Abstract
Non-proliferating cells generate the bulk of cellular ATP by fully oxidizing respiratory substrates in mitochondria. Respiratory substrates cross the mitochondrial outer membrane through only one channel, the voltage dependent anion channel (VDAC). Once in the matrix, respiratory substrates are oxidized in the tricarboxylic acid cycle to generate mostly NADH that is further oxidized in the respiratory chain to generate a proton motive force comprised mainly of membrane potential (ΔΨ) to synthesize ATP. Mitochondrial ΔΨ then drives release of ATP−4 from the matrix in exchange for ADP−3 in the cytosol via the adenine nucleotide translocator (ANT) located in the mitochondrial inner membrane. Thus, mitochondrial function in non-proliferating cells drives a high cytosolic ATP/ADP ratio, essential to inhibit glycolysis. By contrast, the bioenergetics of the Warburg phenotype of proliferating cells is characterized by enhanced aerobic glycolysis and suppression of mitochondrial metabolism. Suppressed mitochondrial function leads to lower production of mitochondrial ATP and hence lower cytosolic ATP/ADP ratios that favor enhanced glycolysis. Thus, cytosolic ATP/ADP ratio is a key feature that determines if cell metabolism is predominantly oxidative or glycolytic. Here, we describe two novel mechanisms to explain the suppression of mitochondrial metabolism in cancer cells: the relative closure of VDAC by free tubulin and inactivation of ANT. Both mechanisms contribute to low ATP/ADP ratios that activate glycolysis.
Keywords: ANT, ATP/ADP ratio, cancer cells, glycolysis, mitochondria, oxidative phosphorylation, VDAC, Warburg
Introduction
Cellular bioenergetics differs between proliferating and non-proliferating cells. Cancer and other proliferating cells display enhanced aerobic glycolysis even in the presence of physiological oxygen (Harvey, et al., 2002;Warburg, 1956). This phenomenon was first described by Otto Warburg in the early 20th century and causes fully aerobic tumors to produce net lactic acid (Warburg, et al., 1927). Lactic acid is usually an end product of anaerobic glycolysis in the cytosol. In aerobic non-proliferating cells, pyruvate generated by glycolysis is not converted to lactic acid but is instead diverted to mitochondria and oxidized to CO2 and H2O by the tricarboxylic acid cycle and the respiratory chain. Lactic acid generation by non-proliferating tissues generally indicates hypoxia. Thus, lactic acid production by aerobic tumors signifies a fundamentally altered metabolism, including suppressed pyruvate oxidation by mitochondria. Indeed, Warburg originally postulated that tumor cells had a defect in what we now call oxidative phosphorylation.
A possible advantage of Warburg metabolism to proliferating cells is that glycolysis furnishes carbon backbones for biomass formation and reductive biosynthesis, whereas oxidative phosphorylation converts glucose and other glycolytic substrates completely to CO2 and H2O (Vander Heiden, et al., 2009;DeBerardinis, et al., 2008). A similar phenomenon occurs in yeast. Grown on a fermentable (glycolytic) substrate like glucose, yeast aerobically generate lactic acid as the end product of glycolysis, which is further metabolized to ethanol. When the fermentable substrate is exhausted or replaced with a non-fermentable substrate like glycerol, a pause in growth occurs as new proteins are expressed, including enzymes of oxidative phosphorylation (diauxic shift) (Galdieri, et al., 2010;Vivier, et al., 1997). Cell proliferation then resumes, but growth is never as rapid as when supported by a fermentable substrate. In a broth, selection is for the most rapidly proliferating cells, and if yeast could grow faster on a non-fermentable substrate, then they would. Arguably then, the Warburg metabolic phenotype favoring more rapid cellular proliferation is why we have beer.
Metabolite exchange across mitochondrial membranes
Mitochondrial ATP synthesis involves oxidation of pyruvate, glutamine, fatty acids and other respiratory substrates by enzymes of the tricarboxylic acid cycle in the mitochondrial matrix to produce mostly NADH, which is then oxidized by the respiratory chain to generate the protonmotive force (Δp) that drives ATP synthesis from ADP and Pi by the reversible F1FO-ATP synthase (Fig. 1). Δp is comprised of a negative-inside membrane potential (ΔΨ) and an alkaline-inside pH gradient (ΔpH) by the relationship (in mV): Δp = ΔΨ - 59ΔpH. In mammalian cardiomyocytes and hepatocytes, ΔΨ is in the range of -120 mV to -150 mV and ΔpH is 0.6-0.8 pH units (Brand and Nicholls, 2011;Chacon, et al., 1994;Emaus, et al., 1986;Hoek, et al., 1980;Nicholls and Ferguson, 2013;Petit, et al., 1990;Rottenberg, 1975;Santo-Domingo and Demaurex, 2012;Zahrebelski, et al., 1995;Mitchell, 2011). Newly synthetized mitochondrial ATP4− in the matrix then exchanges for cytosolic ADP3− via the adenine nucleotide transporter (ANT), and OH− exchanges for Pi− via the phosphate transporter (PT). Both ANT and PT are located in the mitochondrial inner membrane. Similarly, various respiratory substrates cross the inner membrane via separate dedicated carriers. By contrast, movement of hydrophilic anionic metabolites across the mitochondrial outer membrane occurs exclusively via a single channel - the voltage dependent anion channel (VDAC) (Fig. 1). This review addresses altered roles of VDAC and adenine nucleotide exchange in mitochondrial ATP release to the cytosol of proliferating tumor cells.
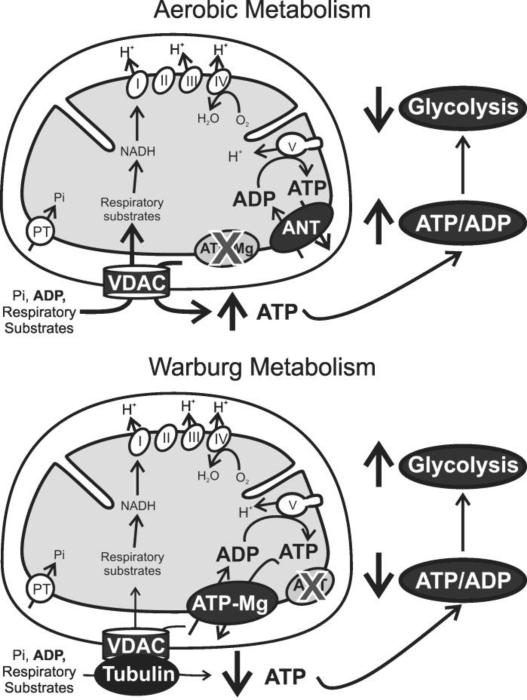
Fig. 1. VDAC closure and inactivation of ANT suppress mitochondrial metabolism and activate glycolysis in the Warburg phenomenon.
Respiratory substrates, ADP and Pi first cross mitochondrial outer membranes via VDAC and then mitochondrial inner membranes via individual transporters, including the ANT. Respiratory substrates generate mostly NADH, which feeds into the respiratory chain (Complexes I-IV). Electron transfer leads to proton translocation from the matrix into the intermembrane space, generating Δp as oxygen is reduced to water. Protons return into the matrix through the F1-F0-ATP synthase (Complex V) driving synthesis of ATP from ADP and Pi. In aerobic metabolism by non-proliferating differentiated cells (top scheme), newly synthesized ATP exchanges for ADP via ANT and subsequently moves into the cytosol through VDAC. A strongly negative mitochondrial ΔΨ drives ANT-mediated outward electrogenic exchange of ATP−4 for inwardly directed ADP−3, which increases cytosolic relative to mitochondrial ATP/ADP ratios by ~100-fold. In proliferating cells (bottom scheme), high free tubulin causes a relative blockade of VDAC conductance. In addition, eletrogenic ATP/ADP exchange by ANT becomes inactivated and is replaced by electroneutral ATP/ADP exchange likely mediated by the ATP-Mg/Pi carrier. Relative VDAC closure and loss of ANT function together produce global suppression of mitochondrial metabolism and decrease cytosolic ATP/ADP ratios that promote Warburg-type aerobic glycolysis.
ANT catalyzes an electrogenic molecule for molecule exchange of ATP4− for ADP3−. Because of the highly negative mitochondrial membrane potential (ΔΨ), ANT acts as a secondary active transport system that pumps ATP4− out and ADP3− into mitochondria. Consequently, ATP/ADP ratios can become 50 to 100 times higher in the cytosol than in the mitochondrial matrix in cells with active aerobic mitochondrial metabolism, such as neurons, cardiomyocytes and hepatocytes (Klingenberg, 2008). High cytosolic ATP/ADP ratios as a consequence of aerobic mitochondrial metabolism and electrogenic ANT exchange suppress glycolysis through inhibition of phosphofructokinase-1 among other possible mechanisms (Fig. 1) (Hers and Van, 1982;Mor, et al., 2011). However, when mitochondrial ATP synthesis becomes compromised as during hypoxia/ischemia, ATP/ADP ratios drop dramatically, which markedly stimulates glycolysis to generate ATP anaerobically.
In proliferating cells, low ATP/ADP ratios are necessary to maintain enhanced glycolysis, which can only occur if mitochondrial metabolism is suppressed or altered. Although considerable research has been devoted to understanding the upregulation of genes and enzymes involved in the glycolytic pathway of cancer cells, much less is known about the basis for mitochondrial metabolic changes in the Warburg effect. Moreover, the ATP/ADP ratio has not been considered a key component in the Warburg metabolism of proliferating cells.
Here, we review evidence that relative closure of VDAC in cancer cells limits access of respiratory substrates and ADP to the matrix, thus decreasing ATP synthesis. We also discuss recent findings that ANT in cancer cells does not exchange ATP for ADP as occurs in non-proliferating cells. Rather, ATP appears to move non-electrogenically via another exchanger, possibly the ATP-Mg/Pi carrier (Joyal and Aprille, 1992;Fiermonte, et al., 2004;Palmieri, 2012). Suppressed mitochondrial function by VDAC closure combined with non-electrogenic ATP-ADP exchange acts to maintain a lower ATP/ADP ratio that is stimulatory of glycolysis. Thus, changes of activity of both VDAC and ANT contribute the aerobic glycolytic Warburg metabolic phenotype of cancer cells.
Energy conversion in non-proliferating cells
In non-proliferating cells, most ATP is formed in the mitochondrial matrix, a highly regulated microenvironment limited by the mitochondrial inner membrane and separated from the cytosol. Mitochondria oxidize respiratory substrates and produce ATP in response to the demand imposed by ATP-consuming reactions in the cytosol. Hence, mitochondria couple an input and an output. The input comprises oxidizable substrates (pyruvate, glutamine, fatty acids and other respiratory substrates), O2, ADP and Pi. Since O2 is the final electron acceptor of the respiratory chain, O2 consumption inhibitable by respiratory chain inhibitors (e.g., cyanide, myxothiazol, rotenone) is a quantitative measurement of mitochondrial respiration in intact cells. In vertebrates, blood flow through a closed circulation fine tunes delivery of O2 and nutrients to match dynamically the energy and metabolic demands of tissues.
The output of mitochondrial energy conversion is ATP, and the free energy made available by ATP hydrolysis to ADP and Pi is the phosphorylation potential (ΔGp = ΔGP°’ + RT ln([ATP]/[ADP][Pi]), where ΔGP° is the standard free energy change of ATP hydrolysis). Since ΔΨ drives mitochondrial ATP-ADP exchange via the electrogenic ANT and ΔpH drives OH−-Pi exchange by the PT, ΔGP in the cytosol becomes amplified relative to the mitochondrial matrix by up to the energetic equivalent of Δp (15-20 kJ/mol). By generating a 33% greater energetic punch to ATP hydrolysis, such ΔGP amplification represents an important advantage of mitochondrial metabolism compared to that of prokaryotes (Lemasters, 1981). This higher ΔGP also exerts a strong brake on glycolysis.
Energy conversion in cancer cells
The ATP yield per mole of glucose is much lower for glycolysis compared to mitochondrial oxidative phosphorylation (2 moles of ATP vs. ~35 moles of ATP per mole of glucose, respectively). The lower efficiency of ATP generation by glycolysis is offset in cancer cells by increased expression of enzymes involved in the glucose catabolism. Although Warburg proposed that mitochondria are damaged in cancer cells and even that mitochondrial damage might be the origin of cancer (Warburg, 1956), numerous studies document that mitochondria isolated from tumor cells can catalyze oxidative phosphorylation effectively, as assessed by ΔΨ formation, respiratory control ratios and activity of respiratory chain components (Nakashima, et al., 1984;Mathupala, et al., 2010a;Singleterry, et al., 2014). Mitochondria also contribute to some extent to ATP generation in cancer cells with the relative contribution differing between cell lines but being consistently much lower than in non-proliferating cells (Griguer, et al., 2005;Moreno-Sanchez, et al., 2007;Zu and Guppy, 2004). Overall, glycolysis contributes to 50 to 70% of total ATP production in cancer cells with the remainder contributed by mitochondrial oxidation of pyruvate, glutamine and fatty acids (DeBerardinis, et al., 2008;Vander Heiden, et al., 2009; Mathupala, et al., 2010).
Voltage dependent anion channel and Warburg metabolism
Metabolites that enter and leave mitochondria for oxidative phosphorylation and other matrix reactions must cross both mitochondrial membranes. Non-polar compounds like oxygen and short chain fatty acids are bilayer-permeant and cross both mitochondrial membranes by diffusion. For polar metabolites, numerous specific transporters in the inner membrane facilitate transport into and out of the matrix space. By contrast, movement of polar metabolites across the outer membrane occurs through one common channel, VDAC.
VDAC, first discovered from Paramecium aurelia and found in all eukaryotic cells, is the most abundant protein in the mitochondrial outer membrane (Sampson, et al., 1997). VDAC in humans and mice comprises three isoforms, VDAC1, VDAC2 and VDAC3, with a molecular mass of approximately 30 kDa and a high degree of sequence homology (Blachly-Dyson and Forte, 2001;Colombini, 2004). VDAC1 and VDAC2 are the most abundant isoforms in most tissues and tumors, except for testis where VDAC3 is most abundant (Sampson, et al., 2001).
As determined by NMR and X-ray crystallography, VDAC1 forms barrels in the lipid bilayer comprised of 19 beta strands, but this non-native structure is disputed by a model suggesting that functional VDAC forms only 13 beta-strands (Bayrhuber, et al., 2008;Colombini, 2009;Hiller, et al., 2008;Ujwal, et al., 2008). Recently, the structure of VDAC2 was resolved showing a similar 19-strand beta barrel (Schredelseker, et al., 2014). The wall of the beta barrel of about 1 nm in thickness surrounds an aqueous channel with an internal diameter or 2.5 nm in the open state and about 1.8 nm in the closed state. An N-terminal alpha-helix lies inside the pore parallel to the membrane plane, which is important for regulation of the flux of metabolites through the channel (Choudhary, et al., 2010;Mannella, 1998;Teijido, et al., 2012). In the open state, solutes up to ~5 kDA can permeate freely through VDAC (Colombini, 1980;Colombini, et al., 1987). In the closed state, most anionic metabolites, including respiratory substrates, creatine phosphate, adenine nucleotides and Pi, cannot cross through VDAC, although small ions like K+, Na+, Ca+2 and Cl− remain permeant (Tan and Colombini, 2007). Since VDAC is the only channel allowing flux of metabolites through the mitochondrial outer membrane, its conductance can control mitochondrial metabolism globally and modulate ATP delivery to the cytosol (Lemasters and Holmuhamedov, 2006). Thus, VDAC opening and closing correspondingly increase and decrease mitochondrial energy conversion. In this way, relative closure of VDAC limits mitochondrial oxidative phosphorylation and lowers cytosolic ATP/ADP ratios to favor the aerobic glycolysis of the Warburg phenomenon, which is the metabolic signature of both normal proliferating cells and malignant cells.
VDAC is gated by voltage with half maximal closure at ±50 mV. Whether ΔΨ closes VDAC in intact cells is not clear. A report of a ΔpH across the outer membrane implies a Donnan potential of ~40 mV, which might be enough to gate VDAC (Porcelli, et al., 2005). Donnan potentials depend on the asymmetrical distribution of non-permeant charged molecules, mainly proteins, and the magnitude of any Donnan potential forming is controversial because charged macromolecules reside on both sides of the outer membrane. Other factors also regulate VDAC conductance, including glutamate, protein kinase A, glycogen synthase 3β, hexokinase II, NADH, acetaldehyde, bcl2 family members, ethanol and free tubulin (Azoulay-Zohar, et al., 2004;Das, et al., 2008;Gincel, et al., 2000;Lee, et al., 1994;Rostovtseva, et al., 2008;Vander Heiden, et al., 2000;Vander Heiden, et al., 2001; Holmuhamedov, et al., 2012;Lemasters, et al., 2012).
Voltage dependent anion channel and mitochondrial metabolism in tumor cells
Mitochondrial ΔΨ is an indicator of mitochondrial metabolism both in proliferating and non-proliferating cells. ΔΨ formation depends on respiration or, alternatively, can be supported by hydrolysis of ATP by the mitochondrial F1FO-ATP synthase acting in reverse (Maldonado, et al., 2010). For example during anoxia, ischemia or respiratory inhibition, ΔΨ can be maintained as long as glycolysis can provide ATP (Nieminen, et al., 1994;Zhang and Lemasters, 2013). To collapse mitochondrial ΔΨ, respiration and ATP supply to mitochondria must be inhibited simultaneously, as with myxothiazol (Complex III respiratory inhibitor) and oligomycin (ATP synthase inhibitor) (Maldonado, et al., 2010).
Free tubulin is an important endogenous regulator of VDAC that induces VDAC closure both in VDAC inserted into lipid bilayers and in isolated mitochondria (Rostovtseva, et al., 2008). In cancer cells such as HepG2 human hepatoma cells, free to polymerized tubulin ratios are high compared to non-transformed hepatocytes. Ratios of free tubulin to polymerized tubulin can be manipulated experimentally with microtubule destabilizers (colchicine, nocodazole) and stabilizers (paclitaxel) to show that mitochondrial ΔΨ in tumor cells increases and decreases as free tubulin decreases and increases. By contrast in hepatocytes with already low free tubulin, microtubule stabilization does not increase ΔΨ, whereas microtubule destabilization to increase free tubulin causes a decline of ΔΨ. Overall, ΔΨ inversely correlates with free to polymerized tubulin ratios. These findings support the conclusion that inhibition of VDAC conductance by free tubulin suppresses ΔΨ formation in intact tumor cells. In non-transformed cells with low free tubulin, such as hepatocytes but presumably other highly aerobic cell types as well, VDAC is constitutively open but may be closed by increased free tubulin (Maldonado, et al., 2010). Tubulin control of VDAC conductance is further regulated by protein kinases. For example, protein kinase A agonists promote VDAC closure and a decrease of ΔΨ, whereas antagonists decrease tubulin-dependent VDAC closure and the depolarizing effects of high tubulin in intact cells (Maldonado, et al., 2010;Sheldon, et al., 2011).
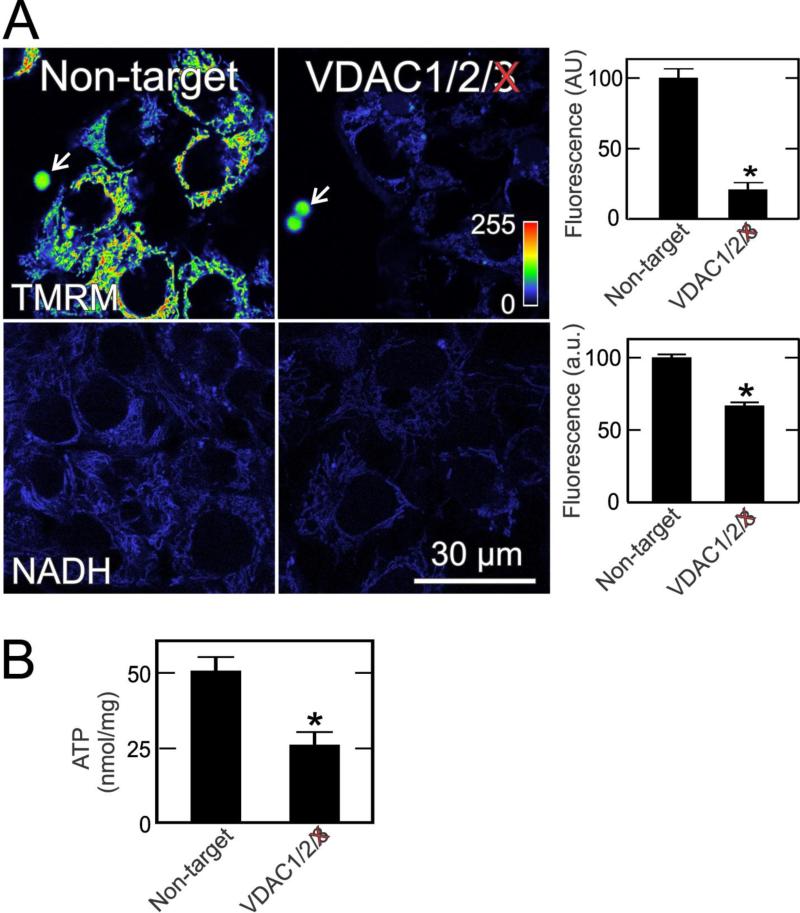
Importantly, VDAC conductance is not completely shut down in tumor cells. Single and double siRNA knockdowns of the three VDAC isoforms in all possible combinations reveal that all VDAC isoforms contribute to maintenance of mitochondrial ΔΨ but that VDAC3 contributes to the greatest extent. As shown in Fig. 2, VDAC3 knockdown decreases uptake of the ΔΨ-indicating fluorophore, tetramethylrhodamine methylester (TMRM), by ~80%. VDAC3 knockdown also decreases by a third the blue autofluorescence of mitochondrial NAD(P)H, a measure of respiratory substrate delivery to mitochondria. Total cellular ATP also decreases by half after VDAC3 knockdown (Fig. 2). Because ADP increases after VDAC3 knockdown (not shown), ATP/ADP decreases even more. These findings illustrate the importance of VDAC, especially VDAC3, in modulating mitochondrial bioenergetics status (Maldonado, et al., 2013).
Fig 2. VDAC3 knockdown decreases mitochondrial membrane potential, NADH and ATP in HepG2 cells.
In A, HepG2 hepatoma cells were transfected with non-target siRNA and siRNA against VDAC3. After 48 h, ΔΨ-indicating TMRM and mitochondrial NAD(P)H-indicating blue autofluorescence were imaged by confocal and multiphoton microscopy, respectively. Note the decrease of TMRM fluorescence and autofluorescence after VDAC3 knockdown, which is quantified in the right panels. Arrows identify 4-μm fiduciary fluorescent beads. In B, total cellular ATP is shown under the same conditions. *, p<0.05. Adapted from (Maldonado, et al., 2013).
When specific VDAC isoforms are inserted into planar lipid bilayers, free tubulin inhibits conductance of VDAC1 and VDAC2 but not that of VDAC3 (Maldonado, et al., 2013). This observation accounts for why in tumor cells VDAC3 is most important of the three isoforms for ΔΨ formation, because high free tubulin inhibits VDAC1 and VDAC2 but not VDAC3. Although VDAC3 is the least abundant isoform, its resistance to inhibition by tubulin makes VDAC3 the most important isoform for ΔΨ formation and other indices of mitochondrial function in unperturbed tumor cells. Nonetheless when tubulin decreases, VDAC1 and VDAC2 conductance increases to upregulate ΔΨ and other aspects of mitochondrial metabolism. Interestingly, erastin, a compound that interacts with VDAC (Yagoda, et al., 2007), blocks and reverses mitochondrial depolarization after microtubule destabilizers in intact cells. This effect is accounted for by the fact that erastin antagonizes tubulin-induced VDAC blockage in planar bilayers. Overall, free tubulin inhibits VDAC1/2 and limits mitochondrial metabolism in tumor cells, thus decreasing ATP/ADP and contributing to the Warburg phenomenon (Fig. 1). Reversal of tubulin-dependent VDAC inhibition by erastin antagonizes Warburg metabolism and restores non-Warburg oxidative mitochondrial metabolism (Maldonado, et al., 2013).
An intriguing question concerns the broader, biologic role of tubulin-dependent inhibition of VDAC. In yeast, aerobic glycolysis supports a higher rate of cell proliferation than aerobic oxidative phosphorylation. Moreover, rapidly dividing cells must maintain a free tubulin reserve for spindle formation at metaphase. Thus, high free tubulin is characteristic of cell proliferation. Although Warburg-type aerobic glycolysis fosters greater proliferation and biomass formation, at mitosis the energy-demanding events of chromosome separation and cytokinesis create an acute need for ATP. As the spindle apparatus forms in prophase, microtubules assemble and free tubulin decreases. Consequently, VDAC inhibition by tubulin is relieved, which turns on aerobic ATP-generating mitochondrial metabolism. In this way, rapidly proliferating cells may transiently suspend Warburg-type aerobic glycolysis in favor of oxidative phosphorylation to meet the uniquely high bioenergetic demands of cells at metaphase. Afterwards, as microtubules of the spindle apparatus depolymerize during telophase, free tubulin again increases and Warburg metabolism becomes restored (Lemasters, et al., 2012).
Adenine nucleotide translocator
Approximately 20 carriers mediate flux of anionic metabolites through the mitochondrial inner membrane whose activities are well characterized in isolated mitochondria and/or reconstituted liposomes (Palmieri, 2012). Most carriers belong to the SLC25 family of nuclear-encoded transporters, also known as the mitochondrial carrier family (Walker and Runswick, 1993). ADP/ATP transport through the inner membrane is catalyzed by ANT, the most abundant inner membrane protein on a molar basis accounting for about 10% of the total. Exchange of ADP and ATP between the matrix and intermembrane space is highly selective and occurs on a 1:1 molar ratio that maintains the adenine nucleotide pool in the matrix constant. ANT translocates specifically free ADP−3 and ATP−4. AMP and Mg+2 complexes of ADP and ATP are not transported by ANT (Klingenberg, 1989;Klingenberg, 2008).
ANT is an electrogenic transporter that exchanges ADP−3 for ATP−4. As a consequence during each cycle of ATP release and ADP uptake, one negative charge is expelled from the matrix to the cytosol. Thus, the negative mitochondrial ΔΨ drives ATP release and ADP uptake such that ATP/ADP and ΔGp are greater in the cytosol than the matrix (Kawamata, et al., 2010;Klingenberg, 2008). In humans, ANT has 4 isoforms, ANT1 through ANT4, that are encoded by different nuclear genes (Palmieri, 2012). The tissue distribution of the isoforms differs from tissue to tissue: ANT1 is expressed in skeletal muscles, heart and brain, ANT2 is expressed mainly in liver and in proliferating tissues, and ANT3 is ubiquitous and expressed at low levels (Stepien, et al., 1992;Chevrollier, et al., 2005). ANT4, recently discovered in humans, is found mainly in liver, testis and brain (Dolce, et al., 2005).
ANT2 expressed in proliferating cells, such as lymphocytes and tumor cells, is considered a marker of cell proliferation, and most non proliferating tissues with the exception of liver have low or very low expression of ANT2 (Barath, et al., 1999;Battini, et al., 1987). In tumor cell lines originating from colon (HT29), breast (MCF7) and liver (HepG2), ANT2 mRNA is more abundant than ANT1 (Giraud, et al., 1998). Increased ANT2 expression is also reported for cancers of the bladder, thyroid gland, lung, ovary, breast and testis (Le, et al., 2006).
In yeast, two isoforms of ANT, AAC1 and AAC2, are expressed only under aerobic conditions, whereas AAC3, the equivalent of ANT2 in mammalian cells, is expressed exclusively during anaerobiosis (Kolarov, et al., 1990;Lawson and Douglas, 1988). AAC3 is essential to maintain yeast cell proliferation on a fermentable substrate, which led to the hypothesis that the AAC3 isoform imports glycolytic ATP into mitochondria to support the anabolic functions of mitochondria (Drgon, et al., 1991). In mice, ANT2 deficiency is embryonically lethal, whereas ANT1 disruption causes mitochondrial myopathy in viable offspring (Kokoszka, et al., 2004).
Adenine nucleotide translocator and mitochondrial membrane potential
Carboxyatractyloside and bongkrekic acid inhibit mitochondrial ATP/ADP exchange by binding respectively to different inhibitory sites on the intermembranous and matrix sides of ANT. Both ANT inhibitors block ADP-stimulated respiration in isolated mitochondria similarly to oligomycin, the F1FO-ATP synthase inhibitor. In intact rat hepatocytes, respiratory inhibition by myxothiazol only slightly decreases ΔΨ, measured by TMRM uptake. Maintenance of ΔΨ despite respiratory inhibition is due to ATP hydrolysis by the F1FO-ATP synthase working in reverse, since subsequent oligomycin addition leads to complete collapse of ΔΨ. Similarly, carboxyatractyloside and bongkrekic acid collapse ΔΨ in myxothiazol-treated hepatocytes, signifying that cytosolic ATP enters mitochondria by ANT to be hydrolyzed by the F1FO-ATP synthase (Maldonado et al., 2013).
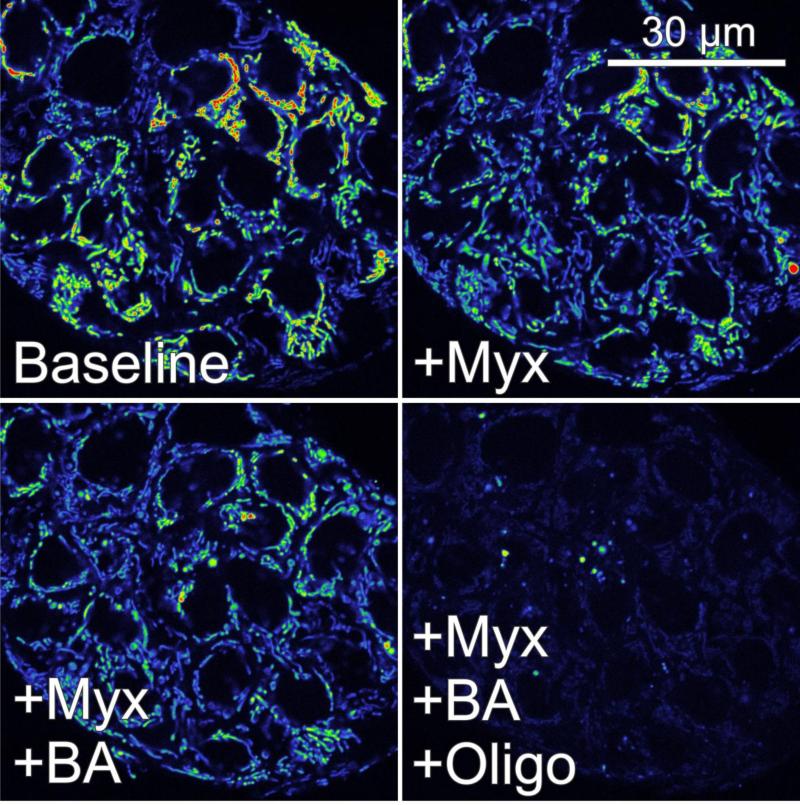
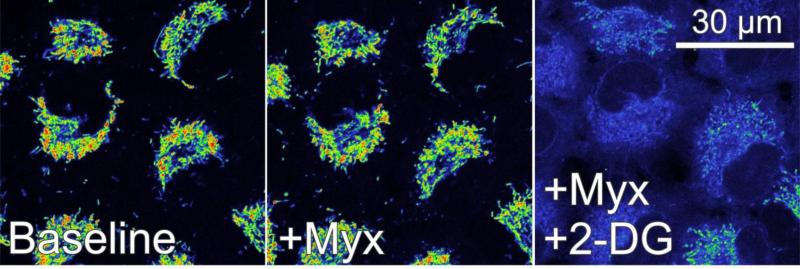
In HepG2 cells and A549 human lung cancer cells, myxothiazol also slightly decreases ΔΨ, and subsequent oligomycin collapses ΔΨ (Fig. 3 and 4, and not shown). In marked contrast to hepatocytes, however, carboxyatractyloside and bongkrekic acid fail to collapse ΔΨ (Fig. 3 and not shown). Nonetheless, subsequent oligomycin does lead to full depolarization (Fig. 3). Similarly, 2-deoxyglucose, a glycolytic inhibitor, added after myxothiazol alone, myxothiazol plus carboxyatractyloside or myxothiazol plus bongkrekic acid leads to ΔΨ collapse (Fig. 4 and not shown). These results show that although mitochondrial hydrolysis of glycolytic ATP can maintain ΔΨ, entry of glycolytic ATP into mitochondria occurs by a pathway other than ANT, possibly through the ATP-Mg/Pi carrier (Joyal and Aprille, 1992;Palmieri, 2012).
Fig. 3. ANT-independent ATP hydrolysis sustains mitochondrial membrane potential in HepG2 hepatoma cells after respiratory inhibition.
HepG2 cells were loaded with TMRM, as described in Fig. 2. Note a small of decrease of TMRM fluorescence after myxothiazol (Myx, 10 μM). Persisting mitochondrial TMRM uptake indicates that ATP hydrolysis supports ΔΨ formation during respiratory inhibition (compare right and left upper panels). Subsequent bongkrekic acid (BA, 5 μM), an ANT inhibitor, does not further decrease ΔΨ, indicating that ATP supply to mitochondria is independent of ANT (bottom left panel). Subsequent oligomycin (Oligo, 10 μg/ml) collapses ΔΨ, confirming that ANT-independent ATP entry into mitochondria maintains ΔΨ after respiratory inhibition (right bottom panel). Additions are 30 min apart. Adapted from (Maldonado, et al., 2013).
Fig. 4. Glycolytic ATP supports mitochondrial membrane potential after respiratory inhibition in A549 cells.
A549 lung cancer cells were loaded with TMRM, as described in Fig. 2. Note that myxothiazol (Myx) slightly decreases TMRM fluorescence similarly to HepG2 cells (compare left and center panel). Subsequent 2-deoxyglucose (2-DG, 50 mM), a glycolytic inhibitor, collapses ΔΨ virtually completely, indicating that mitochondrial hydrolysis of glycolytic ATP supports mitochondrial ΔΨ formation after respiratory inhibition.
A similar pattern is observed from measurements of oxygen uptake. In hepatocytes, oligomycin, carboxyatractyloside and bongkrekic acid each decrease respiration to a comparable extent, signifying inhibition of respiration-linked mitochondrial ATP synthesis and the release of such ATP to the cytosol. By contrast in tumor cell lines, carboxyatractyloside and bongkrekic acid do not inhibit respiration, unlike oligomycin which does. Thus, although ANT2 is abundant in cancer cells, it is inactive and not the principal ATP transporter responsible for mitochondrial uptake of glycolytic ATP required to maintain mitochondrial ΔΨ after respiratory inhibition (Maldonado, et al., 2009;Maldonado, et al, 2013b). Nonetheless, ATP does gain entry into tumor mitochondria by a yet unidentified alternative carrier, possibly via the electroneutral ATP-Mg/Pi carrier (Joyal and Aprille, 1992;Fiermonte, et al., 2004). Overall, the substitution of an electrogenic pathway of ATP/ADP exchange (ANT) for a non-electrogenic pathway (possibly the ATP-Mg/Pi carrier) would mean loss of ΔGP amplification. The resultant lower cytosolic ATP/ADP ratios then favor Warburg-type aerobic glycolysis (Fig. 1).
Conclusion
The ATP/ADP ratio has been neglected as an important regulator of glycolysis in cancer cells despite the importance of ATP/ADP in the control of both glycolysis and oxidative phosphorylation in non-transformed cells. Enhanced aerobic glycolysis confers an anabolic advantage for proliferating cells but can only be sustained if cytosolic ATP/ADP ratios are lower than in non-proliferating cells. Beyond upregulation of genes and enzymes of the glycolytic pathway, mitochondrial ATP production is likely a major factor controlling glycolysis. High ATP/ADP ratios in aerobic non-proliferating cells block glycolysis even in the presence of increased expression of transporters and enzymes involved in glycolysis. In this topical review, we describe two novel mechanisms that contribute to mitochondrial suppression and low ATP/ADP ratios in proliferating cells. The first mechanism is the relative closure of VDAC by the high free tubulin levels characteristic of proliferating cells. Such VDAC closure exerts a global suppression of mitochondrial metabolism. The second mechanism is the loss of function of electrogenic ANT2 and its apparent replacement by a non-electrogenic ATP-ADP exchange pathway, the ATP-Pi/Mg carrier (Fig. 1). The roles of VDAC and ANT in producing Warburg metabolism in cancer cells makes these two proteins potential targets for the development of a new generation of anti-Warburg anti-cancer drugs.
HIGHLIGHTS.
VDAC mediates flux of metabolites across the mitochondrial outer membrane
Electrogenic ANT exchanges matrix ATP for cytosolic ADP across the inner membrane
High cytosolic ATP/ADP generated by oxidative phosphorylation inhibits glycolysis
VDAC closure and ANT inactivation lead to low ATP/ADP in proliferating cells
In cancer cells, low ATP/ADP favors the aerobic glycolytic Warburg phenotype
Acknowledgment
This work was supported, in part, by an American Cancer Society Institutional Research Pilot Program Grant (University of South Carolina) to ENM and by Grants CA138313, DK037034, DK073336 and AA022815 from the National Institutes of Health and Grant 14.Z50.31.0028 from the Ministry of Education and Science of the Russian Federation to JJL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azoulay-Zohar H, Israelson A, Abu-Hamad S, Shoshan-Barmatz V. In self-defence: hexokinase promotes voltage-dependent anion channel closure and prevents mitochondria-mediated apoptotic cell death. Biochem J. 2004;377:347–355. doi: 10.1042/BJ20031465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barath P, Luciakova K, Hodny Z, Li R, Nelson BD. The growth-dependent expression of the adenine nucleotide translocase-2 (ANT2) gene is regulated at the level of transcription and is a marker of cell proliferation. Exp Cell Res. 1999;248:583–588. doi: 10.1006/excr.1999.4432. [DOI] [PubMed] [Google Scholar]
- Battini R, Ferrari S, Kaczmarek L, Calabretta B, Chen ST, Baserga R. Molecular cloning of a cDNA for a human ADP/ATP carrier which is growth-regulated. J Biol Chem. 1987;262:4355–4359. [PubMed] [Google Scholar]
- Bayrhuber M, Meins T, Habeck M, Becker S, Giller K, Villinger S, Vonrhein C, Griesinger C, Zweckstetter M, Zeth K. Structure of the human voltage-dependent anion channel. Proc Natl Acad Sci U S A. 2008;105:15370–15375. doi: 10.1073/pnas.0808115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachly-Dyson E, Forte M. VDAC channels. IUBMB Life. 2001;52:113–118. doi: 10.1080/15216540152845902. [DOI] [PubMed] [Google Scholar]
- Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacon E, Reece JM, Nieminen AL, Zahrebelski G, Herman B, Lemasters JJ. Distribution of electrical potential, pH, free Ca2+, and volume inside cultured adult rabbit cardiac myocytes during chemical hypoxia: a multiparameter digitized confocal microscopic study. Biophys J. 1994;66:942–952. doi: 10.1016/S0006-3495(94)80904-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrollier A, Loiseau D, Chabi B, Renier G, Douay O, Malthiery Y, Stepien G. ANT2 isoform required for cancer cell glycolysis. J Bioenerg Biomembr. 2005;37:307–316. doi: 10.1007/s10863-005-8642-5. [DOI] [PubMed] [Google Scholar]
- Choudhary OP, Ujwal R, Kowallis W, Coalson R, Abramson J, Grabe M. The electrostatics of VDAC: implications for selectivity and gating. J Mol Biol. 2010;396:580–592. doi: 10.1016/j.jmb.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombini M. Structure and mode of action of a voltage dependent anion-selective channel (VDAC) located in the outer mitochondrial membrane. Ann N Y Acad Sci. 1980;341:552–563. doi: 10.1111/j.1749-6632.1980.tb47198.x. [DOI] [PubMed] [Google Scholar]
- Colombini M. VDAC: the channel at the interface between mitochondria and the cytosol. Mol Cell Biochem 256. 2004;257:107–115. doi: 10.1023/b:mcbi.0000009862.17396.8d. [DOI] [PubMed] [Google Scholar]
- Colombini M. The published 3D structure of the VDAC channel: native or not? Trends Biochem Sci. 2009;34:382–389. doi: 10.1016/j.tibs.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Colombini M, Yeung CL, Tung J, Konig T. The mitochondrial outer membrane channel, VDAC, is regulated by a synthetic polyanion. Biochim Biophys Acta. 1987;905:279–286. doi: 10.1016/0005-2736(87)90456-1. [DOI] [PubMed] [Google Scholar]
- Das S, Wong R, Rajapakse N, Murphy E, Steenbergen C. Glycogen synthase kinase 3 inhibition slows mitochondrial adenine nucleotide transport and regulates voltage-dependent anion channel phosphorylation. Circ Res. 2008;103:983–991. doi: 10.1161/CIRCRESAHA.108.178970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: metabolism and tumor cell growth. Curr Opin Genet Dev. 2008;18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolce V, Scarcia P, Iacopetta D, Palmieri F. A fourth ADP/ATP carrier isoform in man: identification, bacterial expression, functional characterization and tissue distribution. FEBS Lett. 2005;579:633–637. doi: 10.1016/j.febslet.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Drgon T, Sabova L, Nelson N, Kolarov J. ADP/ATP translocator is essential only for anaerobic growth of yeast Saccharomyces cerevisiae. FEBS Lett. 1991;289:159–162. doi: 10.1016/0014-5793(91)81059-h. [DOI] [PubMed] [Google Scholar]
- Emaus RK, Grunwald R, Lemasters JJ. Rhodamine 123 as a probe of transmembrane potential in isolated rat-liver mitochondria: spectral and metabolic properties. Biochim Biophys Acta. 1986;850:436–448. doi: 10.1016/0005-2728(86)90112-x. [DOI] [PubMed] [Google Scholar]
- Fiermonte G, De LF, Todisco S, Palmieri L, Lasorsa FM, Palmieri F. Identification of the mitochondrial ATP-Mg/Pi transporter. Bacterial expression, reconstitution, functional characterization, and tissue distribution. J Biol Chem. 2004;279:30722–30730. doi: 10.1074/jbc.M400445200. [DOI] [PubMed] [Google Scholar]
- Galdieri L, Mehrotra S, Yu S, Vancura A. Transcriptional regulation in yeast during diauxic shift and stationary phase. OMICS. 2010;14:629–638. doi: 10.1089/omi.2010.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gincel D, Silberberg SD, Shoshan-Barmatz V. Modulation of the voltage-dependent anion channel (VDAC) by glutamate. J Bioenerg Biomembr. 2000;32:571–583. doi: 10.1023/a:1005670527340. [DOI] [PubMed] [Google Scholar]
- Giraud S, Bonod-Bidaud C, Wesolowski-Louvel M, Stepien G. Expression of human ANT2 gene in highly proliferative cells: GRBOX, a new transcriptional element, is involved in the regulation of glycolytic ATP import into mitochondria. J Mol Biol. 1998;281:409–418. doi: 10.1006/jmbi.1998.1955. [DOI] [PubMed] [Google Scholar]
- Griguer CE, Oliva CR, Gillespie GY. Glucose metabolism heterogeneity in human and mouse malignant glioma cell lines. J Neurooncol. 2005;74:123–133. doi: 10.1007/s11060-004-6404-6. [DOI] [PubMed] [Google Scholar]
- Harvey AJ, Kind KL, Thompson JG. REDOX regulation of early embryo development. Reproduction. 2002;123:479–486. doi: 10.1530/rep.0.1230479. [DOI] [PubMed] [Google Scholar]
- Hers HG, Van SE. Fructose 2,6-bisphosphate 2 years after its discovery. Biochem J. 1982;206:1–12. doi: 10.1042/bj2060001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller S, Garces RG, Malia TJ, Orekhov VY, Colombini M, Wagner G. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science. 2008;321:1206–1210. doi: 10.1126/science.1161302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek JB, Nicholls DG, Williamson JR. Determination of the mitochondrial protonmotive force in isolated hepatocytes. J Biol Chem. 1980;255:1458–1464. [PubMed] [Google Scholar]
- Holmuhamedov EL, Czerny C, Beeson CC, Lemasters JJ. Ethanol Suppresses Ureagenesis in Rat Hepatocytes: role of acetaldehyde. J Biol Chem. 2012;287:7692–7700. doi: 10.1074/jbc.M111.293399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyal JL, Aprille JR. The ATP-Mg/Pi carrier of rat liver mitochondria catalyzes a divalent electroneutral exchange. J Biol Chem. 1992;267:19198–19203. [PubMed] [Google Scholar]
- Kawamata H, Starkov AA, Manfredi G, Chinopoulos C. A kinetic assay of mitochondrial ADP-ATP exchange rate in permeabilized cells. Anal Biochem. 2010;407:52–57. doi: 10.1016/j.ab.2010.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg M. Molecular aspects of the adenine nucleotide carrier from mitochondria. Arch Biochem Biophys. 1989;270:1–14. doi: 10.1016/0003-9861(89)90001-5. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. The ADP and ATP transport in mitochondria and its carrier. Biochim Biophys Acta. 2008;1778:1978–2021. doi: 10.1016/j.bbamem.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolarov J, Kolarova N, Nelson N. A third ADP/ATP translocator gene in yeast. J Biol Chem. 1990;265:12711–12716. [PubMed] [Google Scholar]
- Lawson JE, Douglas MG. Separate genes encode functionally equivalent ADP/ATP carrier proteins in Saccharomyces cerevisiae. Isolation and analysis of AAC2. J Biol Chem. 1988;263:14812–14818. [PubMed] [Google Scholar]
- Le BM, Borgne-Sanchez A, Touat Z, El Dein OS, Deniaud A, Maillier E, Lecellier G, Rebouillat D, Lemaire C, Kroemer G, Jacotot E, Brenner C. Chemosensitization by knockdown of adenine nucleotide translocase-2. Cancer Res. 2006;66:9143–9152. doi: 10.1158/0008-5472.CAN-05-4407. [DOI] [PubMed] [Google Scholar]
- Lee AC, Zizi M, Colombini M. Beta-NADH decreases the permeability of the mitochondrial outer membrane to ADP by a factor of 6. J Biol Chem. 1994;269:30974–30980. [PubMed] [Google Scholar]
- Lemasters JJ. Phosphate potential amplification. Trends Biochem Sci. 1981;6:X. [Google Scholar]
- Lemasters JJ, Holmuhamedov E. Voltage-dependent anion channel (VDAC) as mitochondrial governator--thinking outside the box. Biochim Biophys Acta. 2006;1762:181–190. doi: 10.1016/j.bbadis.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Lemasters JJ, Holmuhamedov EL, Czerny C, Zhong Z, Maldonado EN. Regulation of mitochondrial function by voltage dependent anion channels in ethanol metabolism and the Warburg effect. Biochim Biophys Acta. 2012;1818:1536–1544. doi: 10.1016/j.bbamem.2011.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado EN, Patnaik J, Mullins MR, Lemasters JJ. Free tubulin modulates mitochondrial membrane potential in cancer cells. Cancer Res. 2010;70:10192–10201. doi: 10.1158/0008-5472.CAN-10-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado EN, Sheldon KL, DeHart DN, Patnaik J, Manevich Y, Townsend DM, Bezrukov SM, Rostovtseva TK, Lemasters JJ. Voltage-dependent anion channels modulate mitochondrial metabolism in cancer cells: regulation by free tubulin and erastin. J Biol Chem. 2013a;288:11920–11929. doi: 10.1074/jbc.M112.433847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado EN, Czerny C, Beeson CC, Lemasters JJ. Assessment of respiration-dependent intra- and extramitochondrial ATP turnover: HepG2 cancer cells do not utilize ATP from oxidative phosphorylation in the cytosol. Biophys J. 2009:245a. [Google Scholar]
- Maldonado EN, Vuicich J, DeHart DN, Rodebaugh HS, Lemasters JJ. Translocation of glycolytic ATP into mitochondria of cancer cells does not utilize the ad-enine nucleotide transporter. Biophys J. 2013b;104:303a–304a. [Google Scholar]
- Mannella CA. Conformational changes in the mitochondrial channel protein, VDAC, and their functional implications. J Struct Biol. 1998;121:207–218. doi: 10.1006/jsbi.1997.3954. [DOI] [PubMed] [Google Scholar]
- Mathupala SP, Ko YH, Pedersen PL. The pivotal roles of mitochondria in cancer: Warburg and beyond and encouraging prospects for effective therapies. Biochim Biophys Acta. 2010;1797:1225–1230. doi: 10.1016/j.bbabio.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. 1966. Biochim Biophys Acta. 2011;1807:1507–1538. doi: 10.1016/j.bbabio.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Mor I, Cheung EC, Vousden KH. Control of glycolysis through regulation of PFK1: old friends and recent additions. Cold Spring Harb Symp Quant Biol. 2011;76:211–216. doi: 10.1101/sqb.2011.76.010868. [DOI] [PubMed] [Google Scholar]
- Moreno-Sanchez R, Rodriguez-Enriquez S, Marin-Hernandez A, Saavedra E. Energy metabolism in tumor cells. FEBS J. 2007;274:1393–1418. doi: 10.1111/j.1742-4658.2007.05686.x. [DOI] [PubMed] [Google Scholar]
- Nakashima RA, Paggi MG, Pedersen PL. Contributions of glycolysis and oxidative phosphorylation to adenosine 5′-triphosphate production in AS-30D hepatoma cells. Cancer Res. 1984;44:5702–5706. [PubMed] [Google Scholar]
- Nicholls DG, Ferguson SJ. Bioenergetics 4. Elsevier; London: 2013. [Google Scholar]
- Nieminen AL, Saylor AK, Herman B, Lemasters JJ. ATP depletion rather than mitochondrial depolarization mediates hepatocyte killing after metabolic inhibition. Am J Physiol. 1994;267:C67–C74. doi: 10.1152/ajpcell.1994.267.1.C67. [DOI] [PubMed] [Google Scholar]
- Palmieri F. The mitochondrial transporter family SLC25: Identification, properties and physiopathology. Mol Aspects Med. 2012 doi: 10.1016/j.mam.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Petit PX, O'Connor JE, Grunwald D, Brown SC. Analysis of the membrane potential of rat- and mouse-liver mitochondria by flow cytometry and possible applications. Eur J Biochem. 1990;194:389–397. doi: 10.1111/j.1432-1033.1990.tb15632.x. [DOI] [PubMed] [Google Scholar]
- Porcelli AM, Ghelli A, Zanna C, Pinton P, Rizzuto R, Rugolo M. pH difference across the outer mitochondrial membrane measured with a green fluorescent protein mutant. Biochem Biophys Res Commun. 2005;326:799–804. doi: 10.1016/j.bbrc.2004.11.105. [DOI] [PubMed] [Google Scholar]
- Rostovtseva TK, Sheldon KL, Hassanzadeh E, Monge C, Saks V, Bezrukov SM, Sackett DL. Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proc Natl Acad Sci U S A. 2008;105:18746–18751. doi: 10.1073/pnas.0806303105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of transmembrane electrochemical proton gradients. J Bioenerg. 1975;7:61–74. doi: 10.1007/BF01558427. [DOI] [PubMed] [Google Scholar]
- Sampson MJ, Decker WK, Beaudet AL, Ruitenbeek W, Armstrong D, Hicks MJ, Craigen WJ. Immotile sperm and infertility in mice lacking mitochondrial voltage-dependent anion channel type 3. J Biol Chem. 2001;276:39206–39212. doi: 10.1074/jbc.M104724200. [DOI] [PubMed] [Google Scholar]
- Sampson MJ, Lovell RS, Craigen WJ. The murine voltage-dependent anion channel gene family. Conserved structure and function. J Biol Chem. 1997;272:18966–18973. doi: 10.1074/jbc.272.30.18966. [DOI] [PubMed] [Google Scholar]
- Santo-Domingo J, Demaurex N. Perspectives on: SGP symposium on mitochondrial physiology and medicine: the renaissance of mitochondrial pH. J Gen Physiol. 2012;139:415–423. doi: 10.1085/jgp.201110767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schredelseker J, Paz A, Lopez CJ, Altenbach C, Leung CS, Drexler MK, Chen JN, Hubbell WL, Abramson J. High resolution structure and double electron-electron resonance of the zebrafish voltage-dependent anion channel 2 reveal an oligomeric population. J Biol Chem. 2014;289:12566–12577. doi: 10.1074/jbc.M113.497438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon KL, Maldonado EN, Lemasters JJ, Rostovtseva TK, Bezrukov SM. Phosphorylation of voltage-dependent anion channel by serine/threonine kinases governs its interaction with tubulin. PLoS One. 2011;6:e25539. doi: 10.1371/journal.pone.0025539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleterry J, Sreedhar A, Zhao Y. Components of cancer metabolism and therapeutic interventions. Mitochondrion. 2014;17C:50–55. doi: 10.1016/j.mito.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepien G, Torroni A, Chung AB, Hodge JA, Wallace DC. Differential expression of adenine nucleotide translocator isoforms in mammalian tissues and during muscle cell differentiation. J Biol Chem. 1992;267:14592–14597. [PubMed] [Google Scholar]
- Tan W, Colombini M. VDAC closure increases calcium ion flux. Biochim Biophys Acta. 2007;1768:2510–2515. doi: 10.1016/j.bbamem.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijido O, Ujwal R, Hillerdal CO, Kullman L, Rostovtseva TK, Abramson J. Affixing N-terminal alpha-helix to the wall of the voltage-dependent anion channel does not prevent its voltage gating. J Biol Chem. 2012;287:11437–11445. doi: 10.1074/jbc.M111.314229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujwal R, Cascio D, Colletier JP, Faham S, Zhang J, Toro L, Ping P, Abramson J. The crystal structure of mouse VDAC1 at 2.3 A resolution reveals mechanistic insights into metabolite gating. Proc Natl Acad Sci U S A. 2008;105:17742–17747. doi: 10.1073/pnas.0809634105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Chandel NS, Li XX, Schumacker PT, Colombini M, Thompson CB. Outer mitochondrial membrane permeability can regulate coupled respiration and cell survival. Proc Natl Acad Sci U S A. 2000;97:4666–4671. doi: 10.1073/pnas.090082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Li XX, Gottleib E, Hill RB, Thompson CB, Colombini M. BclxL promotes the open configuration of the voltage-dependent anion channel and metabolite passage through the outer mitochondrial membrane. J Biol Chem. 2001;276:19414–19419. doi: 10.1074/jbc.M101590200. [DOI] [PubMed] [Google Scholar]
- Vivier MA, Lambrechts MG, Pretorius IS. Coregulation of starch degradation and dimorphism in the yeast Saccharomyces cerevisiae. Crit Rev Biochem Mol Biol. 1997;32:405–435. doi: 10.3109/10409239709082675. [DOI] [PubMed] [Google Scholar]
- Walker JE, Runswick MJ. The mitochondrial transport protein superfamily. J Bioenerg Biomembr. 1993;25:435–446. doi: 10.1007/BF01108401. [DOI] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Warburg O, Wind F, Negelein E. THE METABOLISM OF TUMORS IN THE BODY. J Gen Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagoda N, von RM, Zaganjor E, Bauer AJ, Yang WS, Fridman DJ, Wolpaw AJ, Smukste I, Peltier JM, Boniface JJ, Smith R, Lessnick SL, Sahasrabudhe S, Stockwell BR. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447:864–868. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahrebelski G, Nieminen AL, al Ghoul K, Qian T, Herman B, Lemasters JJ. Progression of subcellular changes during chemical hypoxia to cultured rat hepatocytes: a laser scanning confocal microscopic study. Hepatology. 1995;21:1361–1372. [PubMed] [Google Scholar]
- Zhang X, Lemasters JJ. Translocation of iron from lysosomes to mitochondria during ischemia predisposes to injury after reperfusion in rat hepatocytes. Free Radic Biol Med. 2013;63:243–253. doi: 10.1016/j.freeradbiomed.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu XL, Guppy M. Cancer metabolism: facts, fantasy, and fiction. Biochem Biophys Res Commun. 2004;313:459–465. doi: 10.1016/j.bbrc.2003.11.136. [DOI] [PubMed] [Google Scholar]