramidal cells to stimuli of increasing size has proven to be crucial. A first study showed that SOM+ cells are preferentially recruited by horizontal excitatory projections and lack surround suppression (unlike PV+ cells) (Adesnik et al., 2012). Moreover, silencing SOM+ cells with optogenetic tools in V1 decreased surround suppression in pyramidal cells in awake head-fixed mice. A related study using optogenetic activation of PV+ and SOM+ interneurons in sedated mice confirmed and extended these observations by adding information about contrast dependence and spatial integration (Nienborg et al., 2013). Overall, these studies improved our understanding of visual information processing at the level of the primary visual cortex.
Auditory processing
Although the impact of specific interneuron subtypes on the principal cell response properties has not been as extensively studied in the auditory cortex as in the visual cortex, a recent work investigated the impact of VIP+ neurons on auditory responses in awake mice (Pi et al., 2013). Photo-activation of VIP+ neurons had two different effects on distinct populations of principal cells. On one hand, VIP+ cell activation recruited a dis-inhibitory circuit which was able to modulate the gain of a functionally specific subset of pyramidal cells in an additive manner. On the hand, VIP+ cell activation directly inhibited another population of neurons showing a divisive gain modulation. This study also demonstrated that this sub-circuit is recruited during a Go/No-Go discrimination task, at the time of the reinforcement feedback (Pi et al., 2013). Interneurons in the auditory cortex were also studied in the context of auditory fear conditioning (Letzkus et al., 2011- see below), although they were not manipulated in this work.
Somato-sensation
A recent study using in vivo whole cell recordings in awake head-fixed animal showed that SOM+ positive cells in the barrel cortex are hyperpolarized during whisker deflection (Gentet et al., 2012). This hyperpolarization of the SOM+ cells likely originates from the VIP+ cell input, as VIP+ interneurons are recruited by M1 long-range inputs during whisker deflection (Lee et al., 2013). Silencing of SOM+ positive cells, mimicking the natural hyperpolarization of these cells observed during whisker sensing, increased burst firing in nearby excitatory neurons in the in the barrel cortex of awake mice (Gentet et al., 2012). These results are consistent with other observations in the hippocampus in vitro (Lovett-Barron et al., 2012) and in vivo (Royer et al., 2012), showing the impact of dendritic inhibition from SOM+ cells on principal cell bursting properties (Figure 2). Another study used activation of PV+ cells in order to indirectly silence the pyramidal cells in the somatosensory cortex, selectively during the early or the late phase of the sensory responses in a detection task. In both cases, the performance was impaired, revealing a causal role for the late excitation in the stimulus perception (Sachidhanandam et al., 2013).
Olfactory processing
The olfactory bulb constitutes the first central relay in olfactory information processing. Silencing of PV+ cells with pharmacogenetic tools in the awake mice indicated that these cells provide a divisive gain control of the principal cell output, without significantly altering their odor tuning properties (Kato et al., 2013). These observations are consistent with other reports obtained in the visual cortex (Atallah et al., 2012; Wilson et al., 2012; but see Lee et al., 2012 and Lee et al., 2014a). The olfactory bulb is a brain region where theta and gamma oscillations co-exist, the former being mostly coupled to the sniffing-cycle and the latter being enhanced during odor presentation (Kay et al., 2009). Optogenetic manipulations allowed dissecting of the role of two distinct classes of inhibitory interneurons, the periglomerular interneurons and the granule cells, in these two rhythms (Fukunaga et al., 2014). The olfactory bulb is special since new local interneurons are persistently incorporated in its circuits in adult life. Optogenetic-activation of these newborn neurons in the olfactory bulb have been shown to exert powerful inhibition of the mitral cells and to accelerate learning in difficult odor discrimination tasks, when the stimulus is presented a 40Hz and not 10Hz (Alonso et al., 2012).
Interneurons and spatial information coding
As compared to the primary sensory regions described above, circuits of the hippocampal formation are known to encode higher order representation of the external world. The most striking examples are the so-called “place cells” (O’Keefe & Nadel, 1978) and the “grid cells” (Hafting et al., 2005), which are active in specific regions of the environment. The involvement of interneuron subtypes in these complex representations has recently been investigated via optogenetic manipulations in behaving mice. A first study showed that that PV+ and SOM+ in the CA1 region of the hippocampus differentially suppressed the firing rate of the place cells within their place fields: PV+ cells have a major impact at the beginning of the place field whereas SOM+ cells have a stronger effect of at the end (Royer et al., 2012). Optogenetic suppression of inhibition enhances firing rates of pyramidal neurons in their place fields but not outside the place fields. Further difference between PV+ and SOM+ interneuron types was revealed by demonstrated that PV+ cells control the timing of the spikes relative to the ongoing theta oscillation while SOM+ cell suppressed burst firing in pyramidal cells (Gentet et al., 2012; Lovett-Barron et al., 2012; Royer et al., 2012). Another study, performed in the medial entorhinal cortex, shows that PV+ cells modulate the overall firing rate of grid cells and head-direction cells, without affecting their grid firing pattern and their direction selectivity (Buetfering et al., 2014). Combined these observations in the hippocampal system with those in sensory cortices supports the role of PV+ cells in gain control.
Supporting brain oscillations
The implication of interneurons in brain oscillations is now widely recognized, but only few studies causally tested this assumption. Recent technical advances providing precise and closed-loop neuronal control allowed a better understanding of the mechanisms underlying gamma, theta oscillations, sharp wave ripples, sleep spindles and pathological seizures. Early studies showed that, when PV+ interneurons are optogenetically activated under anesthesia, using strong light intensities and repetitive pulses at various frequencies, the LFP responses were enhanced at gamma frequencies (Cardin et al., 2009; Carlen et al., 2012), which effect was attenuated by NMDA receptor blockade (Carlen et al., 2012). Such entrainment was shown to affect the processing of sensory inputs relative to the evoked responses, and enhanced signal transmission in the neocortex (Cardin et al., 2009; Sohal et al., 2009). However, generation of gamma oscillations by continuous or noise stimulation, a key manipulation to demonstrate the critical role of PV+ interneurons in this rhythm (Buzsaki & Wang, 2012), was not attempted in these studies. Another study used PV+ cell activation in the hippocampus and the neocortex to investigated how PV+ cells can contribute to the resonance properties of the network, at the spiking level. It showed that optogenetic activation of PV+ cells was able to induce rebound spiking of the pyramidal cells (i.e., gain), selectively in the theta frequency range, whereas they were mostly suppressed at other stimulation frequencies (Stark et al., 2013) (Figure 3). In the hippocampus, closed-loop optogenetic-activation of the PV+ or the SOM+ cells during sharp wave ripples interrupted the ripple oscillation, due to the indirect silencing of the local pyramidal cells (Stark et al., 2014). In contrast, activation of pyramidal neurons induced fast oscillations in the frequency range of ripples and with several properties of the native ripples oscillations. Activating PV+ cells with moderate light intensity reduced the activity of pyramidal cells but induced fast network oscillations between pyramidal cells and PV+ interneurons, suggesting the critical role of PV-PV interneuron interactions in setting fast oscillation frequency. A combined multi-photon imaging, electrophysiological study showed that dendrites of PV neurons have resonant properties at ripple frequency (Chiovini et al., 2014).
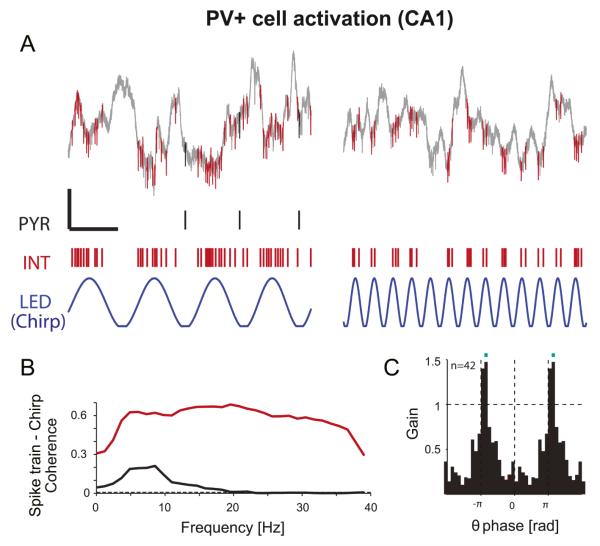
Figure 3.
Theta-resonance of pyramidal cell spiking induced by PV+ cell activation in the hippocampal CA1 region. A. Example traces of local field potentials (LFP) and spikes (1-5000 Hz; calibration: 100 ms, 200 μV) in the CA1 pyramidal layer during two distinct periods (~8Hz - left- and ~25Hz -right-) of a chirp pattern photostimulation of PV+ interneurons (chirp between 0 and 40 Hz;, blue trace). Red: PV+ interneuron spikes (INT). Black: pyramidal cell (PYR) spikes. Note that during PV+ cell activation, the pyramidal cell tended to spike specifically at theta frequency. B. Coherence between chirp pattern and spiking; dashed line shows chance coherence. Note the narrow-band coherence of the PYR at theta frequency (black) and the wide-band coherence of the interneuron (red). During theta-band chirp pattern PV+ cell activation, the PYR spikes specifically at chirp troughs (not shown). C. Mean theta spiking gain for the PYR during PV+ cell activation. For each PYR, firing rates resolved by chirp phase (top) were computed and divided by the baseline rate (in the lack of any light stimulation). Gain=1 thus indicates no change relative to spontaneous activity. Blue bars indicate phase bins for which the number of units with increased spiking (gain>1) exceeds chance level (exact Binomial test, p<0.001). The mean gain is >1 at the chirp theta trough indicating that PYR exhibit excess (“rebound”) spiking. Figure adapted from Stark et al., 2013.
Optogenetic activation of GABAergic neurons of the thalamic reticular nucleus at spindle frequency has been shown to favor spindle occurrence in the neocortex during slow wave sleep (Halassa et al., 2011). Stronger and slower frequency stimulation, on the other hand, induced evoked generalized spike and wave discharges which could be blocked by closed-loop optogenetic activation of PV+ neurons in the neocortex (Berenyi et al., 2012). Tonic optogenetic-activation of reticular thalamic neurons could also reduce focal seizures in the neocortex (Paz et al., 2013). Similarly, spontaneously occurring seizures could be suppressed by optogenetic activation of PV+ interneurons in the hippocampus (Krook-Magnuson et al., 2013).
A role for PV+ cells in network plasticity?
A discussed above, optogenetic methods effectively assisted in revealing the role of interneurons in information processing. Ocular dominance plasticity in the visual cortex during the critical period of development has been studied extensively (Wiesel & Hubel, 1963). Pharmacogenetic suppression of PV+ cells re-introduced the ability of the V1 network to show ocular dominance plasticity, after the natural critical period in adult mice (Kuhlman et al., 2013). The involvement of PV+ cells, and likely the protein PV itself, has also been highlighted in a recent study from Donato and colleagues (Donato et al., 2013). It showed a correlation between the level of PV expression in PV+ interneurons and learning: a switch to a low level of PV expression was observed when plasticity was induced during learning or in an enriched environment. Inhibition of the PV+ cells mediated by VIP+ cells can be a key component in the transitions to the “low-PV expression” network. Silencing of VIP+ neurons (or PV+ cell activation) via pharmacogenetics during learning in the Morris water maze prevented the switch to plasticity-associated low-PV level and impaired learning performance.
Inhibition, fear conditioning and reinforcement learning
The role of specific interneuron types in the amygdala was also studied in the context of fear conditioning. Optogenetic-activation of SOM+ cells in the lateral subdivision of the central amygdala induced freezing in naïve animals. In contrast, silencing of these interneurons impaired conditioned fear expression (Li et al., 2013). Another study indicated that the acquisition of the fear memory trace is bidirectionally controlled by PV+ and the SOM+ positive cells in the basoletaral amygdala, via specific disihibitory circuits. Dendritic disinhibition in the amygdala during auditory fear learning seems to be a crucial process for associative plasticity, similar to the auditory cortex (Letzkus et al., 2011). Indeed, in the auditory cortex, disinhibition occurring selectively during foot shocks (US), via the cholinergic activation of layer 1 interneurons was shown to be required for associative fear learning (Letzkus et al., 2011). In this latter study, optogenetic-activation of PV+ cells during learning impaired the fear response, presumably because it counterbalanced their inhibition by layer 1 interneurons. A related work studied the impact of PV+ interneurons in the prefrontal cortex in the context of fear conditioning and showed that fear expression was conditioned by the phasic inhibition of the PV+ cells, leading to dis-inhibition of the principal cells (Courtin et al., 2014). The role of inhibition during contextual fear conditioning was recently investigated in the hippocampus. At the time of the aversive stimulus (US), cholinergic inputs recruited the dendritic-targeting SOM+ cells, presumably to attenuate the impact of the sensory features of the US. Importantly, inactivation of SOM+ interneurons via pharmacogenetic or optogenetic tools impaired contextual fear learning (Lovett-Barron et al., 2014).
Aversive (and rewarding) stimuli have also been shown to impact neuronal activity in the ventral tegmental area (VTA). This structure contains a mixture of dopaminergic, glutamatergic and GABAergic neurons. The latter neurons provide not only local inhibition but also project to the nucleus accumbens. Optogenetic manipulations of these two inhibitory pathways have allowed dissecting their respective roles in stimulus-outcome learning. Optogenetic activation of VTA GABAergic neurons could mimic contextual aversion typically observed with a foot shock, since it induced conditioned place aversion (Tan et al., 2012). This study also indicated that GABAergic neurons of the VTA inhibit the local dopaminergic neurons via GABAA transmission. These findings are consistent with a related observation that GABAergic neurons of the VTA control the excitability of the local dopaminergic neurons and thereby reduce dopamine release in the nucleus accumbens (van Zessen et al., 2012). Optogenetic-activation of VTA GABAergic neurons but not their projections to the nucleus accumbens, was able to disrupt reward consumption. In another study, the same VTA GABAergic neurons were shown to selectively target cholinergic interneurons in the nuclus accumbens (Brown et al., 2012). Optogenetic activation of these projection neurons was able to enhance discrimination of a salient stimulus that has been associated with an aversive outcome, by controlling the cholinergic tone in the NAc. Non-dopaminergic neurons of the VTA receive glutamatergic and GABAergic inputs from the bed nucleus of the stria terminalis (BNST) (Jennings et al., 2013). Optogenetic stimulation of the BNST GABAergic projections to the VTA brought about rewarding and anxiolytic effects, which could be replicated by direct inhibition of VTA GABAergic neurons (Jennings et al., 2013). Overall, these studies suggest that the activity of the VTA GABAergic neurons may play an important role in encoding stimulus saliency.
In the dorsal striatum, transient optogenetic silencing of choline acetyltransferase (ChAT)-expressing interneurons mimics the pause-excitation population response that can be evoked in by reinforcement signals in the intact animal. The rebound firing exerts a suppression of projection neuron firing rates, presumably due to cholinergic recruitment of GABAergic interneurons, including neuropeptide Y-expressing neurogliaform cells. This feed-forward inhibitory circuit may selectively gate the effect of external stimuli on ongoing behavior by adjusting their salience (English et al., 2012). In the nucleus accumbens, optogenetic activation or silencing of ChAT+ neurons can bidirectionally control medium spiny neuron firing, consistent with an indirect inhibitory action of ChAT+ neurons. In addition, ChAT+ cells can be activated by cocaine and their silencing suppresses cocaine conditioning in behavioral paradigms (Witten et al., 2010). Optogenetic tools were also used to study the relationship between excitation and inhibition for social behavior. Tonic activation of the pyramidal cells in the medial prefrontal cortex altered social exploration behavior in mice, which could be restored by optogenetic activation of PV+ cells (Yizhar et al., 2011).
Although this non-exhaustive list of studies summarized here show that opto- and pharmacogenetics are revolutionary tools for uncovering causal relationships in brain mechanisms, one must keep in mind that such methods provide clearcut interpretations only in linear systems without feedback. In the brain with multiple loops, hierarchical organization and multiple reentries, perturbation at one level may ripple through the entire brain or at least multiple interconnected systems. In such complex systems governed by ‘reciprocal causation’, straightforward explanations of perturbations have to be cautiously considered.
Conclusion
The experiments summarized in our review demonstrate the power of specific cell-type manipulations combined with recording methods for understanding complex neuronal interactions in intact brain circuits. This new knowledge has enriched not only our understanding of the intact brain but also foretell how such methods could be harnessed for clinical applications in the future.
Highlights.
Inhibition secures transient autonomy of principal cells
Inhibitory interneurons are the backbone of brain oscillations
Optogenetics-assisted tagging of interneuron subtypes improves correlative studies
Cell-type specific manipulations clarify the roles of interneurons in computation
Acknowledgements
We thank E. Stark, S. Royer, M. Lovett-Barron, A. Losonczy, L. Gentet and C. Petersen for providing figure material. Research was funded by National Institute of Health Grants NS34994, MH54671 and NS074015, the Human Frontier Science Program, G. Harold and Leila Y. Mathers Charitable Foundation and the J.D. McDonnell Foundation. LR received also support from the Bettencourt Schueller Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adesnik H, Bruns W, Taniguchi H, Huang ZJ, Scanziani M. A neural circuit for spatial summation in visual cortex. Nature. 2012;490:226–231. doi: 10.1038/nature11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A, Kohler C. Evidence for separate projections of hippocampal pyramidal and nonpyramidal neurons to different parts of the septum in the rat brain. Neuroscience letters. 1982;31:209–214. doi: 10.1016/0304-3940(82)90021-0. [DOI] [PubMed] [Google Scholar]
- Alonso A, Kohler C. A study of the reciprocal connections between the septum and the entorhinal area using anterograde and retrograde axonal transport methods in the rat brain. The Journal of comparative neurology. 1984;225:327–343. doi: 10.1002/cne.902250303. [DOI] [PubMed] [Google Scholar]
- Alonso M, Lepousez G, Sebastien W, Bardy C, Gabellec MM, Torquet N, Lledo PM. Activation of adult-born neurons facilitates learning and memory. Nat Neurosci. 2012;15:897–904. doi: 10.1038/nn.3108. [DOI] [PubMed] [Google Scholar]
- Anderson JS, Carandini M, Ferster D. Orientation tuning of input conductance, excitation, and inhibition in cat primary visual cortex. J Neurophysiol. 2000;84:909–926. doi: 10.1152/jn.2000.84.2.909. [DOI] [PubMed] [Google Scholar]
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsaki G, Cauli B, Defelipe J, Fairen A, Feldmeyer D, Fishell G, Fregnac Y, Freund TF, Gardner D, Gardner EP, Goldberg JH, Helmstaedter M, Hestrin S, Karube F, Kisvarday ZF, Lambolez B, Lewis DA, Marin O, Markram H, Munoz A, Packer A, Petersen CC, Rockland KS, Rossier J, Rudy B, Somogyi P, Staiger JF, Tamas G, Thomson AM, Toledo-Rodriguez M, Wang Y, West DC, Yuste R. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atallah BV, Bruns W, Carandini M, Scanziani M. Parvalbumin-expressing interneurons linearly transform cortical responses to visual stimuli. Neuron. 2012;73:159–170. doi: 10.1016/j.neuron.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartho P, Hirase H, Monconduit L, Zugaro M, Harris KD, Buzsaki G. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J Neurophysiol. 2004;92:600–608. doi: 10.1152/jn.01170.2003. [DOI] [PubMed] [Google Scholar]
- Basu J, Srinivas KV, Cheung SK, Taniguchi H, Huang ZJ, Siegelbaum SA. A cortico-hippocampal learning rule shapes inhibitory microcircuit activity to enhance hippocampal information flow. Neuron. 2013;79:1208–1221. doi: 10.1016/j.neuron.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenyi A, Belluscio M, Mao D, Buzsaki G. Closed-loop control of epilepsy by transcranial electrical stimulation. Science. 2012;337:735–737. doi: 10.1126/science.1223154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam JP, Hanley JJ, Booth PA, Bevan MD. Synaptic organisation of the basal ganglia. Journal of anatomy. 2000;196(Pt 4):527–542. doi: 10.1046/j.1469-7580.2000.19640527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Bragin A, Jando G, Nadasdy Z, Hetke J, Wise K, Buzsaki G. Gamma (40-100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci. 1995;15:47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MT, Tan KR, O’Connor EC, Nikonenko I, Muller D, Luscher C. Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature. 2012;492:452–456. doi: 10.1038/nature11657. [DOI] [PubMed] [Google Scholar]
- Buetfering C, Allen K, Monyer H. Parvalbumin interneurons provide grid cell-driven recurrent inhibition in the medial entorhinal cortex. Nat Neurosci. 2014;17:710–718. doi: 10.1038/nn.3696. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Feed-forward inhibition in the hippocampal formation. Progress in neurobiology. 1984;22:131–153. doi: 10.1016/0301-0082(84)90023-6. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Rhythms of the brain. Oxford University Press; New York: 2006. [Google Scholar]
- Buzsaki G. Neural syntax: cell assemblies, synapsembles, and readers. Neuron. 2010;68:362–385. doi: 10.1016/j.neuron.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Chrobak JJ. Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Curr Opin Neurobiol. 1995;5:504–510. doi: 10.1016/0959-4388(95)80012-3. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Eidelberg E. Commissural projection to the dentate gyrus of the rat: evidence for feed-forward inhibition. Brain Res. 1981;230:346–350. doi: 10.1016/0006-8993(81)90413-3. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Eidelberg E. Direct afferent excitation and long-term potentiation of hippocampal interneurons. J Neurophysiol. 1982;48:597–607. doi: 10.1152/jn.1982.48.3.597. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Geisler C, Henze DA, Wang XJ. Interneuron Diversity series: Circuit complexity and axon wiring economy of cortical interneurons. Trends Neurosci. 2004;27:186–193. doi: 10.1016/j.tins.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Penttonen M, Nadasdy Z, Bragin A. Pattern and inhibition-dependent invasion of pyramidal cell dendrites by fast spikes in the hippocampus in vivo. Proc Natl Acad Sci U S A. 1996;93:9921–9925. doi: 10.1073/pnas.93.18.9921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Wang XJ. Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35:203–225. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputi A, Melzer S, Michael M, Monyer H. The long and short of GABAergic neurons. Curr Opin Neurobiol. 2013;23:179–186. doi: 10.1016/j.conb.2013.01.021. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nature protocols. 2010;5:247–254. doi: 10.1038/nprot.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlen M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, Ruhlmann C, Jones SR, Deisseroth K, Sheng M, Moore CI, Tsai LH. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Molecular psychiatry. 2012;17:537–548. doi: 10.1038/mp.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Audinat E, Lambolez B, Angulo MC, Ropert N, Tsuzuki K, Hestrin S, Rossier J. Molecular and physiological diversity of cortical nonpyramidal cells. J Neurosci. 1997;17:3894–3906. doi: 10.1523/JNEUROSCI.17-10-03894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberland S, Salesse C, Topolnik D, Topolnik L. Synapse-specific inhibitory control of hippocampal feedback inhibitory circuit. Frontiers in cellular neuroscience. 2010;4:130. doi: 10.3389/fncel.2010.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiovini B, Turi GF, Katona G, Kaszas A, Palfi D, Maak P, Szalay G, Szabo MF, Szabo G, Szadai Z, Kali S, Rozsa B. Dendritic spikes induce ripples in parvalbumin interneurons during hippocampal sharp waves. Neuron. 2014;82:908–924. doi: 10.1016/j.neuron.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Chiu CQ, Lur G, Morse TM, Carnevale NT, Ellis-Davies GC, Higley MJ. Compartmentalization of GABAergic inhibition by dendritic spines. Science. 2013;340:759–762. doi: 10.1126/science.1234274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsaki G. Selective activation of deep layer (V-VI) retrohippocampal cortical neurons during hippocampal sharp waves in the behaving rat. J Neurosci. 1994;14:6160–6170. doi: 10.1523/JNEUROSCI.14-10-06160.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsaki G. Gamma oscillations in the entorhinal cortex of the freely behaving rat. J Neurosci. 1998;18:388–398. doi: 10.1523/JNEUROSCI.18-01-00388.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482:85–88. doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart R, Dinocourt C, Hirsch JC, Merchan-Perez A, De Felipe J, Ben-Ari Y, Esclapez M, Bernard C. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat Neurosci. 2001;4:52–62. doi: 10.1038/82900. [DOI] [PubMed] [Google Scholar]
- Courtin J, Chaudun F, Rozeske RR, Karalis N, Gonzalez-Campo C, Wurtz H, Abdi A, Baufreton J, Bienvenu TC, Herry C. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature. 2014;505:92–96. doi: 10.1038/nature12755. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat Neurosci. 2007;10:462–468. doi: 10.1038/nn1861. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurko A, Buzsaki G. Reliability and state dependence of pyramidal cell-interneuron synapses in the hippocampus: an ensemble approach in the behaving rat. Neuron. 1998;21:179–189. doi: 10.1016/s0896-6273(00)80525-5. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsaki G. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving Rat. J Neurosci. 1999;19:274–287. doi: 10.1523/JNEUROSCI.19-01-00274.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J, Lopez-Cruz PL, Benavides-Piccione R, Bielza C, Larranaga P, Anderson S, Burkhalter A, Cauli B, Fairen A, Feldmeyer D, Fishell G, Fitzpatrick D, Freund TF, Gonzalez-Burgos G, Hestrin S, Hill S, Hof PR, Huang J, Jones EG, Kawaguchi Y, Kisvarday Z, Kubota Y, Lewis DA, Marin O, Markram H, McBain CJ, Meyer HS, Monyer H, Nelson SB, Rockland K, Rossier J, Rubenstein JL, Rudy B, Scanziani M, Shepherd GM, Sherwood CC, Staiger JF, Tamas G, Thomson A, Wang Y, Yuste R, Ascoli GA. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat Rev Neurosci. 2013;14:202–216. doi: 10.1038/nrn3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K. Optogenetics. Nature methods. 2011;8:26–29. doi: 10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato F, Rompani SB, Caroni P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature. 2013;504:272–276. doi: 10.1038/nature12866. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Ito M, Szentágothai J. The Cerebellum as a Neuronal Machine. Springer-Verlag; New York: 1967. [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- English DF, Ibanez-Sandoval O, Stark E, Tecuapetla F, Buzsaki G, Deisseroth K, Tepper JM, Koos T. GABAergic circuits mediate the reinforcement-related signals of striatal cholinergic interneurons. Nat Neurosci. 2012;15:123–130. doi: 10.1038/nn.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermentrout GB, Kopell N. Fine structure of neural spiking and synchronization in the presence of conduction delays. Proc Natl Acad Sci U S A. 1998;95:1259–1264. doi: 10.1073/pnas.95.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenno LE, Mattis J, Ramakrishnan C, Hyun M, Lee SY, He M, Tucciarone J, Selimbeyoglu A, Berndt A, Grosenick L, Zalocusky KA, Bernstein H, Swanson H, Perry C, Diester I, Boyce FM, Bass CE, Neve R, Huang ZJ, Deisseroth K. Targeting cells with single vectors using multiple-feature Boolean logic. Nature methods. 2014;11:763–772. doi: 10.1038/nmeth.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Yuste R. Dense inhibitory connectivity in neocortex. Neuron. 2011;69:1188–1203. doi: 10.1016/j.neuron.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishell G, Rudy B. Mechanisms of inhibition within the telencephalon: “where the wild things are”. Annu Rev Neurosci. 2011;34:535–567. doi: 10.1146/annurev-neuro-061010-113717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF. Interneuron Diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988;336:170–173. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FE, Bannerman DM, Rozov A, Whittington MA, Traub RD, Rawlins JN, Monyer H. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Fujisawa S, Amarasingham A, Harrison MT, Buzsaki G. Behavior-dependent short-term assembly dynamics in the medial prefrontal cortex. Nat Neurosci. 2008;11:823–833. doi: 10.1038/nn.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga I, Herb JT, Kollo M, Boyden ES, Schaefer AT. Independent control of gamma and theta activity by distinct interneuron networks in the olfactory bulb. Nat Neurosci. 2014 doi: 10.1038/nn.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabernet L, Jadhav SP, Feldman DE, Carandini M, Scanziani M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron. 2005;48:315–327. doi: 10.1016/j.neuron.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Electrical and chemical synapses among parvalbumin fast-spiking GABAergic interneurons in adult mouse neocortex. Proc Natl Acad Sci U S A. 2002;99:12438–12443. doi: 10.1073/pnas.192159599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentet LJ, Kremer Y, Taniguchi H, Huang ZJ, Staiger JF, Petersen CC. Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nat Neurosci. 2012;15:607–612. doi: 10.1038/nn.3051. [DOI] [PubMed] [Google Scholar]
- Germroth P, Schwerdtfeger WK, Buhl EH. GABAergic neurons in the entorhinal cortex project to the hippocampus. Brain Res. 1989;494:187–192. doi: 10.1016/0006-8993(89)90162-5. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Glickfeld LL, Scanziani M. Distinct timing in the activity of cannabinoid-sensitive and cannabinoid-insensitive basket cells. Nat Neurosci. 2006;9:807–815. doi: 10.1038/nn1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Hajos N, Freund TF. Interneurons containing calretinin are specialized to control other interneurons in the rat hippocampus. J Neurosci. 1996;16:3397–3411. doi: 10.1523/JNEUROSCI.16-10-03397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Hajos N, Katona I, Freund TF. Interneurons are the local targets of hippocampal inhibitory cells which project to the medial septum. Eur J Neurosci. 2003;17:1861–1872. doi: 10.1046/j.1460-9568.2003.02630.x. [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Miles R, Sik A, Toth K, Tamamaki N, Freund TF. Hippocampal pyramidal cells excite inhibitory neurons through a single release site. Nature. 1993;366:683–687. doi: 10.1038/366683a0. [DOI] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Siegle JH, Ritt JT, Ting JT, Feng G, Moore CI. Selective optical drive of thalamic reticular nucleus generates thalamic bursts and cortical spindles. Nat Neurosci. 2011;14:1118–1120. doi: 10.1038/nn.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartline HK, Wagner HG, Ratliff F. Inhibition in the eye of Limulus. The Journal of general physiology. 1956;39:651–673. doi: 10.1085/jgp.39.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenstaub A, Shu Y, Haider B, Kraushaar U, Duque A, McCormick DA. Inhibitory postsynaptic potentials carry synchronized frequency information in active cortical networks. Neuron. 2005;47:423–435. doi: 10.1016/j.neuron.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Hebb CO, Konzett H. Difference between morphine and synthetic analgesics in their actions on ganglionic transmission. Nature. 1949;163:720. doi: 10.1038/163720a0. [DOI] [PubMed] [Google Scholar]
- Helmstaedter M, Sakmann B, Feldmeyer D. Neuronal correlates of local, lateral, and translaminar inhibition with reference to cortical columns. Cereb Cortex. 2009;19:926–937. doi: 10.1093/cercor/bhn141. [DOI] [PubMed] [Google Scholar]
- Higo S, Udaka N, Tamamaki N. Long-range GABAergic projection neurons in the cat neocortex. The Journal of comparative neurology. 2007;503:421–431. doi: 10.1002/cne.21395. [DOI] [PubMed] [Google Scholar]
- Hu H, Ma Y, Agmon A. Submillisecond firing synchrony between different subtypes of cortical interneurons connected chemically but not electrically. J Neurosci. 2011;31:3351–3361. doi: 10.1523/JNEUROSCI.4881-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard JR, McCormick DA. Thalamic synchrony and dynamic regulation of global forebrain oscillations. Trends Neurosci. 2007;30:350–356. doi: 10.1016/j.tins.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Hull C, Isaacson JS, Scanziani M. Postsynaptic mechanisms govern the differential excitation of cortical neurons by thalamic inputs. J Neurosci. 2009;29:9127–9136. doi: 10.1523/JNEUROSCI.5971-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomura Y, Harukuni R, Takekawa T, Aizawa H, Fukai T. Microcircuitry coordination of cortical motor information in self-initiation of voluntary movements. Nat Neurosci. 2009;12:1586–1593. doi: 10.1038/nn.2431. [DOI] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496:224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Wang G, Lee AJ, Stornetta RL, Zhu JJ. The organization of two new cortical interneuronal circuits. Nat Neurosci. 2013;16:210–218. doi: 10.1038/nn.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinno S, Klausberger T, Marton LF, Dalezios Y, Roberts JD, Fuentealba P, Bushong EA, Henze D, Buzsaki G, Somogyi P. Neuronal diversity in GABAergic long-range projections from the hippocampus. J Neurosci. 2007;27:8790–8804. doi: 10.1523/JNEUROSCI.1847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato HK, Gillet SN, Peters AJ, Isaacson JS, Komiyama T. Parvalbumin-expressing interneurons linearly control olfactory bulb output. Neuron. 2013;80:1218–1231. doi: 10.1016/j.neuron.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona L, Lapray D, Viney TJ, Oulhaj A, Borhegyi Z, Micklem BR, Klausberger T, Somogyi P. Sleep and movement differentiates actions of two types of somatostatin-expressing GABAergic interneuron in rat hippocampus. Neuron. 2014;82:872–886. doi: 10.1016/j.neuron.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Neurochemical features and synaptic connections of large physiologically-identified GABAergic cells in the rat frontal cortex. Neuroscience. 1998;85:677–701. doi: 10.1016/s0306-4522(97)00685-4. [DOI] [PubMed] [Google Scholar]
- Kay LM, Beshel J, Brea J, Martin C, Rojas-Libano D, Kopell N. Olfactory oscillations: the what, how and what for. Trends Neurosci. 2009;32:207–214. doi: 10.1016/j.tins.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepecs A, Fishell G. Interneuron cell types are fit to function. Nature. 2014;505:318–326. doi: 10.1038/nature12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerlin AM, Andermann ML, Berezovskii VK, Reid RC. Broadly tuned response properties of diverse inhibitory neuron subtypes in mouse visual cortex. Neuron. 2010;67:858–871. doi: 10.1016/j.neuron.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Pignatelli M, Suh J, Kohara K, Yoshiki A, Abe K, Tonegawa S. Island cells control temporal association memory. Science. 2014;343:896–901. doi: 10.1126/science.1244634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H, Hofer SB, Pichler B, Buchanan KA, Sjostrom PJ, Mrsic-Flogel TD. Functional specificity of local synaptic connections in neocortical networks. Nature. 2011;473:87–91. doi: 10.1038/nature09880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler C, Chan-Palay V, Wu JY. Septal neurons containing glutamic acid decarboxylase immunoreactivity project to the hippocampal region in the rat brain. Anatomy and embryology. 1984;169:41–44. doi: 10.1007/BF00300585. [DOI] [PubMed] [Google Scholar]
- Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron. 2010;68:557–569. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Krook-Magnuson E, Armstrong C, Oijala M, Soltesz I. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nature communications. 2013;4:1376. doi: 10.1038/ncomms2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, Olivas ND, Tring E, Ikrar T, Xu X, Trachtenberg JT. A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex. Nature. 2013;501:543–546. doi: 10.1038/nature12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvitsiani D, Ranade S, Hangya B, Taniguchi H, Huang JZ, Kepecs A. Distinct behavioural and network correlates of two interneuron types in prefrontal cortex. Nature. 2013;498:363–366. doi: 10.1038/nature12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapray D, Lasztoczi B, Lagler M, Viney TJ, Katona L, Valenti O, Hartwich K, Borhegyi Z, Somogyi P, Klausberger T. Behavior-dependent specialization of identified hippocampal interneurons. Nat Neurosci. 2012;15:1265–1271. doi: 10.1038/nn.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kruglikov I, Huang ZJ, Fishell G, Rudy B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat Neurosci. 2013;16:1662–1670. doi: 10.1038/nn.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Kwan AC, Dan Y. Interneuron subtypes and orientation tuning. Nature. 2014a;508:E1–2. doi: 10.1038/nature13128. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kwan AC, Zhang S, Phoumthipphavong V, Flannery JG, Masmanidis SC, Taniguchi H, Huang ZJ, Zhang F, Boyden ES, Deisseroth K, Dan Y. Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature. 2012;488:379–383. doi: 10.1038/nature11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Marchionni I, Bezaire M, Varga C, Danielson N, Lovett-Barron M, Losonczy A, Soltesz I. Parvalbumin-Positive Basket Cells Differentiate among Hippocampal Pyramidal Cells. Neuron. 2014b;82:1129–1144. doi: 10.1016/j.neuron.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzkus JJ, Wolff SB, Meyer EM, Tovote P, Courtin J, Herry C, Luthi A. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature. 2011;480:331–335. doi: 10.1038/nature10674. [DOI] [PubMed] [Google Scholar]
- Lewis DA. Neuroplasticity of excitatory and inhibitory cortical circuits in schizophrenia. Dialogues in clinical neuroscience. 2009;11:269–280. doi: 10.31887/DCNS.2009.11.3/dalewis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B. Experience-dependent modification of a central amygdala fear circuit. Nat Neurosci. 2013;16:332–339. doi: 10.1038/nn.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima SQ, Hromadka T, Znamenskiy P, Zador AM. PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PloS one. 2009;4:e6099. doi: 10.1371/journal.pone.0006099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett-Barron M, Kaifosh P, Kheirbek MA, Danielson N, Zaremba JD, Reardon TR, Turi GF, Hen R, Zemelman BV, Losonczy A. Dendritic inhibition in the hippocampus supports fear learning. Science. 2014;343:857–863. doi: 10.1126/science.1247485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett-Barron M, Losonczy A. Behavioral consequences of GABAergic neuronal diversity. Curr Opin Neurobiol. 2014;26:27–33. doi: 10.1016/j.conb.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Lovett-Barron M, Turi GF, Kaifosh P, Lee PH, Bolze F, Sun XH, Nicoud JF, Zemelman BV, Sternson SM, Losonczy A. Regulation of neuronal input transformations by tunable dendritic inhibition. Nat Neurosci. 2012;15:423–430. S421–423. doi: 10.1038/nn.3024. [DOI] [PubMed] [Google Scholar]
- Ma WP, Liu BH, Li YT, Huang ZJ, Zhang LI, Tao HW. Visual representations by cortical somatostatin inhibitory neurons--selective but with weak and delayed responses. J Neurosci. 2010;30:14371–14379. doi: 10.1523/JNEUROSCI.3248-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod K, Backer A, Laurent G. Who reads temporal information contained across synchronized and oscillatory spike trains? Nature. 1998;395:693–698. doi: 10.1038/27201. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Masland RH. Neuronal cell types. Current biology : CB. 2004;14:R497–500. doi: 10.1016/j.cub.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Masquelier T, Hugues E, Deco G, Thorpe SJ. Oscillations, phase-of-firing coding, and spike timing-dependent plasticity: an efficient learning scheme. J Neurosci. 2009;29:13484–13493. doi: 10.1523/JNEUROSCI.2207-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer AP, Cowen SL, Burke SN, Barnes CA, McNaughton BL. Phase precession in hippocampal interneurons showing strong functional coupling to individual pyramidal cells. J Neurosci. 2006;26:13485–13492. doi: 10.1523/JNEUROSCI.2882-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain CJ, Fisahn A. Interneurons unbound. Nat Rev Neurosci. 2001;2:11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Shu Y, Hasenstaub A, Sanchez-Vives M, Badoual M, Bal T. Persistent cortical activity: mechanisms of generation and effects on neuronal excitability. Cereb Cortex. 2003;13:1219–1231. doi: 10.1093/cercor/bhg104. [DOI] [PubMed] [Google Scholar]
- Melzer S, Michael M, Caputi A, Eliava M, Fuchs EC, Whittington MA, Monyer H. Long-range-projecting GABAergic neurons modulate inhibition in hippocampus and entorhinal cortex. Science. 2012;335:1506–1510. doi: 10.1126/science.1217139. [DOI] [PubMed] [Google Scholar]
- Miles R. Synaptic excitation of inhibitory cells by single CA3 hippocampal pyramidal cells of the guinea-pig in vitro. J Physiol. 1990;428:61–77. doi: 10.1113/jphysiol.1990.sp018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R, Toth K, Gulyas AI, Hajos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996;16:815–823. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- Monyer H, Markram H. Interneuron Diversity series: Molecular and genetic tools to study GABAergic interneuron diversity and function. Trends Neurosci. 2004;27:90–97. doi: 10.1016/j.tins.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Moore AK, Wehr M. Parvalbumin-expressing inhibitory interneurons in auditory cortex are well-tuned for frequency. J Neurosci. 2013;33:13713–13723. doi: 10.1523/JNEUROSCI.0663-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CI, Carlen M, Knoblich U, Cardin JA. Neocortical interneurons: from diversity, strength. Cell. 2010;142:189–193. doi: 10.1016/j.cell.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AJ, Sauer JF, Riedel G, McClure C, Ansel L, Cheyne L, Bartos M, Wisden W, Wulff P. Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat Neurosci. 2011;14:297–299. doi: 10.1038/nn.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienborg H, Hasenstaub A, Nauhaus I, Taniguchi H, Huang ZJ, Callaway EM. Contrast dependence and differential contributions from somatostatin- and parvalbumin-expressing neurons to spatial integration in mouse V1. J Neurosci. 2013;33:11145–11154. doi: 10.1523/JNEUROSCI.5320-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford University Press; New York: 1978. [Google Scholar]
- Owen SF, Tuncdemir SN, Bader PL, Tirko NN, Fishell G, Tsien RW. Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature. 2013;500:458–462. doi: 10.1038/nature12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WX, Brown J, Dudman JT. Neural signals of extinction in the inhibitory microcircuit of the ventral midbrain. Nat Neurosci. 2013;16:71–78. doi: 10.1038/nn.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra P, Gulyas AI, Miles R. How many subtypes of inhibitory cells in the hippocampus? Neuron. 1998;20:983–993. doi: 10.1016/s0896-6273(00)80479-1. [DOI] [PubMed] [Google Scholar]
- Paz JT, Davidson TJ, Frechette ES, Delord B, Parada I, Peng K, Deisseroth K, Huguenard JR. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat Neurosci. 2013;16:64–70. doi: 10.1038/nn.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttonen M, Buzsáki G. Natural logarithmic relationship between brain oscillators. Thalamus and Related Systems. 2003:145–152. [Google Scholar]
- Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci. 2013;16:1068–1076. doi: 10.1038/nn.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503:521–524. doi: 10.1038/nature12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo C, Isaacson JS. Odor representations in olfactory cortex: “sparse” coding, global inhibition, and oscillations. Neuron. 2009;62:850–861. doi: 10.1016/j.neuron.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouille F, Marin-Burgin A, Adesnik H, Atallah BV, Scanziani M. Input normalization by global feedforward inhibition expands cortical dynamic range. Nat Neurosci. 2009;12:1577–1585. doi: 10.1038/nn.2441. [DOI] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Routing of spike series by dynamic circuits in the hippocampus. Nature. 2004;429:717–723. doi: 10.1038/nature02615. [DOI] [PubMed] [Google Scholar]
- Renart A, de la Rocha J, Bartho P, Hollender L, Parga N, Reyes A, Harris KD. The asynchronous state in cortical circuits. Science. 2010;327:587–590. doi: 10.1126/science.1179850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux L, Stark E, Sjulson L, Buzsaki G. In vivo optogenetic identification and manipulation of GABAergic interneuron subtypes. Curr Opin Neurobiol. 2014;26C:88–95. doi: 10.1016/j.conb.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Zemelman BV, Barbic M, Losonczy A, Buzsaki G, Magee JC. Multi-array silicon probes with integrated optical fibers: light-assisted perturbation and recording of local neural circuits in the behaving animal. Eur J Neurosci. 2010;31:2279–2291. doi: 10.1111/j.1460-9568.2010.07250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Zemelman BV, Losonczy A, Kim J, Chance F, Magee JC, Buzsaki G. Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat Neurosci. 2012;15:769–775. doi: 10.1038/nn.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol. 2011;71:45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyan CA, Schummers J, Van Wart A, Kuhlman SJ, Wilson NR, Huang ZJ, Sur M. Response features of parvalbumin-expressing interneurons suggest precise roles for subtypes of inhibition in visual cortex. Neuron. 2010;67:847–857. doi: 10.1016/j.neuron.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachidhanandam S, Sreenivasan V, Kyriakatos A, Kremer Y, Petersen CC. Membrane potential correlates of sensory perception in mouse barrel cortex. Nat Neurosci. 2013;16:1671–1677. doi: 10.1038/nn.3532. [DOI] [PubMed] [Google Scholar]
- Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci. 2001;2:539–550. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder CE, Lakatos P. The gamma oscillation: master or slave? Brain topography. 2009;22:24–26. doi: 10.1007/s10548-009-0080-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sik A, Ylinen A, Penttonen M, Buzsaki G. Inhibitory CA1-CA3-hilar region feedback in the hippocampus. Science. 1994;265:1722–1724. doi: 10.1126/science.8085161. [DOI] [PubMed] [Google Scholar]
- Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsaki G. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron. 2008;60:683–697. doi: 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltesz I, Deschenes M. Low- and high-frequency membrane potential oscillations during theta activity in CA1 and CA3 pyramidal neurons of the rat hippocampus under ketaminexylazine anesthesia. J Neurophysiol. 1993;70:97–116. doi: 10.1152/jn.1993.70.1.97. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark E, Eichler R, Roux L, Fujisawa S, Rotstein HG, Buzsaki G. Inhibition-induced theta resonance in cortical circuits. Neuron. 2013;80:1263–1276. doi: 10.1016/j.neuron.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark E, Koos T, Buzsaki G. Diode probes for spatiotemporal optical control of multiple neurons in freely moving animals. J Neurophysiol. 2012;108:349–363. doi: 10.1152/jn.00153.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark E, Roux L, Eichler R, Senzai Y, Royer S, Buzsáki G. Pyramidal cell-interneuron interactions underlie hippocampal ripple oscillations. Neuron. 2014 doi: 10.1016/j.neuron.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes CC, Isaacson JS. From dendrite to soma: dynamic routing of inhibition by complementary interneuron microcircuits in olfactory cortex. Neuron. 2010;67:452–465. doi: 10.1016/j.neuron.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strogatz SH. Nonlinear Dynamics and Chaos: With Applications to Physics, Biology, Chemistry, and Engineering. Westview Press; 1994. [Google Scholar]
- Tamas G, Somogyi P, Buhl EH. Differentially interconnected networks of GABAergic interneurons in the visual cortex of the cat. J Neurosci. 1998;18:4255–4270. doi: 10.1523/JNEUROSCI.18-11-04255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouebe G, Deisseroth K, Tye KM, Luscher C. GABA neurons of the VTA drive conditioned place aversion. Neuron. 2012;73:1173–1183. doi: 10.1016/j.neuron.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Koos T, Wilson CJ. GABAergic microcircuits in the neostriatum. Trends in neurosciences. 2004;27:662–669. doi: 10.1016/j.tins.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Thomson AM, West DC, Wang Y, Bannister AP. Synaptic connections and small circuits involving excitatory and inhibitory neurons in layers 2-5 of adult rat and cat neocortex: triple intracellular recordings and biocytin labelling in vitro. Cereb Cortex. 2002;12:936–953. doi: 10.1093/cercor/12.9.936. [DOI] [PubMed] [Google Scholar]
- Tomioka R, Okamoto K, Furuta T, Fujiyama F, Iwasato T, Yanagawa Y, Obata K, Kaneko T, Tamamaki N. Demonstration of long-range GABAergic connections distributed throughout the mouse neocortex. Eur J Neurosci. 2005;21:1587–1600. doi: 10.1111/j.1460-9568.2005.03989.x. [DOI] [PubMed] [Google Scholar]
- Tomioka R, Rockland KS. Long-distance corticocortical GABAergic neurons in the adult monkey white and gray matter. The Journal of comparative neurology. 2007;505:526–538. doi: 10.1002/cne.21504. [DOI] [PubMed] [Google Scholar]
- Toth K, Borhegyi Z, Freund TF. Postsynaptic targets of GABAergic hippocampal neurons in the medial septum-diagonal band of broca complex. J Neurosci. 1993;13:3712–3724. doi: 10.1523/JNEUROSCI.13-09-03712.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricoire L, Pelkey KA, Erkkila BE, Jeffries BW, Yuan X, McBain CJ. A blueprint for the spatiotemporal origins of mouse hippocampal interneuron diversity. J Neurosci. 2011;31:10948–10970. doi: 10.1523/JNEUROSCI.0323-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubokawa H, Ross WN. IPSPs modulate spike backpropagation and associated [Ca2+]i changes in the dendrites of hippocampal CA1 pyramidal neurons. J Neurophysiol. 1996;76:2896–2906. doi: 10.1152/jn.1996.76.5.2896. [DOI] [PubMed] [Google Scholar]
- Urban NN, Sakmann B. Reciprocal intraglomerular excitation and intra- and interglomerular lateral inhibition between mouse olfactory bulb mitral cells. J Physiol. 2002;542:355–367. doi: 10.1113/jphysiol.2001.013491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vreeswijk C, Abbott LF, Ermentrout GB. When inhibition not excitation synchronizes neural firing. Journal of computational neuroscience. 1994;1:313–321. doi: 10.1007/BF00961879. [DOI] [PubMed] [Google Scholar]
- van Vreeswijk C, Sompolinsky H. Chaos in neuronal networks with balanced excitatory and inhibitory activity. Science. 1996;274:1724–1726. doi: 10.1126/science.274.5293.1724. [DOI] [PubMed] [Google Scholar]
- van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012;73:1184–1194. doi: 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga C, Golshani P, Soltesz I. Frequency-invariant temporal ordering of interneuronal discharges during hippocampal oscillations in awake mice. Proc Natl Acad Sci U S A. 2012;109:E2726–2734. doi: 10.1073/pnas.1210929109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga C, Lee SY, Soltesz I. Target-selective GABAergic control of entorhinal cortex output. Nat Neurosci. 2010;13:822–824. doi: 10.1038/nn.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viney TJ, Lasztoczi B, Katona L, Crump MG, Tukker JJ, Klausberger T, Somogyi P. Network state-dependent inhibition of identified hippocampal CA3 axo-axonic cells in vivo. Nat Neurosci. 2013;16:1802–1811. doi: 10.1038/nn.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426:442–446. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2000;38:315–336. doi: 10.1016/s0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- Wickelgren WA. Webs, cell assemblies, and chunking in neural nets: introduction. Canadian journal of experimental psychology = Revue canadienne de psychologie experimentale. 1999;53:118–131. doi: 10.1037/h0087304. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Effects of Visual Deprivation on Morphology and Physiology of Cells in the Cats Lateral Geniculate Body. J Neurophysiol. 1963;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- Wilent WB, Contreras D. Synaptic responses to whisker deflections in rat barrel cortex as a function of cortical layer and stimulus intensity. J Neurosci. 2004;24:3985–3998. doi: 10.1523/JNEUROSCI.5782-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NR, Runyan CA, Wang FL, Sur M. Division and subtraction by distinct cortical inhibitory networks in vivo. Nature. 2012;488:343–348. doi: 10.1038/nature11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, Gradinaru V, Ramakrishnan C, Deisseroth K. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science. 2010;330:1677–1681. doi: 10.1126/science.1193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff SB, Grundemann J, Tovote P, Krabbe S, Jacobson GA, Muller C, Herry C, Ehrlich I, Friedrich RW, Letzkus JJ, Luthi A. Amygdala interneuron subtypes control fear learning through disinhibition. Nature. 2014;509:453–458. doi: 10.1038/nature13258. [DOI] [PubMed] [Google Scholar]
- Xu H, Jeong HY, Tremblay R, Rudy B. Neocortical somatostatin-expressing GABAergic interneurons disinhibit the thalamorecipient layer 4. Neuron. 2013;77:155–167. doi: 10.1016/j.neuron.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Atallah BV, Scanziani M. Equalizing excitation-inhibition ratios across visual cortical neurons. Nature. 2014;511:596–600. doi: 10.1038/nature13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitsev AV, Lewis DA. Functional properties and short-term dynamics of unidirectional and reciprocal synaptic connections between layer 2/3 pyramidal cells and fast-spiking interneurons in juvenile rat prefrontal cortex. Eur J Neurosci. 2013 doi: 10.1111/ejn.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemelman BV, Lee GA, Ng M, Miesenbock G. Selective photostimulation of genetically chARGed neurons. Neuron. 2002;33:15–22. doi: 10.1016/s0896-6273(01)00574-8. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Tan AY, Schreiner CE, Merzenich MM. Topography and synaptic shaping of direction selectivity in primary auditory cortex. Nature. 2003;424:201–205. doi: 10.1038/nature01796. [DOI] [PubMed] [Google Scholar]