Abstract
Osteoarthritis is a chronic and painful disease of synovial joints. Chondrocytes, synovial cells and other cells in the joint can express and respond to cytokines and chemokines, and all of these molecules can also be detected in synovial fluid of patients with osteoarthritis. The presence of inflammatory cytokines in the osteoarthritic joint raises the question whether they may directly participate in pain generation by acting on innervating joint nociceptors. Here, we first provide a systematic discussion of the known proalgesic effects of cytokines and chemokines that have been detected in osteoarthritic joints, including TNF-α, IL-1, IL-6, IL-15, IL-10, and the chemokines, MCP-1 and fractalkine. Subsequently, we discuss what is known about their contribution to joint pain based on studies in animal models. Finally, we briefly discuss limited data available from clinical studies in human osteoarthritis.
Keywords: osteoarthritis, pain, chemokines, cytokines, animal models
1. Osteoarthritis, a Painful Joint Disease
Osteoarthritis (OA), the most prevalent form of arthritis, is a chronic and painful disease of synovial joints, most commonly the knees, hips, and hands. The prevalence of OA increases with age. OA is a leading cause of disability among older adults in the US [1] and worldwide [2]. Obesity and joint injuries are other major risk factors [3]. The most prominent symptom of OA is pain. Since effective therapies for OA and the associated joint pain are not available, this disease represents an enormous unmet medical need [4, 5].
OA pathology is characterized by progressive cellular and molecular changes in all joint tissues, including articular cartilage, subchondral bone, synovium, ligaments, and peri-articular muscles (Fig.1) [3]. OA was long considered a quintessential “degenerative” joint disease, where mechanical stresses and ageing precipitate cartilage breakdown and bone remodeling – ultimately leading to joint dysfunction. In recent years, however, evidence has been mounting for a critical contribution of inflammatory mechanisms to OA pathogenesis, with a particular role for synovitis [6]. Indeed, synovitis has been shown to correlate with structural progression of OA (cartilage degeneration and osteophyte formation) [7]. Importantly, several studies have demonstrated that pain and joint dysfunction are increased in association with synovitis [7]. In posttraumatic OA, which develops after joint injury and tends to affect younger and middle-aged adults [8], research in animal models and human subjects indicates that several inflammatory cytokines and related inflammatory mediators are elevated following joint injury [9].
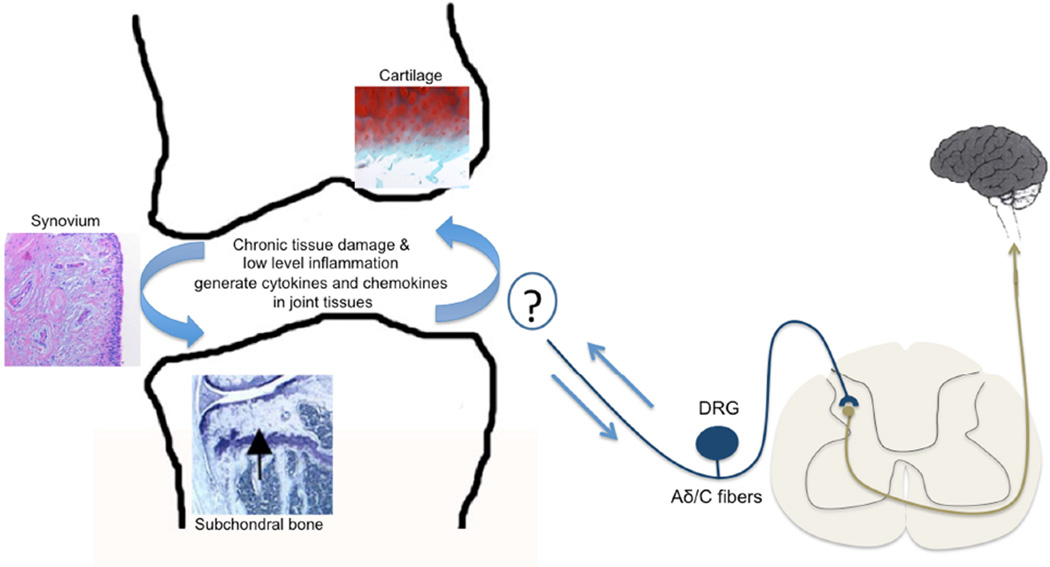
Figure 1.
Nociceptors (medium-sized myelinated Aδ fibers and small unmyelinated C-fibers) detect noxious signals in the innervated tissues and carry them to the dorsal horn of the spinal cord. The cell bodies of these pseudounipolar nociceptors are located in the dorsal root ganglia (DRG) and extend axons to the periphery as well as centrally to the dorsal horn, where the first synapse occurs. In osteoarthritis, all joint tissues participate in driving progressive structural joint damage, including the articular cartilage, subchondral bone, and the synovial membrane. Ongoing chronic tissue damage and low-level inflammation generates cytokines and chemokines that may directly act on innervating nociceptors. The precise nature of this interaction in the arthritic joint has not been elucidated Osteoarthritis is a chronic and painful disease of synovial joints. Osteoarthritic joint tissues produce and respond to cytokines and chemokines. Cytokines promote joint destruction and directly activate innervating nociceptors.
2. Cytokines in Osteoarthritis
Chondrocytes, synovial cells and other cells in the joint can express and respond to cytokines and chemokines, and cytokines can also be detected in synovial fluid of OA patients (reviewed in [10]). Cytokines that have been implicated in OA pathogenesis include tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-6, other common γ-chain cytokines such as IL-2, IL-7, IL-15, and IL-21, and chemokines (reviewed in [6, 10]). The presence of inflammatory cytokines in the OA joint raises the question whether they may directly participate in pain generation by acting on innervating joint nociceptors. In the current review, we first provide a systematic discussion of the known pro-algesic effects of these cytokines, and then discuss what is known about their contribution to joint pain, based on studies in animal models.
3. Nociceptive Actions Of Cytokines
Tissue injury generates noxious stimuli that are sensed by specialized receptors on nociceptors, which are small-diameter unmyelinated C-fiber or medium-diameter thinly myelinated Aδ-fiber sensory neurons that carry pain signals from peripheral tissues to the central nervous system (CNS) (Fig. 1). The cell bodies of these pseudo-unipolar nociceptors are located in the dorsal root ganglia (DRG) and trigeminal ganglia (TRG), and extend axons to the periphery and viscera as well as centrally to the dorsal horn of the spinal cord. The first synapse occurs in the dorsal horn either with interneurons or with supraspinally projecting neurons, which then carry nociceptive information to higher levels of the neuraxis such as the thalamus and cortex.
The major cytokines that are expressed in association with tissue damage and inflammation can affect the pain response at numerous points along the neuraxis. Many cytokines, for instance, can excite DRG neurons directly by a variety of mechanisms, often through very rapid effects that do not require gene transcription but are likely to involve regulation of important conductances such as voltage-gated sodium (VGS) and transient receptor potential (TRP) channels. Moreover, these same cytokines may also have rapid electrophysiological effects on second-order neurons in the dorsal horn. Molecules such as TNF-α, IL-1β, and IL-6 can be produced by cells in the joint, by sensory neurons or associated glial cells in the DRG, and by microglial cells in the dorsal horn. Whatever the cellular source of the cytokines produced in response to injury, increases in sensory neuron excitability are likely to be one of the first cytokine-induced effects that underlie chronic pain. Below, we review the major biological effects on the pain pathway by those cytokines that have been implicated in OA pathogenesis.
3.1 TNF-α
TNF-α has been shown to influence and coordinate the inflammatory response in almost all tissues. Cytokine signaling is organized as a cascade (a primary cytokine acting upon a receptor that leads to expression of one or more secondary cytokines and so on), and TNF-α is often observed near the top of such signaling cascades. In addition to its effect on peripheral tissues, there is good evidence that TNF-α can influence the excitability of nociceptors either directly or through the expression of downstream cytokines – or both. TNF-α can be produced by DRG neurons and can also act on them through a variety of mechanisms, as will be discussed. Some of the long-term changes in nociceptor excitability require alterations in gene transcription and protein expression. However, it is also clear that TNF-α can produce very rapid changes in neuronal excitability that do not seem to require alterations in gene transcription [11] but are the result of the regulation of ion channels that in turn regulate nociceptor electrogenesis, including voltage-gated sodium channels (VGSC) and TRP channels expressed by sensory nerves [12]. Perfusion of DRG with TNF-α produces a rapid increase in the firing rate of both A- and C-fibers [13] and also elicits a rapid increase of calcitonin gene-related peptide (CGRP) release from the peripheral terminals of nociceptors [14]. It appears that the excitatory effects of TNF-α result from the modulation of several important conductances. For example, the ability of an intradermal injection of TNF-α into the plantar surface of the hindpaw to produce thermal, but not mechanical, sensitization is abolished in TRP subfamily V member 1 (TRPV1) knockout mice [11]. This suggests that another conductance might be responsible for the TNF-α-induced mechanical pain hypersensitivity. Indeed, it was observed that application of TNF-α to DRG neurons in culture increased the amplitude of the tetrodotoxin (TTX)-resistant sodium current in these cells [11]. Activation of TNF-α receptors produces a wide array of signaling options, including activation of the MAPK pathway [11]. For example, inhibitors of p38 MAPK selectively abolished TNF-α induced mechanical pain hypersensitivity and enhancement of the DRG TTX-resistant sodium current suggesting a model in which the rapid effects of TNF-α might involve activation of TNFR1 expressed by nociceptors leading to enhanced mechanical hypersensitivity mediated by p38-induced phosphorylation of TTXresistant sodium current subunits, together with thermal hypersensitivity produced by actions on TRPV1. Although it is clear that TNF-α can rapidly excite nociceptors by a number of mechanisms, the cytokine also produced increased TRPV1 protein expression when applied to cultured DRG neurons through extracellular signal-related kinase rather than p38 signaling [15]. However, this effect required chronic treatment of the cells (more than 8 h). Thus, TNF-α can increase nociceptor excitability using both short- and long-term mechanisms.
The observation that TNF-α expression in DRG neurons, as well as in microglia [16], occurs immediately after injury suggests that rapid autocrine excitation of DRG nociceptors by TNF-α may initiate the cytokine-mediated cascade that generates hypersensitivity. Additionally, TNF-α can produce long-term changes in the expression of other important molecules in sensory nerves. For example, one downstream target of the effects of TNF-α is the chemokine, monocyte chemoattractant protein-1 (MCP-1), which is synthesized by DRG neurons in an NFκB-dependent manner [17]. As MCP-1 is itself an important link in the pain behavior signaling pathway (discussed below), such effects are indicative of how a powerful upstream cytokine like TNF-α can manifest its influence in multiple ways. The numerous cellular sources of TNF-α together with the pleiotropic effects it can produce on DRG excitability over a broad time-course are a good illustration of the complex nature of the impact of inflammatory cytokines on the generation of pain.
3.2 Interleukins
As is the case with TNF-α, other important upstream cytokines may also produce rapid excitatory signaling in DRG neurons. Several interleukins have been observed to produce excitation of DRG neurons, including the proinflammatory interleukins IL-1, IL-6 and IL-17, whereas the anti-inflammatory cytokine IL-10 produced the opposite effects [18]. The molecular components of the receptors for all of these interleukins can be expressed by DRG neurons, further suggesting that these cytokines may exert direct effects on nociceptors.
3.2.1 IL-1
IL-1β has been reported to produce both rapid and delayed effects on DRG neurons. Incubation of DRG neurons with IL-1β for several days produced excitatory effects typified by a reduced threshold for depolarization in a subpopulation of DRG neurons [19], and downregulated G protein-coupled receptor kinase 2, thus increasing the responsiveness of multiple G protein– coupled receptors (GPCR) on DRG neurons [20]. Exposure of naïve DRG neurons to IL-1β over two days also resulted in upregulated expression of TRPV1, suggesting a mechanism by which IL-1 may participate in the persistence of thermal hyperalgesia in inflammatory states [21]. In contrast, acute exposure to IL-1β was reported to sensitize DRG neurons to noxious heat [22] and to stimulate heat-activated CGRP release [14], implying that IL-1β can also acutely transactivate TRPV1 expressed by DRG neurons. In the related trigeminal ganglia, the induction of inflammation with complete Freund’s adjuvant (CFA) resulted in expression of IL-1β and its receptor, IL-1R, by satellite glial cells and neuronal cell bodies, respectively [23]. IL-1β produced rapid excitation of these neurons [23] and an IL-1β antagonist reduced CFA-induced neuronal hyperexcitability. Again, this demonstrates how cytokine signaling can contribute to hyperexcitability of pain sensory neurons [23].
In addition to transactivation of TRPV1, IL-1β was also reported to rapidly excite DRG neurons by activating different components of voltage-dependent Na currents - probably consisting of NaV1.8 and NaV1.9. These effects were mediated through activation of p38 kinase [24]. At any rate, IL-1β appears to generally produce excitatory effects although the entire spectrum of its effects may be quite diverse. Indeed, it appears that DRG neurons express all the molecular components required for IL-1β signaling and these can be upregulated in inflammatory pain states [25, 26]. As with TNF-α, IL-1β may also potentiate the production of other algogenic molecules. In particular, intraplantar injection of IL-1β produced thermal hyperalgesia and increased levels of the pro-algesic nerve growth factor (NGF), indicating that IL-1β may play a role in NGF upregulation associated with inflammation [27]. NGF has itself been implicated as a key pain regulator in a variety of models acting primarily through transactivation of TRPV1 [28], further illustrating the complexity of interactions that may occur in the inflammatory environment. Clinical trials testing the effectiveness of anti-NGF in treating osteoarthritis pain are currently ongoing (for review, see [29]).
3.2.2 IL-6
DRG neurons have been shown to express the glycoprotein 130 (gp130) cytokine receptor subunit, a common feature of all cytokine receptors in the IL-6 family [30]. Addition of IL-6 alone, without application of the soluble receptor, was able to acutely induce calcium mobilization in a subset (33%) of cultured DRG neurons, suggesting that this subset expresses IL-6R [31]. In addition, DRG cultures that were pre-treated with IL-6 alone for two days had increased numbers of neurons responding to substance P as well as increased expression of neurokinin (NK)1 receptor [31], which is activated by substance P. In contrast, application of IL-6 to cultured DRG neurons was not effective by itself, but was able to rapidly (minutes) sensitize TRPV1 conductances to heat if the soluble fragment of its receptor was also applied [32]. Application of IL-6 and its soluble receptor to skin also stimulated CGRP release upon exposure to heat [14, 33]. The fact that IL-6 only facilitates sensitization to heat when added together with a soluble form of its binding subunit suggests that it may be activated “in trans” by soluble IL-6 receptors secreted from other cell types in the vicinity. Recently, mice in which the IL-6 family receptor subunit gp130 was selectively deleted from peripheral sensory NaV1.8-expressing neurons were examined in different models of chronic pain. These studies demonstrated that IL-6-induced nociceptor sensitization and transactivation of TRPV1 were absent in these mice [34]. The mice also displayed an ability to recover from mechanical allodynia following nerve injury as opposed to the sustained allodynia seen in wild-type mice [35].
3.2.3 IL-17
Interleukins of the IL-17 inflammatory cytokine family have also been shown to produce excitatory effects on nociceptors. Receptors for these molecules are widely expressed by lumbar DRG neurons [36, 37], and incubation with IL-17A results in a rapid phosphorylation of PKB/Akt and ERK-1/2 in DRG neurons [36]. In addition, experiments on cultured DRG neurons revealed that IL-17 could produce increased expression of TRPV4, whereas TRPV1 was unaffected. Reinforcing the possibility that TRPV4 may be important in mediating mechanical hyperalgesia, Il17a null mice showed protection against mechanical hyperalgesia, but not thermal hyperalgesia, in the zymosan inflammatory pain model [37].
3.2.4 Other common γ-chain cytokines
Two members of the γ-chain cytokine family, IL-2 and IL-15, have also been investigated for a role in generating pain hypersensitivity. IL-2 and its receptor, IL-2R, can be expressed by DRG neurons [38], but mixed effects on pain pathways have been reported. Intraplantar administration of IL-2 in the naïve hindpaw [38] and intrathecal injection post nerve injury [39] increased the time until paw withdrawal upon application of radiant heat. On the other hand, a different series of studies showed a dose-dependent effect of intrathecal IL-2 in naïve rats: a low dose increased heat sensitivity, while a higher dose decreased sensitivity [40]. In addition, the lower dose of IL-2 increased mechanical sensitivity, while the higher dose had no effect [40]. IL-15 has been proposed as a pro-inflammatory cytokine that supports immune infiltration, with possible implications in the development of pain [41]. IL-15 and its receptor have been located on astrocytes and microglia in the spinal cord, and intrathecal injection of an IL-15 antibody prevented macrophage and T-cell recruitment into the sciatic nerve after nerve injury [42]. Intrathecal injection of IL-15 into naïve rats also induced mechanical and thermal sensitivity [40].
3.2.5 IL-10
In contrast to these algogenic actions of interleukins, the anti-inflammatory molecule IL-10 produces quite different effects. Western blot and immunofluorescence have shown that IL-10 receptors are expressed by DRG neurons [43]. Recombinant rat IL-10 not only reduced the densities of TTX-sensitive and insensitive Na currents in control DRG neurons, but also reversed the increase in Na current density induced by rat recombinant TNF-α [43]. Consistent with the electrophysiological results, IL-10 reduced the increase in Na channel expression induced by TNF-α [43]. Moreover, repetitive intrathecal administration of IL-10 for 3 days temporarily attenuated mechanical allodynia in a sciatic nerve chronic constriction injury (CCI) model [44] and profoundly inhibited the excitability of DRG neurons in an L5 spinal nerve ligation model [43]. These results suggest that the down-regulation of the Na channels in DRG neurons might contribute to the therapeutic effect of IL-10 on neuropathic pain.
3.3 Chemokines
The family of cytokines known as CHEMOtactic cytoKINES or chemokines were first described because of their central role in the organization of leukocyte migration and the inflammatory response. It subsequently proved to be the case that some chemokines play an essential role in the development of many tissues including the nervous system. In addition, chemokines and their receptors can be expressed by neurons and can directly affect neuronal excitability. One manifestation of such properties is the proposed role of chemokine signaling in the genesis of chronic pain [45].
Chemokines represent a large family of proteins including several subfamilies. As far as is known all the actions of chemokines are transduced through the activation of a family of G-protein coupled receptors (GPCRs). Originally, Oh et al demonstrated that several different chemokines could excite DRG neurons in culture, indicating the expression of a number of different chemokine receptors by these cells [46]. These original observations suggested that chemokine signaling might play a role in pain behavior and subsequent observations in vivo have supported this hypothesis. Although many chemokines can be synthesized as part of the innate immune response and many of these appear to be able to act directly upon DRG neurons, most investigations have centered on the role of Chemokine (C-C motif) ligand 2 (CCL2 or MCP-1) and its receptor, CCR2. When applied to intact DRG or to acutely isolated DRG neurons after chronic compression of the DRG (CCD model), MCP-1 produced a decrease in the depolarization threshold of DRG neurons [47] and some cells became spontaneously active at resting potential [48]. In voltage clamp, MCP-1 induced an inward current that was not voltage-dependent [48]. The properties of this current were consistent with the possibility that it could represent a TRP channel. In another report, whole-cell patch-clamp recordings demonstrated that MCP-1 concentration-dependently increased TTX-resistant NaV1.8 current densities in both small- and medium-diameter naïve sensory neurons [49]. Incubation with MCP-1 also shifted the activation and steady-state inactivation curves of NaV1.8 in a hyperpolarizing direction in small sensory neurons [49]. As with other cytokines, MCP-1 also produced long-term effects on DRG neuron excitability. Pretreatment with MCP-1 for 24 hours increased the density of capsaicin-induced currents (presumably TRPV1) in small putative DRG nociceptive neurons [50]. MCP-1 also increased the density of TTX-resistant NaV1.8 currents [50]. Neither of these effects was observed in the presence of a specific phosphatidylinositol-3 kinase (PI3K) inhibitor or Akt inhibitor. Although DRG neurons do not normally express MCP-1 or CCR2, MCP-1 and CCR2 expression is upregulated by DRG neurons in association with many different types of experimental pain, and one early investigation demonstrated that chronic pain associated with several different models was ameliorated in Ccr2 null mice [51]. Observations using transgenic reporter mice have demonstrated that in the context of chronic pain, MCP-1 can be released by the cell bodies of DRG neurons within the DRG and activate CCR2 on the same or adjacent cells, thereby spreading excitation throughout the ganglion [52].
The literature makes it clear that MCP-1 may not be the only chemokine involved in states of chronic pain. Other chemokines and their receptors are also upregulated under similar conditions to MCP-1/CCR2 depending on the pain model employed. For instance, the chemokine SDF-1/CXCL12 and its receptor CXCR4 appear to be of particular importance in a number of scenarios such as HIV-1 related pain, opiate-induced hyperalgesia [12] and painful diabetic neuropathy (D. Menichella et al, personal communication). Further, CX3CL1 (fractalkine) appears to have a unique role in the genesis of chronic pain. CX3CL1 is mostly expressed by neurons and exists in a membrane-bound form, which can be cleaved to produce a soluble form of the chemokine by metalloproteinases (ADAM10, ADAM17) [53–55] and cathepsin S [56]. Receptors for CX3CL1, CX3CR1, are generally expressed by microglia in the spinal cord [57]. Indeed, it has been suggested that cathepsin S-mediated cleavage of CX3CL1 in the spinal cord and subsequent activation of CX3CR1 on microglia is an important link in the sequence of events that lead from acute to chronic pain phenotypes [58].
4. Cytokines and Joint Pain: Studies in Animal Models (Table 1)
Table 1.
Cytokines and Joint Pain: Studies in Animal Models
| Type of study | Cytokines Studied | Results | References |
|---|---|---|---|
| Intra-articular cytokine injections | TNF-α, IL-1β, IL-6, IL-17 | Sensitized nociceptive C-fibers to innocuous and noxious knee rotation | Richter et al, 2010; Ebbinghaus et al, 2012; Brenn et al, 2007; Richter et al, 2012 |
| Antigen-induced arthritis (AIA) model | systemic infliximab or etanercept (blocks TNF-α) | Reduced pain behaviors associated with AIA, but neither significantly inhibited joint damage | Boettger et al, 2008 |
| systemic anakinra (blocks IL-1) | Prevented thermal, but not mechanical, hyperalgesia | Ebbinghaus et al, 2012 | |
| systemic soluble gp130 (blocks IL-6) | Attenuated primary mechanical hyperalgesia at days 14 and 21, but did not protect against joint damage | Boettger et al, 2010 | |
| intra-articular soluble gp130 | Significantly reduced joint destruction by day 21 of the model, and reduced primary mechanical hyperalgesia at days 3 and 7 | Boettger et al, 2010 | |
| systemic anti-IL-17 mAb | Improved the guarding score and reduced secondary mechanical hyperalgesia during the acute phase of murine AIA, but it did not significantly inhibit joint swelling | Richter et al, 2012 Pinto et al, 2010 | |
| Destabilization of the medial meniscus (DMM) model | systemic soluble receptor to TNF-α | Reduced NGF mRNA expression in the joint and restored weight-bearing deficits in the first 3 days post surgery, but it had no effect when administered at 16 weeks after surgery | McNamee et al, 2010 |
| systemic CCR2 receptor antagonist | Reversed movement-provoked pain at 9 weeks post surgery | Miller et al, 2012 | |
In order to explore how cytokines may contribute to the sensitization of joint afferents, researchers have injected them directly into the joint cavity of laboratory animals. The main method for determining sensitization is by recording action potentials from innervating nerve fibers while the anesthetized animal is undergoing either innocuous (movement within the normal range) or noxious rotation of the knee joint. TNF-α injected intra-articularly (IA) into rat knees rapidly (1–3 hours) sensitized nociceptive C-fibers in response to both innocuous and noxious outward joint rotation [59]. This also resulted in a small but significant increase in the response of Aδ-fibers to noxious rotation. Coinjection of the recombinant TNF receptor (p75)-Fc fusion protein, etanercept, with TNF-α prevented this increase in sensitivity. Likewise, IA administration of etanercept 90 minutes after injection of TNF-α was able to reverse this sensitization. In addition, a recent follow-up study has demonstrated that IA injection of TNF-α, but not of a vehicle control, increased the response of spinal cord neurons to both innocuous and noxious force applied to knee joint [60]. In the naïve rat knee joint, IL-1β was also able to sensitize nociceptive C-fibers to noxious joint rotation, but it decreased the sensitivity of Aδ-fibers to both innocuous and noxious rotation [21]. Like TNF-α and IL-1β, IA delivery of IL-6 sensitized nociceptive C fibers to noxious joint rotation [61]. Co-administration with the soluble IL-6 receptor significantly increased this response in nociceptors [61]. Unlike for TNF-α/etanercept, only co-delivery of soluble gp130 with IL-6/sIL-6R could prevent these increased responses; if soluble gp130 was delivered after IL-6/sIL-6R, no decrease in nociceptor firing was detected [61]. A single injection of IL-6/sIL-6R into the rat knee joint was also shown to sensitize spinal neurons to both innocuous and noxious joint rotation [62]. Finally, injection of IL9 17 into the knee joint cavity sensitized nociceptive C fibers to both innocuous and noxious joint rotation through neuronal IL-17 receptors [36]. The IL-17A receptor was visualized in most rat DRG neurons while in cultured rat DRG neurons, IL-17A caused rapid phosphorylation of protein kinase B and ERK, and it rapidly enhanced excitability [36]. In addition to TNF-α and the interleukins, excitatory amino acids have also been implicated in promoting joint inflammation through stimulation of cytokine production [63], and in directly inducing pain in the joint. To test the latter hypothesis, glutamate (Glu), aspartate (Asp), and arginate (Arg) were injected intra-articularly into naïve rat knee joints, either separately, or in combination. Injections of these amino acids separately had no effect on hyperalgesia or allodynia. In contrast, within ten minutes of injection of combinations of Asp/Glu, Asp/Arg, or Asp/Glu/Arg, secondary thermal hyperalgesia and mechanical allodynia developed, and these effects lasted for five hours, suggesting that these amino acid combinations may act as chemical mediators of nociception [64]. These in vivo experiments clearly demonstrate that several cytokines rapidly produce pain and hyperalgesia following their injection into joints, suggesting that cytokines can interact with joint nociceptors. Data on the temporospatial distribution of cytokines and chemokines and their receptors in the sensory innervation of the healthy or diseased joint are not yet available.
In order to elucidate the contribution of cytokines to OA joint pain, studies in disease-specific animal models and in human tissues will be needed, but current information on this is still scant. More data have been published using the antigen-induced model of inflammatory arthritis (AIA) [65], which is characterized by acute inflammation leading to rapid joint destruction. In rat AIA, animals display guarding behavior, primary hyperalgesia, and secondary thermal hyperalgesia over the first 14 days post induction [66]. Systemic delivery (beginning 6 hours after induction) of either etanercept or the monoclonal antihuman TNF antibody, infliximab, was able to reduce pain behaviors associated with AIA, but neither significantly inhibited joint damage by day 21 [66]. During the acute phase (day 3), IA etanercept also inhibited C-fiber responses to innocuous and noxious joint rotation [66]. Further, 3 days after induction, lumbar DRG were massively infiltrated with macrophages, and TNF-α blockade was able to inhibit this macrophage infiltration. This indicates that inflammatory arthritis causes TNF-α-dependent infiltration of macrophages into DRG, which correlates with pain-related behavior [67]. By day 7 of the AIA model, IL-1R1 protein expression increased in DRG neurons, and systemic treatment (beginning 5 days prior to induction) with the IL-1 receptor antagonist, anakinra, prevented thermal, but not mechanical, hyperalgesia [21]. Anakinra also reduced TRPV1 expression on DRG neurons by day 21. Intra-articular delivery of soluble gp130 at the time of induction resulted in significantly reduced joint destruction by day 21 of the model, and reduced primary mechanical hyperalgesia at days 3 and 7 [68]. In contrast, multiple systemic injections of soluble gp130 (beginning 6 hours after induction) did not protect against joint damage, but attenuated primary mechanical hyperalgesia at days 14 and 21 [68]. These studies reinforce the fact that the method and timing of inhibiting IL-6 along with the other inflammatory cytokines can have marked effects on structural and symptomatic benefits. Systemic administration of anti-IL-17 mAb prior to induction of the AIA model improved the guarding score and reduced secondary mechanical hyperalgesia during the acute phase of murine AIA [36, 69], but it did not significantly inhibit joint swelling [36]. Finally, intra-articular injection of a glutamate receptor antagonist at the time of induction of the AIA model was able to restore weight-bearing on days 1 and 2 and to reduce knee swelling, synovial inflammation, cartilage degradation, and subchondral bone remodeling through day 21 [70].
While these studies in the AIA model clearly show an important role for selected cytokines in driving inflammatory joint pain, AIA is not a model of OA and it cannot be used to model chronic OA pain due to its very rapid progression, aggressive joint destruction, and excessive inflammation. Studies in disease-specific animal models of OA that capture the slow progression of this disease and the mild inflammation associated with it, are few and far between. The small animal models most commonly used to model pain in association with knee OA are, firstly, the mono-iodoacetate (MIA) model, where IA injection of MIA leads to a chemically induced OA-like pathology, and, secondly, surgical models where instability induced in the knee leads to OA-like structural joint damage (reviewed in [71]). While there is a rapidly growing literature on pain behaviors and associated pathways in these models, studies that evaluate the contribution of cytokines and chemokines are limited. One surgical model that has recently been employed to mimic the slow progression of joint damage in OA is the destabilization of the medial meniscus (DMM) in the mouse knee [72]. Surgical instability triggered by DMM results in a slowly progressive joint damage with histopathological changes in articular cartilage, subchondral bone and synovium that mimic human OA. When monitored over 16 weeks after surgery, mice show longitudinal changes in pain-related behaviors: mechanical allodynia in the ipsilateral hindpaw progresses up to 4 weeks after DMM [73] and is maintained throughout the 16-week follow-up period [74]; locomotive changes indicative of “movement-provoked” pain are first apparent 8 weeks after DMM and continue until week 16 [74, 75]; weight-bearing asymmetry starts around week 12 after surgery [75]. In this model, a soluble receptor to TNF-α reduced NGF mRNA expression in the joint and restored weight-bearing deficits in the first 3 days post surgery, but it had no effect when administered at 16 weeks after surgery [76]. This correlated with TNF-α mRNA expression in the knee joint: levels were elevated in the operated knee 3 days after surgery compared to the contralateral knee, but by 16 weeks post surgery, TNF-α expression levels were not different between both knees [76]. Another 16-week DMM study focused on MCP-1/CCR2 expression in the knee-innervating DRG. Starting at 8 weeks after surgery, DRG neurons showed increased MCP-1/CCR2 production, and this correlated with the development of movement-induced pain [74]. Ccr2 null mice were protected from developing movement-induced pain after DMM surgery and also from maintained secondary mechanical allodynia despite developing similar levels of joint damage. Unlike wild-type mice, Ccr2 null mice displayed no macrophage infiltration into the DRG at 8 and 16 weeks post surgery. Interestingly, a CCR2 receptor antagonist, administered systemically 9 weeks after DMM in wild type mice, was able to reverse movement-provoked pain [74]. As with cytokine receptors, it is not yet known whether peripheral terminals of sensory neurons in the joint express CCR2 (or other chemokine receptors). From studies in animal models, it appears that OA joints produce increased MCP-1 early in the disease (demonstrated as mRNA in the DMM model [77] and as protein in the DMM [78] and MIA models [79]), raising the interesting possibility that locally produced MCP-1 may indeed contribute to driving peripheral sensitization in the OA joint.
5. Cytokines and OA Joint Pain: Evidence From Human Studies
All the cytokines and chemokines discussed above can be produced by joint cells or affect them [10]. Many of them have been detected in synovial fluids of OA patients and subjects suffering from ligament or meniscal damage, including the classic inflammatory cytokines [7] as well as the chemokines, MCP- 1 [80], SDF-1 [81] and fractalkine [80, 82], and the excitatory amino acids glutamate and aspartate [83], but only a limited number of studies report correlations of these protein levels to levels of pain. TNF-α was reportedly correlated with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score in a group of 47 knee OA patients who had not received prior treatment [84]. In addition, synovial fluid levels of IL-6 and MCP-1 were correlated with patient-reported pain scores in 32 patients suffering from acute knee pain believed to be secondary to meniscal damage [85].
The ability of TNF-α and IL-1 inhibitors to abate OA pain has also been tested in small clinical trials, with mixed results to date [86]. Adalimumab, a fully human IgG1 monoclonal antibody directed against TNF-α, was found to reduce pain in an open-label 12-week evaluation of 20 patients with symptomatic radiographic knee OA and evidence of knee effusion [87]. Adalimumab was, however, not efficacious in a randomized, placebo-controlled trial in patients with hand OA who were unresponsive to analgesics and NSAIDs [88]. The primary outcome measure, the response rate (defined as a >50% decrease in pain intensity 6 weeks after the second injection), was not significantly different between the adalimumab-treated (35.1%) and placebo-treated (27.3%) groups (P = 0.48). Likewise, in a multicenter, randomized, double-blind, placebo-controlled study using two different doses of intra-articular IL-1 receptor antagonist, anakinra, 101 patients with symptomatic radiographic knee OA did not significantly benefit over a period of 12 weeks [89]. In a different randomized controlled pilot trial, 6 patients suffering from an acute anterior cruciate ligament (ACL) tear, a condition that represents a strong risk factor for developing post-traumatic OA, reported improved pain over 14 days after IA injection of anakinra [90]. Thus, it is conceivable that early inhibition of the intra-articular inflammatory response after joint injury may improve clinical outcomes for this population.
Lastly, clinical trials.gov lists a randomized, double-blind, placebo-controlled phase 2 study in subjects with osteoarthritic pain of the knee, using a selective CCR2 antagonist [91] but trial resulted are not reported.
Lack of efficacy of cytokine inhibition in OA [86] is in sharp contrast to rheumatoid arthritis, where biologics targeting one cytokine (notably TNF-α) can demonstrate remarkable clinical efficacy [92]. In addition to the anti-inflammatory action of TNF-blockade, functional MRI studies of the brain have suggested that neutralization of TNF-α may affect pain responses in the brain in the context of arthritis, long before it achieves anti-inflammatory effects in the joints [93].
6. Future Directions
In spite of the frequent occurrence of pain in association with OA, there is little information as to the cellular and molecular elements that are responsible for this phenomenon. Current evidence supports the idea that OA pain is generated and maintained through continuous nociceptive peripheral input from the OA joint (reviewed in [4]). Therefore, it is of great interest to define the mediators in the joint that contribute to the sensitization of joint nociceptors, and locally produced inflammatory cytokines and chemokines are certainly likely candidates for this.
To fully appreciate the actual role of cytokine and chemokine signaling in the genesis of OA pain will require elucidating the precise juxtaposition of cells that make and respond to these molecules. For this, we will need detailed analysis of the sensory innervation of the OA joint, and of the temporo-spatial distribution of receptors on these neurons. With the exception of articular cartilage, which is aneural, joint tissues are richly innervated and sensory Aδ and C-fibers terminate as free nerve endings in the fibrous capsule, adipose tissue, ligaments, menisci, and the adjacent periosteum [94]. The sensory innervation of the synovium is less well described, but immunostaining for neuropeptides like CGRP and substance P suggests the presence of sensory fibers [95–99]. Since OA affects all joint tissues, it is likely that nerve endings in these tissues are also affected by the pathology. It has been suggested that inflammatory changes in the OA synovium are associated with destruction of the capillary and neuronal network that is present in normal synovium [100]. Also, osteochondral channels carrying blood vessels and CGRP positive fibres breaching the tidemark and invading the articular cartilage have been demonstrated in human OA joints as well as in animal models [101] – thus, nerves may actually be in direct contact with diseased cartilage. More detailed anatomical studies to map changes in innervation in different joint tissues during OA progression should help unravel how nociceptors are activated by locally generated cytokines and chemokines to generate pain signals.
In conclusion, OA is a chronic slowly progressive painful joint disease, where all joint tissues participate in driving the disease [3]. The microenvironment of the OA joint will therefore be one of great complexity with most of the cell types both producing and responding to inflammatory cytokines and chemokines and other mediators (Fig. 1). Cytokines and chemokines present in the OA joint may not only promote synovitis and joint destruction, they may also directly activate innervating nociceptors. Understanding how these mediators produced at the level of the compromised joint can initiate changes in the DRG and spinal cord, and thus contribute to the pain pathway, is a subject of considerable interest. It can be expected that careful studies of these pathways in disease-specific animal models and human tissues will lead to identification of novel analgesic targets. However, the complex and diverse actions of these molecules on a variety of cell types indicate that the mode of delivery, timing of treatment, and target population must be carefully delineated in order to produce beneficial effects for the patient.
Osteoarthritis is a chronic and painful disease of synovial joints.
Osteoarthritic joint tissues produce and respond to cytokines and chemokines.
Cytokines promote joint destruction and directly activate innervating nociceptors.
Acknowledgments
The authors would like to thank Dr. Carla Scanzello (University of Pennsylvania) for the histology picture of inflamed human OA synovium (in Fig. 1). REM is supported by an Arthritis Foundation Post-Doctoral Fellowship and by grant F32AR062927 from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases. AMM is supported by grant R01AR064251 from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases. RJM is supported by 2 R01 DA013141.
Abbreviations
- AIA
antigen-induced arthritis
- CNS
central nervous system
- DMM
destabilization of the medial meniscus
- DRG
dorsal root ganglia
- GPCR
G-protein coupled receptor
- IL
interleukin
- MCP-1
monocyte chemoattractant protein-1
- MIA
monoiodoacetate model
- NGF
nerve growth factor
- NMDA
N-methyl-d-aspartate
- OA
osteoarthritis
- TNF
tumor necrosis factor
- TRG
trigeminal ganglia
- TRP
transient receptor potential
- TTX
tetrodotoxin
- VGSC
voltage-gated sodium channel
- WOMAC
Western Ontario and McMaster Universities Osteoarthritis Index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guccione AA, Felson DT, Anderson JJ, Anthony JM, Zhang Y, Wilson PW, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. American journal of public health. 1994;84:351–358. doi: 10.2105/ajph.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 3.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis and rheumatism. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malfait AM, Schnitzer TJ. Towards a mechanism-based approach to pain management in osteoarthritis. Nature reviews Rheumatology. 2013;9:654–664. doi: 10.1038/nrrheum.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21:1145–1153. doi: 10.1016/j.joca.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nature reviews Rheumatology. 2010;6:625–635. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 7.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51:249–257. doi: 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20:739–744. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 9.Olson SA, Horne P, Furman B, Huebner J, Al-Rashid M, Kraus VB, et al. The role of cytokines in posttraumatic arthritis. J Am Acad Orthop Surg. 2014;22:29–37. doi: 10.5435/JAAOS-22-01-29. [DOI] [PubMed] [Google Scholar]
- 10.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nature reviews Rheumatology. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 11.Jin X, Gereau RWt. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:246–255. doi: 10.1523/JNEUROSCI.3858-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handbook of experimental pharmacology. 2009:417–449. doi: 10.1007/978-3-540-79090-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schafers M, Lee DH, Brors D, Yaksh TL, Sorkin LS. Increased sensitivity of injured and adjacent uninjured rat primary sensory neurons to exogenous tumor necrosis factor-alpha after spinal nerve ligation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:3028–3038. doi: 10.1523/JNEUROSCI.23-07-03028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Opree A, Kress M. Involvement of the proinflammatory cytokines tumor necrosis factor-alpha, IL-1 beta, and IL-6 but not IL-8 in the development of heat hyperalgesia: effects on heat-evoked calcitonin gene-related peptide release from rat skin. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:6289–6293. doi: 10.1523/JNEUROSCI.20-16-06289.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hensellek S, Brell P, Schaible HG, Brauer R, Segond von Banchet G. The cytokine TNFalpha increases the proportion of DRG neurones expressing the TRPV1 receptor via the TNFR1 receptor and ERK activation. Molecular and cellular neurosciences. 2007;36:381–391. doi: 10.1016/j.mcn.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Ohtori S, Takahashi K, Moriya H, Myers RR. TNF-alpha and TNF-alpha receptor type 1 upregulation in glia and neurons after peripheral nerve injury: studies in murine DRG and spinal cord. Spine. 2004;29:1082–1088. doi: 10.1097/00007632-200405150-00006. [DOI] [PubMed] [Google Scholar]
- 17.Jung H, Miller RJ. Activation of the nuclear factor of activated T-cells (NFAT) mediates upregulation of CCR2 chemokine receptors in dorsal root ganglion (DRG) neurons: a possible mechanism for activity-dependent transcription in DRG neurons in association with neuropathic pain. Molecular and cellular neurosciences. 2008;37:170–177. doi: 10.1016/j.mcn.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen KF, Zhu HQ, Wei XH, Wang J, Li YY, Pang RP, et al. Interleukin-10 down-regulates voltage gated sodium channels in rat dorsal root ganglion neurons. Experimental neurology. 2013;247:466–475. doi: 10.1016/j.expneurol.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 19.Stemkowski PL, Smith PA. Long-term IL-1beta exposure causes subpopulation-dependent alterations in rat dorsal root ganglion neuron excitability. Journal of neurophysiology. 2012;107:1586–1597. doi: 10.1152/jn.00587.2011. [DOI] [PubMed] [Google Scholar]
- 20.von Banchet GS, Fischer N, Uhlig B, Hensellek S, Eitner A, Schaible HG. Molecular effects of interleukin-1beta on dorsal root ganglion neurons: prevention of ligand-induced internalization of the bradykinin 2 receptor and downregulation of G protein-coupled receptor kinase 2. Molecular and cellular neurosciences. 2011;46:262–271. doi: 10.1016/j.mcn.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Ebbinghaus M, Uhlig B, Richter F, von Banchet GS, Gajda M, Brauer R, et al. The role of interleukin-1beta in arthritic pain: main involvement in thermal, but not mechanical, hyperalgesia in rat antigen-induced arthritis. Arthritis and rheumatism. 2012;64:3897–3907. doi: 10.1002/art.34675. [DOI] [PubMed] [Google Scholar]
- 22.Obreja O, Rathee PK, Lips KS, Distler C, Kress M. IL-1 beta potentiates heat-activated currents in rat sensory neurons: involvement of IL-1RI, tyrosine kinase, and protein kinase C. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2002;16:1497–1503. doi: 10.1096/fj.02-0101com. [DOI] [PubMed] [Google Scholar]
- 23.Takeda M, Tanimoto T, Kadoi J, Nasu M, Takahashi M, Kitagawa J, et al. Enhanced excitability of nociceptive trigeminal ganglion neurons by satellite glial cytokine following peripheral inflammation. Pain. 2007;129:155–166. doi: 10.1016/j.pain.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, et al. Nociceptors are interleukin-1beta sensors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:14062–14073. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inoue A, Ikoma K, Morioka N, Kumagai K, Hashimoto T, Hide I, et al. Interleukin-1beta induces substance P release from primary afferent neurons through the cyclooxygenase-2 system. Journal of neurochemistry. 1999;73:2206–2213. [PubMed] [Google Scholar]
- 26.Li M, Shi J, Tang JR, Chen D, Ai B, Chen J, et al. Effects of complete Freund's adjuvant on immunohistochemical distribution of IL-1beta and IL-1R I in neurons and glia cells of dorsal root ganglion. Acta pharmacologica Sinica. 2005;26:192–198. doi: 10.1111/j.1745-7254.2005.00522.x. [DOI] [PubMed] [Google Scholar]
- 27.Safieh-Garabedian B, Poole S, Allchorne A, Winter J, Woolf CJ. Contribution of interleukin-1 beta to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. British journal of pharmacology. 1995;115:1265–1275. doi: 10.1111/j.1476-5381.1995.tb15035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. Embo J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seidel MF, Wise BL, Lane NE. Nerve growth factor: an update on the science and therapy. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21:1223–1228. doi: 10.1016/j.joca.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annual review of immunology. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 31.von Banchet GS, Kiehl M, Schaible HG. Acute and long-term effects of IL-6 on cultured dorsal root ganglion neurones from adult rat. Journal of neurochemistry. 2005;94:238–248. doi: 10.1111/j.1471-4159.2005.03185.x. [DOI] [PubMed] [Google Scholar]
- 32.Obreja O, Biasio W, Andratsch M, Lips KS, Rathee PK, Ludwig A, et al. Fast modulation of heat-activated ionic current by proinflammatory interleukin 6 in rat sensory neurons. Brain : a journal of neurology. 2005;128:1634–1641. doi: 10.1093/brain/awh490. [DOI] [PubMed] [Google Scholar]
- 33.Obreja O, Schmelz M, Poole S, Kress M. Interleukin-6 in combination with its soluble IL-6 receptor sensitises rat skin nociceptors to heat, in vivo. Pain. 2002;96:57–62. doi: 10.1016/s0304-3959(01)00420-1. [DOI] [PubMed] [Google Scholar]
- 34.Andratsch M, Mair N, Constantin CE, Scherbakov N, Benetti C, Quarta S, et al. A key role for gp130 expressed on peripheral sensory nerves in pathological pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:13473–13483. doi: 10.1523/JNEUROSCI.1822-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quarta S, Vogl C, Constantin CE, Uceyler N, Sommer C, Kress M. Genetic evidence for an essential role of neuronally expressed IL-6 signal transducer gp130 in the induction and maintenance of experimentally induced mechanical hypersensitivity in vivo and in vitro. Molecular pain. 2011;7:73. doi: 10.1186/1744-8069-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richter F, Natura G, Ebbinghaus M, von Banchet GS, Hensellek S, Konig C, et al. Interleukin-17 sensitizes joint nociceptors to mechanical stimuli and contributes to arthritic pain through neuronal interleukin-17 receptors in rodents. Arthritis and rheumatism. 2012;64:4125–4134. doi: 10.1002/art.37695. [DOI] [PubMed] [Google Scholar]
- 37.Segond von Banchet G, Boettger MK, Konig C, Iwakura Y, Brauer R, Schaible HG. Neuronal IL-17 receptor upregulates TRPV4 but not TRPV1 receptors in DRG neurons and mediates mechanical but not thermal hyperalgesia. Molecular and cellular neurosciences. 2013;52:152–160. doi: 10.1016/j.mcn.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Song P, Zhao ZQ, Liu XY. Expression of IL-2 receptor in dorsal root ganglion neurons and peripheral antinociception. Neuroreport. 2000;11:1433–1436. doi: 10.1097/00001756-200005150-00016. [DOI] [PubMed] [Google Scholar]
- 39.Yao MZ, Gu JF, Wang JH, Sun LY, Lang MF, Liu J, et al. Interleukin-2 gene therapy of chronic neuropathic pain. Neuroscience. 2002;112:409–416. doi: 10.1016/s0306-4522(02)00078-7. [DOI] [PubMed] [Google Scholar]
- 40.Cata JP, Weng HR, Dougherty PM. Spinal injection of IL-2 or IL-15 alters mechanical and thermal withdrawal thresholds in rats. Neuroscience letters. 2008;437:45–49. doi: 10.1016/j.neulet.2008.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Austin PJ, Moalem-Taylor G. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. Journal of neuroimmunology. 2010;229:26–50. doi: 10.1016/j.jneuroim.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 42.Gomez-Nicola D, Valle-Argos B, Suardiaz M, Taylor JS, Nieto-Sampedro M. Role of IL-15 in spinal cord and sciatic nerve after chronic constriction injury: regulation of macrophage and T-cell infiltration. Journal of neurochemistry. 2008;107:1741–1752. doi: 10.1111/j.1471-4159.2008.05746.x. [DOI] [PubMed] [Google Scholar]
- 43.Shen KF, Zhu HQ, Wei XH, Wang J, Li YY, Pang RP, et al. Interleukin-10 down-regulates voltage gated sodium channels in rat dorsal root ganglion neurons. Experimental neurology. 2013;247:466–475. doi: 10.1016/j.expneurol.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 44.Milligan ED, Langer SJ, Sloane EM, He L, Wieseler-Frank J, O'Connor K, et al. Controlling pathological pain by adenovirally driven spinal production of the anti-inflammatory cytokine, interleukin-10. Eur J Neurosci. 2005;21:2136–2148. doi: 10.1111/j.1460-9568.2005.04057.x. [DOI] [PubMed] [Google Scholar]
- 45.Dawes JM, McMahon SB. Chemokines as peripheral pain mediators. Neuroscience letters. 2013;557(Pt A):1–8. doi: 10.1016/j.neulet.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:5027–5035. doi: 10.1523/JNEUROSCI.21-14-05027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, et al. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14092–14097. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun JH, Yang B, Donnelly DF, Ma C, LaMotte RH. MCP-1 enhances excitability of nociceptive neurons in chronically compressed dorsal root ganglia. Journal of neurophysiology. 2006;96:2189–2199. doi: 10.1152/jn.00222.2006. [DOI] [PubMed] [Google Scholar]
- 49.Belkouch M, Dansereau MA, Reaux-Le Goazigo A, Van Steenwinckel J, Beaudet N, Chraibi A, et al. The chemokine CCL2 increases Nav1.8 sodium channel activity in primary sensory neurons through a Gbetagamma-dependent mechanism. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:18381–18390. doi: 10.1523/JNEUROSCI.3386-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kao DJ, Li AH, Chen JC, Luo RS, Chen YL, Lu JC, et al. CC chemokine ligand 2 upregulates the current density and expression of TRPV1 channels and Nav1.8 sodium channels in dorsal root ganglion neurons. Journal of neuroinflammation. 2012;9:189. doi: 10.1186/1742-2094-9-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, et al. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:7947–7952. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jung H, Bhangoo S, Banisadr G, Freitag C, Ren D, White FA, et al. Visualization of chemokine receptor activation in transgenic mice reveals peripheral activation of CCR2 receptors in states of neuropathic pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:8051–8062. doi: 10.1523/JNEUROSCI.0485-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garton KJ, Gough PJ, Blobel CP, Murphy G, Greaves DR, Dempsey PJ, et al. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1) The Journal of biological chemistry. 2001;276:37993–38001. doi: 10.1074/jbc.M106434200. [DOI] [PubMed] [Google Scholar]
- 54.Hundhausen C, Schulte A, Schulz B, Andrzejewski MG, Schwarz N, von Hundelshausen P, et al. Regulated shedding of transmembrane chemokines by the disintegrin and metalloproteinase 10 facilitates detachment of adherent leukocytes. Journal of immunology (Baltimore, Md: 1950) 2007;178:8064–8072. doi: 10.4049/jimmunol.178.12.8064. [DOI] [PubMed] [Google Scholar]
- 55.Tsou CL, Haskell CA, Charo IF. Tumor necrosis factor-alpha-converting enzyme mediates the inducible cleavage of fractalkine. The Journal of biological chemistry. 2001;276:44622–44626. doi: 10.1074/jbc.M107327200. [DOI] [PubMed] [Google Scholar]
- 56.Clark AK, Yip PK, Grist J, Gentry C, Staniland AA, Marchand F, et al. Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10655–10660. doi: 10.1073/pnas.0610811104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mizutani M, Pino PA, Saederup N, Charo IF, Ransohoff RM, Cardona AE. The Fractalkine Receptor but Not CCR2 Is Present on Microglia from Embryonic Development throughout Adulthood. The Journal of Immunology. 2012;188:29–36. doi: 10.4049/jimmunol.1100421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clark AK, Yip PK, Malcangio M. The liberation of fractalkine in the dorsal horn requires microglial cathepsin S. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:6945–6954. doi: 10.1523/JNEUROSCI.0828-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Richter F, Natura G, Loser S, Schmidt K, Viisanen H, Schaible HG. Tumor necrosis factor causes persistent sensitization of joint nociceptors to mechanical stimuli in rats. Arthritis and rheumatism. 2010;62:3806–3814. doi: 10.1002/art.27715. [DOI] [PubMed] [Google Scholar]
- 60.Konig C, Zharsky M, Moller C, Schaible HG, Ebersberger A. Involvement of Peripheral and Spinal Tumor Necrosis Factor alpha in Spinal Cord Hyperexcitability During Knee Joint Inflammation in Rats. Arthritis & rheumatology. 2014;66:599–609. doi: 10.1002/art.38271. [DOI] [PubMed] [Google Scholar]
- 61.Brenn D, Richter F, Schaible HG. Sensitization of unmyelinated sensory fibers of the joint nerve to mechanical stimuli by interleukin-6 in the rat: an inflammatory mechanism of joint pain. Arthritis and rheumatism. 2007;56:351–359. doi: 10.1002/art.22282. [DOI] [PubMed] [Google Scholar]
- 62.Vazquez E, Kahlenbach J, Segond von Banchet G, Konig C, Schaible HG, Ebersberger A. Spinal interleukin-6 is an amplifier of arthritic pain in the rat. Arthritis and rheumatism. 2012;64:2233–2242. doi: 10.1002/art.34384. [DOI] [PubMed] [Google Scholar]
- 63.McNearney T, Baethge BA, Cao S, Alam R, Lisse JR, Westlund KN. Excitatory amino acids, TNF-alpha, and chemokine levels in synovial fluids of patients with active arthropathies. Clin Exp Immunol. 2004;137:621–627. doi: 10.1111/j.1365-2249.2004.02563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lawand NB, Willis WD, Westlund KN. Excitatory amino acid receptor involvement in peripheral nociceptive transmission in rats. Eur J Pharmacol. 1997;324:169–177. doi: 10.1016/s0014-2999(97)00072-1. [DOI] [PubMed] [Google Scholar]
- 65.Brackertz D, Mitchell GF, Mackay IR. Antigen-induced arthritis in mice. I. Induction of arthritis in various strains of mice. Arthritis and rheumatism. 1977;20:841–850. doi: 10.1002/art.1780200314. [DOI] [PubMed] [Google Scholar]
- 66.Boettger MK, Hensellek S, Richter F, Gajda M, Stockigt R, von Banchet GS, et al. Antinociceptive effects of tumor necrosis factor alpha neutralization in a rat model of antigen-induced arthritis: evidence of a neuronal target. Arthritis and rheumatism. 2008;58:2368–2378. doi: 10.1002/art.23608. [DOI] [PubMed] [Google Scholar]
- 67.Segond von Banchet G, Boettger MK, Fischer N, Gajda M, Brauer R, Schaible HG. Experimental arthritis causes tumor necrosis factor-alpha-dependent infiltration of macrophages into rat dorsal root ganglia which correlates with pain-related behavior. Pain. 2009;145:151–159. doi: 10.1016/j.pain.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 68.Boettger MK, Leuchtweis J, Kummel D, Gajda M, Brauer R, Schaible HG. Differential effects of locally and systemically administered soluble glycoprotein 130 on pain and inflammation in experimental arthritis. Arthritis research & therapy. 2010;12:R140. doi: 10.1186/ar3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pinto LG, Cunha TM, Vieira SM, Lemos HP, Verri WA, Jr, Cunha FQ, et al. IL-17 mediates articular hypernociception in antigen-induced arthritis in mice. Pain. 2010;148:247–256. doi: 10.1016/j.pain.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 70.Bonnet CS, Williams AS, Gilbert SJ, Harvey AK, Evans BA, Mason DJ. AMPA/kainate glutamate receptors contribute to inflammation, degeneration and pain related behaviour in inflammatory stages of arthritis. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-203670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Malfait AM, Little CB, McDougall JJ. A commentary on modelling osteoarthritis pain in small animals. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21:1316–1326. doi: 10.1016/j.joca.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2007;15:1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 73.Malfait AM, Ritchie J, Gil AS, Austin JS, Hartke J, Qin W, et al. ADAMTS-5 deficient mice do not develop mechanical allodynia associated with osteoarthritis following medial meniscal destabilization. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18:572–580. doi: 10.1016/j.joca.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 74.Miller RE, Tran PB, Das R, Ghoreishi-Haack N, Ren D, Miller RJ, et al. CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:20602–20607. doi: 10.1073/pnas.1209294110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Inglis JJ, McNamee KE, Chia SL, Essex D, Feldmann M, Williams RO, et al. Regulation of pain sensitivity in experimental osteoarthritis by the endogenous peripheral opioid system. Arthritis and rheumatism. 2008;58:3110–3119. doi: 10.1002/art.23870. [DOI] [PubMed] [Google Scholar]
- 76.McNamee KE, Burleigh A, Gompels LL, Feldmann M, Allen SJ, Williams RO, et al. Treatment of murine osteoarthritis with TrkAd5 reveals a pivotal role for nerve growth factor in non-inflammatory joint pain. Pain. 2010;149:386–392. doi: 10.1016/j.pain.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 77.Burleigh A, Chanalaris A, Gardiner MD, Driscoll C, Boruc O, Saklatvala J, et al. Joint immobilization prevents murine osteoarthritis and reveals the highly mechanosensitive nature of protease expression in vivo. Arthritis and rheumatism. 2012;64:2278–2288. doi: 10.1002/art.34420. [DOI] [PubMed] [Google Scholar]
- 78.Miller RE, Tran P, Ghoreishi-Haack N, Das R, Malfait AM. A role for chemokines and NGF in pain generation in a murine model of osteoarthritis. Osteoarthritis Cartilage. 2011:S7–S52. [Google Scholar]
- 79.Dawes JM, Kiesewetter H, Perkins JR, Bennett DL, McMahon SB. Chemokine expression in peripheral tissues from the monosodium iodoacetate model of chronic joint pain. Molecular pain. 2013;9:57. doi: 10.1186/1744-8069-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beekhuizen M, Gierman LM, van Spil WE, Van Osch GJ, Huizinga TW, Saris DB, et al. An explorative study comparing levels of soluble mediators in control and osteoarthritic synovial fluid. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21:918–922. doi: 10.1016/j.joca.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 81.Kanbe K, Takagishi K, Chen Q. Stimulation of matrix metalloprotease 3 release from human chondrocytes by the interaction of stromal cell-derived factor 1 and CXC chemokine receptor 4. Arthritis and rheumatism. 2002;46:130–137. doi: 10.1002/1529-0131(200201)46:1<130::aid-art10020>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 82.Ruth JH, Volin MV, Haines GK, 3rd, Woodruff DC, Katschke KJ, Jr, Woods JM, et al. Fractalkine, a novel chemokine in rheumatoid arthritis and in rat adjuvant-induced arthritis. Arthritis and rheumatism. 2001;44:1568–1581. doi: 10.1002/1529-0131(200107)44:7<1568::AID-ART280>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 83.McNearney T, Speegle D, Lawand N, Lisse J, Westlund KN. Excitatory amino acid profiles of synovial fluid from patients with arthritis. J Rheumatol. 2000;27:739–745. [PMC free article] [PubMed] [Google Scholar]
- 84.Orita S, Koshi T, Mitsuka T, Miyagi M, Inoue G, Arai G, et al. Associations between proinflammatory cytokines in the synovial fluid and radiographic grading and pain-related scores in 47 consecutive patients with osteoarthritis of the knee. BMC musculoskeletal disorders. 2011;12:144. doi: 10.1186/1471-2474-12-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cuellar JM, Scuderi GJ, Cuellar VG, Golish SR, Yeomans DC. Diagnostic utility of cytokine biomarkers in the evaluation of acute knee pain. The Journal of bone and joint surgery American volume. 2009;91:2313–2320. doi: 10.2106/JBJS.H.00835. [DOI] [PubMed] [Google Scholar]
- 86.Chevalier X, Eymard F, Richette P. Biologic agents in osteoarthritis: hopes and disappointments. Nature reviews Rheumatology. 2013;9:400–410. doi: 10.1038/nrrheum.2013.44. [DOI] [PubMed] [Google Scholar]
- 87.Maksymowych WP, Russell AS, Chiu P, Yan A, Jones N, Clare T, et al. Targeting tumour necrosis factor alleviates signs and symptoms of inflammatory osteoarthritis of the knee. Arthritis research & therapy. 2012;14:R206. doi: 10.1186/ar4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chevalier X, Ravaud P, Maheu E, Baron G, Rialland A, Vergnaud P, et al. A Randomized, Multicentre, Double Blind, Placebo-Controlled Trial of Anti TNF Alpha (adalimumab) in Refractory Hand Ostoearthritis: The Dora Study. Arthritis & Rheumatology. 2012;64:2472. [Google Scholar]
- 89.Chevalier X, Goupille P, Beaulieu AD, Burch FX, Bensen WG, Conrozier T, et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis and rheumatism. 2009;61:344–352. doi: 10.1002/art.24096. [DOI] [PubMed] [Google Scholar]
- 90.Kraus VB, Birmingham J, Stabler TV, Feng S, Taylor DC, Moorman CT, 3rd, et al. Effects of intraarticular IL1-Ra for acute anterior cruciate ligament knee injury: a randomized controlled pilot trial ( NCT00332254) Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2012;20:271–278. doi: 10.1016/j.joca.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 91.Xue C-B, Wang A, Han Q, Zhang Y, Cao G, Feng H, et al. Discovery of INCB8761/PF-4136309, a Potent, Selective, and Orally Bioavailable CCR2 Antagonist. ACS Medicinal Chemistry Letters. 2011;2:913–918. doi: 10.1021/ml200199c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Williams RO, Paleolog E, Feldmann M. Cytokine inhibitors in rheumatoid arthritis and other autoimmune diseases. Curr Opin Pharmacol. 2007;7:412–417. doi: 10.1016/j.coph.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 93.Hess A, Axmann R, Rech J, Finzel S, Heindl C, Kreitz S, et al. Blockade of TNF-alpha rapidly inhibits pain responses in the central nervous system. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3731–3736. doi: 10.1073/pnas.1011774108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schaible HG, Grubb BD. Afferent and spinal mechanisms of joint pain. Pain. 1993;55:5–54. doi: 10.1016/0304-3959(93)90183-P. [DOI] [PubMed] [Google Scholar]
- 95.Bjurholm A, Kreicbergs A, Ahmed M, Schultzberg M. Noradrenergic and peptidergic nerves in the synovial membrane of the Sprague-Dawley rat. Arthritis and rheumatism. 1990;33:859–865. doi: 10.1002/art.1780330613. [DOI] [PubMed] [Google Scholar]
- 96.Gronblad M, Konttinen YT, Korkala O, Liesi P, Hukkanen M, Polak JM. Neuropeptides in synovium of patients with rheumatoid arthritis and osteoarthritis. J Rheumatol. 1988;15:1807–1810. [PubMed] [Google Scholar]
- 97.Kruger L, Silverman JD, Mantyh PW, Sternini C, Brecha NC. Peripheral patterns of calcitonin-gene-related peptide general somatic sensory innervation: cutaneous and deep terminations. J Comp Neurol. 1989;280:291–302. doi: 10.1002/cne.902800210. [DOI] [PubMed] [Google Scholar]
- 98.Mapp PI, Kidd BL, Gibson SJ, Terry JM, Revell PA, Ibrahim NB, et al. Substance P-, calcitonin gene-related peptide- and C-flanking peptide of neuropeptide Y-immunoreactive fibres are present in normal synovium but depleted in patients with rheumatoid arthritis. Neuroscience. 1990;37:143–153. doi: 10.1016/0306-4522(90)90199-e. [DOI] [PubMed] [Google Scholar]
- 99.Pereira da Silva JA, Carmo-Fonseca M. Peptide containing nerves in human synovium: immunohistochemical evidence for decreased innervation in rheumatoid arthritis. J Rheumatol. 1990;17:1592–1599. [PubMed] [Google Scholar]
- 100.Eitner A, Pester J, Nietzsche S, Hofmann GO, Schaible HG. The innervation of synovium of human osteoarthritic joints in comparison with normal rat and sheep synovium. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21:1383–1391. doi: 10.1016/j.joca.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 101.Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nature reviews Rheumatology. 2012;8:390–398. doi: 10.1038/nrrheum.2012.80. [DOI] [PubMed] [Google Scholar]