Abstract
The use of brightness-mode ultrasound and Doppler ultrasound in physical medicine and rehabilitation has increased dramatically. The continuing evolution of ultrasound technology has also produced ultrasound elastography, a cutting-edge technology that can directly measure the mechanical properties of tissue, including muscle stiffness. Its real-time and direct measurements of muscle stiffness can aid the diagnosis and rehabilitation of acute musculoskeletal injuries and chronic myofascial pain. It can also help monitor outcomes of interventions affecting muscle in neuromuscular and musculoskeletal diseases, and it can better inform the functional prognosis. This technology has implications for even broader use of ultrasound in physical medicine and rehabilitation practice, but more knowledge about its uses and limitations is essential to its appropriate clinical implementation. In this review, we describe different ultrasound elastography techniques for studying muscle stiffness, including strain elastography, acoustic radiation force impulse imaging, and shear-wave elastography. We discuss the basic principles of these techniques, including the strengths and limitations of their measurement capabilities. We review the current muscle research, discuss physiatric clinical applications of these techniques, and note directions for future research.
Keywords: diagnostic imaging, elasticity, elastography, hardness, muscles, ultrasonography
Introduction
Palpation has long played a fundamental role in the physical examination of patients. Diseased, injured, or dysfunctional tissue often demonstrates abnormal mechanical properties. Thus, the evaluation of the mechanical properties of tissue, including the passive and active properties of skeletal muscle, has important clinical applications. Inferences about the mechanical properties of muscle have been made through indirect clinical and research measurements. Indirect clinical measurements are noted on physical examination by documentation of abnormal muscle tone and changes in joint range of motion, strength, or physical functioning. Indirect research measurements of muscle properties include dynamometry, ramp-and-hold tests, and pendulum tests. They provide valuable information about the whole joint, but are unable to isolate the mechanical properties of individual muscles from those of the associated tendons, neurovascular structures, or joint capsule.
Microscopic and macroscopic muscle structures also provide some information about the properties of skeletal muscle. Muscle biopsy can yield detailed information about the microscopic muscle structure of an area of muscle, but it may underestimate or even miss pathologic changes because of sample bias. B-mode (brightness-mode) ultrasound and magnetic resonance imaging reveal the macroscopic structure (ie, anatomy) of individual muscles. Although the microscopic structure and the macroscopic anatomy of muscle provide valuable information about skeletal muscle, they cannot characterize the mechanical properties that affect force generation, joint range of motion, or physical function. Unfortunately, there is a paucity of literature regarding the measurement of the mechanical properties of muscle. However, by combining what is known about microscopic structure, macroscopic anatomy, and tissue mechanical properties, we can objectively evaluate both healthy muscle and pathologic muscle; we can select the best techniques to monitor responses to interventions in patients with functional impairments; and we can perhaps even identify new rehabilitation strategies.
New technologies, including magnetic resonance elastography and ultrasound elastography, show promise for direct measurement of the mechanical properties of muscle. Magnetic resonance elastography uses magnetic resonance imaging to map and quantitate the shear modulus (ie, stiffness) of tissue, including skeletal muscle, when an external force is applied (1–4). However, limitations of this technique are similar to those in magnetic resonance imaging, making it unlikely for it to be incorporated into physical medicine and rehabilitation clinical practice, as B-mode ultrasound has been incorporated. Ultrasound elastography also measures the mechanical properties of tissue (5). This new technology was created in the 1990s, but it has been applied only recently to muscle imaging. Over the years, multiple ultrasound elastography techniques have been described, with each technique producing data that are qualitative, quantitative, or some combination thereof. Clinicians who are unfamiliar with these ultrasound techniques may be unaware of their true measurement capabilities.
Multiple reviews are available that detail the physics and technical aspects of ultrasound elastography (5–11). Unfortunately, these reviews target health care providers with a strong background in ultrasound physics and provide limited discussion of the clinical application and significance of ultrasound elastography with respect to muscle. Thus, they are of little assistance to the typical physical medicine and rehabilitation physician seeking to improve clinical practice by adding ultrasound elastography. Many rehabilitation strategies are aimed at changing the mechanical properties of muscle. However, these changes cannot be measured directly and reliably in the clinic setting. One example of how this technology may have a positive impact on clinical practice is its use for measuring the mechanical properties of myofascial trigger points. This technology can aid diagnosis by providing objective, real-time clinical measurements. Longitudinal measurements during therapeutic interventions may also guide treatment duration or facilitate decisions to alter the therapeutic intervention. With its real-time ability to differentiate between normal and abnormal muscle properties, ultrasound elastography shows promise as a clinical tool to aid in diagnosing muscle abnormalities (12–14), predicting muscle response to treatment (15), and monitoring muscle responses to therapeutic interventions (16,17). The goals of this review are to introduce current ultrasound elastography techniques being used in the study of muscle properties; to outline current research implications for their use in rehabilitation; and to discuss future directions for research on, and potential clinical applications of, ultrasound elastography.
Ultrasound Elastography Principles and Techniques
In general, all methods-testing techniques for determining the material properties of tissue, including mechanical properties, involve measurements of deformation in response to applied stress or force. Ultrasound elastography relies on the same principle. The stress can be produced by internal physiological motions, such as the beating of the heart (18,19) or the pulsation of a blood vessel (20); by external mechanical compression or vibration (21–23); or by a high-intensity long-duration (hundreds of ultrasound cycles) ultrasound “push” beam (a long burst of focused ultrasound pulses) (24–30). Subsequent tissue deformation is then detected by pulse-echo ultrasound. Tissue deformation causes a small time shift in ultrasound echoes, which is used to measure the tissue deformation from applied stress (31,32).
Various ultrasound elastography techniques are available, and each relies on different methods to produce and measure tissue deformation. Three techniques are used to evaluate skeletal muscle: strain elastography (SE), acoustic radiation force impulse imaging (ARFI), and shear-wave elastography (SWE) (Table 1).
Table 1.
Overview of Ultrasound Elastography Techniques
| Technique | Mechanism of Tissue Deformation |
Output | Quantitative | Highlights of Techniques |
|---|---|---|---|---|
| SE | Manual compression | 2D elastogram with strain or strain rate | No | Commercially available (6) and FDA approved Compared to ARFI and SWE, lower reliability and lower repeatability User dependent Lack of modeling for anisotropic tissue such as muscle (ie, accuracy of mathematical models and assumptions for muscle tissue unknown) Better suited for evaluating focal masses rather than diffuse disease changes in tissue |
| ARFI | Ultrasound push beam | 2D elastogram or tissue displacement | No | Commercially available (6) and FDA approved Limited application to muscle Very low frame rate (tissue overheating) |
| SWE (external vibration) | External mechanical vibration | Young modulus (a single number) Can be 1D or 2D: 1D number of TE, 2D map for CW vibration (display is 2D elastogram with shear-wave speed) |
Yes | Commercially available (6) and FDA approved Lower reliability No B-mode imaging for 1D TE For CW vibration method: complicated boundary conditions and possible shear-wave reflection Inconvenient compared to SE, SSI, and ARFI (ie, a large, heavy shaker must be held in one hand while the probe is held in the other hand) |
| SWE (supersonic shear imaging) | Ultrasound push beam–induced shear-wave propagation | 2D elastogram, Young modulus, or shear-wave speed | Yes | Commercially available (6) and FDA approved Compared to SE, ARFI, and SWE-EV: higher reliability and repeatability, less user dependent Good B-mode imaging quality as guidance Only available on 1 commercial machine Limited by small region of interest from which the Young modulus is measured Frame rate fast (real-time imaging) and continuing to improve |
Abbreviations: ARFI, acoustic radiation force impulse imaging; B-mode, brightness-mode; CW, constant wave; 1D, 1-dimensional; 2D, 2-dimensional; FDA, US Food and Drug Administration; SE, strain elastography; SWE, shear-wave elastography; SWE-EV, shear-wave elastography–external vibration; TE, transient elastography
Strain Elastography
In SE, the sonographer manually compresses the ultrasound transducer against the patient’s body surface. Tissue deformation, represented by strain, is measured in a 2-dimensional (2D) region under the transducer (typically the full field of view) and is displayed as an elastogram (Figure 1). Given the same amount of applied stress, softer tissue in the elastogram has more deformation and therefore experiences larger strain than stiffer tissue. The elastogram shows the strain differences qualitatively by variations in color or gray scale, depending on the type of ultrasound machine (make and manufacturer) (33). Moving from this qualitative assessment to a quantitative measure using the Young modulus requires quantification of the applied stress, which is challenging in practice. Although the stress distribution within the tissue is unknown with SE, there are methods to convert from qualitative to semiquantitative data.
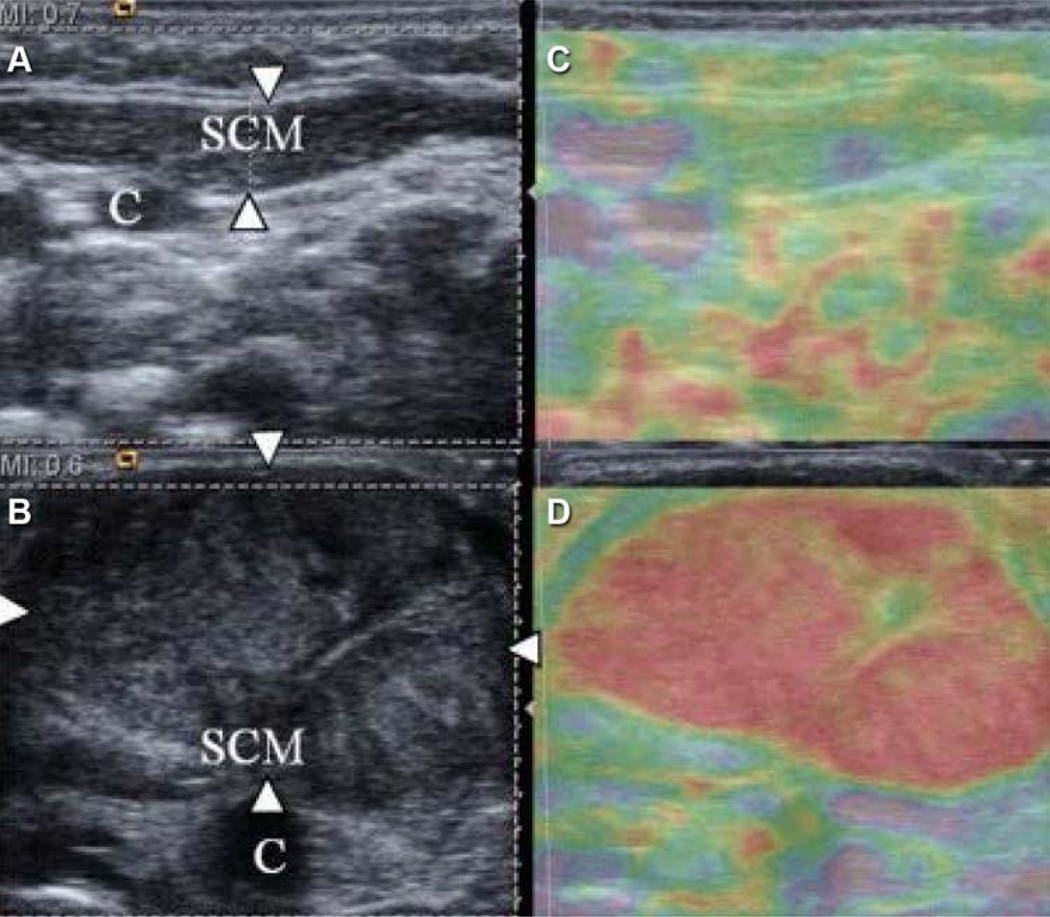
Figure 1.
Images of the sternocleidomastoid (SCM) muscle. A and B, B-mode ultrasound images. C and D, Ultrasound elastograms. Normal muscle (arrowheads in part A) is mostly soft. Mass in an affected SCM (arrowheads in part B) shows increased hardness from normal. C indicates carotid artery; purple, soft tissue; red, hard tissue. (Adapted from Kwon and Park [15]. Used with permission.)
One semiquantitative method is the strain ratio, which compares 2 separate areas in the same elastogram (33). The elastography software first calculates the average strain in the normal (ie, reference) area and then divides it by the average strain in the diseased area, which is typically an area of a focal mass. The higher the strain ratio, the more likely it is that the area of interest will be malignant (34). Other semiquantitative methods include placing references of known hardness over the tissue and using them to calculate a strain ratio in a similar way (Figure 2) (35), or using a visual scoring system that compares the color of the compressed area to the color of the surrounding area (15). These semiquantitative techniques are not truly quantitative because the stress distribution within muscle tissue is neither known nor uniform (as is assumed for this method), thus rendering the Young modulus calculations unreliable (35). In addition to concerns about assumptions for semiquantitative techniques, variable tissue compression by the ultrasound operator calls into question the reliability of all SE measurements (33).
Figure 2.
Ultrasound elastogram (left) and B-mode scan (right) of the medial gastrocnemius. Left image shows the soft and hard references used to calculate a semiquantitative strain ratio. (Adapted from Chino et al [35]. Used under the terms of the Creative Commons Attribution License.)
Acoustic Radiation Force Impulse
In ARFI, a single ultrasound transducer generates a push beam within the tissue to apply stress. The same transducer then measures the tissue displacement along the push beam. As with traditional materials-testing techniques, the push beam produces more displacement of softer tissue than of stiffer tissue. Multiple push-detection data acquisitions are used to form a 2D ARFI image of the area of interest as the push and detection beams are translated or steered to cover the entire 2D imaging area. Like SE images, ARFI images are qualitative maps of tissue stiffness, because tissue displacement depends on the stress produced by the ultrasound push beam, the extent of which is unknown for ARFI (28). ARFI differs from SE in that it uses the dynamic tissue response produced by ultrasound push beams, whereas the tissue compression method for SE is static. ARFI measurements may be more reliable than SE measurements because tissue displacement with ARFI is caused by fixed ultrasound waves, rather than by tissue compression by the sonographer. Unfortunately, creating a series of push beams in this manner requires considerable electrical power, which can lead to transducer and tissue overheating that limits the frame rate and the extent of data acquisition.
Shear-Wave Elastography
Lastly, SWE is an ultrasound elastography technique that uses shear waves to measure tissue stiffness quantitatively. The shear waves travel perpendicularly through tissue to the direction of the particle motion. The relationship between physical shear-wave properties and tissue mechanical properties was first studied in the 1960s (36). Unlike the use of SE and ARFI, the use of SWE to characterize tissue stiffness does not require knowledge of applied stress. By solving the shear-wave equations, one can quantitatively estimate tissue mechanical properties in units of Pascal (24,37,38). SWE takes advantage of the nice property of shear waves, and various methods have been developed to induce shear waves in tissue, such as ultrasound push beams (supersonic shear imaging [SSI]) or external mechanical vibrations (transient elastography [TE]). Shear waves are tracked by pulse-echo ultrasound and can be used quantitatively to calculate the tissue modulus (ie, stiffness); as the stiffness of underlying tissue increases, shear-wave speed increases (6).
Quantifying tissue stiffness on the basis of shear-wave propagation requires advanced mathematical modeling (9,24), which is beyond the scope of this paper. Additionally, most SWE techniques assume that the underlying tissue is isotropic, elastic, and locally homogenous—such as that of breast, liver, or thyroid. Muscle, however, is anisotropic: the mechanical properties along muscle fibers differ from those across muscle fibers. This anisotropy requires orientation of the transducer for all SWE techniques to be longitudinal to muscle fibers in order to achieve accurate and reliable measurements (39). Despite the anisotropy, the shear modulus (a stiffness measure that assumes isotropy) measured from shear-wave speed displays good agreement with the Young modulus (a stiffness measure that assumes isotropy and incompressibility) throughout the range of normal physiologic tension of skeletal muscle (40). In the medical literature, the shear modulus (or shear elastic modulus) and the Young modulus have both been used in reporting outcomes. The Young modulus is often the output from commercial machines, but it can be converted to the shear modulus by dividing by 3. Since muscle is compressible but may be considered transversely isotropic (41), the most accurate measure is the shear modulus.
Transient Elastography
Traditional TE uses a single-element transducer, making it 1-dimensional (22). Therefore, a TE device cannot produce conventional 2D B-mode ultrasound images, because that requires an array ultrasound transducer with multiple elements. TE is also limited in obese patients by the difficulty of achieving depth penetration with a standard probe (6). Two-dimensional TE, also known as 2D SWE, has some benefits over traditional TE. The most notable is B-mode guidance. Unlike the transient shear waves of traditional TE, 2D SWE produces continuous-wave vibrations that can be captured with slower ultrasound frame rates (Figure 3) (42). However, continuous-wave vibrations are complicated by boundary conditions (the vibration wave can be affected or altered by traveling through skin and fat) and by the interference of reflected shear waves with measurements (9).
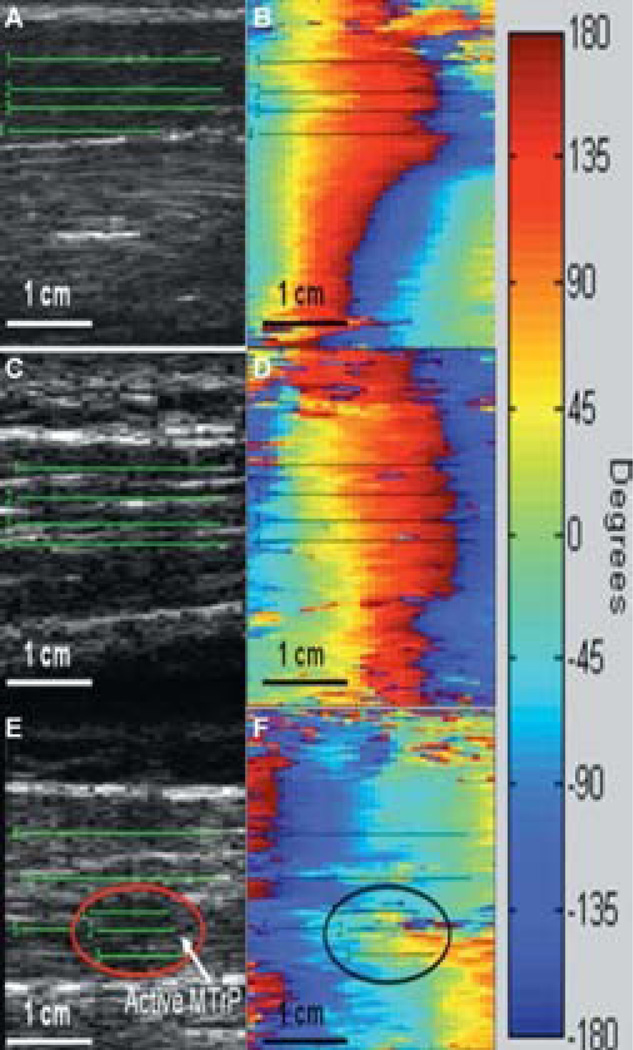
Figure 3.
Images produced from custom transient elastography with continuous shear waves using a linear array (B-mode) transducer and a mechanical handheld vibrator. B-mode (gray scale) and phase plot images of shear-wave vibrations for the biceps brachii (A and B), normal upper trapezius (C and D), and upper trapezius with an active myofascial trigger point (MTrP) (E and F). Numbered lines represent areas where phase lag was measured. Note the limited gray-scale image quality. (Adapted from Ballyns et al [13]. Used with permission.)
Supersonic Shear Imaging
Compared with TE, SSI creates shear waves over a greater range of depth by using multiple push beams focused at different depths within the tissue (24). The multiple push beams facilitate the detection of shear waves in a larger area for imaging. SSI uses an ultrafast plane-wave imaging technique for shear-wave detection with a high frame rate (6,39). This high frame rate allows the capturing of 2D shear-wave propagation and enables SSI to perform real-time SWE. SSI produces 2D quantitative images of the Young modulus superimposed on B-mode images (Figure 4). Although the region of interest, or measurement area, in SWE is small, newer techniques are being developed that permit dynamic measurements and increase the region of interest (30,43). Since tissue deformation in SSI relies on highly controlled ultrasound push beams rather than on tissue compression by the sonographer, SSI is considerably less operator dependent than SE. However, compression of tissue through the ultrasound transducer can increase tissue stiffness (an effect due to nonlinearity of tissue rather than to measurement bias) (44). Therefore, minimal compression should be used to maintain transducer contact with the body surface. In addition, the upper limits of shear-wave speed (ie, stiffness) that can be measured, despite the ultrafast frame rate, tend to limit the evaluation of muscle with activation beyond 40% maximal voluntary contraction (45,46).
Figure 4.
Representative shear-wave elastograms at 20° (A), 0° plantar flexion (B), and 10° dorsiflexion (C) in a pediatric patient’s left lateral gastrocnemius muscle. The blue-shaded box over the lateral gastrocnemius muscle represents the area of measurement. Purple indicates softer tissue (lower Young modulus) and green indicates stiffer tissue (higher Young modulus). Numbers indicate the lateral gastrocnemius (no. 1), the soleus (no. 2), and the flexor hallucis longus (no. 3).
Ultrasound Elastography and Muscle
Both passive muscle stiffness and active muscle stiffness contribute to physical function. The measurement of passive and active stiffness of individual muscles is challenging, because common techniques used for musculoskeletal measurements are not capable of measuring individual muscles in isolation. These techniques are gross measurements of the entire joint, muscle, tendon, or neurovascular complex. However, certain conditions (eg, muscular dystrophy, collagen disorders, cerebral palsy, prolonged bed rest, or chronic compartment syndrome) affect muscle directly and impact function. By measuring the viscoelastic properties of muscle (ie, the mechanical properties of muscle), we can gain information on the effect of these conditions on static (passive), dynamic (as with stretch), and active muscle properties. Viscoelastic properties also provide a link to the histopathologic properties of muscle without the need for biopsy. For more information on ultrasound elastography muscle studies, see Table 2.
Table 2.
Results Form Select Ultrasound Elastography Muscle Studies
| Author | Technique | Participants | Shear-Wave Velocity, m/s | Shear Modulus, kPa | Young Modulus, kPa | Findings |
|---|---|---|---|---|---|---|
| Unloaded muscle (normal) | ||||||
| Arda et al (47) | SSI | 127 healthy subjects (38 M; age 17–63 y) | Masseter M: 10.8±3.9 F: 10.3±3.6 Gastrocnemius M: 11.4±4.1 F: 11.0±4.0 |
No significant difference between sexes for masseter or gastrocnemius | ||
| Kot et al (44) | SSI | 20 healthy subjects (14 M; age 21–33 y) | Rectus femoris Light: 12.78±3.56 Mod: 18.51±6.71 Hard: 32.29±14.17 |
Significant difference in stiffness with change in external pressure (light, moderate, or hard) on ultrasound transducer (P<.05) | ||
| Unloaded muscle (pathology) | ||||||
| Niitsu et al (12) | SE | 6 M post-exercise; age 21–36 y | Strain ratio peaked 2 days following exercise; correlated well with durometer measurements; followed expected pattern of delayed-onset muscle soreness | |||
| Ballyns et al (13) | SWE | 13 subjects with palpable trigger points (5 M; age 41±13 y); 9 healthy subjects (6 M; age 30±5 y) | Trapezius Normal: 3.38 Trigger point: 3.5 Near trigger: 2.14 |
Both active trigger points and surrounding tissue were significantly stiffer than normal tissue (P<.05) | ||
| Chan et al (14) | SE | 12 M with chronic low back pain (age 22–52 y); 12 healthy M (age 21–34 y) | Subjects with low back pain had greater L4 multifidus stiffness in all positions evaluated (prone, standing, 25° stoop, 45° stoop) | |||
| Kuo et al (48) | ARFI | 20 healthya subjects (9 M; age 22–39 y) | Sternocleidomastoid 0.9688±0.0994 Scalene 1.1193±0.1701 Trapezius 2.0865±0.4480 Levator scapulae 1.2093±0.2977 |
Significant difference between all 4 muscles (P<.001); trapezius significantly stiffer in chronic neck pain (P=.008) | ||
| Normal values, passive stretch | ||||||
| Nordez et al (49) | TE | 9 healthy M; age 25±3 y | Medial gastrocnemius throughout stretch ratio of max/min hardness: 2.62±0.46 | Passive gastrocnemius stiffness increases with passive stretch | ||
| Gennisson et al (39) | SSI | 5 healthy subjects (sex and age not reported) | Biceps brachii 90°: 2.73±0.02 165°: 5.68±0.03 Brachialis 90°: 3.11±0.02 165°: 5.56±0.09 |
Biceps brachii and brachialis stiffness increased with stretch or loading; anisotropy affected shear-wave speed measurements | ||
| Akagi et al (50) | SE | 12 healthy M; age 25±4 y | Medial gastrocnemius muscle hardness significantly different between neutral and 20° PF (P<.01) and 30°DF and neutral (P<.05); no change in medial gastrocnemius thickness with stretch | |||
| Chino et al (35) | SE | 10 healthy M; age 25.3±4.3 y | Repeated measures of strain ratio for medial gastrocnemius at 30° PF, anatomic neutral, and 20° DF found low coefficient of variation (<12%, acceptable) and high ICC (>0.75) within and between investigators, demonstrating favourable reliability and validity for SE | |||
| Maïsetti et al (51) | SSI | 7 healthy M; age 27.6±6 y | Medial gastrocnemius shear modulus-muscle length relationship matches well to force-length relationship with passive stretch | Passive gastrocnemius stiffness increases exponentially with stretch; shear modulus may be used to estimate slack length or indirectly estimate muscle force | ||
| Normal values, active | ||||||
| Nightingale et al (52) | ARFI | 3 healthy subjects (2 M; age 35–48 y) | Measures of vastus medialis stiffness demonstrate significant differences between subjects (P<.01) and between loading levels (P<.001); demonstrate feasibility of using ARFI to quantify real-time tissue stiffness | |||
| Gennisson et al (53) | TE | 10 healthy subjects; age 26.8±3.2 y (sex not reported) | Biceps brachii Rest: 0.92±0.55 Biceps hardness index: Rest: 3.08±2.20 Activated: 1.31±0.22 |
Change in shear modulus with activation depends on resting level; linear relationship between surface EMG and shear modulus; nonlinear relationship between surface EMG and torque, indicating other muscles involved in torque production | ||
| Shinohara et al (54) | SSI | 1 healthy M; age 42 y | Tibialis anterior Rest: 40.6±1.0 30% MVC: 268±25.0 Medial gastrocnemius Rest: 16.5±1.0 30% MVC Knee flex: 41.2±2.0 Knee ext: 225.4±41.0 Standing: 112.5±5.0 Soleus Rest: 14.5±2.0 30% MVC Knee flex: 76.8±7.0 Knee ext: 55.0±5.0 Standing: 36.3±17.0 |
Increased muscle stiffness with activity; comparable to expected level of activity | ||
| Bouillard et al (45) | SSI | 11 healthy M; age 25±2.7 y | First dorsal interosseous shear modulus/torque R2: 0.986±0.007 Abductor digiti minimi shear modulus/torque R2: 0.977±0.016 | Strong linear relationship between shear modulus and torque surpasses that of surface EMG and torque for both muscles examined, indicating torque is more accurately measured by shear modulus than by surface EMG | ||
| Bouillard et al (55) | SSI | E1: 12 healthy subjects (8 M [age 23.1±2.2 y]; 4 F [age 24.8±2.2 y]) during fatigue E2: 8 healthy M (age 27.3±5.4 y) during fatigue |
E1: Abductor digiti minimi pre- to post-fatigue RMSdev: 3.7±2.6% MVC E2: vastus lateralis, vastus medialis, rectus femoris during fatigue opposite changes in shear moduli |
E1: Shear elastic modulus significantly more accurate than EMG for measuring muscle force during fatigue; E2: Load sharing among muscles during fatigue varies among individuals |
||
| Leong et al (56) | SSI | 28 healthy subjects (15 M; age 18–55 y) | Upper trapezius Rest: 17.11±5.82 30° active abduction: 26.56±12.32 | Significant difference for active abduction; excellent intra- and interoperator reliability (ICC>0.75) | ||
| Pediatric population | ||||||
| Brandenburg et al (57) | SSI | 2 healthy subjects (1 M); age 5–11 y | Lateral gastrocnemius 20° PF F, R: 19.2±3.8 F, L: 19.5±5.0 M, R: 25.6±1.9 M, L: 33.1±6.7 10° dorsiflexion F, R: 59.6±6.7 F, L: 86.0±5.9 M, R: 107.9±32.8 M, L: 96.0±3.8 |
Demonstrates feasibility of application to pediatric population; possible age- or sex-related effects | ||
| Drakonaki and Allen (58) | SE | 1 M with Bethlem myopathy; age 15 y | Center of vastus lateralis and rectus femoris appears stiffer than periphery; elastogram of vastus lateralis and rectus femoris subjectively stiffer than in asymptomatic 20-year-old | |||
| Vasilescu et al (16) | SE | 7 children with CP; age 3–10 y | Subjective reduction in passive stiffness after botulinum toxin injection | |||
| Kwon et al (59) | SE; ARFI | 15 children with CP (10 M; age 58.7±20.6 mo); 13 typically developing children (4 M; age 46.9±20.2 mo) | Medial gastrocnemius Spastic: 2.5±0.7 Normal: 1.3±0.4 Soleus Spastic: 1.7±0.3 Normal: 1.5±0.1 |
Medial gastrocnemius stiffness and shear-wave velocity greater in children with CP (P<.01); positive correlation with MAS and shear-wave velocity (r=0.712); negative correlation with MAS and strain ratio (r=−0.766) | ||
| Kwon et al (17) | SE | 1 M with CP; age 28 mo | Medial gastrocnemius strain ratio increased 4 weeks after botulinum toxin injection and intensive rehabilitation treatment | |||
| Kwon and Park (15) | SE | 50 infants with torticollis: 20 severe (age 28.7±10.6 d); 30 moderate (age 32.9±11.2 d) | Greater stiffness in infants with severe torticollis (P=.001) |
Abbreviations: ARFI, acoustic radiation force impulse; CP, cerebral palsy; DF, dorsiflexion; EMG, electromyography; ext, extension; F, female; flex, flexion; ICC, intraclass coefficient; kPa, kilopascals; L, left; M, male; MAS, modified Ashworth scale; max, maximum; min, minimum; m/s, meters per second; mod, moderate; MVC, maximal voluntary contraction; PF, plantar flexion; R, right; rest, resting; RMSdev, root mean squared deviation; R2, coefficient of determination; SE, strain elastography; SSI, supersonic shear imaging; SWE, shear-wave elastography; TE, transient elastography.
Three reported chronic neck pain.
Passive Muscle Stiffness
Numerous authors have described using the ultrasound elastography techniques outlined above to measure passive stiffness in various healthy skeletal muscles (14,47,48,54,56). Early studies have not shown a significant difference in passive muscle stiffness in healthy persons by age or sex (47). The reliability of passive stiffness measurements appears to be poorer for TE, SE, and ARFI than for SSI (28,49–51). In addition, the assumptions and additional calculations required for semiquantitative analysis using SE make it more challenging for real-time results. Real-time results are important if this technology is to be incorporated as a clinical tool. Unfortunately, it is difficult to compare results from different studies because they lack consistency in probe positioning, use different ultrasound elastography techniques, and report stiffness measures differently. For example, SSI measurements include shear-wave speed, elastic modulus, shear elastic modulus, the Young modulus, and elasticity value (39,44,47,56,60). Additional normative data are needed on how to identify normal passive stiffness with standard measurement protocols; on the use of surface electromyography to monitor muscle relaxation during measurements; and on the standard reporting of ultrasound elastography measures.
The measurement of passive muscle stiffness with ultrasound elastography is perhaps the simplest measurement to capture, but it must be done in a standardized manner. For example, orientation of the transducer probe can affect the reliability of measurements (39). It is also not certain how reliable these measurements are in muscles with disrupted fascicles or severe fibrosis, such as muscular dystrophy. Moreover, although muscles with various pennation patterns have been studied, the influence of muscle pennation on the reliability of measurements is unknown. For instance, in a multipennate soleus muscle, is it possible to maintain a truly longitudinal probe position, particularly as muscle is being dynamically stretched or activated? Does location of the probe affect passive stiffness properties near origin, mid-belly, or tendinous transition? These concerns apply not only to passive stiffness measurements but also to measurements of dynamic stiffness and active stiffness. Optimizing techniques for measuring passive muscle stiffness in healthy muscle will assist with recognition of abnormalities in diseased or disordered muscle.
As with work-optimizing measurement techniques, ultrasound elastography shows promise for identifying abnormal passive muscle stiffness in neuromuscular and musculoskeletal disorders (15,58,59). For example, one study of SE found passive stiffness of muscle to be elevated in children with cerebral palsy (59). From a histopathologic standpoint, this finding corroborates the results of a recent biopsy study showing increased collagen content and fewer sarcomeres in muscle in a series of children with cerebral palsy (61). Although the SE study had some methodology limitations, such as the use of a semiquantitative technique and the lack of monitoring for muscle relaxation, it shows promise for the direct study of disordered muscle. Ultrasound elastography may eventually be used to measure response to spasticity treatment or to target specific areas of increased stiffness within a muscle (16,17). With the ongoing debate about the effects of spasticity treatment on passive muscle stiffness (62,63), direct measurement of muscle might be the key to improved understanding. In another study using SE technique, infants with congenital muscular torticollis showed improvements in the passive stiffness of the involved sternocleidomastoid muscle that corresponded to improvements in the torticollis (Figure 1) (15). This finding demonstrates how ultrasound elastography might be useful for longitudinal follow-up of therapeutic interventions in even very young patients. More rigorous studies evaluating the effects of neuromuscular disorders on passive muscle properties may provide insights on longitudinal changes to the muscle. Ultimately, such studies may assist with targeting treatment or therapy.
Passive muscle stiffness may be caused not only by the effects of disease but also by acute pain or chronic pain. In persons with delayed-onset muscle soreness (DOMS), SE measurements have been shown to have increasing stiffness over the first 2 days (12). Measures began to decrease by day 3, but did not return to baseline. These results correlate well with the expected course of DOMS, suggesting that ultrasound elastography is sensitive to changes in stiffness secondary to acute inflammation and swelling. Thus, ultrasound elastography may be useful for measuring and monitoring DOMS, and it may provide real-time clinical measurement for tracking the effects of DOMS on muscle. Ultrasound elastography shows further promise for potential applications to the evaluation of acute muscle injuries or to the measurement of changes in passive stiffness with chronic compartment syndrome.
For persons with chronic muscular pain trigger points, ultrasound elastography appears to be able to detect changes in muscle stiffness (Figure 3) (13,14,48). Although the exact etiology of trigger points is unknown, one theory is that chronic muscle overuse leads to inflammation (64). These early results suggest that histologic changes within the muscle in response to acute pain or chronic pain may correspond to changes in mechanical properties. These changes can be detected with ultrasound elastography. Further studies in larger groups of persons with more homogenous pain syndromes are needed to help characterize the muscle stiffness changes associated with pain. Ultrasound elastography may also be useful for monitoring response to therapeutic pain procedures, such as dry needling, massage, or botulinum toxin therapies for trigger points.
Dynamic Muscle Stiffness
Muscle measurements with passive stretch and hold positioning show a pattern of exponentially increasing stiffness (Figures 2 and 4) (35,50,57). These results are similar to the typical in vivo loading curves for many skeletal muscles. Ultrasound elastography studies of dynamic muscle stretch—measurements taken during passive stretch of muscle—have indicated that the resistance to passive stretch correlates with passive muscle stiffness (49,51,52). However, the 2 techniques used, ARFI and TE, displayed poor reliability (49,52). Poor reliability may result from the slow acquisition time with ARFI and from the use of external vibrators with TE. Thus far, only the loading curve of skeletal muscle in stretch has been evaluated, leaving many clinically relevant questions unanswered. Do the stretch and relaxation of living muscle compare to those documented in the laboratory setting? Do stretch and relaxation vary in different muscles? Does the stretch and relaxation response predispose to injury?
Active Muscle Stiffness
Active muscle stiffness—the stiffness that results from active muscle contraction—is an especially exciting area for clinical integration of ultrasound elastography. Previously, direct measurements of active muscle stiffness were limited to ex vivo studies. These studies included evaluation of muscle force as a function of actin-myosin crossbridge formation, with minimal direct relevance to the clinical setting. In biomechanics research, the ability to measure active stiffness has been limited to multiple, whole-joint muscle groups. The total force generated by these muscle groups requires mathematical modeling techniques to estimate the contribution of individual muscles to this force. This type of technique is not conducive to clinical use. However, ultrasound has been used to directly measure individual muscle stiffness during active muscle contraction, showing increasing stiffness with increasing force (45,52–54,56). However, maximally activated skeletal muscle can be quite stiff. Current studies are limited to about 40% maximal voluntary contraction, because greater contraction results in high-speed shear waves that are too fast to be detected (45). This limitation means that current SWE techniques are unlikely to identify the subtle changes in strength typically seen at the level of maximal voluntary contraction. Despite these limitations, valuable information has been reported on patterns of muscle failure in submaximal sustained isometric contraction (55). This information provides insight for targeted strengthening to reduce fatigue in patients with muscle disorders. Identifying normal patterns of muscle fatigue may also help diagnose disease states, such as myasthenia gravis or other neuromuscular disorders, or it may provide insight for optimizing endurance training in athletes.
Future Directions
The real-time, direct measurement of the passive and active properties of individual muscles has already begun to advance our basic understanding of skeletal muscle. Improvements in the rehabilitation of patients with these conditions can be expected with greater understanding of the relation between muscle properties and physical function. As this technology and these muscle measurements are being explored, questions remain regarding the measurement capabilities of ultrasound elastography. How does the location of the ultrasound probe on a given muscle affect muscle stiffness measurements? Could imaging a larger area of muscle improve the reliability of measurements?
Ultrasound elastography techniques under development include the measurement of larger areas of tissue with shorter data acquisition time. This would increase both the area of muscle that can be studied and the sensitivity of measurements (30). Another potential future direction of SWE technique development might focus on capturing the high-speed shear waves seen during maximal muscle activation and spastic catch.
Ultrasound elastography could be instrumental in detecting subtle changes in the muscle properties that occur early in the course of muscle injury, disease, or disorder. Earlier detection could improve athletic training and rehabilitation strategies. It could also alert the physician to an impending functional decline in a progressive muscle disorder. Real-time measurements of passive and active stiffness of muscles may assist with designing focused, individualized rehabilitation strategies, such as in a patient on prolonged bed rest. Through serial measurements, ultrasound elastography could provide a more sensitive measurement of strength changes in patients on inpatient rehabilitation units. Correlation of ultrasound elastography measurements with function may give providers greater insights into predicting length of stay and setting inpatient rehabilitation goals. Other uses by rehabilitation providers include the identification of muscle disease or muscle property changes suggestive of impending athletic injury; the performance of real-time, noninvasive diagnostic testing in patients with chronic compartment syndrome; the tracking of the therapeutic response to rehabilitation interventions for trigger points or muscular back pain; and the monitoring of the longitudinal response of muscle to spasticity interventions.
Conclusion
As ultrasound elastography evolves, we must strive to understand its varied techniques—including their strengths, limitations, and anticipated clinical applications for use in muscle measurements. Ultrasound elastography provides the opportunity to further our understanding of the interaction between muscle structure and function by its measurement of individual muscle mechanical properties. Direct measurement has the potential to quantify previously subjective clinical examination measurements and diagnoses. Other applications include tracking outcomes of treatments and therapies that target muscle. This technology will arm physical medicine and rehabilitation researchers and providers with a new tool for furthering the understanding of muscle properties and the impact of these properties on function.
Acknowledgments
Acknowledgment of Presentation: None.
Financial Support: We would like to thank the National Institutes of Health (KL2TR000136-07) and the Department of Physical Medicine and Rehabilitation at Mayo Clinic, Rochester, Minnesota, for supporting J.E.B. in the preparation of this manuscript. S.F.E. was supported by the National Institutes of Health through grants from the National Institute on Aging (F30 AG044075) and the National Institute of General Medical Sciences (T32 GM 65841).
Other Acknowledgments: None.
Abbreviations
- ARFI
acoustic radiation force impulse imaging
- B-mode
brightness-mode
- DOMS
delayed-onset muscle soreness
- 2D
2-dimensional
- SE
strain elastography
- SSI
supersonic shear imaging
- SWE
shear-wave elastography
- TE
transient elastography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Zhao, Chen, and Song disclose royalties from General Electric Medical Systems and Samsung Electronics Company, Ltd for ultrasound elastography technology. Chen receives consultation fees from Sonoscape, Inc. The authors disclose the following patents (no.): Zhao, Chen: System and Method for Correcting Errors in Shear Wave Measurements Arising from Ultrasound Beam Geometry (8,734,350); Chen: Vibration Generation and Detection in Shear Wave Dispersion Ultrasound Vibrometry with Large Background Motions (8,659,975), Method for Ultrasound Vibrometry using Orthogonal Basis Functions (8,602,994), Detection of Motion in Vibro-Acoustography (7,785,259), Ultrasound Vibrometry (7,753,847), Method and Apparatus for Shear Property Characterization from Resonance Induced by Oscillatory Radiation Force (7,713,201); An: Expandable Screw Apparatus and Method (6,668,688), Spinal Fixation Support Device and Methods of Using (7,060,066), Temporomandibular Joint Fossa-Eminence Prosthesis (8,211,180), Doppler Ultrasound for Identifying Material Properties of a Carpal Tunnel Anatomy (8,216,148).
References
- 1.Basford JR, Jenkyn TR, An KN, Ehman RL, Heers G, Kaufman KR. Evaluation of healthy and diseased muscle with magnetic resonance elastography. Arch Phys Med Rehabil. 2002 Nov;83(11):1530–1536. doi: 10.1053/apmr.2002.35472. [DOI] [PubMed] [Google Scholar]
- 2.Chen Q, Bensamoun S, Basford JR, Thompson JM, An KN. Identification and quantification of myofascial taut bands with magnetic resonance elastography. Arch Phys Med Rehabil. 2007 Dec;88(12):1658–1661. doi: 10.1016/j.apmr.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Debernard L, Robert L, Charleux F, Bensamoun SF. Analysis of thigh muscle stiffness from childhood to adulthood using magnetic resonance elastography (MRE) technique. Clin Biomech (Bristol, Avon) 2011 Oct;26(8):836–840. doi: 10.1016/j.clinbiomech.2011.04.004. Epub 2011 May 14. [DOI] [PubMed] [Google Scholar]
- 4.Mariappan YK, Glaser KJ, Ehman RL. Magnetic resonance elastography: a review. Clin Anat. 2010 Jul;23(5):497–511. doi: 10.1002/ca.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ophir J, Cespedes I, Ponnekanti H, Yazdi Y, Li X. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991 Apr;13(2):111–134. doi: 10.1177/016173469101300201. [DOI] [PubMed] [Google Scholar]
- 6.Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F, Cantisani V, Correas JM, D’Onofrio M, Drakonaki EE, Fink M, Friedrich-Rust M, Gilja OH, Havre RF, Jenssen C, Klauser AS, Ohlinger R, Saftoiu A, Schaefer F, Sporea I, Piscaglia F. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013 Apr;34(2):169–184. doi: 10.1055/s-0033-1335205. Epub 2013 Apr 4. [DOI] [PubMed] [Google Scholar]
- 7.Cosgrove D, Piscaglia F, Bamber J, Bojunga J, Correas JM, Gilja OH, Klauser AS, Sporea I, Calliada F, Cantisani V, D’Onofrio M, Drakonaki EE, Fink M, Friedrich-Rust M, Fromageau J, Havre RF, Jenssen C, Ohlinger R, Saftoiu A, Schaefer F, Dietrich CF. EFSUMB. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall Med. 2013 Jun;34(3):238–253. doi: 10.1055/s-0033-1335375. Epub 2013 Apr 19. [DOI] [PubMed] [Google Scholar]
- 8.Drakonaki EE, Allen GM, Wilson DJ. Ultrasound elastography for musculoskeletal applications. Br J Radiol. 2012 Nov;85(1019):1435–1445. doi: 10.1259/bjr/93042867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenleaf JF, Fatemi M, Insana M. Selected methods for imaging elastic properties of biological tissues. Annu Rev Biomed Eng. 2003;5:57–78. doi: 10.1146/annurev.bioeng.5.040202.121623. Epub 2003 Apr 10. [DOI] [PubMed] [Google Scholar]
- 10.Hall TJ. AAPM/RSNA physics tutorial for residents: topics in US: beyond the basics: elasticity imaging with US. Radiographics. 2003 Nov-Dec;23(6):1657–1671. doi: 10.1148/rg.236035163. [DOI] [PubMed] [Google Scholar]
- 11.Sarvazyan A, Hall TJ, Urban MW, Fatemi M, Aglyamov SR, Garra BS. An overview of elastography: an emerging branch of medical imaging. Curr Med Imaging Rev. 2011 Nov;7(4):255–282. doi: 10.2174/157340511798038684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niitsu M, Michizaki A, Endo A, Takei H, Yanagisawa O. Muscle hardness measurement by using ultrasound elastography: a feasibility study. Acta Radiol. 2011 Feb 1;52(1):99–105. doi: 10.1258/ar.2010.100190. [DOI] [PubMed] [Google Scholar]
- 13.Ballyns JJ, Turo D, Otto P, Shah JP, Hammond J, Gebreab T, Gerber LH, Sikdar S. Office-based elastographic technique for quantifying mechanical properties of skeletal muscle. J Ultrasound Med. 2012 Aug;31(8):1209–1219. doi: 10.7863/jum.2012.31.8.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan ST, Fung PK, Ng NY, Ngan TL, Chong MY, Tang CN, He JF, Zheng YP. Dynamic changes of elasticity, cross-sectional area, and fat infiltration of multifidus at different postures in men with chronic low back pain. Spine J. 2012 May;12(5):381–388. doi: 10.1016/j.spinee.2011.12.004. Epub 2011 Dec 23. [DOI] [PubMed] [Google Scholar]
- 15.Kwon DR, Park GY. Diagnostic value of real-time sonoelastography in congenital muscular torticollis. J Ultrasound Med. 2012 May;31(5):721–727. doi: 10.7863/jum.2012.31.5.721. [DOI] [PubMed] [Google Scholar]
- 16.Vasilescu D, Vasilescu D, Dudea S, Botar-Jid C, Sfrangeu S, Cosma D. Sonoelastography contribution in cerebral palsy spasticity treatment assessment, preliminary report: a systematic review of the literature apropos of seven patients. Med Ultrason. 2010 Dec;12(4):306–310. [PubMed] [Google Scholar]
- 17.Kwon DR, Park GY, Kwon JG. The change of intrinsic stiffness in gastrocnemius after intensive rehabilitation with botulinum toxin a injection in spastic diplegic cerebral palsy. Ann Rehabil Med. 2012 Jun;36(3):400–403. doi: 10.5535/arm.2012.36.3.400. Epub 2012 Jun 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanai H. Propagation of spontaneously actuated pulsive vibration in human heart wall and in vivo viscoelasticity estimation. IEEE Trans Ultrason Ferroelectr Freq Control. 2005 Nov;52(11):1931–1942. doi: 10.1109/tuffc.2005.1561662. [DOI] [PubMed] [Google Scholar]
- 19.Olsen DA, Song P, Glaser KJ, Ehman RL. Cardiac-gated hepatic MR elastography with intrinsic transient waveforms. Proc Intl Soc Mag Reson Med. 2011;19:43. [Google Scholar]
- 20.Weaver JB, Pattison AJ, McGarry MD, Perreard IM, Swienckowski JG, Eskey CJ, Lollis SS, Paulsen KD. Brain mechanical property measurement using MRE with intrinsic activation. Phys Med Biol. 2012 Nov 21;57(22):7275–7287. doi: 10.1088/0031-9155/57/22/7275. Epub 2012 Oct 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muthupillai R, Lomas DJ, Rossman PJ, Greenleaf JF, Manduca A, Ehman RL. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science. 1995 Sep 29;269(5232):1854–1857. doi: 10.1126/science.7569924. [DOI] [PubMed] [Google Scholar]
- 22.Sandrin L, Tanter M, Gennisson JL, Catheline S, Fink M. Shear elasticity probe for soft tissues with 1-D transient elastography. IEEE Trans Ultrason Ferroelectr Freq Control. 2002 Apr;49(4):436–446. doi: 10.1109/58.996561. [DOI] [PubMed] [Google Scholar]
- 23.Wu Z, Taylor LS, Rubens DJ, Parker KJ. Sonoelastographic imaging of interference patterns for estimation of the shear velocity of homogeneous biomaterials. Phys Med Biol. 2004 Mar 21;49(6):911–922. doi: 10.1088/0031-9155/49/6/003. [DOI] [PubMed] [Google Scholar]
- 24.Bercoff J, Tanter M, Fink M. Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control. 2004 Apr;51(4):396–409. doi: 10.1109/tuffc.2004.1295425. [DOI] [PubMed] [Google Scholar]
- 25.Hah Z, Hazard C, Mills B, Barry C, Rubens D, Parker K. Integration of crawling waves in an ultrasound imaging system. Part 2: signal processing and applications. Ultrasound Med Biol. 2012 Feb;38(2):312–323. doi: 10.1016/j.ultrasmedbio.2011.10.014. Epub 2011 Dec 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hazard C, Hah Z, Rubens D, Parker K. Integration of crawling waves in an ultrasound imaging system. Part 1: system and design considerations. Ultrasound Med Biol. 2012 Feb;38(2):296–311. doi: 10.1016/j.ultrasmedbio.2011.10.026. Epub 2011 Dec 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nightingale K, McAleavey S, Trahey G. Shear-wave generation using acoustic radiation force: in vivo and ex vivo results. Ultrasound Med Biol. 2003 Dec;29(12):1715–1723. doi: 10.1016/j.ultrasmedbio.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Nightingale KR, Palmeri ML, Nightingale RW, Trahey GE. On the feasibility of remote palpation using acoustic radiation force. J Acoust Soc Am. 2001 Jul;110(1):625–634. doi: 10.1121/1.1378344. [DOI] [PubMed] [Google Scholar]
- 29.Sarvazyan AP, Rudenko OV, Swanson SD, Fowlkes JB, Emelianov SY. Shear wave elasticity imaging: a new ultrasonic technology of medical diagnostics. Ultrasound Med Biol. 1998 Nov;24(9):1419–1435. doi: 10.1016/s0301-5629(98)00110-0. [DOI] [PubMed] [Google Scholar]
- 30.Song P, Zhao H, Manduca A, Urban MW, Greenleaf JF, Chen S. Comb-push ultrasound shear elastography (CUSE): a novel method for two-dimensional shear elasticity imaging of soft tissues. IEEE Trans Med Imaging. 2012 Sep;31(9):1821–1832. doi: 10.1109/TMI.2012.2205586. Epub 2012 Jun 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lubinski MA, Emelianov SY, O’Donnell M. Speckle tracking methods for ultrasonic elasticity imaging using short-time correlation. IEEE Trans Ultrason Ferroelectr Freq Control. 1999;46(1):82–96. doi: 10.1109/58.741427. [DOI] [PubMed] [Google Scholar]
- 32.Pinton GF, Dahl JJ, Trahey GE. Rapid tracking of small displacements with ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control. 2006 Jun;53(6):1103–1117. doi: 10.1109/tuffc.2006.1642509. [DOI] [PubMed] [Google Scholar]
- 33.Carlsen JF, Ewertsen C, Lonn L, Nielsen MB. Strain elastography ultrasound: an overview with emphasis on breast cancer diagnosis. Diagnostics. 2013;3:117–125. doi: 10.3390/diagnostics3010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas A, Degenhardt F, Farrokh A, Wojcinski S, Slowinski T, Fischer T. Significant differentiation of focal breast lesions: calculation of strain ratio in breast sonoelastography. Acad Radiol. 2010 May;17(5):558–563. doi: 10.1016/j.acra.2009.12.006. Epub 2010 Feb 20. [DOI] [PubMed] [Google Scholar]
- 35.Chino K, Akagi R, Dohi M, Fukashiro S, Takahashi H. Reliability and validity of quantifying absolute muscle hardness using ultrasound elastography. PLoS One. 2012;7(9):e45764. doi: 10.1371/journal.pone.0045764. Epub 2012 Sep 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarvazian AP, Pasechnik VI, Schnol SE. Biofizika. 4. Vol. 13. Russian: 1968. Jul-Aug. [Low sonic velocity in gels and protoplasmic structures: possible biological significance of this phenomenon] pp. 587–594. [PubMed] [Google Scholar]
- 37.Manduca A, Oliphant TE, Dresner MA, Mahowald JL, Kruse SA, Amromin E, Felmlee JP, Greenleaf JF, Ehman RL. Magnetic resonance elastography: non-invasive mapping of tissue elasticity. Med Image Anal. 2001 Dec;5(4):237–254. doi: 10.1016/s1361-8415(00)00039-6. [DOI] [PubMed] [Google Scholar]
- 38.Oliphant TE, Manduca A, Ehman RL, Greenleaf JF. Complex-valued stiffness reconstruction for magnetic resonance elastography by algebraic inversion of the differential equation. Magn Reson Med. 2001 Feb;45(2):299–310. doi: 10.1002/1522-2594(200102)45:2<299::aid-mrm1039>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 39.Gennisson JL, Deffieux T, Mace E, Montaldo G, Fink M, Tanter M. Viscoelastic and anisotropic mechanical properties of in vivo muscle tissue assessed by supersonic shear imaging. Ultrasound Med Biol. 2010 May;36(5):789–801. doi: 10.1016/j.ultrasmedbio.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Eby SF, Song P, Chen S, Chen Q, Greenleaf JF, An KN. Validation of shear wave elastography in skeletal muscle. J Biomech. 2013 Sep 27;46(14):2381–2387. doi: 10.1016/j.jbiomech.2013.07.033. Epub 2013 Jul 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrow DA, Haut Donahue TL, Odegard GM, Kaufman KR. Transversely isotropic tensile material properties of skeletal muscle tissue. J Mech Behav Biomed Mater. 2010 Jan;3(1):124–129. doi: 10.1016/j.jmbbm.2009.03.004. Epub 2009 Apr 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandrin L, Tanter M, Catheline S, Fink M. Shear modulus imaging with 2-D transient elastography. IEEE Trans Ultrason Ferroelectr Freq Control. 2002 Apr;49(4):426–435. doi: 10.1109/58.996560. [DOI] [PubMed] [Google Scholar]
- 43.Song P, Urban MW, Manduca A, Zhao H, Greenleaf JF, Chen S. Comb-push ultrasound shear elastography (CUSE) with various ultrasound push beams. IEEE Trans Med Imaging. 2013 Aug;32(8):1435–1447. doi: 10.1109/TMI.2013.2257831. Epub 2013 Apr 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kot BC, Zhang ZJ, Lee AW, Leung VY, Fu SN. Elastic modulus of muscle and tendon with shear wave ultrasound elastography: variations with different technical settings. PLoS One. 2012;7(8):e44348. doi: 10.1371/journal.pone.0044348. Epub 2012 Aug 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bouillard K, Nordez A, Hug F. Estimation of individual muscle force using elastography. PLoS One. 2011;6(12):e29261. doi: 10.1371/journal.pone.0029261. Epub 2011 Dec 21. Erratum in: PLoS One. 2012;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nordez A, Hug F. Muscle shear elastic modulus measured using supersonic shear imaging is highly related to muscle activity level. J Appl Physiol (1985) 2010 May;108(5):1389–1394. doi: 10.1152/japplphysiol.01323.2009. Epub 2010 Feb 18. [DOI] [PubMed] [Google Scholar]
- 47.Arda K, Ciledag N, Aktas E, Aribas BK, Kose K. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. AJR Am J Roentgenol. 2011 Sep;197(3):532–536. doi: 10.2214/AJR.10.5449. [DOI] [PubMed] [Google Scholar]
- 48.Kuo WH, Jian DW, Wang TG, Wang YC. Neck muscle stiffness quantified by sonoelastography is correlated with body mass index and chronic neck pain symptoms. Ultrasound Med Biol. 2013 Aug;39(8):1356–1361. doi: 10.1016/j.ultrasmedbio.2012.11.015. Epub 2013 May 15. [DOI] [PubMed] [Google Scholar]
- 49.Nordez A, Gennisson JL, Casari P, Catheline S, Cornu C. Characterization of muscle belly elastic properties during passive stretching using transient elastography. J Biomech. 2008 Jul 19;41(10):2305–2311. doi: 10.1016/j.jbiomech.2008.03.033. Epub 2008 Jun 9. [DOI] [PubMed] [Google Scholar]
- 50.Akagi R, Chino K, Dohi M, Takahashi H. Relationships between muscle size and hardness of the medial gastrocnemius at different ankle joint angles in young men. Acta Radiol. 2012 Apr 1;53(3):307–311. doi: 10.1258/ar.2011.110481. Epub 2012 Feb 1. [DOI] [PubMed] [Google Scholar]
- 51.Maïsetti O, Hug F, Bouillard K, Nordez A. Characterization of passive elastic properties of the human medial gastrocnemius muscle belly using supersonic shear imaging. J Biomech. 2012 Apr 5;45(6):978–984. doi: 10.1016/j.jbiomech.2012.01.009. Epub 2012 Feb 9. [DOI] [PubMed] [Google Scholar]
- 52.Nightingale K, Nightingale R, Stutz D, Trahey G. Acoustic radiation force impulse imaging of in vivo vastus medialis muscle under varying isometric load. Ultrason Imaging. 2002 Apr;24(2):100–108. doi: 10.1177/016173460202400203. [DOI] [PubMed] [Google Scholar]
- 53.Gennisson JL, Cornu C, Catheline S, Fink M, Portero P. Human muscle hardness assessment during incremental isometric contraction using transient elastography. J Biomech. 2005 Jul;38(7):1543–1550. doi: 10.1016/j.jbiomech.2004.07.013. Epub 2004 Nov 24. [DOI] [PubMed] [Google Scholar]
- 54.Shinohara M, Sabra K, Gennisson JL, Fink M, Tanter M. Real-time visualization of muscle stiffness distribution with ultrasound shear wave imaging during muscle contraction. Muscle Nerve. 2010 Sep;42(3):438–441. doi: 10.1002/mus.21723. [DOI] [PubMed] [Google Scholar]
- 55.Bouillard K, Hug F, Guevel A, Nordez A. Shear elastic modulus can be used to estimate an index of individual muscle force during a submaximal isometric fatiguing contraction. J Appl Physiol (1985) 2012 Nov;113(9):1353–1361. doi: 10.1152/japplphysiol.00858.2012. Epub 2012 Sep 13. [DOI] [PubMed] [Google Scholar]
- 56.Leong HT, Ng GY, Leung VY, Fu SN. Quantitative estimation of muscle shear elastic modulus of the upper trapezius with supersonic shear imaging during arm positioning. PLoS One. 2013 Jun 25;8(6):e67199. doi: 10.1371/journal.pone.0067199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brandenburg JE, Eby S, Landry BW, Hoffman AM, Chen S, Zhao H, Song P, Kingsley-Berg S, An K-N. Shear wave elastography for noninvasive quantification of passive muscle stiffness in typically developing children [poster] PM&R. 2013;5(9S):S199. [Google Scholar]
- 58.Drakonaki EE, Allen GM. Magnetic resonance imaging, ultrasound and real-time ultrasound elastography of the thigh muscles in congenital muscle dystrophy. Skeletal Radiol. 2010;39(4):391–396. doi: 10.1007/s00256-009-0861-0. [DOI] [PubMed] [Google Scholar]
- 59.Kwon DR, Park GY, Lee SU, Chung I. Spastic cerebral palsy in children: dynamic sonoelastographic findings of medial gastrocnemius. Radiology. 2012 Jun;263(3):794–801. doi: 10.1148/radiol.12102478. Epub 2012 Apr 10. [DOI] [PubMed] [Google Scholar]
- 60.Akagi R, Takahashi H. Acute effect of static stretching on hardness of the gastrocnemius muscle. Med Sci Sports Exerc. 2013 Jul;45(7):1348–1354. doi: 10.1249/MSS.0b013e3182850e17. [DOI] [PubMed] [Google Scholar]
- 61.Smith LR, Lee KS, Ward SR, Chambers HG, Lieber RL. Hamstring contractures in children with spastic cerebral palsy result from a stiffer extracellular matrix and increased in vivo sarcomere length. J Physiol. 2011 May 15;589(Pt 10):2625–2639. doi: 10.1113/jphysiol.2010.203364. Epub 2011 Mar 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alhusaini AA, Crosbie J, Shepherd RB, Dean CM, Scheinberg A. No change in calf muscle passive stiffness after botulinum toxin injection in children with cerebral palsy. Dev Med Child Neurol. 2011 Jun;53(6):553–558. doi: 10.1111/j.1469-8749.2011.03930.x. [DOI] [PubMed] [Google Scholar]
- 63.Tedroff K, Granath F, Forssberg H, Haglund-Akerlind Y. Long-term effects of botulinum toxin A in children with cerebral palsy. Dev Med Child Neurol. 2009 Feb;51(2):120–127. doi: 10.1111/j.1469-8749.2008.03189.x. [DOI] [PubMed] [Google Scholar]
- 64.Bron C, Dommerholt JD. Etiology of myofascial trigger points. Curr Pain Headache Rep. 2012 Oct;16(5):439–444. doi: 10.1007/s11916-012-0289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]