Abstract
There is a wealth of knowledge about how different Ser/Thr protein kinases participate in Toll-like receptor (TLR) signaling. In many cases, we know the identities of the Ser/Thr residues of various components of the TLR-signaling pathways that are phosphorylated, the functional consequences of the phosphorylation and the responsible protein kinases. In contrast, the analysis of Tyr-phosphorylation of TLRs and their signaling proteins is currently incomplete, because several existing analyses are not systematic or they do not rely on robust experimental data. Nevertheless, it is clear that many TLRs require, for signaling, ligand-dependent phosphorylation of specific Tyr residues in their cytoplasmic domains; the list includes TLR2, TLR3, TLR4, TLR5, TLR8 and TLR9. In this article, we discuss the current status of knowledge on the effect of Tyr-phosphorylation of TLRs and their signaling proteins on their biochemical and biological functions, the possible identities of the relevant protein tyrosine kinases (PTKs) and the nature of regulations of PTK-mediated activation of TLR signaling pathways.
Keywords: TLR, Tyrosine phosphorylation, Protein Tyrosine kinases, EGFR, Src, Btk, PI3K, IRFs, TRIF, MyD88
Introduction
In the past two decades, there has been significant advancement in our knowledge of how the host recognizes and mounts innate immune response to a wide range of microbial infections. Host cells are equipped with distinct classes of cellular sensors, such as, Toll-like receptors (TLRs), RIG-I like receptors (RLRs), Nod like receptors (NLRs) and cytoplasmic DNA sensors, which can detect specific type of microbial components and trigger cellular antimicrobial responses. Among these sensors, the TLRs were the first to be identified and have been most extensively studied. Using the powerful genetic system in Drosophila, the Toll receptor was first identified as a protector against fungal infection (1). Following this discovery in Drosophila, thirteen members of mammalian TLRs have been identified so far. TLRs function primarily by inducing the synthesis of anti-microbial proteins. In uninfected cells, the TLRs are maintained in inactive states to avoid continuous and undesired synthesis of the TLR-induced proteins, which include many inflammatory cytokines. In addition to microbial infections, endogenous ligands, such as nucleic acids from damaged cells, can also activate TLRs and initiate auto-immune diseases. Pathogen insult results in the activation of TLRs, which, by recruiting TLR-specific cytoplasmic adaptors and a variety of additional cytoplasmic proteins, activates several transcription factors e.g. NF-κB and IRFs, that induce transcription of the target genes (2, 3). The biochemistry of the TLR-signaling pathways involves cascades of protein phosphorylation and the roles of specific serine/threonine protein kinases, in this process, have been clearly delineated. In contrast, although several TLRs require tyrosine phosphorylation for their activation (4), our knowledge of the roles that protein tyrosine kinases (PTK) play in TLR signaling, is relatively weak. In this review, we discuss what is known about how tyrosine phosphorylation of TLRs and their signaling components, and specific PTKs regulate TLR signaling and the resultant biological effects.
TLRs: the sensors of pathogens
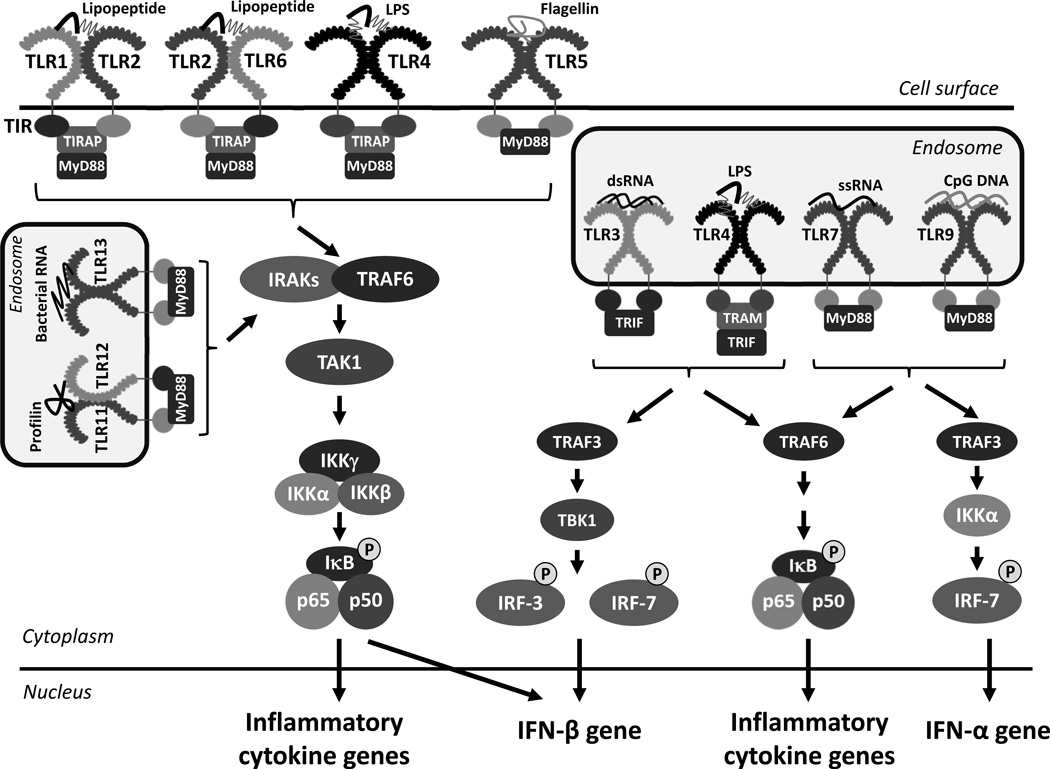
TLRs are type I transmembrane glycoproteins expressed on the plasma membrane or the endosomal membrane. In mammals, thirteen members of TLR family have been identified till now (Figure 1). All members of TLR have an ectodomain containing leucine-rich-repeats (LRR), a transmembrane domain, and a short cytoplasmic region, containing Toll/interleukin-1 receptor (TIR) domain (2, 5). The ectodomain of TLRs is responsible for the recognition of ligands; specific LRRs are responsible for the interaction with respective ligands. The TIR domain is required for the interaction with cytoplasmic adaptor proteins. A linker region in the cytoplasmic domain determines the sub-cellular localization of the endosomal TLRs (6). The plasma membrane-expressing TLRs include TLR1, TLR2, TLR5, TLR6 and TLR10 and the endosomal TLRs are TLR3, TLR7, TLR8, TLR9, TLR11, TLR12 and TLR13. TLR4 is expressed both on the plasma membrane and on the endosomal membrane. Several endosomal TLRs are transported to the endosomal membranes from the endoplasmic reticulum by the chaperone protein, UNC93B1, followed by proteolytic processing of the TLRs, which is required for effective downstream signaling (3, 7, 8). Not all TLRs are expressed in all cell types, the myeloid cells e.g. macrophages and dendritic cells are the primary sources of the TLRs. The TLRs provide both protective and pathological functions, such as, antiviral and antibacterial immunity, bacterial septic shock, autoimmune disorders and anti-parasitic responses.
Figure 1. TLRs, their ligands, adaptors and cellular signaling.
The cell surface and endosomal TLRs, their ectodomains and TIR domains, specific cytoplasmic adaptor molecules, and respective PAMPs are shown. The activation of TLRs by the respective PAMPs triggers downstream signaling pathways, involving pathway-specific Ub ligases (the TRAFs), and Ser/Thr kinases (IKK, TBK1 and others not shown), leading to the activation of NF-κB and IRFs. Co-ordinated action of these transcription factors lead to the transcription of inflammatory and antiviral genes including IFNs. Many known components of the signaling pathways are not shown here, because of simplicity.
TLR Ligands
TLRs recognize a variety of chemicals, known as Pathogen Associated Molecular Patterns (PAMPs), e.g. lipids, sugars, lipopolysaccharides, lipoproteins, proteins, and nucleic acids generated by a wide range of microbes such as bacteria, viruses, parasites, fungi or mammalian cells (Table 1). TLR1, TLR2, TLR6 and TLR10 can recognize microbial cell wall components, such as lipoproteins and peptidoglycans, whereas TLR4 and TLR5 can detect bacterial lipopolysaccharides (LPS) and flagellin, respectively. The endosomal TLRs recognize nucleic acids of viral, bacterial or cellular origin or a parasitic protein. TLR3 recognizes viral double-stranded RNA (dsRNA), TLR7 and TLR8 recognize viral single stranded RNA (ssRNA), TLR9 recognizes CpG DNA, TLR11 and TLR12 can recognize a parasitic protein, profilin, from Toxoplasma gondii, and TLR13 can detect bacterial 23S ribosomal RNA (9). In addition to detecting foreign nucleic acids, the TLRs can also recognize endogenous ligands, the DAMPs (10, 11). DAMPs are damage-associated molecular patterns, which are non-PAMP molecules, produced by uninfected, damaged cells; DAMPs include both nucleic acids and proteins from the damaged cells. TLR3 has been shown to detect cellular RNA generated from necrotic cells; TLR7, TLR8 and TLR9 can detect self RNA or DNA, leading to autoimmune diseases. Secreted cellular proteins, such as HMGB1 and S100 can activate TLR4 signaling (12, 13). The sub-cellular localization of the TLRs is important for the recognition of foreign ligands as well as tolerance to the self-molecules (14).
Table 1. Specific tyrosine residues and the protein tyrosine kinases involved in TLR signaling.
The TLRs and their signaling components, which are known to be tyrosine phosphorylated, are shown here. The critical tyrosine residues and the kinases involved in their functions are indicated.
| TLRs | Localization | Ligands |
Required Tyrosine residues |
Involved Tyrosine kinases |
References |
| TLR2 | Cell surface | Lipoproteins, Peptidoglycans, Pam3CSK4 | Tyr616, Tyr761 | Btk, Fyn | (65–67) |
| TLR3 | Endosome | dsRNA, polyI:C | Tyr759, Tyr858 | EGFR, Src, Btk | (46–52) |
| TLR4 | Cell surface and endosome | LPS, RSV fusion protein, HMGB1, Hsp70 | Tyr674, Tyr680 | Btk, Lyn, Syk, Hck | (59–62) |
| TLR5 | Cell surface | Bacterial flagellin | Tyr798 | 68 | |
| TLR8 | Endosome | ssRNA, Imidazoquinolines | Tyr898, Tyr904, Tyr1048 | Btk | 69 |
| TLR9 | Endosome | CpG DNA, Plasmodium, hemozoin | Tyr888 | Src, Syk, Btk | (70–73) |
| TLR components | Specific TLRs involved |
Required Tyrosine residues |
Involved Tyrosine kinases |
References | |
| MD-2 | TLR4 | Tyr22, Tyr131 | SFK, Lyn | 74 | |
| TIRAP/MAL | TLR2, TLR4 | Tyr86, Tyr106, Tyr159 | Btk | (75–77) | |
| TRIF | TLR3, TLR4 | Syk | 78 | ||
| MyD88 | All TLRs except TLR3 | Syk | 78 | ||
| Caveolin-1 | TLR4 | Tyr14 | 79 | ||
TLR signaling pathways
Upon interaction with the respective ligands, the TLRs get activated by conformational changes, which allow them to homo- or hetero-dimerize and make their cytoplasmic domains accessible to binding signaling proteins. The cytoplasmic domains of the TLRs contain the TIR signature motif, which is responsible for the interaction with cytoplasmic TIR-domain containing adaptor proteins, such as MyD88, TRIF, TIRAP/Mal, TRAM etc (15). The recruitment of the adaptor proteins leads to the formation of signalosomes, which through a series of ubiquitin ligase and kinase signaling cascades, activate the downstream transcription factors, such as NF-κB, IRF and AP-1. MyD88 is used as an essential adaptor by all members of the TLR, except TLR3, whereas TRIF is used only by TLR3 and TLR4 (16). A recent study reported that DOCK8 functions as an adaptor protein for TLR9 signaling in B cells; DOCK8-dependent signaling is required for B cell differentiation and proliferation (17).
The MyD88-depdendent pathway
All plasma membrane TLRs and selective endosomal TLRs, TLR7, TLR8, TLR9, activate downstream signaling upon interactions with MyD88 (Figure 1). TLR2 and TLR4 require TIRAP, as an additional adaptor protein for this interaction. Upon binding to the TLRs, MyD88 recruits the signaling proteins such as the kinases IRAK1, IRAK4, the activation of which results in the interaction with the E3 ubiquitin ligase, TRAF6. Both IRAK1 and TRAF6 are activated by K63-linked ubiquitination, which recruits the protein kinase TAK1. Activated TAK1 phosphorylates IKKβ, which in turn phosphorylates IκBα, a repressor of the NF-κB subunits, p65 and p50, in the canonical NF-κB pathway. Phosphorylation of IκBα leads to its proteasomal degradation allowing the nuclear translocation of the p65:p50 heterodimer, which binds to the κB sites on the promoters of the target genes. TAK1, recruited to MyD88, also activates the MAP kinase (MAPK) signaling pathway, by activating Erk1, Erk2, p38 and JNK. The MyD88-dependent activation of NF-κB and the MAPKs results in the production of inflammatory cytokines. The MyD88-dependent TLR7 and TLR9 signaling pathways also lead to the induction of type I IFNs in plasmacytoid DCs (pDCs) (18). This requires the recruitment of TRAF3 and IKKα to the MyD88/IRAK/TRAF6 complex, which subsequently activates the transcription factor, IRF-7 (19).
The TRIF-dependent pathway
The TRIF-dependent pathway is triggered by the endosomal TLR3 and TLR4, which culminate in the activation of both NF-κB and IRFs (Figure 1). Although TRIF can be directly recruited to the TIR domain of TLR3, it requires the adaptor protein, TRAM for the interaction with endosomal TLR4. TRIF recruits RIP1 and TRAF6, which are activated by K63-linked ubiquitination to activate TAK1. Other adaptor proteins and E3 ubiquitin ligases, such as TRADD and Pellino-1, are also required for the activation of TAK1 (20). TAK1 activates NF-κB using mechanisms similar to the MyD88-dependent pathway. TRIF recruits TRAF3, TANK, and the kinases TBK1 or IKKε, which phosphorylate IRF-3. Inactive IRF-3 has a closed conformation, in which its DNA binding domain, which also contains the nuclear localization signal, remains inaccessible (21, 22). Phosphorylation of specific C-terminal Ser/Thr residues of IRF-3, by TBK1/IKKε, leads to the changes in conformation, followed by its dimerization. Dimeric IRF-3 is translocated to the nucleus, where it binds to a specific sequence, the ISRE, near the promoters of the target genes and interacts with essential co-activators, β-catenin and CBP, to induce transcription of the IFN-β gene and the interferon stimulated genes (ISGs) (22, 23). Moreover, HDAC6-mediated deacetylation of β-catenin is essential for the interaction of IRF-3 with CBP and subsequent assembly of the transcriptional complex; consequently, in HDAC6 deficient cells, there is no induction of the IRF-3-driven genes by either the TLR3 or the TLR4 signaling pathway (24).
The role of TLRs in pathogenesis
TLRs perform dual roles in mammalian pathogenesis. For protective functions of TLRs in infections, they are activated transiently by microbial PAMPs to trigger the synthesis of cytokines and chemokines, which activate various cells of the host immune system to clear the infection. However, overproduction of inflammatory cytokines, upon massive infections, often causes diseases. The detrimental roles of specific TLRs are also manifested in uninfected hosts; when TLRs are continuously activated by cellular DAMPs, they cause chronic inflammatory and auto-immune diseases (25). Because inflammation and cancer are closely connected, TLR-signaling affects cancer progression as well (26). The causal connections, between specific TLRs and specific diseases, have been established by their experimental manipulations as well as by observing disease propensities of humans and mice carrying mutations in genes encoding specific TLRs or their signaling proteins (27). TLR antagonists are used currently for treating patients with specific inflammatory diseases (28). TLR signaling is associated with both pro- and anti-tumor activities. TLR4 signaling is involved in the establishment of colon, liver and skin cancer and a specific MyD88 mutation (L265P) causes increased NF-κB signaling and survival of tumor cells (29). Treatment of tumor cells, with various TLR agonists, show increased migration and angiogenic properties (28).
The TLR2 polymorphism, that causes R753Q mutation in its TIR domain, is associated with susceptibility to leprosy and tuberculosis (30–32). A polymorphism in the TIRAP gene has also been linked to susceptibility to tuberculosis (33). Mutations associated with IRAK4 have been linked with increased bacterial infection and poor inflammatory responses (34). TLR3, the sensor of virally generated extracellular dsRNA, has both pro- and anti-viral functions against specific viruses. A dominant negative mutant of TLR3 has been identified in humans with susceptibility to HSV-1 infection (35). Anti-angiogenic effects of TLR3 signaling, probably mediated by apoptotic activity, have been demonstrated in various animal models (36, 37). TLR3 signaling also plays a protective role in Age-Related Macular Degeneration (AMD). A variant of TLR3 is protective against AMD, probably through suppression of apoptotic activity in retinal pigmented epithelial cells (38). TLR4 senses LPS from Gram-negative bacteria and promotes their successful elimination by inducing anti-microbial genes. A mutation in human TLR4 gene, D299G, causes reduced LPS-response, leading to increased bacterial infection (39). In contrast to the protective anti-microbial functions, aberrant TLR4 signaling results in uncontrolled inflammatory responses, causing bacterial sepsis (39, 40). A specific cytokine, IFN-β, induced by TLR4 signaling, is associated with TLR4-mediated sepsis (41). An ISG, Ifit2, has recently been shown to be a potential effector of TLR4-mediated sepsis (42). The expression of TLR3 and TLR4 in intestinal epithelium is altered in active IBD and specific polymorphism in the TLR4 gene has been associated with Crohn’s disease and ulcerative colitis (43). TLR4-mediated inflammatory responses lead to neuronal damage after retinal ischemic-reperfusion (I/R) injury, with NF-κB activation playing a critical role in this process (44). Autoimmune diseases, e.g. Systemic Lupus Erythematosus (SLE), are directly associated with two nucleic acid-sensing TLRs, TLR7 and TLR9. The hallmark of SLE is the elevation of autoreactive B cells, which produce large quantities of autoantibodies that form immune complexes with self-nucleic acids present in the SLE serum. These self-nucleic acids can activate TLR7 and TLR9, thereby producing additional signal which contributes to the SLE pathogenesis (45). Inhibitors of TLR7 and TLR9 signaling are being tested as therapeutic agents against SLE (28).
Tyrosine phosphorylation in TLR signaling pathways
In general, it is thought that tyrosine phosphorylation of the cytoplasmic domains of selected TLRs is required for the recruitment of adaptor proteins and subsequent activation of the downstream signaling cascades. Moreover, in several cases, it has been shown that tyrosine phosphorylation results in the recruitment of the Ser/Thr kinase, PI3 Kinase (PI3K), to the signaling complex, a step that is essential for the activation of downstream transcription factors. Thus, Tyr phosphorylation may provide docking sites for other proteins, which may regulate TLR signaling. In the following sections, we discuss what is known about Tyr phosphorylation of specific TLRs and its functional consequences.
Tyrosine phosphorylation of TLR3
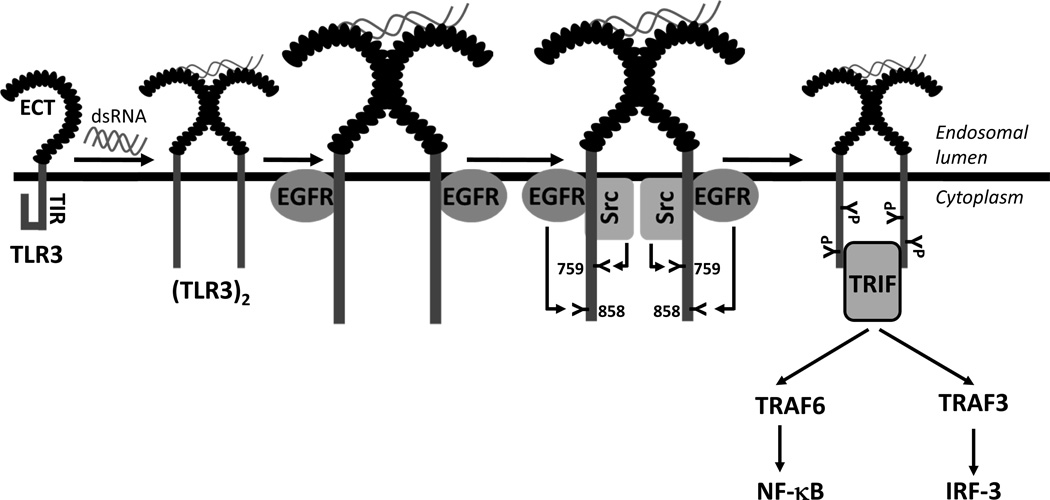
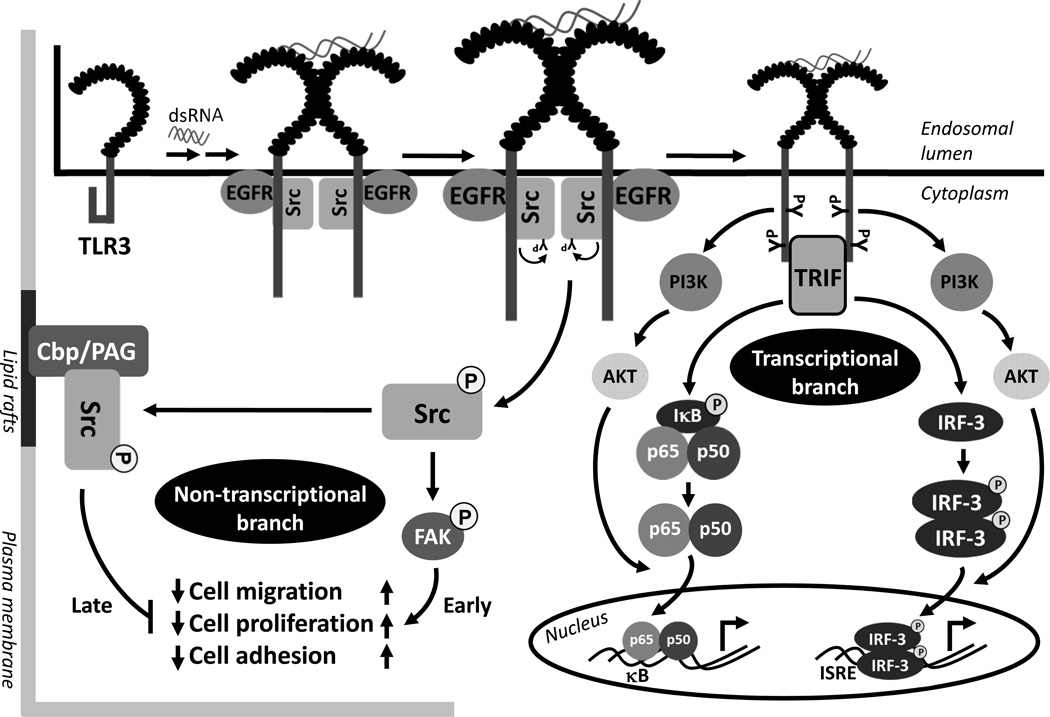
Our in-depth investigation of the early steps of dsRNA-induced TLR3 activation has provided a detailed view of how TLR3 is tyrosine phosphorylated and why it is critical for the downstream signaling (Figure 2). The cytoplasmic domain of TLR3 remains in a closed conformation, which blocks the undesired activation of TLR3. In the endosomal lumen, dsRNA binding to the ectodomain of TLR3 causes its dimerization. TLR3 dimerization leads to changes in the conformation of TLR3 cytoplasmic domain, thereby allowing the specific tyrosine kinases, EGFR and Src, to be recruited to TLR3 (46, 47); both EGFR and Src are abundant in the endosomal membranes of cells growing in normal medium. The cytoplasmic domain of TLR3 has five tyrosine residues and phosphorylation of at least two of them, Tyr759 and Tyr858, present in the cytoplasmic TIR domain, is required for TLR3 signaling (48, 49). Mutation of either of these two critical tyrosine residues produces TLR3 mutants that cannot signal. A TLR3 mutant, which harbors only these two tyrosine residues in the cytoplasmic domain, is tyrosine phosphorylated and functional for downstream activation of transcription factors. These results indicate that these two tyrosine residues are not only necessary, but sufficient, for TLR3 activation. The tyrosine residues in the TLR3 cytoplasmic domain are not required for its interaction with the tyrosine kinases, because they bind to the linker region of TLR3, which is located in between the trans-membrane domain and the TIR domain. In un-stimulated cells, the linker region is kept inaccessible to the kinases, presumably, because of an interaction with the TIR domain; the conformational change caused by dsRNA-binding to the ectodomain breaks this interaction and allows access to the linker domain. In support of the above model, a TLR3 mutant, from which the TIR domain has been deleted, can bind EGFR without dsRNA-activation. Between the two protein tyrosine kinases, EGFR binds to TLR3 first before Src joins the complex; hence, it is not clear whether the direct contact of Src is with EGFR or TLR3. The Src recruitment does not require the kinase activity of EGFR; neither does it require the SH2 domain of Src, which recognizes phospho-tyrosine residues of its binding partners. Although both the ErbB1 and the ErbB2 isoforms of EGFR can bind to activated TLR3, functionally, only ErbB1 can trigger TLR3 signaling. There is a high degree of specificity of the targets of the two PTKs, EGFR and Src; EGFR phosphorylates Tyr858 of TLR3 directly whereas Src phosphorylates Tyr759 directly. Phosphorylation of both residues is required for TRIF recruitment to TLR3. They may be required for the recruitment of other proteins to the signaling complex as well, PI3K being a prime candidate. PI3K, recruited to the activated TLR3, is required for complete transcriptional activation of IRF-3. Chemical inhibitors of PI3K or a dominant negative mutant of AKT, a downstream target of PI3K, do not inhibit the IRF-3 activation required for its nuclear translocation; however, they abolish the promoter binding and the transcriptional abilities of IRF-3, indicating that PI3K-AKT activity is required for full activation of this transcription factor (Figure 3). The activation of the NF-κB signaling branch of TLR3 is also dependent on its tyrosine phosphorylation and PI3K activity (50). In the absence of PI3K activity, although IκBα is degraded and p65 is translocated to the nucleus, there is no gene induction by NF-κB. Most of the above studies were done with HEK293 cells, but it has been demonstrated that Src interacts with TLR3 and phosphorylates the target Tyr759 residue in mouse macrophages as well (51). Another tyrosine kinase, Btk, can phosphorylate Tyr759 of TLR3 in primary macrophages (52). Btk-deficient macrophages are defective in TLR3 tyrosine phosphorylation, and transcriptional induction of IRF-3 and NF-κB-dependent genes. Consequently, the Btk-deficient macrophages are deficient in clearing dengue virus infection. In vivo, intraperitoneal administration of poly (I:C), in combination with D-galactosamine, induces septic shock due to increased production of TNF-α. The Btk-deficient mice are resistant to poly (I:C)-induced septic shock, suggesting Btk-dependent tyrosine phosphorylation of TLR3 plays an essential role in both antiviral and pro-inflammatory functions. Thus, it is clear that tyrosine phosphorylation of TLR3 is essential for its ability to signal and multiple tyrosine kinases may target the critical tyrosine residues in a cell-type specific manner.
Figure 2. Activation of early steps of TLR3 signaling by tyrosine phosphorylation.
Inactive TLR3 remains in a closed conformation that maintains the TIR domain inaccessible to cytoplasmic proteins. DsRNA activation causes dimerization and conformational changes of TLR3, which sequentially recruits EGFR and Src to the active TLR3 complex. EGFR and Src simultaneously phosphorylate two individual tyrosine residues, Tyr858 and Tyr759, respectively. Phosphorylation of these tyrosine residues is critical for the recruitment of the cytoplasmic adaptor protein, TRIF to TLR3 and subsequent downstream signaling via NF-κB and IRF-3.
Figure 3. Transcriptional and non-transcriptional effects of TLR3 signaling.
DsRNA-activated TLR3 triggers both gene transcription-dependent and independent pathways via recruitment of Src. The transcriptional branch of TLR3 is activated by EGFR and Src-mediated tyrosine phosphorylation of TLR3 and subsequent activation of both NF-κB and IRF-3. PI3K is recruited to the activated TLR3 and via activation of AKT produces fully active NF-κB and IRF-3. A TRIF-independent branch of TLR3 signaling is mediated by the activation of Src upon interacting with TLR3. Src activation is mediated by its autophosphorylation on Tyr416, which causes an early activation of downstream kinases such as FAK, an essential mediator of various cellular activities e.g. migration, adhesion and proliferation. Later, TLR3-activated Src is depleted by sequestration in the lipid rafts by the resident Cbp/PAG complex, thereby resulting in the inhibition of all Src-mediated cellular activities.
Tyrosine phosphorylation of TLR4
TLR4 signaling is complex because of the existence of two distinct signaling branches triggered from two subcellular locations of the receptor. LPS from Gram-negative bacteria is transported by the coordinated action of CD14 and MD-2 to the cell surface associated TLR4 (53). CD14, in addition to transporting LPS to TLR4, controls the endosomal trafficking of TLR4 (54). Activated TLR4 recruits TIRAP/Mal at the cell surface which facilitates its interaction with MyD88 and the assembly of a multi-protein signaling complex causing activation of the NF-κB and the MAPK signaling cascades. In the other branch, TLR4 signals from the endosomal membrane, where, upon activation, it recruits the adaptors, TRAM and TRIF, to initiate the TRIF-dependent signaling pathway (16, 55). The TRIF-dependent TLR4-branch activates, in addition to the NF-κB and MAPK signaling pathways, the IRF-3-dependent pathway. Because the induction of type I IFN genes requires active IRF-3, only the TRIF-branch elicits IFN response to TLR4 activation (56). Tyrosine kinase activity is required for the activation of both branches of TLR4 signaling; chemical inhibitors of tyrosine kinases block the expression of all TLR4-induced genes (57, 58). Tyr-phosphorylation of TLR4 itself may be essential for signaling, because TLR4 TIR domain mutants Y674A and Y680A are defective in tyrosine phosphorylation and activation of NF-κB and MAPK (59). Moreover, a point mutation (P712H) in the TIR domain results in an inactive TLR4, which shows impaired tyrosine phosphorylation and defective downstream signaling (59). Although it is not clear who phosphorylates these key residues of TLR4, a number of PTKs have been implicated in TLR4 signaling. Specifically, upon LPS stimulation, interactions of Src, Btk, Lyn and Syk with TLR4 have been reported (60–62). Continuous exposure to LPS, which causes LPS-tolerance, reduces TLR4 tyrosine phosphorylation, presumably due to the loss of TLR4-associated Lyn during LPS-tolerization (59). Syk plays opposing role in the activation of the two branches of TLR4 signaling: it inhibits signaling from the plasma membrane but promotes that from the endosomal membrane (63). Although recruited to both TLR4 signaling complexes, Syk differentially regulates the K63 ubiquitination of TRAF6 and TRAF3, which are required, respectively, for the MyD88 and the TRIF branches of TLR4 signaling. It promotes TRAF3 ubiquitination but impairs TRAF6 ubiquitination. Our unpublished results indicate that complete TLR4 signaling requires EGFR activity; induction of only a specific subset, but not all, of the TLR4-induced genes is strongly inhibited by chemical inhibitors of EGFR. A recent study further indicates that although EGFR activity in macrophages is essential for DSS-induced colitis, it is not required for TLR4-induced NF-κB activation and TNF-α production (64). Further studies will be needed for a better understanding of how different tyrosine kinases regulate the complex nature of TLR4 signaling.
Tyrosine phosphorylation of TLR2 and TLR5
Infection of monocytes with live or heat-inactivated Staphylococcus aureus induces rapid tyrosine phosphorylation of TLR2 (65). Two tyrosine residues in the TLR2 cytoplasmic domain, Y616 and Y761, are critical for TLR2-dependent NF-κB activation; mutation of these residues to alanine results in reduced production of IL8 and IL18. Tyrosine phosphorylated TLR2 recruits the p85 subunit of PI3K and Rac1 and the TLR2/PI3K/Rac1 complex activates AKT, which regulates the downstream activation of NF-κB (65). The TLR2/PI3K signaling pathway provides protection against I/R injury to cardiomyocytes in vivo (66). Administration of TLR2 ligands to mice induces tyrosine phosphorylation of TLR2, interaction with PI3K, followed by activation of AKT, resulting in cardioprotection. Inhibition of PI3K by chemical inhibitors in Wt mice shows reduced cardioprotection upon I/R injury; the same is true for kinase-deficient AKT transgenic mice, without the use of any inhibitor. A mutation, R753Q, in the TIR domain of TLR2 results in impaired tyrosine phosphorylation of TLR2, which leads to reduced hetero-dimerization with TLR6 and the recruitment of TIRAP and MyD88 to the signaling complex (32). TLR2 R753Q polymorphism is associated with increased incidence of tuberculosis and other infectious diseases (31). PBMCs from patients with XLA, which are defective in Btk activity, show impaired induction of TNF-α and IL-1β, but not IL-6, upon TLR2 signaling (67). TLR5 is apparently tyrosine phosphorylated upon stimulation by bacterial flagellin (68). Mass Spectrometric analyses of TLR5 isolated from flagellin-treated cells identified the phospho-tyrosine residue as Tyr798 in the TLR5 cytoplasmic domain. Mutation of Tyr798 results in reduced activation of NF-κB and p38 upon flagellin treatment of cells. Bioinformatics analyses suggest that Tyr798 may provide a docking site for the interaction with PI3K and downstream activation of AKT.
Tyrosine phosphorylation of TLR8 and TLR9
Human TLR8 has been shown to be tyrosine phosphorylated upon stimulation by an immunomodulator, 3M-003 (69). Mutational analyses revealed that among thirteen tyrosine residues in the cytoplasmic domain of TLR8, Tyr898, Tyr904 and Tyr1048 are critical for NF-κB activation. Tyrosine phosphorylation of TLR8 is important for its interaction with PI3K subunit, p85; mutation of Tyr898 and Tyr904 abolished both tyrosine phosphorylation, and PI3K recruitment, of TLR8. CpG stimulation of TLR9, in human monocytes and B cells, activates several PTKs (70–72); however, how these tyrosine kinases regulate TLR9 signaling is not clear. Rapid tyrosine phosphorylation of TLR9, upon CpG treatment of the human monocytic cell line, THP-1, causes the recruitment of an activated Spleen tyrosine kinase (Syk) (72). Treatment of cells with chemical inhibitors of Src family kinases (SFKs) inhibits both tyrosine phosphorylation and Syk-interaction of TLR9, indicating that SFKs could be the direct PTKs for TLR9. A tyrosine motif in the cytoplasmic domain of TLR9 is selectively required for the activation of NF-κB signaling, but not type I IFN production (73). A specific Tyr of TLR9, Tyr888, may be structurally required for the intracellular trafficking of TLR9 to the endolysomal compartment, where TLR9 activates its NF-κB signaling branch. Substitution of Tyr888 by alanine, but not phenylalanine, inhibits the tyrosine phosphorylation of TLR9 and the subsequent downstream signaling, indicating that Tyr888 is not a direct target of a PTK, but it structurally regulates TLR9 functions.
Tyrosine phosphorylation of the components of TLR signaling pathways
In addition to the receptors, other components of the TLR signaling pathways, e.g. the adaptors, also undergo tyrosine phosphorylation upon stimulation with TLR ligands (Table 1). These phosphorylation events provide regulatory mechanisms to fine-tune the TLR signaling responses. MD-2, an essential component of TLR4 signaling, undergoes tyrosine phosphorylation upon LPS-stimulation, thereby providing an additional regulatory step in TLR4 signaling (74). Chemical inhibitors of endocytosis block MD-2 tyrosine phosphorylation, suggesting that the phosphorylation takes place during the endocytic trafficking of MD-2. Two specific tyrosine residues of MD-2, Tyr22, Tyr131, could be the targets because mutation of these residues results in impaired NF-κB activation by TLR4 signaling (74). The SFK, Lyn, interacts with LPS-stimulated MD-2 and a Lyn-binding peptide inhibitor abolishes the tyrosine phosphorylation of MD-2, indicating Lyn as a potential PTK for MD-2. TIRAP, which is required for the recruitment of MyD88 to TLR2 and TLR4, is tyrosine phosphorylated upon LPS-stimulation, and regulates its interaction with TLR4 (75). Tyr86, Tyr106 and Tyr159 residues in the TIR domain of TIRAP are critical for its tyrosine phosphorylation; mutation of these residues diminishes TIRAP tyrosine phosphorylation, and downstream activation of the NF-κB and p38 signaling pathways. Btk may be a relevant PTK because it can phosphorylate the tyrosine residues in TIRAP TIR domain in human monocytes, it interacts with TIRAP upon LPS stimulation and a chemical inhibitor of Btk blocks the tyrosine phosphorylation of TIRAP (76). Tyrosine phosphorylation of TIRAP leads to its eventual proteasomal degradation triggered by SOCS1, a mechanism that is used to terminate TLR4 signaling (77). The TLR adaptors, TRIF and MyD88 have been shown to be tyrosine phosphorylated by Syk causing down-regulation of TLR signaling (78). The tyrosine phosphorylation of TRIF and MyD88 is mediated by TLR-activated integrin αM, which activates Syk and Src in macrophages. The integrin-activated Syk phosphorylates TRIF and Myd88, leading to their proteasomal degradation, triggered by the E3 ubiquitin ligase Cbl-b, to shut off the TLR signaling. Caveolin-1 is a component of TLR4 signaling and LPS-induced phosphorylation of Tyr14 of Caveolin-1 is required for TLR4/MyD88 interaction (79). Expression of Tyr14Phe mutant of Caveolin-1 in Caveolin-1 knockout cells results in impaired MyD88-dependent NF-κB activation by TLR4 signaling. TRIM21 is a downstream component of TLR3 and TLR4 signaling and tyrosine phosphorylation of three Tyr residues in TRIM21 is required for TLR-induced IRF-3 activation (80).
Additional biological consequences of TLR-PTK interactions
We have discussed, at length, how PTKs affect TLR functions by interacting with their signaling components. However, in addition, at least in some cases, the binary interactions also affect the PTKs’ functions. The most well studied example is the TLR3/Src interaction (81). Src phosphorylates Tyr759 of TLR3, an essential step of TLR3 signaling that requires the binding of Src to the TLR3-EGFR complex. This protein-protein interaction changes the conformation of Src and allows its activation by auto-phosphorylation of its Tyr416. Thus, TLR3 activation by its ligand causes Src activation at a step prior to TRIF recruitment to the receptor and the initiation of any transcriptional signaling. TLR3-activated Src has major effects on several cellular processes; as expected, these effects are TLR3-dependent but TRIF-independent. TLR3-activated Src promotes cell migration by downstream activation of another kinase, FAK (Figure 3). The cell migration effect of TLR3/Src signaling branch is biphasic, the cell migration is enhanced in the early phase, and is inhibited at the later phase. The early phase of enhanced cell migration is mediated by the TLR3-activated Src, whereas in the late phase, Src is deactivated, leading to the inhibition of cell migration. TLR3-activated Src rapidly translocates to lipid rafts which depletes the cytoplasmic pool of active Src. The effect of dsRNA-activation of TLR3 on cell migration has been observed in many cell types, an effect that requires Src, but not Fyn or Yes, which are two other SFKs. The in vivo effects of TLR3 on angiogenesis, could be explained, at least in part, by the observed inhibitory effects of dsRNA on the migration of vascular endothelial cells to form microtube networks which are required for angiogenesis. Src activation by TLR3 also affects cell adhesion and cell proliferation in a biphasic way. These studies clearly indicate that many biological functions of Src, a PTK, can be regulated by TLR3 signaling by an effect that is independent of its canonical, TRIF-dependent action. With regards to EGFR-TLR3 and EGFR-TLR4 interactions, EGFR inhibitors are clinically used for treating various types of cancer; it is therefore anticipated that these patients may exhibit a reduced response to TLR3-dependent anti-microbial functions and TLR4-induced septic shock response (82). Reciprocally, the discovery of EGFR as a binding partner of activated TLR3 raises the possibility that this interaction can affect the functions of EGFR as well. Although the connection is not yet clear, EGFR has been shown to be transactivated by TLR4 agonists or Helicobacter pylori infection (83, 84). H. pylori is a major causative agent for gastrointestinal diseases e.g. gastric ulcer, gastric adenocarcinoma, gastric lymphoma. A pathogenic role of TLR4-activated EGFR contributes to these gastrointestinal diseases (83). EGFR is activated upon TLR4 stimulation in intestinal epithelial cells by a p38, MMP-dependent mechanism and the activated EGFR is required for the expression of COX-2, which promotes cell survival, proliferation and migration (84). These cellular functions lead to a diseased state, known as necrotizing enterocolitis, a major cause of gastrointestinal morbidity.
Negative regulation of PTK-activated TLR signaling
Protein tyrosine phosphatases catalyze the dephosphorylation of tyrosine phosphorylated proteins and regulate various steps of innate and adaptive immune responses (85). Although tyrosine phosphatases, that directly dephosphoryate TLRs, have not yet been identified, they are involved in the negative regulation of TLR signaling. A cytoplasmic tyrosine phosphatase, SH2-containing protein tyrosine phosphatase 2 (SHP-2) inhibits the TRIF-dependent signaling pathways (86). TLR3 and TLR4-mediated, TRIF-dependent induction of interferons and inflammatory cytokines are inhibited by SHP-2 in macrophages. The detailed mechanism is not yet clear, but SHP-2 interacts with the kinase domain of TBK1, and inhibits its kinase activity. Another tyrosine phosphatase, SHP-1 differentially regulates TLR-dependent NF-κB and IRF-3 activation (87). SHP-1 inhibits TLR-induced NF-κB-dependent genes, whereas, promotes the induction of IFN-β upon TLR stimulation. Mechanistically, SHP-1 interacts with, and inhibits the activity of IRAK1, which promotes NF-κB activation, but inhibits IRF-3 activation by TLRs. Surprisingly none of the SHP-1 and SHP-2-dependent regulatory steps of TLR signaling is dependent on their tyrosine phosphatase activities. Another tyrosine phosphatase, PTP-PEST, has been shown to inhibit NF-kB activation by TLR3, TLR4 and TLR9 signaling in macrophages (88). PTP-PEST interacts with and inhibits the activity of IKKβ, but not TBK1. This provides a selective regulation of TLR signaling by PTP-PEST. Proteins, other than phosphatases, can also inhibit PTK actions in TLR signaling. A recent study indicates the role of CEACAM-1, an ITIM (immunoreceptor tyrosine-based inhibitory motif)-containing neutrophil receptor, to negatively regulate the TLR4/Syk-activated inflammasome (89). CEACAM-1 is recruited to the TLR4-signaling complex and inhibits the activity of Syk and thus, down-regulates IL-1β secretion. Microbes often take advantage of the negative regulation of the host inflammatory responses, to favor their replication. A group of bacteria, including Neisseria, activates the CEACAM-1-mediated pathway to inhibit the inflammatory responses. Similarly, an ITIM-containing E. coli protein, Tir, interacts with cellular SHP-1 and inhibits the TLR-dependent NF-κB and MAPK activation (90). The negative regulatory action of Tir is mediated by a tripartite complex containing Tir-SHP-1-TRAF6, thereby inhibiting the E3 ubiquitin ligase activity of TRAF6. Future studies may identify tyrosine phosphatases that can regulate the early activation steps of TLRs by causing dephosphorylation of tyrosine phosphorylated TIR domain of TLRs.
Future perspectives
Much remains to be learned about the role of PTKs in TLR signaling. The genetic, immuno-histochemical and biochemical approaches, that have been successfully used to answer similar questions for other receptors, have proven to be difficult to execute for studying TLRs. Molecular genetic studies have been hampered by the inability to reproduce TLRs’, especially the endosomal ones’, full physiological activities and their regulations in experimental cell culture lines that are convenient for such studies. This is not surprising given the fact that natural TLR expression and signaling are restricted to only a few cell types and the laboratory lines may not be expressing all the essential signaling proteins. Moreover, the TLRs need to be transported within the cell to the right subcellular compartments and some of them require proteolytic processing for signaling; these processes may be cell–type specific as well. Another major block for biochemical studies is the scarcity of good antibodies for different TLRs, which are essential for analyzing the signaling pathways in cells that express TLRs naturally. For studying the role of a specific PTK in TLR signaling, decisive in vivo experiments are difficult to do because many PTKs are essential for the survival of the mouse because of their roles in other pathways. In spite of these difficulties, progress is being made by designing approaches that circumvent the problems. We anticipate that more complete descriptions of the biochemistry of different TLR signaling pathways will be forthcoming and it will be interesting to know whether they reveal any cell-type specific component. For example, are different PTKs used, for a specific TLR-signaling pathway, in myeloid cells and epithelial cells? Studies on positive or negative cross-talks among different TLRs are quite likely because many share the same signaling proteins. Similarly, cross-talks between TLRs and other signaling receptors, such as cytokine and growth factor receptors, are expected to be studied in the near future. Another fertile field will explore how rapidly evolving microbes find ways to evade the innate host defense mediated by different TLRs.
Acknowledgements
We apologize to our colleagues whose relevant work could not be cited because of space constraints. We thank other members of our laboratory for helpful discussion. Our research is supported by National Institutes of Health grants AI073303, CA068782 and CA062220.
Biographies

Saurabh Chattopadhyay
Saurabh Chattopadhyay is an Assistant Professor of Case Western Reserve University and Project Staff in the Department of Molecular Genetics at Lerner Research Institute, Cleveland Clinic. He received his Ph.D. in 2002 from Indian Institute of Technology, Delhi, in Biotechnology. He worked as a Research Fellow in the Department of Molecular Cardiology at Lerner Research Institute. He joined the laboratory of Ganes Sen in 2005, when he started to work on the role of IRF-3 in mediating virus-induced apoptosis. His studies revealed a novel role of IRF-3 in virus-induced cell death. He is a recipient of Milstein Young Investigator Award in 2010 by International Society for Interferon and Cytokine Research (ISICR) and Boltzmann Award in 2008 by ISICR and International Cytokine Society (ICS).

Ganes C. Sen
Ganes C. Sen is the Thomas Lord Chair and Professor of Molecular Genetics at Cleveland Clinic. He received his Ph.D. in Biochemistry from McMaster University, Canada. During his postdoctoral training with Peter Lengyel at Yale University, USA, he began investigating the role of interferon (IFN) system in antiviral functions. He has expanded his activities in this area of research in his own laboratory, first at the Memorial Sloan-Kettering Cancer Center, USA, and then at Lerner Research Institute, where he nucleated the formation of a strong cytokine research group. He has published extensively on the mechanism of actions of IFN-induced proteins, their mode of induction, actions of double-stranded RNA-stimulated genes and signaling by endosomal TLRs. For his contributions to IFN research, he received the prestigious Milstein Award in 2002 from International Society for Interferon and Cytokine Research, and Boltzmann Award from European Cytokine Society in 2008. He has been elected a Fellow of American Academy of Microbiology and a Fellow of the American Association for Advancement of Science for his scientific achievements and original research contributions. He is a consultant for the National Institutes of Health (NIH) and Editor-in-Chief of the Journal of Interferon and Cytokine Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 2.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nature immunology. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 3.McGettrick AF, O'Neill LA. Localisation and trafficking of Toll-like receptors: an important mode of regulation. Current opinion in immunology. 2010;22:20–27. doi: 10.1016/j.coi.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Johnson P, Cross JL. Tyrosine phosphorylation in immune cells: direct and indirect effects on toll-like receptor-induced proinflammatory cytokine production. Critical reviews in immunology. 2009;29:347–367. doi: 10.1615/critrevimmunol.v29.i4.50. [DOI] [PubMed] [Google Scholar]
- 5.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Nishiya T, Kajita E, Miwa S, Defranco AL. TLR3 and TLR7 are targeted to the same intracellular compartments by distinct regulatory elements. The Journal of biological chemistry. 2005;280:37107–37117. doi: 10.1074/jbc.M504951200. [DOI] [PubMed] [Google Scholar]
- 7.Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature. 2008;452:234–238. doi: 10.1038/nature06726. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Cattaneo A, Gobert FX, Muller M, Toscano F, Flores M, Lescure A, Del Nery E, Benaroch P. Cleavage of Toll-like receptor 3 by cathepsins B and H is essential for signaling. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:9053–9058. doi: 10.1073/pnas.1115091109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oldenburg M, Kruger A, Ferstl R, Kaufmann A, Nees G, Sigmund A, Bathke B, Lauterbach H, Suter M, Dreher S, Koedel U, Akira S, Kawai T, Buer J, Wagner H, Bauer S, Hochrein H, Kirschning CJ. TLR13 recognizes bacterial 23S rRNA devoid of erythromycin resistance-forming modification. Science. 2012;337:1111–1115. doi: 10.1126/science.1220363. [DOI] [PubMed] [Google Scholar]
- 10.Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. Journal of leukocyte biology. 2007;81:28–37. doi: 10.1189/jlb.0306170. [DOI] [PubMed] [Google Scholar]
- 11.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, Hua J, An LL, Audoly L, La Rosa G, Bierhaus A, Naworth P, Marshak-Rothstein A, Crow MK, Fitzgerald KA, Latz E, Kiener PA, Coyle AJ. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nature immunology. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 12.Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, Stolz DB, Geller DA, Rosengart MR, Billiar TR. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. The Journal of experimental medicine. 2007;204:2913–2923. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rohde D, Schon C, Boerries M, Didrihsone I, Ritterhoff J, Kubatzky KF, Volkers M, Herzog N, Mahler M, Tsoporis JN, Parker TG, Linke B, Giannitsis E, Gao E, Peppel K, Katus HA, Most P. S100A1 is released from ischemic cardiomyocytes and signals myocardial damage via Toll-like receptor 4. EMBO molecular medicine. 2014 doi: 10.15252/emmm.201303498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nature reviews. Immunology. 2009;9:535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nature reviews. Immunology. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 17.Jabara HH, McDonald DR, Janssen E, Massaad MJ, Ramesh N, Borzutzky A, Rauter I, Benson H, Schneider L, Baxi S, Recher M, Notarangelo LD, Wakim R, Dbaibo G, Dasouki M, Al-Herz W, Barlan I, Baris S, Kutukculer N, Ochs HD, Plebani A, Kanariou M, Lefranc G, Reisli I, Fitzgerald KA, Golenbock D, Manis J, Keles S, Ceja R, Chatila TA, Geha RS. DOCK8 functions as an adaptor that links TLR-MyD88 signaling to B cell activation. Nature immunology. 2012;13:612–620. doi: 10.1038/ni.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, Taniguchi T. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 19.Hoshino K, Sugiyama T, Matsumoto M, Tanaka T, Saito M, Hemmi H, Ohara O, Akira S, Kaisho T. IkappaB kinase-alpha is critical for interferon-alpha production induced by Toll-like receptors 7 and 9. Nature. 2006;440:949–953. doi: 10.1038/nature04641. [DOI] [PubMed] [Google Scholar]
- 20.Jiang Z, Johnson HJ, Nie H, Qin J, Bird TA, Li X. Pellino 1 is required for interleukin-1 (IL-1)-mediated signaling through its interaction with the IL-1 receptor-associated kinase 4 (IRAK4)-IRAK-tumor necrosis factor receptor-associated factor 6 (TRAF6) complex. The Journal of biological chemistry. 2003;278:10952–10956. doi: 10.1074/jbc.M212112200. [DOI] [PubMed] [Google Scholar]
- 21.Lin R, Heylbroeck C, Pitha PM, Hiscott J. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Molecular and cellular biology. 1998;18:2986–2996. doi: 10.1128/mcb.18.5.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiscott J. Triggering the innate antiviral response through IRF-3 activation. The Journal of biological chemistry. 2007;282:15325–15329. doi: 10.1074/jbc.R700002200. [DOI] [PubMed] [Google Scholar]
- 23.Yang P, An H, Liu X, Wen M, Zheng Y, Rui Y, Cao X. The cytosolic nucleic acid sensor LRRFIP1 mediates the production of type I interferon via a beta-catenin-dependent pathway. Nature immunology. 2010;11:487–494. doi: 10.1038/ni.1876. [DOI] [PubMed] [Google Scholar]
- 24.Chattopadhyay S, Fensterl V, Zhang Y, Veleeparambil M, Wetzel JL, Sen GC. Inhibition of viral pathogenesis and promotion of the septic shock response to bacterial infection by IRF-3 are regulated by the acetylation and phosphorylation of its coactivators. mBio. 2013;4 doi: 10.1128/mBio.00636-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasare C, Medzhitov R. Toll-like receptors: balancing host resistance with immune tolerance. Current opinion in immunology. 2003;15:677–682. doi: 10.1016/j.coi.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nature reviews. Cancer. 2009;9:57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 27.Netea MG, Wijmenga C, O'Neill LA. Genetic variation in Toll-like receptors and disease susceptibility. Nature immunology. 2012;13:535–542. doi: 10.1038/ni.2284. [DOI] [PubMed] [Google Scholar]
- 28.Hennessy EJ, Parker AE, O'Neill LA. Targeting Toll-like receptors: emerging therapeutics? Nature reviews. Drug discovery. 2010;9:293–307. doi: 10.1038/nrd3203. [DOI] [PubMed] [Google Scholar]
- 29.Ngo VN, Young RM, Schmitz R, Jhavar S, Xiao W, Lim KH, Kohlhammer H, Xu W, Yang Y, Zhao H, Shaffer AL, Romesser P, Wright G, Powell J, Rosenwald A, Muller-Hermelink HK, Ott G, Gascoyne RD, Connors JM, Rimsza LM, Campo E, Jaffe ES, Delabie J, Smeland EB, Fisher RI, Braziel RM, Tubbs RR, Cook JR, Weisenburger DD, Chan WC, Staudt LM. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang TJ, Chae GT. Detection of Toll-like receptor 2 (TLR2) mutation in the lepromatous leprosy patients. FEMS immunology and medical microbiology. 2001;31:53–58. doi: 10.1111/j.1574-695X.2001.tb01586.x. [DOI] [PubMed] [Google Scholar]
- 31.Ogus AC, Yoldas B, Ozdemir T, Uguz A, Olcen S, Keser I, Coskun M, Cilli A, Yegin O. The Arg753GLn polymorphism of the human toll-like receptor 2 gene in tuberculosis disease. The European respiratory journal. 2004;23:219–223. doi: 10.1183/09031936.03.00061703. [DOI] [PubMed] [Google Scholar]
- 32.Xiong Y, Song C, Snyder GA, Sundberg EJ, Medvedev AE. R753Q polymorphism inhibits Toll-like receptor (TLR) 2 tyrosine phosphorylation, dimerization with TLR6, and recruitment of myeloid differentiation primary response protein 88. The Journal of biological chemistry. 2012;287:38327–38337. doi: 10.1074/jbc.M112.375493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khor CC, Chapman SJ, Vannberg FO, Dunne A, Murphy C, Ling EY, Frodsham AJ, Walley AJ, Kyrieleis O, Khan A, Aucan C, Segal S, Moore CE, Knox K, Campbell SJ, Lienhardt C, Scott A, Aaby P, Sow OY, Grignani RT, Sillah J, Sirugo G, Peshu N, Williams TN, Maitland K, Davies RJ, Kwiatkowski DP, Day NP, Yala D, Crook DW, Marsh K, Berkley JA, O'Neill LA, Hill AV. A Mal functional variant is associated with protection against invasive pneumococcal disease, bacteremia, malaria and tuberculosis. Nature genetics. 2007;39:523–528. doi: 10.1038/ng1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ku CL, von Bernuth H, Picard C, Zhang SY, Chang HH, Yang K, Chrabieh M, Issekutz AC, Cunningham CK, Gallin J, Holland SM, Roifman C, Ehl S, Smart J, Tang M, Barrat FJ, Levy O, McDonald D, Day-Good NK, Miller R, Takada H, Hara T, Al-Hajjar S, Al-Ghonaium A, Speert D, Sanlaville D, Li X, Geissmann F, Vivier E, Marodi L, Garty BZ, Chapel H, Rodriguez-Gallego C, Bossuyt X, Abel L, Puel A, Casanova JL. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. The Journal of experimental medicine. 2007;204:2407–2422. doi: 10.1084/jem.20070628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang SY, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, Segal D, Sancho-Shimizu V, Lorenzo L, Puel A, Picard C, Chapgier A, Plancoulaine S, Titeux M, Cognet C, von Bernuth H, Ku CL, Casrouge A, Zhang XX, Barreiro L, Leonard J, Hamilton C, Lebon P, Heron B, Vallee L, Quintana-Murci L, Hovnanian A, Rozenberg F, Vivier E, Geissmann F, Tardieu M, Abel L, Casanova JL. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- 36.Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, Albuquerque RJ, Yamasaki S, Itaya M, Pan Y, Appukuttan B, Gibbs D, Yang Z, Kariko K, Ambati BK, Wilgus TA, DiPietro LA, Sakurai E, Zhang K, Smith JR, Taylor EW, Ambati J. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paone A, Galli R, Gabellini C, Lukashev D, Starace D, Gorlach A, De Cesaris P, Ziparo E, Del Bufalo D, Sitkovsky MV, Filippini A, Riccioli A. Toll-like receptor 3 regulates angiogenesis and apoptosis in prostate cancer cell lines through hypoxia-inducible factor 1 alpha. Neoplasia. 2010;12:539–549. doi: 10.1593/neo.92106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Z, Stratton C, Francis PJ, Kleinman ME, Tan PL, Gibbs D, Tong Z, Chen H, Constantine R, Yang X, Chen Y, Zeng J, Davey L, Ma X, Hau VS, Wang C, Harmon J, Buehler J, Pearson E, Patel S, Kaminoh Y, Watkins S, Luo L, Zabriskie NA, Bernstein PS, Cho W, Schwager A, Hinton DR, Klein ML, Hamon SC, Simmons E, Yu B, Campochiaro B, Sunness JS, Campochiaro P, Jorde L, Parmigiani G, Zack DJ, Katsanis N, Ambati J, Zhang K. Toll-like receptor 3 and geographic atrophy in age-related macular degeneration. The New England journal of medicine. 2008;359:1456–1463. doi: 10.1056/NEJMoa0802437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, Frees K, Watt JL, Schwartz DA. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nature genetics. 2000;25:187–191. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- 40.Lorenz E, Mira JP, Frees KL, Schwartz DA. Relevance of mutations in the TLR4 receptor in patients with gram-negative septic shock. Archives of internal medicine. 2002;162:1028–1032. doi: 10.1001/archinte.162.9.1028. [DOI] [PubMed] [Google Scholar]
- 41.Karaghiosoff M, Steinborn R, Kovarik P, Kriegshauser G, Baccarini M, Donabauer B, Reichart U, Kolbe T, Bogdan C, Leanderson T, Levy D, Decker T, Muller M. Central role for type I interferons and Tyk2 in lipopolysaccharide-induced endotoxin shock. Nature immunology. 2003;4:471–477. doi: 10.1038/ni910. [DOI] [PubMed] [Google Scholar]
- 42.Siegfried A, Berchtold S, Manncke B, Deuschle E, Reber J, Ott T, Weber M, Kalinke U, Hofer MJ, Hatesuer B, Schughart K, Gailus-Durner V, Fuchs H, Hrabe de Angelis M, Weber F, Hornef MW, Autenrieth IB, Bohn E. IFIT2 is an effector protein of type I IFN-mediated amplification of lipopolysaccharide (LPS)-induced TNF-alpha secretion and LPS-induced endotoxin shock. Journal of immunology. 2013;191:3913–3921. doi: 10.4049/jimmunol.1203305. [DOI] [PubMed] [Google Scholar]
- 43.Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infection and immunity. 2000;68:7010–7017. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishizuka F, Shimazawa M, Inoue Y, Nakano Y, Ogishima H, Nakamura S, Tsuruma K, Tanaka H, Inagaki N, Hara H. Toll-like receptor 4 mediates retinal ischemia/reperfusion injury through nuclear factor-kappaB and spleen tyrosine kinase activation. Investigative ophthalmology & visual science. 2013;54:5807–5816. doi: 10.1167/iovs.13-11932. [DOI] [PubMed] [Google Scholar]
- 45.Celhar T, Magalhaes R, Fairhurst AM. TLR7 and TLR9 in SLE: when sensing self goes wrong. Immunologic research. 2012;53:58–77. doi: 10.1007/s12026-012-8270-1. [DOI] [PubMed] [Google Scholar]
- 46.Yamashita M, Chattopadhyay S, Fensterl V, Saikia P, Wetzel JL, Sen GC. Epidermal growth factor receptor is essential for Toll-like receptor 3 signaling. Science signaling. 2012;5:ra50. doi: 10.1126/scisignal.2002581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnsen IB, Nguyen TT, Ringdal M, Tryggestad AM, Bakke O, Lien E, Espevik T, Anthonsen MW. Toll-like receptor 3 associates with c-Src tyrosine kinase on endosomes to initiate antiviral signaling. The EMBO journal. 2006;25:3335–3346. doi: 10.1038/sj.emboj.7601222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarkar SN, Smith HL, Rowe TM, Sen GC. Double-stranded RNA signaling by Toll-like receptor 3 requires specific tyrosine residues in its cytoplasmic domain. The Journal of biological chemistry. 2003;278:4393–4396. doi: 10.1074/jbc.C200655200. [DOI] [PubMed] [Google Scholar]
- 49.Sarkar SN, Peters KL, Elco CP, Sakamoto S, Pal S, Sen GC. Novel roles of TLR3 tyrosine phosphorylation and PI3 kinase in double-stranded RNA signaling. Nature structural & molecular biology. 2004;11:1060–1067. doi: 10.1038/nsmb847. [DOI] [PubMed] [Google Scholar]
- 50.Sarkar SN, Elco CP, Peters KL, Chattopadhyay S, Sen GC. Two tyrosine residues of Toll-like receptor 3 trigger different steps of NF-kappa B activation. The Journal of biological chemistry. 2007;282:3423–3427. doi: 10.1074/jbc.C600226200. [DOI] [PubMed] [Google Scholar]
- 51.Hsieh MY, Chang MY, Chen YJ, Li YK, Chuang TH, Yu GY, Cheung CH, Chen HC, Maa MC, Leu TH. The inducible nitric-oxide synthase (iNOS)/Src axis mediates Toll-like receptor 3 tyrosine 759 phosphorylation and enhances its signal transduction, leading to interferon-beta synthesis in macrophages. The Journal of biological chemistry. 2014;289:9208–9220. doi: 10.1074/jbc.M113.508663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee KG, Xu S, Kang ZH, Huo J, Huang M, Liu D, Takeuchi O, Akira S, Lam KP. Bruton's tyrosine kinase phosphorylates Toll-like receptor 3 to initiate antiviral response. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5791–5796. doi: 10.1073/pnas.1119238109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nagai Y, Akashi S, Nagafuku M, Ogata M, Iwakura Y, Akira S, Kitamura T, Kosugi A, Kimoto M, Miyake K. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nature immunology. 2002;3:667–672. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- 54.Zanoni I, Ostuni R, Marek LR, Barresi S, Barbalat R, Barton GM, Granucci F, Kagan JC. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell. 2011;147:868–880. doi: 10.1016/j.cell.2011.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T, Takeuchi O, Takeda K, Akira S. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nature immunology. 2003;4:1144–1150. doi: 10.1038/ni986. [DOI] [PubMed] [Google Scholar]
- 56.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nature immunology. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen LY, Zuraw BL, Zhao M, Liu FT, Huang S, Pan ZK. Involvement of protein tyrosine kinase in Toll-like receptor 4-mediated NF-kappa B activation in human peripheral blood monocytes. American journal of physiology. Lung cellular and molecular physiology. 2003;284:L607–L613. doi: 10.1152/ajplung.00116.2002. [DOI] [PubMed] [Google Scholar]
- 58.Smolinska MJ, Horwood NJ, Page TH, Smallie T, Foxwell BM. Chemical inhibition of Src family kinases affects major LPS-activated pathways in primary human macrophages. Molecular immunology. 2008;45:990–1000. doi: 10.1016/j.molimm.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 59.Medvedev AE, Piao W, Shoenfelt J, Rhee SH, Chen H, Basu S, Wahl LM, Fenton MJ, Vogel SN. Role of TLR4 tyrosine phosphorylation in signal transduction and endotoxin tolerance. The Journal of biological chemistry. 2007;282:16042–16053. doi: 10.1074/jbc.M606781200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Avila M, Martinez-Juarez A, Ibarra-Sanchez A, Gonzalez-Espinosa C. Lyn kinase controls TLR4-dependent IKK and MAPK activation modulating the activity of TRAF-6/TAK-1 protein complex in mast cells. Innate immunity. 2012;18:648–660. doi: 10.1177/1753425911435265. [DOI] [PubMed] [Google Scholar]
- 61.Jefferies CA, Doyle S, Brunner C, Dunne A, Brint E, Wietek C, Walch E, Wirth T, O'Neill LA. Bruton's tyrosine kinase is a Toll/interleukin-1 receptor domain-binding protein that participates in nuclear factor kappaB activation by Toll-like receptor 4. The Journal of biological chemistry. 2003;278:26258–26264. doi: 10.1074/jbc.M301484200. [DOI] [PubMed] [Google Scholar]
- 62.Miller YI, Choi SH, Wiesner P, Bae YS. The SYK side of TLR4: signalling mechanisms in response to LPS and minimally oxidized LDL. British journal of pharmacology. 2012;167:990–999. doi: 10.1111/j.1476-5381.2012.02097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin YC, Huang DY, Chu CL, Lin YL, Lin WW. The tyrosine kinase Syk differentially regulates Toll-like receptor signaling downstream of the adaptor molecules TRAF6 and TRAF3. Science signaling. 2013;6:ra71. doi: 10.1126/scisignal.2003973. [DOI] [PubMed] [Google Scholar]
- 64.Lu N, Wang L, Cao H, Liu L, Van Kaer L, Washington MK, Rosen MJ, Dube PE, Wilson KT, Ren X, Hao X, Polk DB, Yan F. Activation of the epidermal growth factor receptor in macrophages regulates cytokine production and experimental colitis. Journal of immunology. 2014;192:1013–1023. doi: 10.4049/jimmunol.1300133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arbibe L, Mira JP, Teusch N, Kline L, Guha M, Mackman N, Godowski PJ, Ulevitch RJ, Knaus UG. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nature immunology. 2000;1:533–540. doi: 10.1038/82797. [DOI] [PubMed] [Google Scholar]
- 66.Ha T, Hu Y, Liu L, Lu C, McMullen JR, Kelley J, Kao RL, Williams DL, Gao X, Li C. TLR2 ligands induce cardioprotection against ischaemia/reperfusion injury through a PI3K/Akt-dependent mechanism. Cardiovascular research. 2010;87:694–703. doi: 10.1093/cvr/cvq116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Horwood NJ, Page TH, McDaid JP, Palmer CD, Campbell J, Mahon T, Brennan FM, Webster D, Foxwell BM. Bruton's tyrosine kinase is required for TLR2 and TLR4-induced TNF, but not IL-6, production. Journal of immunology. 2006;176:3635–3641. doi: 10.4049/jimmunol.176.6.3635. [DOI] [PubMed] [Google Scholar]
- 68.Ivison SM, Khan MA, Graham NR, Bernales CQ, Kaleem A, Tirling CO, Cherkasov A, Steiner TS. A phosphorylation site in the Toll-like receptor 5 TIR domain is required for inflammatory signalling in response to flagellin. Biochemical and biophysical research communications. 2007;352:936–941. doi: 10.1016/j.bbrc.2006.11.132. [DOI] [PubMed] [Google Scholar]
- 69.Rajagopal R, Waller AS, Mendoza JD, Wightman PD. The covalent modification and regulation of TLR8 in HEK-293 cells stimulated with imidazoquinoline agonists. The Biochemical journal. 2008;409:275–287. doi: 10.1042/BJ20070519. [DOI] [PubMed] [Google Scholar]
- 70.Lee KG, Xu S, Wong ET, Tergaonkar V, Lam KP. Bruton's tyrosine kinase separately regulates NFkappaB p65RelA activation and cytokine interleukin (IL)-10/IL-12 production in TLR9-stimulated B Cells. The Journal of biological chemistry. 2008;283:11189–11198. doi: 10.1074/jbc.M708516200. [DOI] [PubMed] [Google Scholar]
- 71.Doyle SL, Jefferies CA, Feighery C, O'Neill LA. Signaling by Toll-like receptors 8 and 9 requires Bruton's tyrosine kinase. The Journal of biological chemistry. 2007;282:36953–36960. doi: 10.1074/jbc.M707682200. [DOI] [PubMed] [Google Scholar]
- 72.Sanjuan MA, Rao N, Lai KT, Gu Y, Sun S, Fuchs A, Fung-Leung WP, Colonna M, Karlsson L. CpG-induced tyrosine phosphorylation occurs via a TLR9-independent mechanism and is required for cytokine secretion. The Journal of cell biology. 2006;172:1057–1068. doi: 10.1083/jcb.200508058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chockalingam A, Rose WA, 2nd, Hasan M, Ju CH, Leifer CA. Cutting edge: a TLR9 cytoplasmic tyrosine motif is selectively required for proinflammatory cytokine production. Journal of immunology. 2012;188:527–530. doi: 10.4049/jimmunol.1102713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gray P, Dagvadorj J, Michelsen KS, Brikos C, Rentsendorj A, Town T, Crother TR, Arditi M. Myeloid differentiation factor-2 interacts with Lyn kinase and is tyrosine phosphorylated following lipopolysaccharide-induced activation of the TLR4 signaling pathway. Journal of immunology. 2011;187:4331–4337. doi: 10.4049/jimmunol.1100890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Piao W, Song C, Chen H, Wahl LM, Fitzgerald KA, O'Neill LA, Medvedev AE. Tyrosine phosphorylation of MyD88 adapter-like (Mal) is critical for signal transduction and blocked in endotoxin tolerance. The Journal of biological chemistry. 2008;283:3109–3119. doi: 10.1074/jbc.M707400200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gray P, Dunne A, Brikos C, Jefferies CA, Doyle SL, O'Neill LA. MyD88 adapter-like (Mal) is phosphorylated by Bruton's tyrosine kinase during TLR2 and TLR4 signal transduction. The Journal of biological chemistry. 2006;281:10489–10495. doi: 10.1074/jbc.M508892200. [DOI] [PubMed] [Google Scholar]
- 77.Mansell A, Smith R, Doyle SL, Gray P, Fenner JE, Crack PJ, Nicholson SE, Hilton DJ, O'Neill LA, Hertzog PJ. Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nature immunology. 2006;7:148–155. doi: 10.1038/ni1299. [DOI] [PubMed] [Google Scholar]
- 78.Han C, Jin J, Xu S, Liu H, Li N, Cao X. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nature immunology. 2010;11:734–742. doi: 10.1038/ni.1908. [DOI] [PubMed] [Google Scholar]
- 79.Jiao H, Zhang Y, Yan Z, Wang ZG, Liu G, Minshall RD, Malik AB, Hu G. Caveolin-1 Tyr14 phosphorylation induces interaction with TLR4 in endothelial cells and mediates MyD88-dependent signaling and sepsis-induced lung inflammation. Journal of immunology. 2013;191:6191–6199. doi: 10.4049/jimmunol.1300873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stacey KB, Breen E, Jefferies CA. Tyrosine phosphorylation of the E3 ubiquitin ligase TRIM21 positively regulates interaction with IRF3 and hence TRIM21 activity. PloS one. 2012;7:e34041. doi: 10.1371/journal.pone.0034041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yamashita M, Chattopadhyay S, Fensterl V, Zhang Y, Sen GC. A TRIF-independent branch of TLR3 signaling. Journal of immunology. 2012;188:2825–2833. doi: 10.4049/jimmunol.1103220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burtness B, Marur S, Bauman JE, Golemis EA, Mehra R, Cohen SJ. Comment on "epidermal growth factor receptor is essential for toll-like receptor 3 signaling". Science signaling. 2012;5:lc5. doi: 10.1126/scisignal.2003734. [DOI] [PubMed] [Google Scholar]
- 83.Basu S, Pathak SK, Chatterjee G, Pathak S, Basu J, Kundu M. Helicobacter pylori protein HP0175 transactivates epidermal growth factor receptor through TLR4 in gastric epithelial cells. The Journal of biological chemistry. 2008;283:32369–32376. doi: 10.1074/jbc.M805053200. [DOI] [PubMed] [Google Scholar]
- 84.McElroy SJ, Hobbs S, Kallen M, Tejera N, Rosen MJ, Grishin A, Matta P, Schneider C, Upperman J, Ford H, Polk DB, Weitkamp JH. Transactivation of EGFR by LPS induces COX-2 expression in enterocytes. PloS one. 2012;7:e38373. doi: 10.1371/journal.pone.0038373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mustelin T, Vang T, Bottini N. Protein tyrosine phosphatases and the immune response. Nature reviews. Immunology. 2005;5:43–57. doi: 10.1038/nri1530. [DOI] [PubMed] [Google Scholar]
- 86.An H, Zhao W, Hou J, Zhang Y, Xie Y, Zheng Y, Xu H, Qian C, Zhou J, Yu Y, Liu S, Feng G, Cao X. SHP-2 phosphatase negatively regulates the TRIF adaptor protein-dependent type I interferon and proinflammatory cytokine production. Immunity. 2006;25:919–928. doi: 10.1016/j.immuni.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 87.An H, Hou J, Zhou J, Zhao W, Xu H, Zheng Y, Yu Y, Liu S, Cao X. Phosphatase SHP-1 promotes TLR- and RIG-I-activated production of type I interferon by inhibiting the kinase IRAK1. Nature immunology. 2008;9:542–550. doi: 10.1038/ni.1604. [DOI] [PubMed] [Google Scholar]
- 88.Zhang P, Liu X, Li Y, Zhu X, Zhan Z, Meng J, Li N, Cao X. Protein tyrosine phosphatase with proline-glutamine-serine-threonine-rich motifs negatively regulates TLR-triggered innate responses by selectively inhibiting IkappaB kinase beta/NF-kappaB activation. Journal of immunology. 2013;190:1685–1694. doi: 10.4049/jimmunol.1202384. [DOI] [PubMed] [Google Scholar]
- 89.Lu R, Pan H, Shively JE. CEACAM1 negatively regulates IL-1beta production in LPS activated neutrophils by recruiting SHP-1 to a SYK-TLR4-CEACAM1 complex. PLoS pathogens. 2012;8:e1002597. doi: 10.1371/journal.ppat.1002597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yan D, Wang X, Luo L, Cao X, Ge B. Inhibition of TLR signaling by a bacterial protein containing immunoreceptor tyrosine-based inhibitory motifs. Nature immunology. 2012;13:1063–1071. doi: 10.1038/ni.2417. [DOI] [PubMed] [Google Scholar]