Abstract
BACKGROUND
Management decisions and parental counseling after pediatric cardiac arrest depend on the ability of physicians to make accurate and timely predictions regarding neurological recovery. We evaluated neurologists and intensivists performing neuroprognostication after cardiac arrest to determine prediction agreement, accuracy, and confidence.
METHODS
Pediatric neurologists (n = 10) and intensivists (n = 9) reviewed 18 cases of children successfully resuscitated from a cardiac arrest and managed in the pediatric intensive care unit. Cases were sequentially presented (after arrest day 1, days 2–4, and days 5–7), with updated examinations, neurophysiologic data, and neuroimaging data. At each time period, physicians predicted outcome by Pediatric Cerebral Performance Category and specified prediction confidence.
RESULTS
Predicted discharge Pediatric Cerebral Performance Category versus actual hospital discharge Pediatric Cerebral Performance Category outcomes were compared. Exact (Predicted Pediatric Cerebral Performance Category – Actual Pediatric Cerebral Performance Category = 0) and close (Predicted Pediatric Cerebral Performance Category – Actual Pediatric Cerebral Performance Category = ±1) outcome prediction accuracies for all physicians improved over successive periods (P < 0.05). Prediction accuracy did not differ significantly between physician groups at any period or overall. Agreement improved over time among neurologists (day 1 Kappa [κ], 0.28; days 2–4 κ, 0.43; days 5–7 κ, 0.68) and among intensivists (day 1 κ, 0.30; days 2–4 κ, 0.44; days 5–7 κ, 0.57). Prediction confidence increased over time (P < 0.001) and did not differ between physician groups.
CONCLUSIONS
Inter-rater agreement among neurologists and among intensivists improved over time and reached moderate levels. For all physicians, prediction accuracy and confidence improved over time. Further prospective research is needed to better characterize how physicians objectively and subjectively estimate neurological recovery after acute brain injury.
Keywords: neuroprognostication, pediatric cardiac arrest, outcome prediction
Introduction
Prognosis after cardiopulmonary arrest in children is determined based on multiple pre-arrest and arrest characteristics. Among children who achieve sustained return of spontaneous circulation, a favorable neurological outcome is observed in about 5% with an out-of-hospital cardiac arrest and 15–45% with an in-hospital cardiac arrest.1–5 Prognostication after pediatric cardiac arrest is complex because no single test reliably predicts neurological outcome, especially when performed soon after the arrest. Furthermore, the time frame when objective clinical, laboratory, imaging, and neurophysiologic predictors are the most accurate for long-term outcome prognostication is unclear.6,7
In spite of these prognostication uncertainties, management decisions and parental counseling depend on the ability of physicians to make accurate and timely predictions regarding neurological recovery. An overly pessimistic prediction may lead to withdrawal of supportive care in a child with a potentially good quality of life, while an overly optimistic prediction may lead to survival of a neurologically devastated child with poor quality of life. The aims of the present study were to (1) characterize the accuracy and agreement of neurological outcome prognoses in children resuscitated from cardiac arrest among and between pediatric neurologists and pediatric intensivists and (2) determine the accuracy and agreement of neurological outcome prognoses over the first 7 days after resuscitation from cardiac arrest.
Materials and Methods
This study was conducted at the Children’s Hospital of Philadelphia. The study received Institutional Review Board’s approval, and informed consent was obtained from all participants.
Subjects
Pediatric neurology (n = 10) and pediatric intensive care attendings (n = 9) were enrolled. Demographic information including gender and number of years practicing after fellowship was collected. Physicians were compensated (gift card) for their participation after study completion.
Clinical vignettes
Each subject reviewed 18 clinical vignettes. Each vignette described a child successfully resuscitated from either in-hospital or out-of-hospital cardiac arrest who was managed in the pediatric intensive care unit (PICU) and survived until at least 7 days after arrest. The cases were compiled from a cardiac arrest database between 2007 and 2009 for a study of induced therapeutic hypothermia for hypoxic-ischemic brain injury after cardiac arrest. Cases were de-identified, and some were modified slightly to avoid potential recognition by treating physicians. Cases were presented in a random order to each subject, although the same randomization sequence was used for neurologists and intensivists. Participants were instructed to complete no more than half of the vignettes in a single sitting to prevent fatigue.
The patients described in these cases varied in (1) age: median 0.9 (interquartile range [IQR], 0.34–3.4) years; (2) gender: 10 girls and eight boys; (3) the presence of chronic pre-existing medical conditions; (4) baseline neurological function (prearrest Pediatric Cerebral Performance Category [PCPC] scores: 1 [n = 11]; 3 [n = 5]; 4 [n = 2]); (5) cardiac arrest characteristics (Cardiopulmonary resuscitation [CPR] duration and medications administered); and (6) outcome as measured by the PCPC score at hospital discharge.8 All patients underwent an induced therapeutic hypothermia protocol and survived to at least 7 days after arrest.9
Case-specific data were sequentially presented to physicians at three standardized post-arrest periods: day 1 (initial presentation), days 3–5, and days 5–7 (see Appendix for sample cases). On initial presentation, the child’s medical background, cardiac arrest details, and initial post-return of spontaneous circulation physical examination were provided. Head computed tomography results were provided if available (n = 12). On days 3–5, the case was updated with relevant interval history, an updated physical examination, continuous electroencephalography (cEEG) report (n = 18), and a brain magnetic resonance imaging (MRI) scan report if available (n = 6). On days 5–7, the case was updated with relevant interval history, an updated physical examination, and a brain MRI scan report if available (n = 10). Patients in half of the cases had seizures with two patients having only clinical seizures before cEEG monitoring and seven patients demonstrating electrographic seizures with or without clinical correlates (two of these seven patients had myoclonic seizures). Seizures were aggressively treated in all patients with the most common antiepileptic agents being levetiracetam, phenytoin, and phenobarbital. All physical examination reports were derived from neurology consultation progress notes and described the patient’s neurological examination and whether the patient was intubated, sedated, and/or paralyzed.
For each period, subjects reported their assessment of predicted neurological prognosis as a PCPC score (Predicted PCPC) and their confidence in their predicted neurological prognosis. The PCPC categories are 1 = normal, 2 = mild disability, 3 = moderate disability, 4 = severe disability, 5 = coma and vegetative state, and 6 = death.8 Confidence was reported using a 1–5 Likert scale. At the conclusion of each case, subjects selected which factors (e.g., clinical history, physical examination, cEEG, MRI, and so forth) most influenced their prognoses. All assessments were completed anonymously.
Data analysis
Accuracy
Accuracy was assessed by comparing physicians’ Predicted PCPC scores at each period (day 1, days 3–5, and days 5–7) to the patient’s actual hospital discharge PCPC score (Actual PCPC). To assess accuracy within and between the neurologist and intensivist physician groups, outcome was defined as a binary score which was equal to exactly accurate if a physician’s Actual PCPC – Predicted PCPC = 0; closely accurate if a physician’s Actual PCPC – Predicted PCPC=−1, 0, 1; or not accurate if physician’s Actual PCPC – Predicted PCPC = >1 or <−1.We used a quasi-least squares logistic regression model with a logit link to observe the difference in percent accuracy between physician specialty groups and over time while accounting for the correlation within-physician specialty groups and within time points. We also assessed the accuracy between the more- and less-experienced physicians, adjusting for each case number, using a repeated logistic regression analysis. One case was omitted from accuracy analyses because that child survived her cardiac arrest with mild neurological impairments but died of an unrelated complication later in her hospital course. This case was included in the agreement analyses.
Agreement
Agreement was defined as the reliability physicians within the same specialty had in predicting outcome PCPC scores. To observe within physician specialty group agreement of Predicted PCPC scores, a Kappa (κ) statistic was calculated at each time point. A Kappa statistic was also calculated at each time point after grouping the Predicted PCPC scores (PCPC scores groups 1 and 2, 3 and 4, and 5 and 6). Kappa statistic values <0 were interpreted as indicating no agreement, and 0–0.20 as slight, 0.21–0.40 as fair, 0.41–0.60 as moderate, 0.61–0.80 as substantial, and 0.81–1 as almost perfect agreement between physicians.10 Repeated ordinal logistic regression was used to evaluate agreement of PCPC scores between physician specialty groups at each time point, adjusting for each case number.
Confidence
Repeated ordinal logistic regression was performed to assess the probability of obtaining confidence scores within and between physicians over time, adjusting for each case number.
Results
Demographics
Pediatric neurologists (n = 10; five women and five men) and pediatric intensivists (n = 9; four women and five men) completed the 18 cases. The median clinical experience was 16 years (IQR = 5–25) for the neurologists and 1 year (IQR = 1–10) for the intensivists.
Accuracy
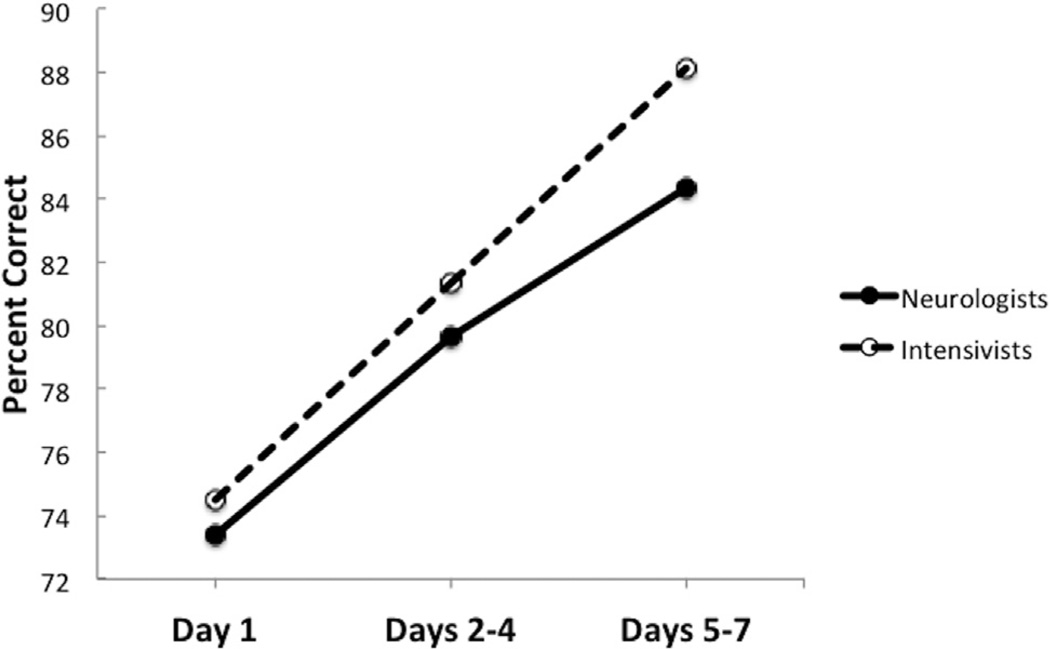
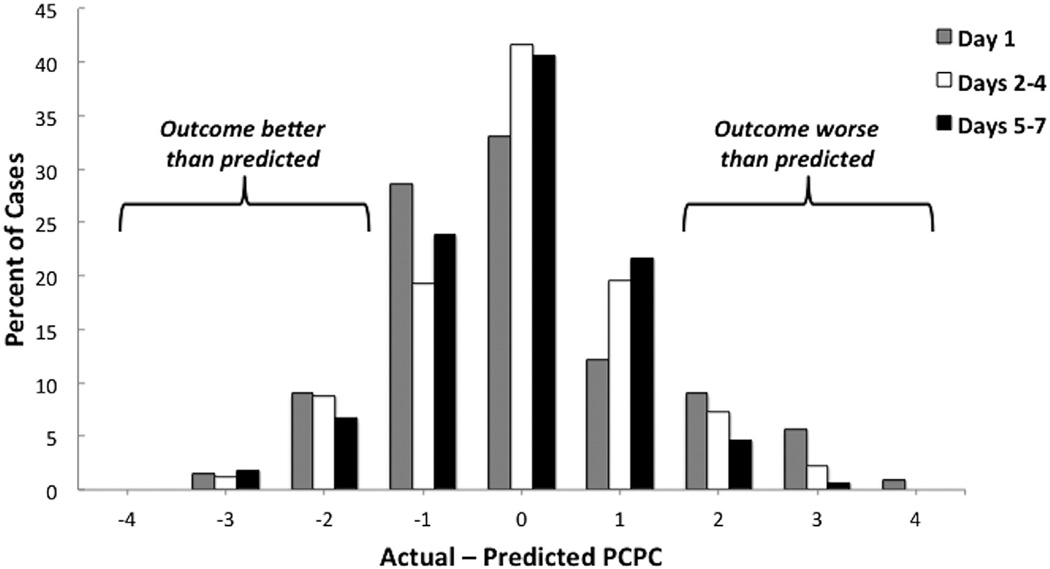
Physician outcome prediction accuracy (exact and close) significantly improved over time from day 1 to days 5–7 after arrest (Fig 1; P < 0.05). Exact predictions for all physicians at day 1, days 2–4, and days 5–7 after arrest occurred for 33%, 42%, and 41% of cases, respectively. Similarly, close predictions occurred for 74%, 80%, and 86% of cases. The percent of cases where outcome predictions were either overly optimistic (Actual PCPC – Predicted PCPC, ≥2) or pessimistic (Actual PCPC – Predicted PCPC, ≤−2) decreased over time (Fig 2). There was no difference in accuracy between neurologists and intensivists at any of the three periods (P = 0.78, 0.52, and 0.28) or overall (P = 0.32). Additionally, there was no difference in accuracy between more- and less-experienced physicians (P = 0.51).
FIGURE 1.
Percent close accuracy (Actual PCPC – Predicted PCPC = −1, 0, 1) by physician specialty group over time. PCPC, Pediatric Cerebral Performance Category.
FIGURE 2.
Distribution of the difference between Predicted and Actual PCPC scores for each time period. Data from neurologists and intensivists are averaged together. PCPC, Pediatric Cerebral Performance Category. (Color version of this figure is available in the online edition.)
Agreement
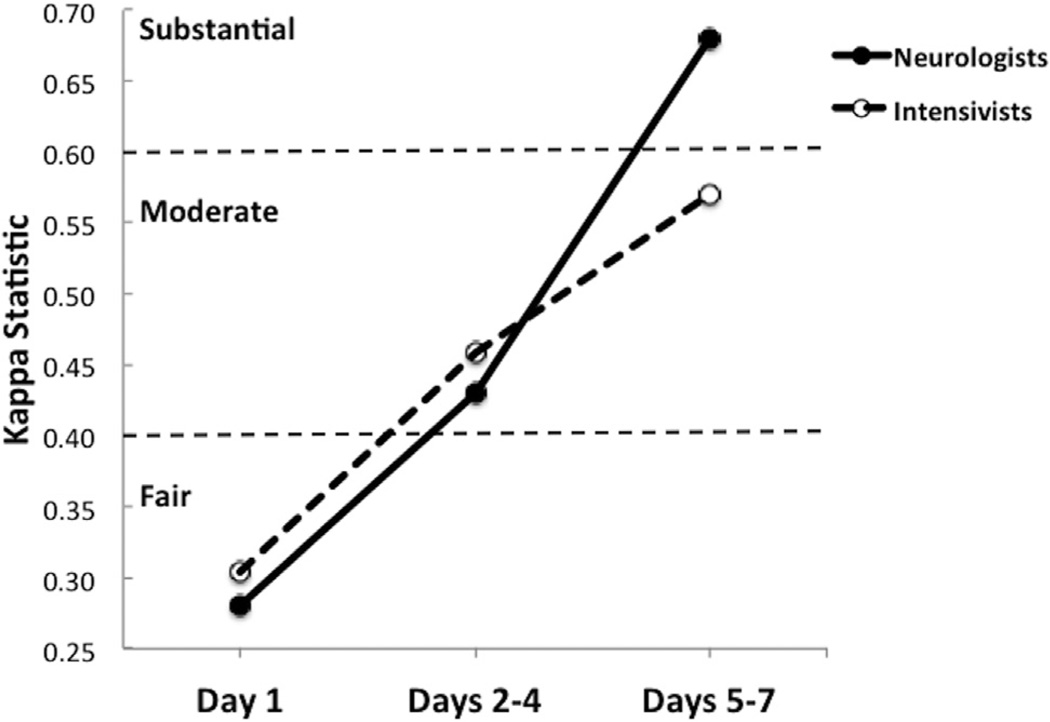
Inter-rater agreement improved over time to moderate-to-substantial levels among neurologists (day 1 κ, 0.28; days 2–4 κ, 0.43; days 5–7 κ, 0.68) and among intensivists (day 1 κ, 0.30; days 2–4 κ, 0.44; days 5–7 κ, 0.57) (Fig 3). Neurologist and intensivist groups significantly differed from each other only on day 1 (P = 0.045).
FIGURE 3.
Inter-rater agreement among neurologists and inter-rate agreement among intensivists over time as determined by Kappa statistics. Agreement only differed between the two physician specialty groups on day 1 (P = 0.045).
Confidence
Table 1 displays the average confidence scores for physician specialty groups. Confidence scores did not differ between physician specialty groups at any period (day 1: P = 0.308; days 3–5: P = 0.286; and days 5–7: P = 0.552). However, for all physicians, confidence scores increased over time (P < 0.0001). Physicians’ confidence improved over the first 7 days, whether their prognoses were accurate or inaccurate (Table 2).
TABLE 1.
Confidence Scores Reported as Mean and Standard Error
| Physician Specialty Group | Day 1 | Days 2–4 | Days 5–7 |
|---|---|---|---|
| Neurologists | 2.73 (0.08) | 3.26 (0.08) | 3.69 (0.07) |
| Intensivists | 2.78 (0.07) | 3.23 (0.06) | 3.73 (0.06) |
Confidence was rated on a scale of 1 (not confident) to 5 (very confident).
TABLE 2.
Percentage of Accurate (Actual PCPC – Predicted PCPC = −1, 0, 1) and Inaccurate Responses Over Time With Associated Confidence Ratings
| TIme after cardiac arrest |
Accurate (Actual PCPC – Predicted PCPC = −1, 0, 1) | Inaccurate (Actual PCPC – Predicted PCPC ≠ −1, 0, 1) | ||||
|---|---|---|---|---|---|---|
| Low Confidence | Somewhat Confident | Very Confident | Low Confidence | Somewhat Confident | Very Confident | |
| Day 1 | 35.44 | 40.93 | 23.63 | 47.62 | 35.71 | 16.67 |
| Days 2–4 | 12.35 | 42.63 | 45.02 | 30.64 | 41.94 | 27.42 |
| Days 5–7 | 5.55 | 24.81 | 69.63 | 11.36 | 34.09 | 54.54 |
| PCPC | – Pediatric Cerebral Performance Category |
| Low confidence | = 1 + 2 |
| somewhat confident | = 3 |
| very confident | = 4 + 5. |
Influential factors
Both neurologists and intensivists reported that the most influential factor for prognostication was the patient’s physical examination at days 5–7. Other important factors were the brain MRI scan and cEEG for neurologists and the cardiac arrest characteristics and brain MRI scan for the intensivists.
Discussion
This study used actual cases of children who survived a cardiac arrest with known outcomes assessed by PCPC score to characterize the accuracy and agreement of neurological outcome prognoses among and between pediatric neurologists and intensivists. Our data demonstrate that prediction accuracy was poor on initial presentation, prediction accuracy and agreement among physician specialty groups improved over the first 7 days of care, and prediction accuracy was not different between neurologists and intensivists. Physician confidence in their predictions also significantly improved over time, both for accurate and inaccurate outcome predictions. Much of the postcardiac arrest prognosis literature focuses on evaluating the effectiveness of particular metrics in predicting outcome such as clinical examinations, imaging, EEG background and seizures, and somatosensory evoked potentials. However, there are few studies that evaluate the accuracy and timing of neurological outcome prediction by physicians and none in pediatrics.11
In our study, physician outcome prediction accuracy was exact (Actual PCPC – Predicted PCPC = 0) 33% of the time on day 1 and improved to 41% by days 5–7 after arrest. When prediction accuracy was defined as close (Actual PCPC – Predicted PCPC= −1, 0, 1), accuracy at days 5–7 improved to 86%. Although the PCPC scale is not a linear measure of neurological function, outcome predictions that differ by a single point are not likely to significantly alter clinical management decisions, with the possible exception of predicting a patient to be in a coma or vegetative state (PCPC = 5) when the actual outcome would be severe disability (PCPC = 4). This particular situation occurred at least once for 90% of neurologists and 86% of intensivists and as late as days 5–7 after arrest. These data imply that physicians, independent of experience or specialty are susceptible to generating inaccurate prognoses that could have a profound impact on clinical management decisions regarding withdrawal technological support, and family counseling.
Prognostication regarding neurological recovery after a brain injury is one of the most challenging tasks that intensivists and neurologists face. Management recommendations that physicians offer to families including the aggressiveness or limitations of care are frequently based on complex assessments that can be influenced by many factors including available scientific evidence, a physician’s prior clinical experience, subjective impressions, personal biases, and ethics. They can also vary depending on factors such as a physician’s level of training, clinical experience, age, and medical specialty.12 Especially in pediatrics where the scientific evidence is limited,6 these additional factors may play a more substantial role in outcome and quality of life predictions.
Some physicians may overestimate the likelihood of a favorable neurological recovery so as not to miss a patient with a potentially acceptable long-term neuro-developmental outcome. In this study, 10% of predictions were at least two PCPC points better than actual outcome. Similarly, some physicians may underestimate the likelihood of a favorable recovery to avoid having a child survive in a severely disabled state. In our study, actual outcomes were better than predicted by at least two PCPC points in 15% of cases on day 1 and in 5% of cases at days 5–7. Every physician made such overly pessimistic predictions at least once on day 1 and 65% percent made overly pessimistic predictions on days 5–7 after arrest. Similarly, in another study of neuroprognostication, 7% of outcome predictions for adults at postinjury day 4 were overly pessimistic by ≥2 points on the seven-point modified Rankin scale compared with actual functional outcomes.13
The optimal timing of postcardiac arrest neuro-prognostication may be impacted by ongoing clinical interventions such as therapeutic hypothermia. Prematurely prognosticating before the completion of a neuroprotective therapy may contribute to inappropriate decisions to limit care.11,14 All the children in our study received 24 hours of induced therapeutic hypothermia (32°C–34°C) as part of their postcardiac arrest clinical care. This intervention was included in each case presentation, and it is unclear how it influenced physician outcome prognoses. When queried for which factors were most influential in determining prognoses, not a single physician selected therapeutic hypothermia for any of the cases. Therapeutic hypothermia reduces drug clearance, allowing sedative and paralytic medications to accumulate and extend their duration of action, which may impact the reliability and validity of a physical examination at earlier time points after return of spontaneous circulation, which physicians identified as highly influential for prognostication. Thus, sedative medications, which are almost universally used in pediatric postcardiac arrest care may be important cofounders to accurate neuroprognostication.
There is a known association between outcome prediction and confidence.12,15,16 In this study, physicians were asked to rate their confidence in their prognosis at each period, and we found that both physician prognostic accuracy and confidence improved significantly over time. Qualitatively, physician experience was somewhat associated with confidence. Physicians practicing for less than 5 years were infrequently very certain in their initial prognosis. Of the four physicians with ≥20 years of experience, two of them were very confident in their initial prognoses for most of the cases, whereas the other two physicians were rarely confident. We also dichotomized accuracy (Actual PCPC – Predicted PCPC = −1, 0, 1 as accurate and all other responses as inaccurate) to evaluate whether confidence varied differently when physicians’ prognoses were accurate (Table 2). While both accuracy and confidence improved over time, in more than half of cases where outcome predictions were inaccurate at days 5–7, physicians were very confident in their assessments. Although the interaction between accuracy and confidence is difficult to quantify, these data indicate that physicians should use caution in providing overly precise or overly confident neurological outcome prognoses after hypoxic-ischemic brain injury.
Both neurologists and intensivists reported that the most influential factor for estimating neurological outcome was the patient’s physical examination at days 5–7. Additionally, outcome prediction for neurologists was influenced by the patient’s brain MRI scan and cEEG, whereas outcome prediction for intensivists was influenced by the patient’s cardiac arrest characteristics and brain MRI scan. These results stress the importance of the neurological examination in assessing postcardiac arrest patients and suggest that emphasis should be placed on teaching the skills required to conduct and interpret quality neurological examinations.17,18
This study had limitations. First, cases were derived from actual patients managed in our PICU and presented as clinical vignettes, which limited the amount of information being presented. cEEG, head computerized tomography, and MRI data were presented as written reports, and actual images were not provided for interpretation. It is possible that providing more detailed information would have impacted physician predictions. Second, there was no long-term follow-up neurological function for survivors, and it is possible that the short-term outcomes used in this study do not fully reflect long-term outcomes. Third, cases were chosen for this study only if children survived to at least 7 days from their cardiac arrest to identify a group of patients where neuroprognostication was clinically relevant. Therefore, these cases represent a select population of postcardiac arrest patient survivors and may not generalize to all patients resuscitated from cardiac arrest. Fourth, because these were retrospective cases, the clinicians who had cared for these patients had already prognosticated early on and made the decision to continue aggressive care. Fifth, in this study, the Kappa statistic was used to assess agreement between physician prognoses. This statistical approach has been criticized as being a relative measure of observer variation rather than an absolute measure of observed agreement.19 Last, this study only examined children with hypoxic-ischemic brain injury secondary to cardiac arrest; therefore, these outcome prediction results may not be generalizable to acute brain injury from other mechanisms that have different trajectories for neurological recovery.
Conclusions
In this case-based study, neurological prognoses after pediatric cardiac arrest by neurologists and intensivists are inaccurate early after arrest and improve over the first week after resuscitation. For all physicians, prediction accuracy and physician confidence improved over time. Further prospective research is needed to better characterize how physicians objectively and subjectively estimate neurological recovery after acute brain injury and communicate this information to families.
Acknowledgments
This work was supported by the American Academy of Neurology Ethics, Law, and Humanities Committee’s Neurologist-in-Training Clinical Ethics Elective Award (N.S.A. and J.I., faculty mentors). Nicholas Abend is funded by National Institutes of Health K23NS076550. Alexis Topjian is funded by National Institutes of Health career development award K23NS075363 and U01HL094345. Judy Illes is supported by Neuro Dev Net, Inc. The authors thank Rebecca Seltzer, MD for assistance with extracting case information and writing cases, Sarah Sanchez, BA for assistance with data entry and study coordination, Justine Schultz, PhD for statistical support, and Robert Berg, MD and Vinay Nadkarni, MD for their guidance and critiques of earlier versions of this manuscript.
Appendix
Sample case 1
A) Initial presentation (day 1)
A 6-month-old otherwise-healthy boy with history of recurrent otitis media presented to an outside hospital in respiratory distress secondary to an upper respiratory infection. He was intubated for respiratory distress and subsequently required reintubation because of persistent desaturations. After the reintubation, he continued to desaturate and became profoundly bradycardic. CPR was initiated, and he was administered six rounds of epinephrine and two rounds of atropine before his hemodynamics stabilized. Total CPR time was 20 minutes. After resuscitation, he was tried briefly on high-frequency oscillatory ventilation secondary to difficulty with ventilation and oxygenation. He was transferred to the Children’s Hospital of Philadelphia PICU and initiated on the therapeutic hypothermia protocol.
Examination
Patient was intubated. Patient was pharmacologically sedated and paralyzed. His pupils were small but equal and reactive to light.
Head ultrasound
Mild and nonspecific increase of the extra-axial spaces.
B) Days 2–4
He was administered surfactant for his acute respiratory distress syndrome and covered broadly with antibiotics. He had an echocardiogram, which revealed a shortening fraction of 28% and a bicuspid aortic valve. He required pressors for hypotension. He was cooled and rewarmed without complications or seizures, and paralysis was weaned after rewarming. He was able to wean from high-frequency oscillatory ventilation to conventional ventilation. He was found to be positive for rhinovirus. He was tolerating advancing nasogastric tube feeds.
Electroencephalography
The background contained slow delta activity that was continuous and reactive to stimulation but without organization or sleep architecture. There were no sharps or electrographic seizures. According to the electroencephalography (EEG) report, his EEG was consistent with a moderate nonspecific encephalopathy.
Examination
Patient was intubated. Patient was pharmacologically sedated. He appeared very edematous. His pupils were equal and reactive to light. He responded to stimulation and seemed to be calmed by parent’s voice. He withdrew to pain in all extremities.
C) Days 5–7
He continued to require aggressive mechanical ventilation for his acute respiratory distress syndrome and pulmonary hypertension. He continued to require pressors for intermittent hypotension. His laboratories revealed evidence of agammaglobulinemia, and thus he was treated with intravenous immunoglobulin therapy. A complete immunological workup was in progress.
Examination
Patient was intubated. Patient was receiving sedating medications but was awake. He was breathing over the ventilator. Overall, he appeared very edematous. He opened his eyes to stimulation, but not spontaneously, which was difficult secondary to his diffuse edema. His pupils were equal and reactive to light, and he had corneal reflexes. Vestibular-ocular reflexes were difficult secondary to his edema. He had no cough and no gag reflex. He grimaced and withdrew to pain in all extremities.
Magnetic resonance imaging
Enlarged cerebrospinal fluid spaces of uncertain etiology.
Sample case 2
A) Initial presentation (day 1)
A 4-month-old girl with no significant past medical history had a cardiac arrest at home. Her parents found her unresponsive; face down in her playpen and not breathing. She had been in the care of a babysitter for 20 minutes. Dad performed CPR for 5 minutes before the arrival of emergency medical services (EMS) personnel. EMS personnel found her pulseless and continued CPR for an additional 2–3 minutes. She had return of spontaneous circulation after one round of epinephrine. She was intubated and taken to an outside hospital where she was noted to have seizure-like movements in her right arm and eye twitching. She received two doses of lorazepam and was put on dopamine before transfer to the Children’s Hospital of Philadelphia PICU where she was initiated on the therapeutic hypothermia protocol.
Examination
Patient was intubated. Patient was pharmacologically sedated. She was unarousable, and her pupils were 1–2 mm and sluggishly reactive to light. She had absent vestibular-ocular reflexes. She had a weak cough and weak gag reflex. She had no facial asymmetry and no spontaneous movements. She had decreased tone throughout and no withdrawal or grimace to pain. Deep tendon reflexes were normal.
Head computed tomography
Interpreted as left transverse sinus thrombosis by outside hospital but reinterpreted as normal by Children’s Hospital of Philadelphia radiology.
B) Days 2–4
She developed refractory seizures during the hypothermia protocol. She was hemodynamically stable on a dopamine infusion.
Electroencephalography
Her EEG recording initially revealed no definite cerebral activity. Myoclonic jerks were observed but did not have any electrographic correlate. At 72 hours of recording, the background developed some highly attenuated and slow activity and was not clearly reactive to stimulation. Some myoclonic jerks were associated with epileptiform sharps. Levetiracetam was loaded and subsequently rebolused without improvement. Phenytoin was added, and she eventually required a midazolam infusion to control the seizures. According to the EEG report, her EEG was consistent with severe nonspecific encephalopathy and myoclonic seizures.
Examination
Patient was intubated. Patient was pharmacologically sedated. She was breathing over the ventilator. She had sluggish pupils bilaterally and her corneal, vestibularocular, and gag reflexes were absent. She had slight movement of her extremities with painful stimulation. She had normal deep tendon reflexes.
C) Days 5–7
Dopamine was weaned, and she had a normal echocardiogram and electrocardiogram. She was weaned off of the midazolam and had a recurrent episode of status epilepticus, which was treated with levetiracetam and phenytoin boluses and a transient ketamine infusion. She had periodic chewing and bicycling movements of the arms, but these did not correlate with seizures on EEG. She had no further seizure activity. She was successfully extubated and taken off sedation. Ophthalmologic evaluation revealed no evidence of retinal hemorrhages; however, she did have decreased vision and a poor blink response. She was tolerating advancing nasogastric feeds.
Examination
Patient was awake, with occasional spontaneous eye opening. Her pupils were equal and reactive, but she did not fix or follow and she had no blink to threat. Her vestibular-ocular reflexes were intact. Her face was symmetric. Her tone was increased throughout. She had cortical thumbs as well as palmar and plantar grasp reflexes. She moved all extremities spontaneously. Occasional lip smacking and bicycling movements of her extremities were observed, as well as occasional extensor posturing of her extremities. Deep tendon reflexes were normal.
Magnetic resonance imaging
Multifocal areas of restricted diffusion in the posterior limbs of the internal capsules and the cerebral peduncles bilaterally. The magnetic resonance imaging report described this was compatible with hypoxic-ischemic injury.
Footnotes
These data were presented at the American Heart Association Resuscitation Science Symposium 2012.
References
- 1.Topjian AA, Berg RA, Nadkarni VM. Advances in recognition, resuscitation, and stabilization of the critically ill child. Pediatr Clin North Am. 2013;60:605–620. doi: 10.1016/j.pcl.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Topjian AA, Berg RA, Nadkarni VM. Pediatric cardiopulmonary resuscitation: advances in science, techniques, and outcomes. Pediatrics. 2008;122:1086–1098. doi: 10.1542/peds.2007-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donoghue AJ, Nadkarni V, Berg RA, et al. Out-of-hospital pediatric cardiac arrest: an epidemiologic review and assessment of current knowledge. Ann Emerg Med. 2005;46:512–522. doi: 10.1016/j.annemergmed.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 4.Girotra S, Spertus JA, Li Y, Berg RA, Nadkarni VM, Chan PS. Survival trends in pediatric in-hospital cardiac arrests: an analysis from Get With the Guidelines-Resuscitation. Circ Cardiovasc Qual Outcomes. 2013;6:42–49. doi: 10.1161/CIRCOUTCOMES.112.967968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitamura T, Iwami T, Kawamura T, et al. Conventional and chest-compression-only cardiopulmonary resuscitation by bystanders for children who have out-of-hospital cardiac arrests: a prospective, nationwide, population-based cohort study. Lancet. 2010;375:1347–1354. doi: 10.1016/S0140-6736(10)60064-5. [DOI] [PubMed] [Google Scholar]
- 6.Abend NS, Licht DJ. Predicting outcome in children with hypoxic ischemic encephalopathy. Pediatr Crit Care Med. 2008;9:32–39. doi: 10.1097/01.PCC.0000288714.61037.56. [DOI] [PubMed] [Google Scholar]
- 7.Wijdicks EF, Hijdra A, Young GB, Bassetti CL, Wiebe S. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67:203–210. doi: 10.1212/01.wnl.0000227183.21314.cd. [DOI] [PubMed] [Google Scholar]
- 8.Fiser DH, Long N, Roberson PK, Hefley G, Zolten K, Brodie-Fowler M. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000;28:2616–2620. doi: 10.1097/00003246-200007000-00072. [DOI] [PubMed] [Google Scholar]
- 9.Topjian A, Hutchins L, DiLiberto MA, et al. Induction and maintenance of therapeutic hypothermia after pediatric cardiac arrest: efficacy of a surface cooling protocol. Pediatr Crit Care Med. 2011;12:e127–e135. doi: 10.1097/PCC.0b013e3181e28717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 11.Perman SM, Kirkpatrick JN, Reitsma AM, et al. Timing of neuroprognostication in postcardiac arrest therapeutic hypothermia. Crit Care Med. 2012;40:719–724. doi: 10.1097/CCM.0b013e3182372f93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Racine E, Dion MJ, Wijman CA, Illes J, Lansberg MG. Profiles of neurological outcome prediction among intensivists. Neurocrit Care. 2009;11:345–352. doi: 10.1007/s12028-009-9225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finley Caulfield A, Gabler L, Lansberg MG, et al. Outcome prediction in mechanically ventilated neurologic patients by junior neurointensivists. Neurology. 2010;74:1096–1101. doi: 10.1212/WNL.0b013e3181d8197f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peberdy MA, Callaway CW, Neumar RW, et al. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18) Suppl 3:S768–S786. doi: 10.1161/CIRCULATIONAHA.110.971002. [DOI] [PubMed] [Google Scholar]
- 15.Marcin JP, Pollack MM, Patel KM, Sprague BM, Ruttimann UE. Prognostication and certainty in the pediatric intensive care unit. Pediatrics. 1999;104(4 Pt 1):868–873. doi: 10.1542/peds.104.4.868. [DOI] [PubMed] [Google Scholar]
- 16.Marcin JP, Pretzlaff RK, Pollack MM, Patel KM, Ruttimann UE. Certainty and mortality prediction in critically ill children. J Med Ethics. 2004;30:304–307. doi: 10.1136/jme.2002.001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abend NS, Topjian AA, Kessler SK, et al. Outcome prediction by motor and pupillary responses in children treated with therapeutic hypothermia after cardiac arrest. Pediatr Crit Care Med. 2012;13:32–38. doi: 10.1097/PCC.0b013e3182196a7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bratton SL, Jardine DS, Morray JP. Serial neurologic examinations after near drowning and outcome. Arch Pediatr Adolesc Med. 1994;148:167–170. doi: 10.1001/archpedi.1994.02170020053008. [DOI] [PubMed] [Google Scholar]
- 19.de Vet HC, Mokkink LB, Terwee CB, Hoekstra OS, Knol DL. Clinicians are right not to like Cohen’s kappa. BMJ. 2013;346:f2125. doi: 10.1136/bmj.f2125. [DOI] [PubMed] [Google Scholar]