Abstract
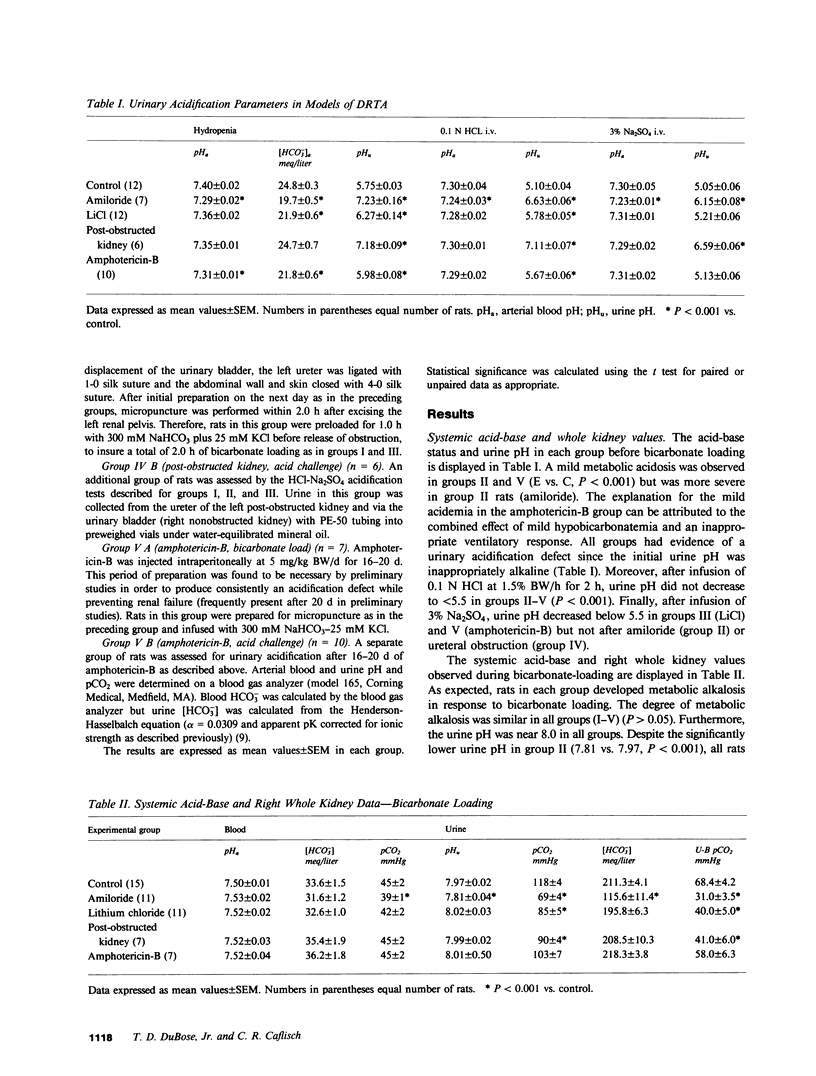
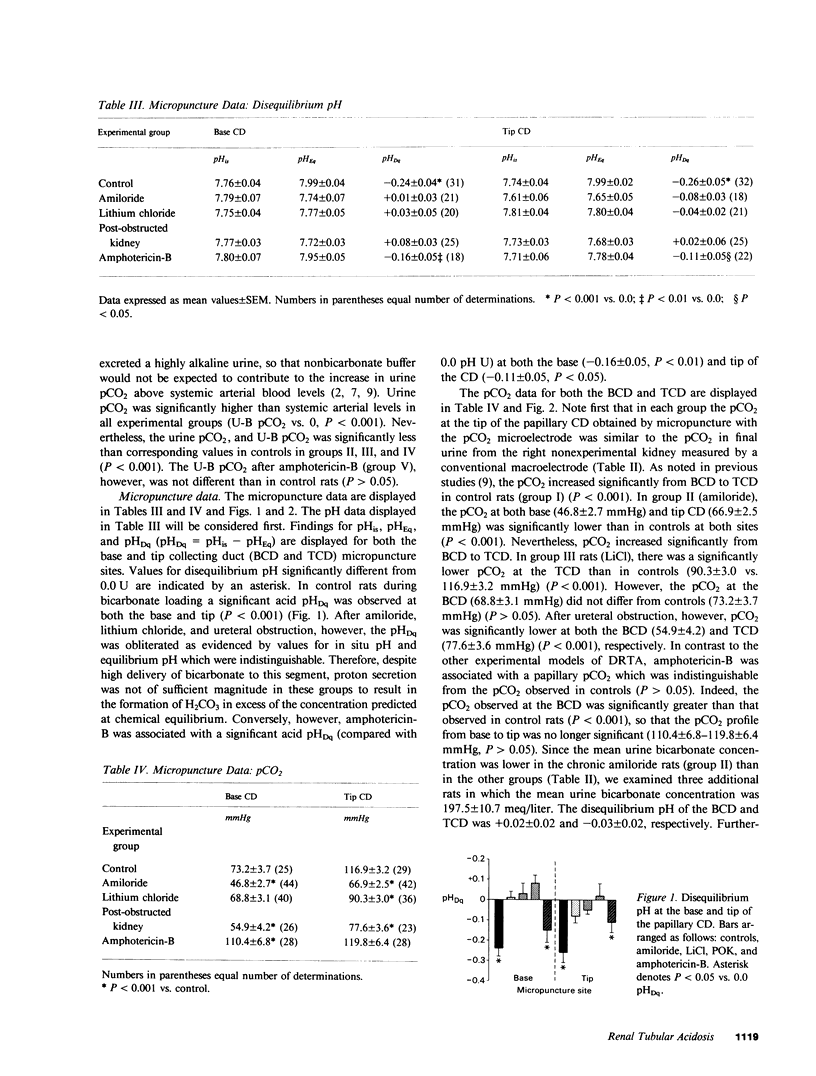
Recent classifications of the several pathophysiologic types of distal renal tubular acidosis (secretory, voltage dependent, and gradient) have been based on the response of acidification parameters to a series of provocative maneuvers in vivo and in vitro. A reduction in the difference in urine and blood CO2 tension during bicarbonate loading (U-B pCO2 gradient), a widely applied parameter, has been employed as an index of reduced distal nephron proton secretion. This study was designed to test the validity of the U-B pCO2 gradient in a variety of experimental models of distal renal tubular acidosis by measuring and comparing disequilibrium pH (a direct technique to detect H+ secretion in situ) with the pCO2 in the papillary collecting duct of the rat in vivo during bicarbonate loading. Chronic amiloride, lithium chloride, and amphotericin-B administration, and the post-obstructed kidney models were employed. Amiloride resulted in an acidification defect which did not respond to sulfate infusion (urine pH = 6.15 +/- 0.08), and was associated with an obliteration of the acid disequilibrium pH (-0.26 +/- 0.05- -0.08 +/- 0.03) and reduction in papillary pCO2 (116.9 +/- 3.2 - 66.9 +/- 2.5 mmHg). The defect induced by lithium administration responded to Na2SO4 (urine pH = 5.21 +/- 0.06) but was similar to amiloride with respect to the observed reduction in disequilibrium pH (-0.04 +/- 0.02) and pCO2 (90.3 +/- 3.0 mmHg). The post-obstructed kidney model was characterized by an abnormally alkaline urine pH unresponsive to sulfate (6.59 +/- 0.06) and a reduction in disequilibrium pH (+0.02 +/- 0.06) and pCO2 (77.6 +/- 3.6 mmHg). Amphotericin-B resulted in a gradient defect as characterized by excretion of an acid urine after infusion of sodium sulfate (5.13 +/- 0.06). Unlike other models, however, amphotericin-B was associated with a significant acid disequilibrium pH (-0.11 +/- 0.05) and an appropriately elevated urine pCO2 (119.8 +/- 6.4 mmHg) which did not differ from the respective values in control rats. Thus, these findings support the use of the U-B pCO2 as a reliable means of demonstrating impaired distal nephron proton secretion in secretory and voltage-dependent forms of distal renal tubular acidosis (RTA) and supports the view that proton secretion is not impaired in gradient forms of distal RTA.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arruda J. A., Dytko G., Mola R., Kurtzman N. A. On the mechanism of lithium-induced renal tubular acidosis: studies in the turtle bladder. Kidney Int. 1980 Feb;17(2):196–204. doi: 10.1038/ki.1980.23. [DOI] [PubMed] [Google Scholar]
- Arruda J. A., Kurtzman N. A. Mechanisms and classification of deranged distal urinary acidification. Am J Physiol. 1980 Dec;239(6):F515–F523. doi: 10.1152/ajprenal.1980.239.6.F515. [DOI] [PubMed] [Google Scholar]
- Arruda J. A., Nascimento L., Kumar S. K., Kurtzman N. A. Factors influencing the formation of urinary carbon dioxide tension. Kidney Int. 1977 May;11(5):307–317. doi: 10.1038/ki.1977.48. [DOI] [PubMed] [Google Scholar]
- Arruda J. A., Nascimento L., Mehta P. K., Rademacher D. R., Sehy J. T., Westenfelder C., Kurtzman N. A. The critical importance of urinary concentrating ability in the generation of urinary carbon dioxide tension. J Clin Invest. 1977 Oct;60(4):922–935. doi: 10.1172/JCI108847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda J. A., Subbarayudu K., Dytko G., Mola R., Kurtzman N. A. Voltage-dependent distal acidification defect induced by amiloride. J Lab Clin Med. 1980 Mar;95(3):407–416. [PubMed] [Google Scholar]
- Batlle D. C., Sehy J. T., Roseman M. K., Arruda J. A., Kurtzman N. A. Clinical and pathophysiologic spectrum of acquired distal renal tubular acidosis. Kidney Int. 1981 Sep;20(3):389–396. doi: 10.1038/ki.1981.151. [DOI] [PubMed] [Google Scholar]
- DuBose T. D., Jr Application of the disequilibrium pH method to investigate the mechanism of urinary acidification. Am J Physiol. 1983 Nov;245(5 Pt 1):F535–F544. doi: 10.1152/ajprenal.1983.245.5.F535. [DOI] [PubMed] [Google Scholar]
- DuBose T. D., Jr, Caflisch C. R., Bidani A. Role of metabolic CO2 production in the generation of elevated renal cortical PCO2. Am J Physiol. 1984 May;246(5 Pt 2):F592–F599. doi: 10.1152/ajprenal.1984.246.5.F592. [DOI] [PubMed] [Google Scholar]
- DuBose T. D., Jr Hydrogen ion secretion by the collecting duct as a determinant of the urine to blood PCO2 gradient in alkaline urine. J Clin Invest. 1982 Jan;69(1):145–156. doi: 10.1172/JCI110425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBose T. D., Jr, Lucci M. S. Effect of carbonic anhydrase inhibition on superficial and deep nephron bicarbonate reabsorption in the rat. J Clin Invest. 1983 Jan;71(1):55–65. doi: 10.1172/JCI110751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBose T. D., Jr, Pucacco L. R., Carter N. W. Determination of disequilibrium pH in the rat kidney in vivo: evidence of hydrogen secretion. Am J Physiol. 1981 Feb;240(2):F138–F146. doi: 10.1152/ajprenal.1981.240.2.F138. [DOI] [PubMed] [Google Scholar]
- Garg L. C. Lack of effect of amphotericin B on urine-blood pCO2 gradient in spite of urinary acidification defect. Pflugers Arch. 1979 Aug;381(2):137–142. doi: 10.1007/BF00582344. [DOI] [PubMed] [Google Scholar]
- Gougoux A., Vinay P., Lemieux G., Richardson R. M., Tam S., Goldstein M. B., Stinebaugh B. J., Halperin M. L. Effect of blood pH on distal nephron hydrogen ion secretion. Kidney Int. 1980 May;17(5):615–621. doi: 10.1038/ki.1980.72. [DOI] [PubMed] [Google Scholar]
- Halperin M. L., Goldstein M. B., Haig A., Johnson M. D., Stinebaugh B. J. Studies on the pathogenesis of type I (distal) renal tubular acidosis as revealed by the urinary PCO2 tensions. J Clin Invest. 1974 Mar;53(3):669–677. doi: 10.1172/JCI107604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley M. J., Davidson K. Isolated nephron segments from rabbit models of obstructive nephropathy. J Clin Invest. 1982 Jan;69(1):165–174. doi: 10.1172/JCI110427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R. H., Yarger W. E. Renal function after release of unilateral ureteral obstruction in rats. Am J Physiol. 1974 Oct;227(4):806–815. doi: 10.1152/ajplegacy.1974.227.4.806. [DOI] [PubMed] [Google Scholar]
- Hulter H. N., Licht J. H., Glynn R. D., Sebastian A., Ilnicki L. P. Pathophysiology of chronic renal tubular acidosis induced by administration of amiloride. J Lab Clin Med. 1980 May;95(5):637–653. [PubMed] [Google Scholar]
- Husted R. F., Steinmetz P. R. The effects of amiloride and ouabain on urinary acidification by turtle bladder. J Pharmacol Exp Ther. 1979 Aug;210(2):264–268. [PubMed] [Google Scholar]
- Julka N. K., Arruda J. A., Kurtzman N. A. The mechanism of amphotericin-induced distal acidification defect in rats. Clin Sci (Lond) 1979 Jan;56(6):555–562. doi: 10.1042/cs0560555. [DOI] [PubMed] [Google Scholar]
- Kurtzman N. A. Acquired distal renal tubular acidosis. Kidney Int. 1983 Dec;24(6):807–819. doi: 10.1038/ki.1983.233. [DOI] [PubMed] [Google Scholar]
- Laski M. E., Kurtzman N. A. Characterization of acidification in the cortical and medullary collecting tubule of the rabbit. J Clin Invest. 1983 Dec;72(6):2050–2059. doi: 10.1172/JCI111170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard W. E., Kokko J. P., Jacobson H. R. Bicarbonate transport in cortical and outer medullary collecting tubules. Am J Physiol. 1983 Mar;244(3):F289–F296. doi: 10.1152/ajprenal.1983.244.3.F289. [DOI] [PubMed] [Google Scholar]
- Lönnerholm G., Ridderstråle Y. Intracellular distribution of carbonic anhydrase in the rat kidney. Kidney Int. 1980 Feb;17(2):162–174. doi: 10.1038/ki.1980.20. [DOI] [PubMed] [Google Scholar]
- Maren T. H. Carbon dioxide equilibria in the kidney: the problems of elevated carbon dioxide tension, delayed dehydration, and disequilibrium pH. Kidney Int. 1978 Nov;14(5):395–405. doi: 10.1038/ki.1978.144. [DOI] [PubMed] [Google Scholar]
- McKinney T. D., Burg M. B. Bicarbonate secretion by rabbit cortical collecting tubules in vitro. J Clin Invest. 1978 Jun;61(6):1421–1427. doi: 10.1172/JCI109061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento L., Rademacher D. R., Hamburger R., Arruda J. A., Kurtzman A. On the mechanism of lithium-induced renal tubular acidosis. J Lab Clin Med. 1977 Mar;89(3):455–462. [PubMed] [Google Scholar]
- REYNOLDS T. B. Observations on the pathogenesis of renal tubular acidosis. Am J Med. 1958 Oct;25(4):503–515. doi: 10.1016/0002-9343(58)90040-8. [DOI] [PubMed] [Google Scholar]
- Roscoe J. M., Goldstein M. B., Halperin M. L., Schloeder F. X., Stinebaugh B. J. Effect of amphotercin B on urine acidification in rats: implications for the pathogenesis of distal renal tubular acidosis. J Lab Clin Med. 1977 Mar;89(3):463–470. [PubMed] [Google Scholar]
- Roscoe J. M., Goldstein M. B., Halperin M. L., Wilson D. R., Stinebaugh B. J. Lithium-induced impairment of urine acidification. Kidney Int. 1976 Apr;9(4):344–350. doi: 10.1038/ki.1976.40. [DOI] [PubMed] [Google Scholar]
- Steinmetz P. R., Lawson L. R. Defect in urinary acidification induced in vitro by amphotericin B. J Clin Invest. 1970 Mar;49(3):596–601. doi: 10.1172/JCI106270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinebaugh B. J., Esquenazi R., Schloeder F. X., Suki W. N., Goldstein M. B., Halperin M. L. Control of the urine-blood PCO2 gradient in alkaline urine. Kidney Int. 1980 Jan;17(1):31–39. doi: 10.1038/ki.1980.4. [DOI] [PubMed] [Google Scholar]
- Stone D. K., Seldin D. W., Kokko J. P., Jacobson H. R. Mineralocorticoid modulation of rabbit medullary collecting duct acidification. A sodium-independent effect. J Clin Invest. 1983 Jul;72(1):77–83. doi: 10.1172/JCI110986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirakomen K., Kozlov N., Arruda J. A., Kurtzman N. A. Renal hydrogen ion secretion after release of unilateral ureteral obstruction. Am J Physiol. 1976 Oct;231(4):1233–1239. doi: 10.1152/ajplegacy.1976.231.4.1233. [DOI] [PubMed] [Google Scholar]
- Ullrich K. J., Papavassiliou F. Sodium reabsorption in the papillary collecting duct of rats. Effect of adrenalectomy, low Na+ diet, acetazolamide, HCO-3-free solutions and of amiloride. Pflugers Arch. 1979 Feb 14;379(1):49–52. doi: 10.1007/BF00622904. [DOI] [PubMed] [Google Scholar]
- Walls J., Buerkert J. E., Purkerson M. L., Klahr S. Nature of the acidifying defect after the relief of ureteral obstruction. Kidney Int. 1975 May;7(5):304–316. doi: 10.1038/ki.1975.43. [DOI] [PubMed] [Google Scholar]


