Abstract
The tight junction creates an intercellular barrier limiting paracellular movement of solutes and material across epithelia. Currently many proteins have been identified as components of the tight junction and understanding their architectural organization and interactions is critical to understanding the biology of the barrier. In general the architecture can be conceptualized into compartments with the transmembrane barrier proteins (claudins, occludin, JAM-A, etc.), linked to peripheral scaffolding proteins (such as ZO-1, afadin, MAGI1, etc.) which are in turned linked to actin and microtubules through numerous linkers (cingulin, myosins, protein 4.1, etc.). Within this complex network are associated many signaling proteins that affect the barrier and broader cell functions. The PDZ domain is a commonly used motif to specifically link individual junction protein pairs. Here we review some of the key proteins defining the tight junction and general themes of their organization with the perspective that much will be learned about function by characterizing the detailed architecture and subcompartments within the junction.
Keywords: tight junction, epithelium, ZO-1, claudin, occludin, actin
Introduction to Tight Junctions
Transporting epithelia require a paracellular seal to allow the directional transport of ions and solutes across cell layers. This seal is formed by the tight junction, the apical-most junction of a series of cell contacts that form lateral connections between adjacent cells. The ultrastructure of epithelial junctions was first described in a seminal paper by Farquhar and Palade over 50 years ago [1]. A few years later, freeze fracture electron microscopy (FFEM) was used to visualize the tight junction-containing plasma membranes, Fig. 1; using this method, tight junctions were observed to consist of rows of membrane contacts [2] that varied in number and morphology among different tissues [3]. In parallel, there was a gradual recognition among physiologists studying epithelial transport that the tight junction, which had been considered to be an impermeable structure, was actually variably permeable to ions and solutes [4]. These observations together led to a remarkable period of investigation in which tight junctions in different tissues were compared and characterized by electron microscopic and physiologic methods and led to the conclusion that the barrier varied widely among epithelia in its physiology. It was assumed that tissue-specific barrier differences were the result of the variations in protein composition and architecture.
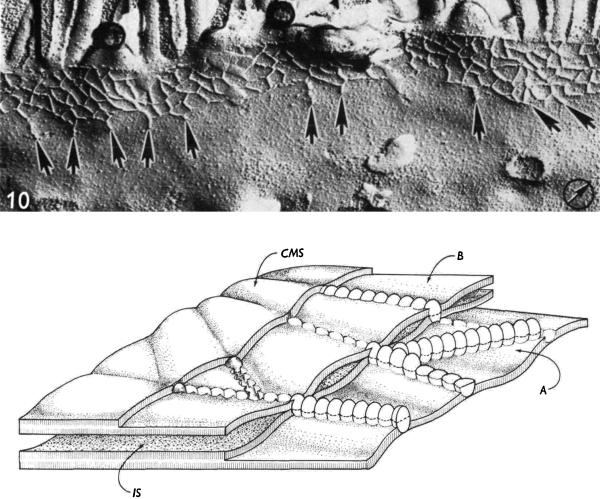
Fig. 1.
Freeze fracture electron microscopic image and diagram of a tight junction. Top, FFEM image from rat jejunum showing a gradient of strand organization from the lateral to apical side of the junction. One continuous apical strand is typically followed by a network of interconnected strands and on the basal side the strands can be free and unconnected to the network. Bottom, An artist's view of a tight junction as seen in FFEM. The diagram is of two fracture planes in apposed tight junction membranes. (A) Rows of particles in one fracture plane that become a meshwork after glutaraldehyde fixation and (B) grooves, modeled in the front fibrils of this second fracture plane. IS, intercellular space and CMS, cytoplasmic membrane surface. Reproduced with permission from Staehelin, 1973.
However, it was not until 1986 that ZO-1, the first protein component of the tight junction was identified and localized to the tight junction by immuno-electron microscopy (immuno EM), Fig.2A [5]. This discovery was soon followed by the identification of two ZO-1-related proteins that could co-immunoprecipitate with ZO-1, termed ZO-2 [6] and ZO-3 [7] and by the discovery of an unrelated protein termed cingulin [8]. However, since all four of these were found to be peripheral membrane proteins, none could directly create the intercellular barrier. In a tour de force, the Tsukita laboratory, using a similar biochemical fractionation method as had Stevenson and Goodenough, identified the first tight junction transmembrane protein, occludin [9]. When mouse knock-out studies demonstrated occludin was dispensable for barrier formation [10], this group went on to identify several members of the claudin family of proteins [11]. Morphologic and functional studies subsequently demonstrated that claudins were the critical barrier forming proteins [12].
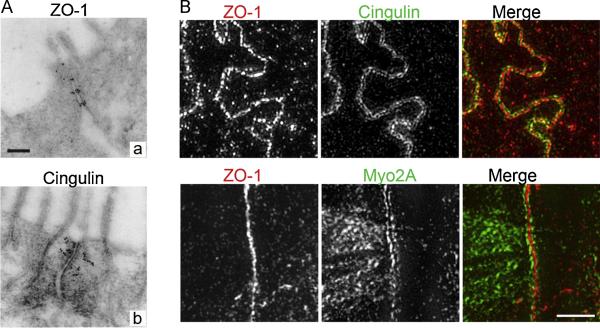
Fig. 2.
Different subjunctional localization of cingulin, ZO-1, and Myo2A are revealed by immune-gold EM labeling (A) and high-resolution Structured Illumination Microscopy (SIM) (B). Cingulin (Aa) labeling is chick intestine is displaced from the membrane contacts and decorates actin filaments that radiate out from the junction contact sites. In contrast, ZO-1 (Ac) in cells is closely apposed to plasma membrane contacts corresponding to intermittent claudin-based strands. These relative positions are confirmed by immunolocalization in polarized Caco-2 cells by SIM (B) for cingulin (top), which in part co-localizes with ZO-1 but is also positions lateral to the ZO-1 signal. Myo2A (bottom) which binds cingulin, like cingulin, is positioned farther from the contacts. EM images were reproduced with permission from Stevenson, et al. 1986. SIM images produced by C.M. Van Itallie. Cells were fixed with 1% paraformaldehyde and incubated with (top) monoclonal ZO-1 (Life Technologies) and polyclonal cingulin (gift from Dr. Sandra Citi, University of Geneva) antibodies and (bottom) monoclonal ZO-1 and polyclonal non-muscle myosin 2A (Covance) antibodies; secondary antibodies were from Jackson Immunoresearch. Images were acquired on a using a GE OMX Blaze V4 Ultrafast Structured Illumination Microscope equipped with 4 sCMOS cameras using a 60X 1.42NA lens using 488 and 561 laser lines; images were acquired using Deltavision OMX software and adjusted and cropped in Adobe Photoshop. Unpublished results, bar=1 μm.
The identification of these proteins was just the beginning of an extensive enumeration of tight junction components that continues today. Identification of proteins has occurred though both systematic efforts to enumerate the junction proteome [13-15] and from serendipitous discovery of single proteins. Some cataloging represents isolated reports of an antigen that co-localized with ZO-1 or another known tight junction protein. Others represent recognition that previously characterized proteins are also at the junction, for example, many well-defined junctional signaling or cytoskeletal proteins. The current list of proteins is likely incomplete and the 3-dimensional architecture and functional interactions of these proteins are not well defined.
The goal of this review is to develop a more complete and nuanced model of tight junction functional and structural compartments based on the variety of techniques that have been used to probe protein interactions and localization. To do this, we will consider interactions among and spatial compartmentalization of the core tight junction proteins and some relevant cytoskeletal proteins. Mixed within this network are dozens of signaling proteins that control junction function and provide differentiation signals to the cell. We will not only highlight the relationships between these different groups of proteins but also pose important unanswered questions in tight junction structure and function.
1. Core components of the Tight Junction: Integral membrane and scaffolding proteins
1.1 Integral Membrane Proteins: Claudins, TAMPs and JAMs
1.1.1 Claudins
There is overwhelming evidence that the main freeze-fracture fibril forming proteins are the 25-plus members of the claudin family [16]. When expressed in fibroblasts which do not normally form tight junctions and the transfected cells are examined by FFEM, these small, 20-25kDa, integral membrane proteins can recapitulate fibrils similar to those of epithelial cell tight junctions [11]. In addition, much physiologic evidence supports the idea that claudins form the paracellular seal (reviewed in [17]).
1.1.2 Claudin architecture
Although tight junction strands that can be visualized by FFEM are a hallmark of epithelial tissues, the appearance of these strands differs in different tissue in terms of their number and the degree of crosslinking between strands [18]. Within most tight junctions, there exists a polarity to strand organization. FFEM images typically reveal one continuous apical-most strand, variably cross-linked medial strands and looser, less well organized and sometimes discontinuous basal strands [19, 20], Fig. 1. How these strands are organized and the basis for their structural gradients is not well understood, but the strand organization may reflect a maturation process from basolateral to apical. As early as 1973, this gradient of organization led Staehelin to suggest that the seal formed by a “zippering up” of cell-cell contacts in the lateral to apical direction [19]. All claudins (except claudin-12) end in a carboxyl terminal PDZ binding motif; these motifs interact with the first of the three PDZ domains of the tight junction scaffolding proteins ZO-1, -2 and -3 [21] and this interaction contributes to strand organization. Binding to other PDZ domain-containing proteins has also been reported, including MPDZ (MUPP1, Multi PDZ domain protein 1) [22] and PatJ (Protein associated with tight junctions) [23]. In addition, preferential interactions between distinct claudins and different PDZ domain-containing proteins have been reported [23, 24], although this remains to be more fully investigated. There does appear to be a requirement for PDZ-dependent interactions in setting up a tight junction, since for example in the mouse breast epithelial cell line, Eph4 cells, the scaffolding proteins ZO-1 and ZO-2 are required to create the claudin-based tight junction strands [25]. It is notable however, that depletion (as opposed to knockout) of greater than 90% of ZO-1 and ZO-2 does not result in major tight junction fibril disruption [26], suggesting that only a small amount of ZO protein is required for nucleating claudin strand assembly. It is possible that an apical/basal gradient in localization of ZO (or other) scaffolding proteins within the junction or the fact that different claudins may have variable affinities for their scaffolds may contribute to a nonhomogeneous strand organization, but there is no direct evidence for this. Additionally, it is also possible that posttranslational modification of either claudins or the scaffolding proteins may factor into their relative affinities and thus organization [27].
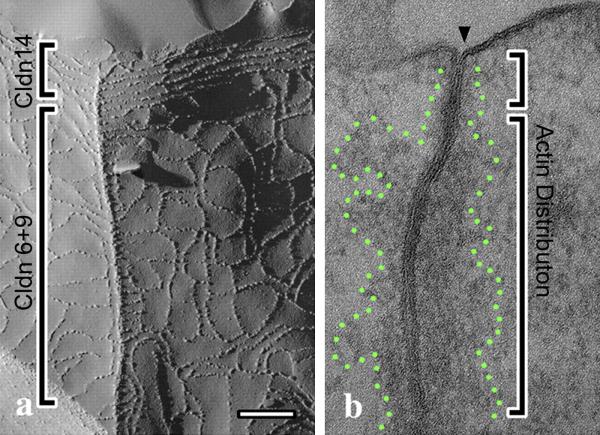
Although scaffolding proteins may be required to set up tight junctions, it is also clear that claudins have the capacity to self-organize into different strand architectures [28], an ability that has not only structural but physiologic and pathologic consequences [29]. Claudins lacking PDZ binding motifs form patches of tight junction fibrils when expressed in fibroblasts and, in epithelial cells, claudins lacking this motif will still accumulate at tight junctions, suggesting claudin oligomerization is not dependent on interaction with scaffolding proteins [30]. Differing affinities for both cis and trans homo and hetero-oligomerization among different claudins have been well documented [31-33], so that stand maturation might result in claudin “sorting”. This could result in different strand architecture in more mature strands where claudin-claudin interactions with the highest affinity would dominate. Differing affinities may explain one dramatic example seen in hybrid junctions formed between sensory and nonsensory cells in the inner ear [34]. In this special case, immuno-EM demonstrates ZO-1 expression extends all the way down the hybrid junction, while claudin-14 distribution is limited to very apical, mostly parallel tight junction strands. In contrast, claudins-6 and -9 are found in a looser, more basal network below claudin-14 but still within the domain of the ZO-1 distribution, Fig.3(left panel). Occludin distribution follows ZO-1 and thus is not specifically associated with either claudin-14 or claudin-6/9 localization. There is co-distribution of claudin-6 and -9 and α-, β- and p120 catenins and increased junctional actin, Fig. 3(right panel). The authors postulate that this unusual organization is a specific response to the continuous shear stress to which this junction is exposed.
Fig. 3.
Two subdomains (brackets) of a tricellular (a, left) and bicellular (b, right) intercellular junction between sensory and non-sensory cells of the mouse inner ear revealed by freeze fracture and transmission EM. Left, a, Strand morphology is distinctly difference at the apical versus basal end of the junction. This correlates with restricted expression of claudin-14 to the top zone and claudin-6 and -9 to the bottom zone; in contrast, ZO-1 and occludin are expressed in both zones. Right, b, significantly more perijunctional actin is accumulated in bottom zone, outlined by green dots. Modified and reproduced with permission from Nunes, et al., 2006.
Overall, it seems most likely that scaffolding and self-organization both contribute to tight junction architecture. A minimal scaffold at the junction may be sufficient to set up the initial claudin fibrils, while further refinement of this organization may be more dependent on claudin-claudin interactions than on their continued binding to scaffold proteins. The idea that claudin organization may be partially independent of scaffold proteins is consistent with FRAP (Fluorescence recovery after photobleaching) data showing different recovery times for ZO proteins compared with claudins, suggesting that a majority of claudins are not tightly linked to scaffolding proteins once they are at tight junctions [35]. Additionally, it has been demonstrated that claudins can be endocytosed transcellularly unaccompanied by ZO-1 or occludin [36].
The structure of a single claudin was recently solved [37] and although it gives insights into residues that line the paracellular pore space, it does not provide clear evidence as to how this protein forms oligomers. The size of individual freeze fracture particles is approximately 10nm (for example see Fig. 3) [38] and seems likely to consist of a higher order claudin multimer. Retaining protein interactions in Blue Native PAGE electrophoresis reveals a fundamental claudin unit is the dimer [39, 40] but the higher order assembly above dimers has not been defined.
Although claudins are the strand-forming proteins, there is considerable evidence for extra-junctional claudin distribution. Several claudins are localized to basolateral cell membranes, including claudin-7 [41] and in some tissues, claudin-1 [42] and -4 [43] among others. Claudin-7 is competent to form tight junction strands [28], but when localized to the basolateral membrane, it does not contribute to strand formation [44]. It is restrained on the basolateral surface via an interaction with the cell adhesion protein, Ep-Cam [45], since Ep-Cam depletion results in claudin-7 relocalization to tight junctions [46]. Claudin-1, which is found in tight junctions but also along lateral membrane in some cells, is associated with the tetraspanin CD81 at the lateral membrane but not at tight junction [47]. The biologic function of the non-tight junction claudins is unclear at present, but it would be a mistake to think of claudins as purely tight junction proteins.
1.2 The TAMPs: Occludin, Tricellulin and MarvelD3
TAMPs, or Tight Junction-Associated Marvel domain-containing proteins are characterized by the four transmembrane domains that share some homology with MAL (Myelin and lymphocyte-associated protein); this homology was observed by the Matter and Balda laboratories [48] and the Turner laboratory [49]. The three members of this family that are associated with tight junctions include occludin [9], tricellulin [50] and MarvelD3 [48]. Occludin was the first transmembrane protein identified [9], but its role at the tight junction is still controversial. Occludin knockout animals have normal paracellular permeability and normal appearing junctions, although they also exhibit some unexplained phenotypes that may represent an indirect role for this protein in permeability regulation [51]. The occludin-related protein, tricellulin, is concentrated at tricellular junctions, a specialized domain of the tight junction where freeze-fracture fibrils extend more basally than they do at bicellular junctions [50] (see Fig. 3 left) and variably distributed along bicellular junctions, [52]. It is an essential tight junction protein for hearing [53] but its mechanistic contribution to barrier function in epithelial cell lines is controversial [49, 54, 55]. MarvelD3, expressed as two splice forms in cells and tissues, is widely expressed in epithelia and in part co-localizes with occludin at bi-cellular junctions [48, 49]. It has a longer N-terminal and shorter C-terminal domain relative to occludin and can be co-immunoprecipitated with occludin and with tricellulin; in contrast, occludin does not immunoprecipitate with tricellulin [49]. MarvelD3 knockdown has been reported to have variable physiological effects, but it seems likely that occludin, tricellulin and marvelD3 have both independent and overlapping functions [49].
1.2.1 TAMP architecture
Although most occludin is concentrated at the tight junction, a smaller fraction of it is also distributed on the lateral membrane; it is a phosphoprotein and tight junction localization is positively correlated with the degree of phosphorylation [56]. Extra-junctional localization could represent a step in occludin trafficking, since occludin is first delivered to the lateral cell membrane and from there traffics laterally to the tight junction [57], possibly after being phosphorylated. This lateral targeting is reported to require the occludin MARVEL domain which also mediates cis-oligomerization [58]. Like claudins, the carboxyl terminal sequences of occludin binds to ZO-1 but not through a PDZ-dependent interaction. Instead, a positively charged surface in the last 150 amino acids of occludin, identified by structural analysis, contains the binding site for ZO-1, and charge reversing mutations on this surface disrupt its co-localization with ZO-1 at cell contacts in fibroblasts [59]. However, as is the case for claudins, deletion of the ZO-1 binding region does not alter trafficking to preformed epithelial tight junctions [60], suggesting that other interactions are sufficient to recruit it to the junction.
When transfected into fibroblasts, none of the TAMPs can form freeze fracture strands but when co-transfected with claudins, they are then recruited to the continuous claudin-based strands [33, 61]. Occludin, but not tricellulin or marvelD3 forms homophilic transcellular interactions [61], and tricellulin shows neither cis nor trans interactions with occludin although marvelD3 can interact in cis with both [49, 61]. This is consistent with the co-distribution of marvelD3 with both occludin and with the segregation of tricellulin to tricellular contacts. In some cell types, siRNA-mediated depletion of occludin results in redistribution of tricellulin to bi-cellular junctions [62]. FRAP analysis has demonstrated that recovery kinetics for claudins, occludin and ZO-1 are all different, suggesting that their interactions are quite labile or that only a small fraction of each is in a stable complex [35]. The reason for this disconnect in protein dynamics is unclear but may allow for dynamic remodeling among junction proteins during cell-cell movements.
The Furuse laboratory has recently identified a family of integral membrane proteins that is required for localization of tricellulin to tricellular contacts [63, 64]. These proteins, called angulins, are single span immunoglobulin superfamily members and include LSR (the Lipolysis-Stimulated Lipoprotein Receptor), ILDR1 and ILDR2 (Immunoglobulin-like domain-containing receptor 1 and 2). The three members of this family are differentially expressed at tricellular junctions in most epithelial tissues and knockdowns/overexpression have variable effects on barrier function [64]. All three co-immunoprecipitate with tricellulin and their knockdown disrupts tricellulin localization.
Although as stated above, expression of the TAMPs in fibroblasts does not result in fibril formation, co-expression of any of the three with claudins can alter the morphology of the freeze fracture fibrils relative to those seen in cells expressing claudins alone [61]. For example, co-expression of tricellulin with claudin-1 in fibroblasts induces the formation of sharply angled branches between the freeze fracture fibrils, suggesting that this TAMP in particular is involved in strand organization [61, 62]. Therefore, although strands are differentially nucleated depending on the claudin composition [28, 34], the TAMPs are capable of modifying strand organization [61, 62], and both scaffolding proteins and the angulins also likely confer specific architectural features to the tight junction.
1.3 JAMs
We will focus on JAM-A (Junctional Adhesion Molecule-A) which is a member of the immunoglobulin superfamily; junction-associated relatives include JAM-B, -C and the more distantly related JAM-4, JAM-L and CAR (Coxsackie and Adenovirus receptor), reviewed in [65]. Although many of these proteins have been implicated in regulation of barrier function, most published studies have focused on the junctional role of JAM-A. This protein is concentrated at epithelial and endothelial tight junctions [66, 67], with some localization to lateral cell membranes [67]. By immuno-FFEM, it localizes to claudin-based tight junction fibrils in epithelial cells [68]. It has been implicated in regulation of endothelial cell migration and it is also expressed on immune cells, where expression mediates immune cell transcytosis across epithelia though the junctions [66]; cis-dimerization is required for these functions. JAM-A protein contains two extracellular immunoglobulin-like loops, a single transmembrane and a cytoplasmic domain ending in a PDZ binding motif. The more membrane distal extracellular Ig loop mediates homodimerization between JAM-A proteins on the same cell and might mediate trans interactions [69, 70]. The PDZ binding motif of JAM-A has been reported to interact with Afadin, ZO-1 [71], ZO-2 [72] and with PDZ-GEF2 (Guanine exchange factor-2)[73]; the ZO-2/Afadin/PDZ-GEF2 interactions are required for Rap activation and physiologic effects [72, 73]. JAM-A also interacts with some members of the cell polarity complex [68, 74] and has thus been implicated in the establishment of cell polarity. Phosphorylation of JAM-A at serine 285 by atypical protein kinase C (aPKC) is associated with exclusive tight junction localization and phosphorylation at this site is required for normal kinetics in the formation of fully functional tight junctions [75]. JAMs do not appear critical for regulation junction architecture but contribute to adhesion and signaling.
1.4 Scaffolding proteins of the tight junction: ZO-1, -2, -3, Cingulin and Cingulin-like protein 1 and others
1.4.1 ZO-1, -2 and -3
ZO-1 was the first identified tight junction protein [5] and it remains one of the best studied. ZO-1 and its relatives, ZO-2 and -3 are multidomain proteins each with three N-terminal PDZ domains, a central SH3 (Src homology 3) domain and a region with homology to guanylate kinase [6, 7, 76]. ZO-1 can dimerize with itself, ZO-2 or ZO-3; dimerization is mediated through interaction (domain-swapping) at the second PDZ domain [77]. The members of the ZO family of proteins are peripheral membrane proteins; immuno-EM of ZO-1 shows that it is positioned immediately below the tight junction membrane contact points [5] (Fig. 2A, top). Ultrastructural localization of ZO-2 and ZO-3 are indistinguishable from that of ZO-1 [6, 7]. The ZO proteins may be in part targeted to or stabilized at the plasma membrane by the ability of their second PDZ domains to bind membrane phosphoinositides [78], but they are well characterized for their binding to the transmembrane claudins and occludin. All three ZO proteins bind actin [79, 80], which in the case of ZO-1 has been localized to a 220 amino acid region in the carboxyl half of the protein [81]. In addition, ZO proteins bind a number of actin binding proteins (reviewed in [82]).
1.4.2 Cingulin
Cingulin [8] is a coil-coiled domain containing peripheral membrane protein that binds directly to ZO-1. It clearly has both structural and signaling roles at the tight junction; for example, cingulin phosphorylation promotes the junctional association of microtubules [83]. Cingulin is also involved in the junction recruitment of GTPase regulatory proteins and in junction formation [84, 85]. Comparison of the immuno-EM localization of cingulin (Fig. 2A, bottom) with that of ZO-1 (Fig. 2A top) reveals that it extends further from the membrane than do the ZO proteins (Fig. 2A). Although this difference in localization is not detectable by conventional light microscope immunofluorescence [86], the lateral displacement of cingulin from the ZO proteins is evident by super resolution, SIM (Structured Illumination Microscopic) analysis (Fig. 2B, top). Nonmuscle myosin 2A appears similarly, but slightly farther displaced from the membrane than cingulin (Fig. 2B, bottom) supporting the idea that ZO-1 forms a link between the transmembrane proteins and the cytoskeletal compartment.
1.4.3 Afadin
Afadin is a PDZ domain-containing protein that plays a critical role in the early polarization of the apical junctional complex [87]. It has been localized both to tight [88] and adherens [89] junctions by immuno-EM: its distribution likely overlaps both junctions. Afadin interacts with JAM-A [73] and with elements of the polarity complex; these proteins together with nectin and ZO-1 appear to be required for the proper apical positioning of the tight junction relative to the adherens junction [90]. Afadin can interact with ZO-1 when tight junctions are disrupted as after calcium removal, but this interaction is not detectable in polarized cells [91]. As afadin binds both to transmembrane proteins and to actin, its distribution means it is unusually well positioned for functional coordination between tight and adherens junctions, particularly during junction development and dynamic remodeling.
1.4.4 MAGI-1 and -3
Both MAGI-1 (membrane-associated guanylate kinase inverted-1) [92] and MAGI-3 [93] are PDZ domain-containing scaffolding proteins concentrated at epithelial tight junctions. MAGI1 [92] and MAGI3 [93] were localized at tight junctions by immuno-EM, but MAGI3 was found in other cellular locations as well. MAGI-3 has been implicated as a phosphoprotein scaffold and as a scaffold for the WNT signaling pathway receptor, Frizzled-4 [94], while MAGI-1 interacts with JAM-4 [95], the lipid phosphatase PTEN (Phosphatase and tensin homolog) [96], the rap activator PDZ-GEF1 [97] and the actin binding proteins α-actinin-4 and synaptopodin [98]. In spite of their tight junction localization and demonstration of numerous protein interactions, the role of MAGI-1 and -3 in tight junction physiology is yet not clear.
1.4.5 MPDZ
MPDZ (Multiple PDZ domain protein, aka MUPP-1) is yet another PDZ domain scaffolding protein that has been localized to tight junctions [22]; it contains 13 PDZ domains and interacts with a large number of tight junction proteins, including claudins and JAMs [22, 24]. MPDZ plays a clear role as a synaptic scaffold and is variably associated with tight junctions. One unusual interaction is that it apparently acts to scaffold somatostatin receptors at some epithelial tight junctions [99].
2. Cytoskeletal proteins of the tight junction
Actin [100-102], non-muscle myosin 2A, 2B, 2C [103] and microtubules [83, 104] all clearly play critical roles in tight junction architecture and physiology. Coupling to the perijunctional cytoskeleton is required for assembly of the junction and for maintenance of the barrier. A common feature of physiologic and pathologic alteration of the barrier is alteration of the junction-associated actin cytoskeleton; not surprisingly the number of cytoskeletal proteins at the junction is large. Cytoskeletal connections scaffold the forming contacts, provide stability against shear and serve as a matrix for signaling pathways.
A number of actin polymerizing proteins have been localized to tight junctions, including Arp2/3 [105], N-WASP [106], cortactin [107] and VASP [108]. In addition, many regulators of actin organization have been implicated in tight junction biology, including members of the RhoA, Rac and Cdc42 family of small GTPase; their roles at the junction have been the subject of excellent recent reviews [109, 110] as has trafficking of tight junction components [111], so these proteins will not be explicitly covered here. In addition to actin effectors, numerous tight junction-associated actin binding proteins have been identified and their importance implicated in tight junction biology, these have been recently reviewed as well [112]. Many of these proteins are involved in linking the transmembrane proteins of the junction to the cytoskeleton; these include the ZO proteins themselves, which bind actin directly and also a number of actin binding proteins [82]. Examples of ZO interacting actin binding proteins include the actin-crosslinking protein, α-actinin-4 [113], the tension sensitive actin-binding relatives vinculin [114] and α-catenin [115], and actin/spectrin interacting protein 4.1 [116]. A recently described role for the protein anillin involves regulation of actin and myosin at the tight junction [117]; anillin binds MgcRacGAP which interacts with cingulin and has been shown to play a role in the coordination of junctional GTPase activities [85]. Another particularly important actin regulatory protein is shroom2, which interacts with ZO proteins and regulates apical actin, myosin and microtubule organization with significant consequences for junctional organization and cell shape [118].
Non-muscle myosin II (NMII) is enriched at the apical junction [119] and plays a critical role both in maintenance of normal junctional tone [120] and in driving junction assembly and disassembly (reviewed in [121]). The anatomic relationship between NMII and apical junctions was recently elegantly described by Kachar and co-workers [122]. They demonstrated that junctional NMII was arranged in a periodic array of bipolar NMII filaments interwoven with actin and α-actinin; this was organized into sarcomeric-like units arranged in a belt around the apical pole of epithelial cells. The sarcomeres were aligned across cell membranes to allow organized contraction across a monolayer. This structure overlapped the basal half of the tight junction and apical end of the adherens junctions.
Links between microtubules and tight junctions were most clearly demonstrated in a recent study [83], in which the use of super resolution microscopy was revealed a previously undescribed planar apical network of microtubules at the tight junction. These microtubules bound to phosphorylated cingulin and their association with junctions was compromised in cingulin knockdown cells. While control cells formed spherical cysts in three dimensional cultures, cingulin knockdown cells or cell expressing non phosphorylatable cingulin formed asymmetric anisotropic cysts, supporting a previously unappreciated role for junction-associated microtubules in normal epithelial architecture.
3. Subdomains of tight junction
When viewed at low resolution, for example by immunolocalization of its core proteins, the tight junction appears to be a homogeneous zone of cell-cell contact. However, similar to the synapse [123] or to focal adhesions [124], the architecture of the tight junction is likely to contain structurally and functionally segregated zones. In fact, using a range of higher resolution methods including immuno-EM, FFEM and super-resolution light microscopy another picture emerges in which the junction is compartmentalized along the apical to lateral axis and from the plasma membrane to the cell’s interior. For example strand morphology can vary between the most apical strands compared to more basal fibrils, corresponding with the segregation of individual claudins toward top or bottom of the junction (Fig.1). Other proteins and complexes are concentrated at the bottom of the tight junction and the top of the adherens junction [91, 122], and still others proteins are apparently equivalently distributed along the entire tight junction and into adherens junctions [34]. Par3 represents an unusual case; it is found localized to the top and the bottom (but not the middle) of tight junctions [125]. A second gradient of localization is in the x-y plane, that is laterally from the transmembrane sealing proteins (e.g. claudin), to their scaffolds (e.g. ZO-1) to intermediate linkers (e.g. cingulin) to actin filaments and microtubules.
In order to gain more insight into these positional subtleties, and expand knowledge of the tight junction proteome, we recently employed a proximity-based proteomic method to find proteins near known junction proteins [13]. In this method an engineered form of biotin ligase is fused to a junction protein, expressed in cultured epithelial cells, and proteins within about 25Å of the fusion protein become labeled and can be recovered on a streptavidin–matrix and identified by mass spectrometry. When this method was applied to ZO-1 several hundred proteins were tagged although when ranked by frequency of recovery, many of those previously known to be close to ZO-1 were at the top of the list, for example ZO-1, ZO-2, claudins and occludin. Unexpectedly we found integral membrane proteins more heavily tagged when the ligase was fused to the N-terminus of ZO-1 while cytoskeletal proteins were more heavily tagged when the ligase was fused to the C-terminus. In retrospect this is consistent with known binding sites for claudins, occludin and JAM-A in the N-terminal half while actin and cortactin binds in the C-terminal half of ZO-1. The unexpected spatial resolution of tagging suggests the approach could be used to map the location of many proteins around the junction. When we positioned the ligase at the C-terminus of E-cadherin, the core protein of the adherens junction [126], the most highly tagged proteins were the catenins while very few tight junction proteins were tagged. Afadin, however, was high on both ZO-1 and E-cadherin lists, consistent with its interactions with components of both junctions. In contrast, members of the WNT signaling pathway, like the disheveled homolog proteins were much more heavily tagged by ZO-1 than E-cadherin, suggesting unexpected spatial compartmentalization in this signaling pathway.
In the future it will be important to continue to apply a range of methods to define the organizational subtleties of the tight junction. Beyond simple microscopic localization of proteins, proximity-based proteomic approaches seem a promising approach to define spatial compartments. Finally, there is evidence from methods like FRAP that we should be thinking about kinetically distinguishable compartments of junction proteins. These spatial and temporal compartments will have currently unappreciated consequences for function.
Highlights.
A large number of proteins are located at the tight junction.
General themes of their organizational architecture can be defined.
Structural and functional compartments exist along the junction and from the membrane toward the cell interior.
Much remains to be learned about the interaction and spatial organization within the junction.
Acknowledgements
The authors are supported by the Division of Intramural Research (CMV) and the Office of the Director (JMA), National Institutes of Health, USA. We apologize to all colleagues whose work could not be cited because of lack of space. We acknowledge the help of Dr. Xufeng Wu in the NHLBI Light Microscopy Core with the SIM images.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalcroft JP, Bullivant S. An interpretation of liver cell membrane and junction structure based on observation of freeze-fracture replicas of both sides of the fracture. J Cell Biol. 1970;47:49–60. doi: 10.1083/jcb.47.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claude P, Goodenough DA. Fracture faces of zonulae occludentes from "tight" and "leaky" epithelia. J Cell Biol. 1973;58:390–400. doi: 10.1083/jcb.58.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fromter E, Diamond J. Route of passive ion permeation in epithelia. Nat New Biol. 1972;235:9–13. doi: 10.1038/newbio235009a0. [DOI] [PubMed] [Google Scholar]
- 5.Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–66. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jesaitis LA, Goodenough DA. Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophila discs-large tumor suppressor protein. J Cell Biol. 1994;124:949–61. doi: 10.1083/jcb.124.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haskins J, Gu L, Wittchen ES, Hibbard J, Stevenson BR. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol. 1998;141:199–208. doi: 10.1083/jcb.141.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Citi S, Sabanay H, Jakes R, Geiger B, Kendrick-Jones J. Cingulin, a new peripheral component of tight junctions. Nature. 1988;333:272–6. doi: 10.1038/333272a0. [DOI] [PubMed] [Google Scholar]
- 9.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–88. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saitou M, Fujimoto K, Doi Y, Itoh M, Fujimoto T, Furuse M, et al. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J Cell Biol. 1998;141:397–408. doi: 10.1083/jcb.141.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furuse M, Sasaki H, Fujimoto K, Tsukita S. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol. 1998;143:391–401. doi: 10.1083/jcb.143.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol. 2001;153:263–72. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Itallie CM, Aponte A, Tietgens AJ, Gucek M, Fredriksson K, Anderson JM. The N and C termini of ZO-1 are surrounded by distinct proteins and functional protein networks. J Biol Chem. 2013;288:13775–88. doi: 10.1074/jbc.M113.466193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamazaki Y, Okawa K, Yano T, Tsukita S. Optimized proteomic analysis on gels of cell-cell adhering junctional membrane proteins. Biochemistry. 2008;47:5378–86. doi: 10.1021/bi8002567. [DOI] [PubMed] [Google Scholar]
- 15.Tang VW. Proteomic and bioinformatic analysis of epithelial tight junction reveals an unexpected cluster of synaptic molecules. Biol Direct. 2006;1:37. doi: 10.1186/1745-6150-1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mineta K, Yamamoto Y, Yamazaki Y, Tanaka H, Tada Y, Saito K, et al. Predicted expansion of the claudin multigene family. FEBS Lett. 2011;585:606–12. doi: 10.1016/j.febslet.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 17.Gunzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiol Rev. 2013;93:525–69. doi: 10.1152/physrev.00019.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friend DS, Gilula NB. Variations in tight and gap junctions in mammalian tissues. J Cell Biol. 1972;53:758–76. doi: 10.1083/jcb.53.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staehelin LA. Further observations on the fine structure of freeze-cleaved tight junctions. J Cell Sci. 1973;13:763–86. doi: 10.1242/jcs.13.3.763. [DOI] [PubMed] [Google Scholar]
- 20.Schneeberger EE. Heterogeneity of tight junction morphology in extrapulmonary and intrapulmonary airways of the rat. Anat Rec. 1980;198:193–208. doi: 10.1002/ar.1091980207. [DOI] [PubMed] [Google Scholar]
- 21.Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–63. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamazaki Y, Itoh M, Sasaki H, Furuse M, Tsukita S. Multi-PDZ domain protein 1 (MUPP1) is concentrated at tight junctions through its possible interaction with claudin-1 and junctional adhesion molecule. J Biol Chem. 2002;277:455–61. doi: 10.1074/jbc.M109005200. [DOI] [PubMed] [Google Scholar]
- 23.Poliak S, Matlis S, Ullmer C, Scherer SS, Peles E. Distinct claudins and associated PDZ proteins form different autotypic tight junctions in myelinating Schwann cells. J Cell Biol. 2002;159:361–72. doi: 10.1083/jcb.200207050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeansonne B, Lu Q, Goodenough DA, Chen YH. Claudin-8 interacts with multi-PDZ domain protein 1 (MUPP1) and reduces paracellular conductance in epithelial cells. Cell Mol Biol (Noisy-le-grand) 2003;49:13–21. [PubMed] [Google Scholar]
- 25.Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, et al. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–54. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 26.Fanning AS, Van Itallie CM, Anderson JM. Zonula occludens-1 and -2 regulate apical cell structure and the zonula adherens cytoskeleton in polarized epithelia. Mol Biol Cell. 2012;23:577–90. doi: 10.1091/mbc.E11-09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dravis C, Henkemeyer M. Ephrin-B reverse signaling controls septation events at the embryonic midline through separate tyrosine phosphorylation-independent signaling avenues. Dev Biol. 2011;355:138–51. doi: 10.1016/j.ydbio.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamazaki Y, Tokumasu R, Kimura H, Tsukita S. Role of claudin species-specific dynamics in reconstitution and remodeling of the zonula occludens. Mol Biol Cell. 2011;22:1495–504. doi: 10.1091/mbc.E10-12-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Capaldo CT, Farkas AE, Hilgarth RS, Krug SM, Wolf MF, Benedik JK, et al. Proinflammatory cytokine-induced Tight Junction remodeling through dynamic self-assembly of claudins. Mol Biol Cell. 2014 doi: 10.1091/mbc.E14-02-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruffer C, Gerke V. The C-terminal cytoplasmic tail of claudins 1 and 5 but not its PDZ-binding motif is required for apical localization at epithelial and endothelial tight junctions. Eur J Cell Biol. 2004;83:135–44. doi: 10.1078/0171-9335-00366. [DOI] [PubMed] [Google Scholar]
- 31.Piontek J, Fritzsche S, Cording J, Richter S, Hartwig J, Walter M, et al. Elucidating the principles of the molecular organization of heteropolymeric tight junction strands. Cell Mol Life Sci. 2011;68:3903–18. doi: 10.1007/s00018-011-0680-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daugherty BL, Ward C, Smith T, Ritzenthaler JD, Koval M. Regulation of heterotypic claudin compatibility. J Biol Chem. 2007;282:30005–13. doi: 10.1074/jbc.M703547200. [DOI] [PubMed] [Google Scholar]
- 33.Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol. 1999;147:891–903. doi: 10.1083/jcb.147.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nunes FD, Lopez LN, Lin HW, Davies C, Azevedo RB, Gow A, et al. Distinct subdomain organization and molecular composition of a tight junction with adherens junction features. J Cell Sci. 2006;119:4819–27. doi: 10.1242/jcs.03233. [DOI] [PubMed] [Google Scholar]
- 35.Yu D, Marchiando AM, Weber CR, Raleigh DR, Wang Y, Shen L, et al. MLCK-dependent exchange and actin binding region-dependent anchoring of ZO-1 regulate tight junction barrier function. Proc Natl Acad Sci U S A. 2010;107:8237–41. doi: 10.1073/pnas.0908869107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsuda M, Kubo A, Furuse M, Tsukita S. A peculiar internalization of claudins, tight junction-specific adhesion molecules, during the intercellular movement of epithelial cells. J Cell Sci. 2004;117:1247–57. doi: 10.1242/jcs.00972. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki H, Nishizawa T, Tani K, Yamazaki Y, Tamura A, Ishitani R, et al. Crystal structure of a claudin provides insight into the architecture of tight junctions. Science. 2014;344:304–7. doi: 10.1126/science.1248571. [DOI] [PubMed] [Google Scholar]
- 38.Anderson JM. Molecular structure of tight junctions and their role in epithelial transport. News Physiol Sci. 2001;16:126–30. doi: 10.1152/physiologyonline.2001.16.3.126. [DOI] [PubMed] [Google Scholar]
- 39.Rossa J, Ploeger C, Vorreiter F, Saleh T, Protze J, Gunzel D, et al. Claudin-3 and claudin-5 protein folding and assembly into the tight junction are controlled by non-conserved residues in the transmembrane 3 (TM3) and extracellular loop 2 (ECL2) segments. J Biol Chem. 2014;289:7641–53. doi: 10.1074/jbc.M113.531012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Itallie CM, Mitic LL, Anderson JM. Claudin-2 forms homodimers and is a component of a high molecular weight protein complex. J Biol Chem. 2011;286:3442–50. doi: 10.1074/jbc.M110.195578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li WY, Huey CL, Yu AS. Expression of claudin-7 and -8 along the mouse nephron. Am J Physiol Renal Physiol. 2004;286:F1063–71. doi: 10.1152/ajprenal.00384.2003. [DOI] [PubMed] [Google Scholar]
- 42.Gregory M, Dufresne J, Hermo L, Cyr D. Claudin-1 is not restricted to tight junctions in the rat epididymis. Endocrinology. 2001;142:854–63. doi: 10.1210/endo.142.2.7975. [DOI] [PubMed] [Google Scholar]
- 43.Rahner C, Mitic LL, Anderson JM. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology. 2001;120:411–22. doi: 10.1053/gast.2001.21736. [DOI] [PubMed] [Google Scholar]
- 44.Inai T, Sengoku A, Hirose E, Iida H, Shibata Y. Claudin-7 expressed on lateral membrane of rat epididymal epithelium does not form aberrant tight junction strands. Anat Rec (Hoboken) 2007;290:1431–8. doi: 10.1002/ar.20597. [DOI] [PubMed] [Google Scholar]
- 45.Lei Z, Maeda T, Tamura A, Nakamura T, Yamazaki Y, Shiratori H, et al. EpCAM contributes to formation of functional tight junction in the intestinal epithelium by recruiting claudin proteins. Dev Biol. 2012;371:136–45. doi: 10.1016/j.ydbio.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Wu CJ, Mannan P, Lu M, Udey MC. Epithelial Cell Adhesion Molecule (EpCAM) Regulates Claudin Dynamics and Tight Junctions. J Biol Chem. 2013 doi: 10.1074/jbc.M113.457499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harris HJ, Davis C, Mullins JG, Hu K, Goodall M, Farquhar MJ, et al. Claudin association with CD81 defines hepatitis C virus entry. J Biol Chem. 2010;285:21092–102. doi: 10.1074/jbc.M110.104836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steed E, Rodrigues NT, Balda MS, Matter K. Identification of MarvelD3 as a tight junction-associated transmembrane protein of the occludin family. BMC Cell Biol. 2009;10:95. doi: 10.1186/1471-2121-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raleigh DR, Marchiando AM, Zhang Y, Shen L, Sasaki H, Wang Y, et al. Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol Biol Cell. 2010;21:1200–13. doi: 10.1091/mbc.E09-08-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005;171:939–45. doi: 10.1083/jcb.200510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, et al. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–42. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kojima T, Ninomiya T, Konno T, Kohno T, Taniguchi M, Sawada N. Expression of tricellulin in epithelial cells and non-epithelial cells. Histol Histopathol. 2013;28:1383–92. doi: 10.14670/HH-28.1383. [DOI] [PubMed] [Google Scholar]
- 53.Riazuddin S, Ahmed ZM, Fanning AS, Lagziel A, Kitajiri S, Ramzan K, et al. Tricellulin is a tight-junction protein necessary for hearing. Am J Hum Genet. 2006;79:1040–51. doi: 10.1086/510022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krug SM, Amasheh S, Richter JF, Milatz S, Gunzel D, Westphal JK, et al. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol Biol Cell. 2009;20:3713–24. doi: 10.1091/mbc.E09-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Itallie CM, Fanning AS, Holmes J, Anderson JM. Occludin is required for cytokine-induced regulation of tight junction barriers. J Cell Sci. 2010;123:2844–52. doi: 10.1242/jcs.065581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong V. Phosphorylation of occludin correlates with occludin localization and function at the tight junction. Am J Physiol. 1997;273:C1859–67. doi: 10.1152/ajpcell.1997.273.6.C1859. [DOI] [PubMed] [Google Scholar]
- 57.Balda MS, Flores-Maldonado C, Cereijido M, Matter K. Multiple domains of occludin are involved in the regulation of paracellular permeability. J Cell Biochem. 2000;78:85–96. [PubMed] [Google Scholar]
- 58.Yaffe Y, Shepshelovitch J, Nevo-Yassaf I, Yeheskel A, Shmerling H, Kwiatek JM, et al. The MARVEL transmembrane motif of occludin mediates oligomerization and targeting to the basolateral surface in epithelia. J Cell Sci. 2012;125:3545–56. doi: 10.1242/jcs.100289. [DOI] [PubMed] [Google Scholar]
- 59.Li Y, Fanning AS, Anderson JM, Lavie A. Structure of the conserved cytoplasmic C-terminal domain of occludin: identification of the ZO-1 binding surface. J Mol Biol. 2005;352:151–64. doi: 10.1016/j.jmb.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 60.Subramanian VS, Marchant JS, Ye D, Ma TY, Said HM. Tight junction targeting and intracellular trafficking of occludin in polarized epithelial cells. Am J Physiol Cell Physiol. 2007;293:C1717–26. doi: 10.1152/ajpcell.00309.2007. [DOI] [PubMed] [Google Scholar]
- 61.Cording J, Berg J, Kading N, Bellmann C, Tscheik C, Westphal JK, et al. In tight junctions, claudins regulate the interactions between occludin, tricellulin and marvelD3, which, inversely, modulate claudin oligomerization. J Cell Sci. 2013;126:554–64. doi: 10.1242/jcs.114306. [DOI] [PubMed] [Google Scholar]
- 62.Ikenouchi J, Sasaki H, Tsukita S, Furuse M. Loss of occludin affects tricellular localization of tricellulin. Mol Biol Cell. 2008;19:4687–93. doi: 10.1091/mbc.E08-05-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Masuda S, Oda Y, Sasaki H, Ikenouchi J, Higashi T, Akashi M, et al. LSR defines cell corners for tricellular tight junction formation in epithelial cells. J Cell Sci. 2011;124:548–55. doi: 10.1242/jcs.072058. [DOI] [PubMed] [Google Scholar]
- 64.Higashi T, Tokuda S, Kitajiri S, Masuda S, Nakamura H, Oda Y, et al. Analysis of the 'angulin' proteins LSR, ILDR1 and ILDR2--tricellulin recruitment, epithelial barrier function and implication in deafness pathogenesis. J Cell Sci. 2013;126:966–77. doi: 10.1242/jcs.116442. [DOI] [PubMed] [Google Scholar]
- 65.Luissint AC, Nusrat A, Parkos CA. JAM-related proteins in mucosal homeostasis and inflammation. Semin Immunopathol. 2014;36:211–26. doi: 10.1007/s00281-014-0421-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–27. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Y, Nusrat A, Schnell FJ, Reaves TA, Walsh S, Pochet M, et al. Human junction adhesion molecule regulates tight junction resealing in epithelia. J Cell Sci. 2000;113:2363–74. doi: 10.1242/jcs.113.13.2363. Pt 13. [DOI] [PubMed] [Google Scholar]
- 68.Itoh M, Sasaki H, Furuse M, Ozaki H, Kita T, Tsukita S. Junctional adhesion molecule (JAM) binds to PAR-3: a possible mechanism for the recruitment of PAR-3 to tight junctions. J Cell Biol. 2001;154:491–7. doi: 10.1083/jcb.200103047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kostrewa D, Brockhaus M, D'Arcy A, Dale GE, Nelboeck P, Schmid G, et al. X-ray structure of junctional adhesion molecule: structural basis for homophilic adhesion via a novel dimerization motif. Embo J. 2001;20:4391–8. doi: 10.1093/emboj/20.16.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Severson EA, Jiang L, Ivanov AI, Mandell KJ, Nusrat A, Parkos CA. Cis-dimerization mediates function of junctional adhesion molecule A. Mol Biol Cell. 2008;19:1862–72. doi: 10.1091/mbc.E07-09-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ebnet K, Schulz CU, Meyer Zu, Brickwedde MK, Pendl GG, Vestweber D. Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J Biol Chem. 2000;275:27979–88. doi: 10.1074/jbc.M002363200. [DOI] [PubMed] [Google Scholar]
- 72.Monteiro AC, Sumagin R, Rankin CR, Leoni G, Mina MJ, Reiter DM, et al. JAM-A associates with ZO-2, afadin, and PDZ-GEF1 to activate Rap2c and regulate epithelial barrier function. Mol Biol Cell. 2013;24:2849–60. doi: 10.1091/mbc.E13-06-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Severson EA, Lee WY, Capaldo CT, Nusrat A, Parkos CA. Junctional adhesion molecule A interacts with Afadin and PDZ-GEF2 to activate Rap1A, regulate beta1 integrin levels, and enhance cell migration. Mol Biol Cell. 2009;20:1916–25. doi: 10.1091/mbc.E08-10-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ebnet K, Suzuki A, Horikoshi Y, Hirose T, Meyer Zu, Brickwedde MK, Ohno S, et al. The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM) Embo J. 2001;20:3738–48. doi: 10.1093/emboj/20.14.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iden S, Misselwitz S, Peddibhotla SS, Tuncay H, Rehder D, Gerke V, et al. aPKC phosphorylates JAM-A at Ser285 to promote cell contact maturation and tight junction formation. J Cell Biol. 2012;196:623–39. doi: 10.1083/jcb.201104143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Willott E, Balda MS, Fanning AS, Jameson B, Van Itallie C, Anderson JM. The tight junction protein ZO-1 is homologous to the Drosophila discs-large tumor suppressor protein of septate junctions. Proc Natl Acad Sci U S A. 1993;90:7834–8. doi: 10.1073/pnas.90.16.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fanning AS, Lye MF, Anderson JM, Lavie A. Domain swapping within PDZ2 is responsible for dimerization of ZO proteins. J Biol Chem. 2007;282:37710–6. doi: 10.1074/jbc.M707255200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meerschaert K, Tun MP, Remue E, De Ganck A, Boucherie C, Vanloo B, et al. The PDZ2 domain of zonula occludens-1 and -2 is a phosphoinositide binding domain. Cell Mol Life Sci. 2009;66:3951–66. doi: 10.1007/s00018-009-0156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wittchen ES, Haskins J, Stevenson BR. Protein interactions at the tight junction. Actin has multiple binding partners, and ZO-1 forms independent complexes with ZO-2 and ZO-3. J Biol Chem. 1999;274:35179–85. doi: 10.1074/jbc.274.49.35179. [DOI] [PubMed] [Google Scholar]
- 80.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–53. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 81.Fanning AS, Ma TY, Anderson JM. Isolation and functional characterization of the actin binding region in the tight junction protein ZO-1. Faseb J. 2002;16:1835–7. doi: 10.1096/fj.02-0121fje. [DOI] [PubMed] [Google Scholar]
- 82.Fanning AS, Anderson JM. Zonula occludens-1 and -2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann N Y Acad Sci. 2009;1165:113–20. doi: 10.1111/j.1749-6632.2009.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yano T, Matsui T, Tamura A, Uji M, Tsukita S. The association of microtubules with tight junctions is promoted by cingulin phosphorylation by AMPK. J Cell Biol. 2013;203:605–14. doi: 10.1083/jcb.201304194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aijaz S, D'Atri F, Citi S, Balda MS, Matter K. Binding of GEF-H1 to the tight junction-associated adaptor cingulin results in inhibition of Rho signaling and G1/S phase transition. Dev Cell. 2005;8:777–86. doi: 10.1016/j.devcel.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 85.Guillemot L, Guerrera D, Spadaro D, Tapia R, Jond L, Citi S. MgcRacGAP interacts with cingulin and paracingulin to regulate Rac1 activation and development of the tight junction barrier during epithelial junction assembly. Mol Biol Cell. 2014;25:1995–2005. doi: 10.1091/mbc.E13-11-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stevenson BR, Heintzelman MB, Anderson JM, Citi S, Mooseker MS. ZO-1 and cingulin: tight junction proteins with distinct identities and localizations. Am J Physiol. 1989;257:C621–8. doi: 10.1152/ajpcell.1989.257.4.C621. [DOI] [PubMed] [Google Scholar]
- 87.Huang RY, Guilford P, Thiery JP. Early events in cell adhesion and polarity during epithelial-mesenchymal transition. J Cell Sci. 2012;125:4417–22. doi: 10.1242/jcs.099697. [DOI] [PubMed] [Google Scholar]
- 88.Yamamoto T, Harada N, Kano K, Taya S, Canaani E, Matsuura Y, et al. The Ras target AF-6 interacts with ZO-1 and serves as a peripheral component of tight junctions in epithelial cells. J Cell Biol. 1997;139:785–95. doi: 10.1083/jcb.139.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mandai K, Nakanishi H, Satoh A, Obaishi H, Wada M, Nishioka H, et al. Afadin: A novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J Cell Biol. 1997;139:517–28. doi: 10.1083/jcb.139.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yamada T, Kuramitsu K, Rikitsu E, Kurita S, Ikeda W, Takai Y. Nectin and junctional adhesion molecule are critical cell adhesion molecules for the apico-basal alignment of adherens and tight junctions in epithelial cells. Genes Cells. 2013;18:985–98. doi: 10.1111/gtc.12091. [DOI] [PubMed] [Google Scholar]
- 91.Ooshio T, Kobayashi R, Ikeda W, Miyata M, Fukumoto Y, Matsuzawa N, et al. Involvement of the interaction of afadin with ZO-1 in the formation of tight junctions in Madin-Darby canine kidney cells. J Biol Chem. 2010;285:5003–12. doi: 10.1074/jbc.M109.043760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ide N, Hata Y, Nishioka H, Hirao K, Yao I, Deguchi M, et al. Localization of membrane-associated guanylate kinase (MAGI)-1/BAI-associated protein (BAP) 1 at tight junctions of epithelial cells. Oncogene. 1999;18:7810–5. doi: 10.1038/sj.onc.1203153. [DOI] [PubMed] [Google Scholar]
- 93.Adamsky K, Arnold K, Sabanay H, Peles E. Junctional protein MAGI-3 interacts with receptor tyrosine phosphatase beta (RPTP beta) and tyrosine-phosphorylated proteins. J Cell Sci. 2003;116:1279–89. doi: 10.1242/jcs.00302. [DOI] [PubMed] [Google Scholar]
- 94.Yao R, Natsume Y, Noda T. MAGI-3 is involved in the regulation of the JNK signaling pathway as a scaffold protein for frizzled and Ltap. Oncogene. 2004;23:6023–30. doi: 10.1038/sj.onc.1207817. [DOI] [PubMed] [Google Scholar]
- 95.Hirabayashi S, Tajima M, Yao I, Nishimura W, Mori H, Hata Y. JAM4, a junctional cell adhesion molecule interacting with a tight junction protein, MAGI-1. Mol Cell Biol. 2003;23:4267–82. doi: 10.1128/MCB.23.12.4267-4282.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu Y, Dowbenko D, Spencer S, Laura R, Lee J, Gu Q, et al. Interaction of the tumor suppressor PTEN/MMAC with a PDZ domain of MAGI3, a novel membrane-associated guanylate kinase. J Biol Chem. 2000;275:21477–85. doi: 10.1074/jbc.M909741199. [DOI] [PubMed] [Google Scholar]
- 97.Sakurai A, Fukuhara S, Yamagishi A, Sako K, Kamioka Y, Masuda M, et al. MAGI-1 is required for Rap1 activation upon cell-cell contact and for enhancement of vascular endothelial cadherin-mediated cell adhesion. Mol Biol Cell. 2006;17:966–76. doi: 10.1091/mbc.E05-07-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Patrie KM, Drescher AJ, Welihinda A, Mundel P, Margolis B. Interaction of two actin-binding proteins, synaptopodin and alpha-actinin-4, with the tight junction protein MAGI-1. J Biol Chem. 2002;277:30183–90. doi: 10.1074/jbc.M203072200. [DOI] [PubMed] [Google Scholar]
- 99.Liew CW, Vockel M, Glassmeier G, Brandner JM, Fernandez-Ballester GJ, Schwarz JR, et al. Interaction of the human somatostatin receptor 3 with the multiple PDZ domain protein MUPP1 enables somatostatin to control permeability of epithelial tight junctions. FEBS Lett. 2009;583:49–54. doi: 10.1016/j.febslet.2008.11.048. [DOI] [PubMed] [Google Scholar]
- 100.Baranwal S, Naydenov NG, Harris G, Dugina V, Morgan KG, Chaponnier C, et al. Nonredundant roles of cytoplasmic beta- and gamma-actin isoforms in regulation of epithelial apical junctions. Mol Biol Cell. 2012;23:3542–53. doi: 10.1091/mbc.E12-02-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hull BE, Staehelin LA. The terminal web. A reevaluation of its structure and function. J Cell Biol. 1979;81:67–82. doi: 10.1083/jcb.81.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hirokawa N, Tilney LG. Interactions between actin filaments and between actin filaments and membranes in quick-frozen and deeply etched hair cells of the chick ear. J Cell Biol. 1982;95:249–61. doi: 10.1083/jcb.95.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu KC, Cheney RE. Myosins in cell junctions. Bioarchitecture. 2012;2:158–70. doi: 10.4161/bioa.21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Glotfelty LA, Zahs A, Iancu C, Shen L, Hecht GA. Microtubules Are Required for Efficient Epithelial Tight Junction Homeostasis and Restoration. Am J Physiol Cell Physiol. 2014 doi: 10.1152/ajpcell.00336.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhou K, Muroyama A, Underwood J, Leylek R, Ray S, Soderling SH, et al. Actin-related protein2/3 complex regulates tight junctions and terminal differentiation to promote epidermal barrier formation. Proc Natl Acad Sci U S A. 2013;110:E3820–9. doi: 10.1073/pnas.1308419110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ivanov AI, Hunt D, Utech M, Nusrat A, Parkos CA. Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol Biol Cell. 2005;16:2636–50. doi: 10.1091/mbc.E05-01-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Katsube T, Takahisa M, Ueda R, Hashimoto N, Kobayashi M, Togashi S. Cortactin associates with the cell-cell junction protein ZO-1 in both Drosophila and mouse. J Biol Chem. 1998;273:29672–7. doi: 10.1074/jbc.273.45.29672. [DOI] [PubMed] [Google Scholar]
- 108.Lawrence DW, Comerford KM, Colgan SP. Role of VASP in reestablishment of epithelial tight junction assembly after Ca2+ switch. Am J Physiol Cell Physiol. 2002;282:C1235–45. doi: 10.1152/ajpcell.00288.2001. [DOI] [PubMed] [Google Scholar]
- 109.Citi S, Spadaro D, Schneider Y, Stutz J, Pulimeno P. Regulation of small GTPases at epithelial cell-cell junctions. Mol Membr Biol. 2011;28:427–44. doi: 10.3109/09687688.2011.603101. [DOI] [PubMed] [Google Scholar]
- 110.Citalan-Madrid AF, Garcia-Ponce A, Vargas-Robles H, Betanzos A, Schnoor M. Small GTPases of the Ras superfamily regulate intestinal epithelial homeostasis and barrier function via common and unique mechanisms. Tissue Barriers. 2013;1:e26938. doi: 10.4161/tisb.26938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chalmers AD, Whitley P. Continuous endocytic recycling of tight junction proteins: how and why? Essays Biochem. 2012;53:41–54. doi: 10.1042/bse0530041. [DOI] [PubMed] [Google Scholar]
- 112.Miyoshi J, Takai Y. Structural and functional associations of apical junctions with cytoskeleton. Biochim Biophys Acta. 2008;1778:670–91. doi: 10.1016/j.bbamem.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 113.Chen VC, Li X, Perreault H, Nagy JI. Interaction of zonula occludens-1 (ZO-1) with alpha-actinin-4: application of functional proteomics for identification of PDZ domain-associated proteins. J Proteome Res. 2006;5:2123–34. doi: 10.1021/pr060216l. [DOI] [PubMed] [Google Scholar]
- 114.Zemljic-Harpf AE, Godoy JC, Platoshyn O, Asfaw EK, Busija AR, Domenighetti AA, et al. Vinculin directly binds zonula occludens-1 and is essential for stabilizing connexin-43-containing gap junctions in cardiac myocytes. J Cell Sci. 2014;127:1104–16. doi: 10.1242/jcs.143743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Itoh M, Nagafuchi A, Moroi S, Tsukita S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J Cell Biol. 1997;138:181–92. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mattagajasingh SN, Huang SC, Hartenstein JS, Benz EJ., Jr. Characterization of the interaction between protein 4.1R and ZO-2. A possible link between the tight junction and the actin cytoskeleton. J Biol Chem. 2000;275:30573–85. doi: 10.1074/jbc.M004578200. [DOI] [PubMed] [Google Scholar]
- 117.Reyes CC, Jin M, Breznau EB, Espino R, Delgado-Gonzalo R, Goryachev AB, et al. Anillin regulates cell-cell junction integrity by organizing junctional accumulation of Rho-GTP and actomyosin. Curr Biol. 2014;24:1263–70. doi: 10.1016/j.cub.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hildebrand JD. Shroom regulates epithelial cell shape via the apical positioning of an actomyosin network. J Cell Sci. 2005;118:5191–203. doi: 10.1242/jcs.02626. [DOI] [PubMed] [Google Scholar]
- 119.Drenckhahn D, Dermietzel R. Organization of the actin filament cytoskeleton in the intestinal brush border: a quantitative and qualitative immunoelectron microscope study. J Cell Biol. 1988;107:1037–48. doi: 10.1083/jcb.107.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ivanov AI, Bachar M, Babbin BA, Adelstein RS, Nusrat A, Parkos CA. A unique role for nonmuscle myosin heavy chain IIA in regulation of epithelial apical junctions. PLoS One. 2007;2:e658. doi: 10.1371/journal.pone.0000658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ivanov AI. Actin motors that drive formation and disassembly of epithelial apical junctions. Front Biosci. 2008;13:6662–81. doi: 10.2741/3180. [DOI] [PubMed] [Google Scholar]
- 122.Ebrahim S, Fujita T, Millis BA, Kozin E, Ma X, Kawamoto S, et al. NMII forms a contractile transcellular sarcomeric network to regulate apical cell junctions and tissue geometry. Curr Biol. 2013;23:731–6. doi: 10.1016/j.cub.2013.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sudhof TC. Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron. 2013;80:675–90. doi: 10.1016/j.neuron.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, et al. Nanoscale architecture of integrin-based cell adhesions. Nature. 2010;468:580–4. doi: 10.1038/nature09621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hirose T, Izumi Y, Nagashima Y, Tamai-Nagai Y, Kurihara H, Sakai T, et al. Involvement of ASIP/PAR-3 in the promotion of epithelial tight junction formation. J Cell Sci. 2002;115:2485–95. doi: 10.1242/jcs.115.12.2485. [DOI] [PubMed] [Google Scholar]
- 126.Van Itallie CM, Tietgens AJ, Aponte A, Fredriksson K, Fanning AS, Gucek M, et al. Biotin ligase tagging identifies proteins proximal to E-cadherin, including lipoma preferred partner, a regulator of epithelial cell-cell and cell-substrate adhesion. J Cell Sci. 2014;127:885–95. doi: 10.1242/jcs.140475. [DOI] [PMC free article] [PubMed] [Google Scholar]