Abstract
Little is known about the tissue tropism of KI polyomavirus (KIPyV), and there are no studies to date describing any specific cell types it infects. The limited knowledge of KIPyV tropism has hindered study of this virus and understanding of its potential pathogenesis in humans. We describe tissues from two immunocompromised patients that stained positive for KIPyV antigen using a newly developed immunohistochemical assay targeting the KIPyV VP1 (KVP1) capsid protein. In the first patient, a pediatric bone marrow transplant recipient, KVP1 was detected in lung tissue. Double immunohistochemical staining demonstrated that approximately 50% of the KVP1-positive cells were CD68-positive cells of the macrophage/monocyte lineage. In the second case, an HIV-positive patient, KVP1 was detected in spleen and lung tissues. These results provide the first identification of a specific cell type in which KVP1 can be detected and expand our understanding of basic properties and in vivo tropism of KIPyV.
Keywords: KI polyomavirus, tissue tropism, immunohistochemistry, HIV, macrophage
Introduction
KI polyomavirus (KIPyV) was discovered in 2007 in patients with respiratory tract infections (1). Subsequent studies have detected KIPyV in respiratory tract secretions, blood, stool and tonsil tissue (2) and have suggested a seroprevalence of 55-70% (3, 4), with infection occurring most frequently in childhood. KIPyV is not currently associated with any human disease(s). However, other polyomaviruses are known to be important human pathogens. BK polyomavirus (BKPyV) causes BK nephropathy and hemorrhagic cystitis, while JC polyomavirus (JCPyV) is the etiological agent of progressive multifocal leukoencephalopathy. For both BKPyV and JCPyV, disease only manifests when the host is immunocompromised (5). As the more recently discovered Merkel cell carcinoma polyomavirus and Trichodysplasia spinulosa-associated polyomavirus are also thought to cause disease in the context of immunosuppression (6, 7), a significant question is whether KIPyV follows this paradigm and causes disease in immunocompromised patients.
Since little is known about the in vivo tropism of KIPyV, it is not yet possible to accurately identify potential diseases with which it may be associated. The virus has most commonly been detected in respiratory secretions, but there have been only minimal efforts to explore additional specimen types. In addition, prior studies have relied exclusively on PCR approaches to detect viral genomes in bulk-extracted nucleic acid, making it impossible to define the specific cell type(s) that harbor KIPyV. There have also been no published reports describing the detection of KIPyV antigens in tissues. In order to define the tissue and cell tropism of KIPyV and as a step toward understanding the role of KIPyV in human disease, we developed an immunohistochemical (IHC) assay targeting KIPyV VP1 (KVP1), the viral capsid protein. We applied this assay to tissue specimens from patients positive for KIPyV by PCR described in the published literature (8, 9). Tissues from two different patients were positive. KVP1 was detected in the lung tissue of a pediatric bone marrow transplant recipient, and a portion of the positive cells was identified as being CD68-positive using a double immunohistochemical stain. In addition, KVP1 was detected in the spleen and lung tissues of a deceased HIV-positive patient. These results provide the first insights into a specific cell type in which KIPyV can be detected.
Materials and Methods
Development of anti-KVP1 monoclonal antibodies
To develop anti-KVP1 monoclonal antibodies, the KVP1 Gateway pENTR/SD/D-TOPO construct previously described (3) was transferred into the Gateway pDEST17 plasmid (Life Technologies, Carlsbad, CA) and expressed in E. coli. The resulting recombinant His-tagged KVP1 protein was purified via an affinity Ni-NTA column (Pierce Biotechnology, Rockford, IL). A BALB/c mouse was immunized with three consecutive doses of the purified antigen. Its spleen was harvested for hybridoma fusion with the murine myeloma line P3X63Ag8.653 (Sigma-Aldrich, St. Louis, MO). We screened for clones producing anti-KVP1 antibody by ELISA and Western blot using purified GST-tagged KVP1 as the target antigen. Positive clones were then screened by ELISA using GST-tagged WVP1 (3), and those demonstrating cross reactivity to WVP1 were excluded. Two rounds of limiting dilutions were performed to achieve clonality. The anti-KVP1 monoclonal antibody used in the following experiments, NN-Ab03, was isotype IgG2b. The anti-WVP1 monoclonal antibody (NN-Ab06) used in Figure 1e was developed in an analogous manner and was also isotype IgG2b.
Figure 1.
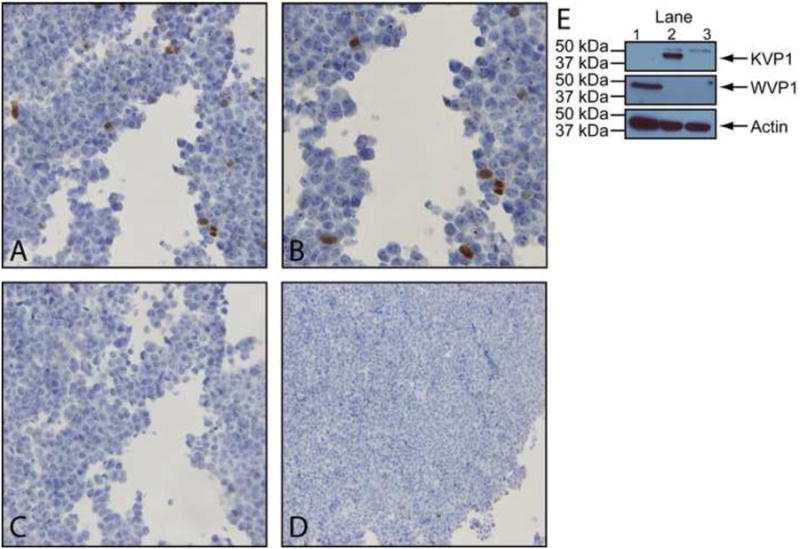
Validation of anti-KVP1 monoclonal antibody specificity. Immunohistochemistry (IHC) of 293T cells transfected with pDEST26-KVP1 and stained with (A, B) a KVP1 monoclonal antibody or with (C) an isotype control. (D) IHC of mock transfected 293T cells stained with a KVP1 monoclonal antibody. Panels A and C are at 400×; panel B is at 600×, and panel D is at 200×. (E) Western blot using a WVP1 monoclonal antibody of protein lysates from mock transfected 293T cells (lane 3) or cells transfected with pDEST26-KVP1 (lane 2) or pDEST26-WVP1 (lane 1). The blot was stripped and re-blotted using a KVP1 monoclonal antibody.
Generation of a KVP1 positive control
We generated a positive control for the immunohistochemistry assay by transfecting 293T cells with a plasmid (pDEST26, Life Technologies, Carlsbad, CA) encoding KVP1. Cells were harvested three days after transfection, and a portion was fixed in 10% neutral buffered formalin for 24 hours, embedded in paraffin, and sections of the cell block were transferred to glass slides.
Immunohistochemistry (IHC)
Tissue sections were deparaffinized in three changes of xylene and then rehydrated in a series of graded ethanol solutions. Antigen retrieval was accomplished in citrate buffer pH 6.0 (10mM citric acid, 0.05% Tween 20) at 95°C in a water bath for 35 minutes. Slides were then stained using the Histostain®-Plus 3rd Generation IHC Detection kit (Life Technologies, Carlsbad, CA) according to the manufacturer's instructions, with Superblock T20 (Thermo #37516, Rockford, IL) used as the blocking agent. The aforementioned KVP1 monoclonal antibody or an isotype control (mouse IgG2b, BD Biosciences #557351, San Jose, CA) was used as the primary antibody. After completion of the Histostain protocol, the slides were counterstained with hematoxylin and dehydrated in a series of graded ethanol solutions and xylene.
Slight modifications to this assay were used for staining performed on the pediatric transplant recipient's tissue. Tissue sections were deparaffinized in xylene for 15 minutes and then rehydrated in a series of graded ethanol solutions. Endogenous peroxidases were quenched in 3% hydrogen peroxide for 15 minutes. Antigen retrieval was accomplished in citrate buffer in a pre-warmed pressure cooker (Nesco PC6-25, Two Rivers, WI) for three minutes on the high setting. After blocking in 1.5% normal horse serum (Vector Labs #S-200, Burlingame, CA), the tissues were incubated first in primary antibody and then in secondary antibody (biotinylated anti-mouse IgG, Vector BA-2000, Burlingame, CA). The staining was developed using the Vectastain standard ABC kit (Vector Labs #PK-6100, Burlingame, CA) and DAB (Vector Labs #SK-4100, Burlingame, CA), counterstained with hematoxylin and dehydrated in a series of graded ethanol solutions and xylene.
Double immunohistochemistry (dIHC)
Double staining of the tissues utilized a protocol similar to that mentioned above for the transplant recipient's tissues with the addition of several steps. Following chromagen development of the first antibody using either DAB or ImmPACT SG (Vector Labs #SK-4705, Burlingame, CA), tissues were blocked with avidin and biotin (Vector Labs #SP-2001, Burlingame, CA) and a second time with 1.5% normal horse serum. They were incubated with the second primary and secondary antibodies and then developed as per the above IHC protocol. Other primary monoclonal antibodies used were against CD45 (BD Biosciences #555480, San Jose, CA), CD68 (Dako #M081401, Glostrup, Denmark) and CD31 (Dako #M0823, Glostrup, Denmark). Citrate buffer was used for antigen retrieval in the double IHC assays with CD45 and CD68, while Tris-EDTA buffer pH 9.0 (10mM Tris, 1mM EDTA, 0.05% Tween 20) was utilized for the CD31 double IHC assay.
Image manipulation
Images were cropped to squares. The resolution was changed to 500dpi in Photoshop with constrained proportions and no resampling. No other image manipulation was conducted.
Human studies
This study was approved by the Human Research Protection Office of Washington University in St. Louis under IRB 201108413.
Results
Establishment of a KVP1-specific immunohistochemistry assay
To study the cell and tissue tropism of KIPyV, we established an IHC assay using a newly developed monoclonal anti-KVP1 antibody. To validate the new IHC assay and evaluate its specificity, we developed a positive control cell pellet. Figure 1a shows 293T cells transfected with the pDEST26-KVP1 construct, which expressed KVP1 from a CMV promoter, and stained with the KVP1 antibody. Several cells showed prominent dark brown staining (Figure 1a, b), while cells from both a sequentially cut slide stained with the isotype control (Figure 1c) or mock transfected 293T cells stained with the KVP1 antibody (Figure 1d) did not.
To independently evaluate the specificity of the KVP1 monoclonal antibody, a subset of the transfected cells were lysed to extract proteins for Western blot analysis (Figure 1e). As negative controls, lysates of 293T cells from a mock transfection and from a transfection of an analogous plasmid expressing the VP1 protein of WU polyomavirus (pDEST26-WVP1) were included. We first blotted with a primary antibody against WU polyomavirus VP1 (WVP1), which is the most closely related virus to KIPyV and has also been detected in respiratory tract secretions. A single band was detected in the WVP1 lysate, while no band was seen in the KVP1 lysate or in the mock transfected cells (Figure 1e). After the membrane was stripped, we blotted with the KVP1 monoclonal antibody. A single band corresponding to the predicted size of KVP1 was detected in the KVP1 lysate, while no band is seen in mock transfected cells or in the WVP1 lysate (Figure 1e). The blot was stripped a second time and blotted for actin (Millipore #MAB1501, Billerica, MA) as a loading control. Given these data, we concluded the KVP1 antibody is specific for KI polyomavirus, and the KVP1 IHC assay can detect KI polyomavirus antigen in formalin-fixed paraffin-embedded cell pellets. Potential cross reactivity of the KVP1 assay with human polyomaviruses 6 and 7, which are the next most closely related polyomaviruses to KIPyV, was not explicitly tested. However, we believe cross reactivity with these viruses to be highly unlikely as they are much more divergent from KIPyV than WUPyV.
Detection of KVP1 in a pediatric transplant recipient
During the course of a recent study to evaluate the prevalence of human polyomaviruses in a prospective pediatric transplant cohort (8), we identified one patient (#3001) whose nasopharyngeal aspirate sample (NPA) was strongly positive for KIPyV by real-time PCR. The patient's clinical parameters have been described in detail (8). In brief, the patient was a 17-month-old child who received a bone marrow transplant as treatment for Fanconi anemia in April 2009. The patient's disease course was complicated by recurrent pulmonary hemorrhage, severe graft-versus-host disease (GVHD), and renal failure. The patient ultimately died of acute respiratory failure and extensive pulmonary hemorrhage several months later. The autopsy of the lung revealed evidence of chronic pulmonary hemorrhage with numerous hemosiderin-laden macrophages in the alveolar spaces. Diffuse alveolar hemorrhage leading to respiratory failure was the listed likely cause of death. Infection was considered less likely due to the negative results of routine microbiology testing (via culture and/or PCR). There was no significant inflammation or airway fibrosis to suggest GVHD in the lungs. A NPA sample collected 24 days prior to the death of the patient was strongly positive for KIPyV (1.3 × 109 genome copies per mL of transport media) (8).
Tissue blocks obtained at autopsy from 17 different body sites were available for KVP1 IHC testing. These included skin, liver, lung, esophagus, stomach, small intestine, large intestine, pancreas, spleen, right kidney, left kidney, bladder, left ventricle, right ventricle and pituitary, right adrenal and left adrenal glands. Of these, only the lung was positive by IHC (Figure 2). Two patterns of cellular staining were seen—strong, dark nuclear staining (arrows) and weaker, granular staining exclusively in the cytoplasm (arrow heads). Several controls were performed on serial sections to analyze this staining pattern, including a corresponding IgG2b isotype control (Figure 2a) and staining performed without the primary antibody or without both the primary and secondary antibodies (not shown). The weaker cytoplasmic staining was occasionally seen in controls and thus was potentially non-specific, but the strong nuclear staining was exclusively seen in the KVP1 stained tissue. Our subsequent analyses focused on these strong, nuclear staining cells. There were positive cells scattered throughout the section (similar to Figure 2b and c) with a few localized regions containing a high density of positive staining cells.
Figure 2.

Immunohistochemical staining of human lung with a KVP1 monoclonal antibody. Tissue stained with (A) an isotype control or (B) the KVP1 antibody (both at 200×). (C) Higher magnification (600×) of KVP1 staining in panel B.
Detection of KVP1 in CD68-positive cells
We used a double IHC (dIHC) staining approach to identify the cell type(s) that were KVP1-positive. Many of the positive cells were found within the alveolar spaces and were morphologically consistent with immune cells, so we began our analysis by establishing a dIHC assay using the KVP1 monoclonal antibody and an antibody against human CD45, which marks all cells of hematopoietic origin. Double IHC using the CD45/KVP1 assay showed clearly staining double positive cells throughout the tissue (Figure 3b,c,e). Of the 105 KVP1-positive cells counted in one tissue section, 51 (49%) were also CD45-positive. An isotype control (IgG2b for the KVP1 antibody) performed on a serial section (Figure 3a,d) was negative. A hematoxylin and eosin stain showed dark staining “grape-like” clusters, morphologically consistent with phagocytic cells (Figure 3f). Based on these data, we hypothesized that the CD45/KVP1-positive cells may be alveolar macrophages
Figure 3.

Detection of KIPyV VP1 in cells of hematopoietic origin in the lung. Lung tissue stained with a double IHC assay using antibodies against (A,D) CD45 (brown) and IgG2b (blue) as an isotype control or (B,C,E) CD45 (brown) and KVP1 (blue). (F) Hematoxylin and eosin-stained lung tissue. Panels A and B are at 400×; panel C is at 1000×; panels D and E are at 600×, and panel F is at 200×.
To test this hypothesis, we established a second dIHC assay using the KVP1 monoclonal antibody and an antibody against human CD68, which primarily marks macrophages and monocytes, and applied it to the lung tissue from patient 3001. Clear double staining was seen in ∼48% of KVP1-positive cells (Figure 4a-c), while no staining was seen in the isotype control (not shown). This demonstrated a subset of the KVP1-positive cells were likely to be alveolar macrophages. In addition, the morphology of a positive staining cell presented in Figure 4c resembles that of a foamy macrophage, a specific morphotype of macrophage loaded with lipid droplets (10).
Figure 4.

KIPyV VP1 detection in CD68-positive cells. Double IHC staining with antibodies against CD68 (brown) and KVP1 (blue) at (A) 400× and (B) 1000×. (C) A second double positive cell from another field of the tissue at 1000×.
We next attempted to identify the remaining subset of KVP1-positive cells that were CD45-negative. Based on the observation that KVP1-positive cells lined the alveoli in another case described below, we established a dIHC assay using antibodies against KVP1 and cytokeratin, which labels epithelial cells. While cells staining single positive for either KVP1 or cytokeratin were evident, no double positive cells were seen (Supplementary Figure 1), suggesting KIPyV was not present in epithelial cells in this lung specimen. One caveat to this interpretation is the absence of a gold standard positive control (i.e. epithelial cells known to express KVP1 in lung tissue) to validate the cytokeratin/KVP1 double stain in the context of lung tissue.
Detection of KVP1 in an HIV-positive patient
Sharp et al. previously screened 97 autopsy samples of lymphoid tissue by PCR and identified four samples positive for KIPyV, three from AIDS patients and one from an HIV-negative patient (9). A spleen sample from one patient had KVP1-positive staining cells scattered throughout the section with a few localized regions containing a high density of positive staining cells (Figure 5a-c). This sample was derived from a 42-year-old HIV-positive male who had initially presented with peripheral neuropathy and atypical mycobacterial infection. He was re-admitted four months later for cytomegalovirus (CMV) retinitis, and his condition continued to deteriorate until death. Autopsy showed disseminated mycobacterial infection, CMV esophagitis, low grade CMV encephalitis and vacuolar myelopathy.
Figure 5.

Immunohistochemical staining of human spleen and lung with a KVP1 monoclonal antibody. Tissue surrounding a splenic blood vessel consistent with splenic white pulp stained with (A) the isotype control or (B) the KVP1 antibody (both at 400×). (C) Hematoxylin and eosin staining of the same splenic area at 200×. Gas exchange tissue near a bronchiole stained with (D) the isotype control at 200× or with (E) the KVP1 antibody at 200× and (F) 600×.
Most of the KVP1 positive cells were in areas of white pulp, which also showed poorly formed granulomas attributed to a mycobacterial infection, according to the autopsy report. One such example with positive cells surrounding a blood vessel consistent with splenic white pulp is shown in Figure 5b-c. The isotype matched control antibody (J6.36) targeting the E2 glycoprotein of Hepatitis C virus (11) yielded no staining, consistent with specific anti-KVP1 staining (Figure 5a). Based on morphology and the detection of KVP1 in CD68-positive cells in the previously described case, we suspected hematopoietic cells may harbor KIPyV. Double staining of KVP1 with CD45 or CD68, however, yielded only single positive cells (Supplementary Figure 2). Given these negative results, we hypothesized that the KVP1-positive cells may be endothelial cells, so we established a dIHC assay with the anti-KVP1 antibody and an antibody against CD31, which primarily marks endothelial cells. However, only single positive cells were seen (Supplementary Figure 3). Therefore, the identity of the positive staining cells in the spleen is currently unknown.
We obtained sections of all other available tissues from this patient from the tissue bank for further analysis. These included the adrenal gland, carotid artery, colon, heart, ileum, kidney, liver, lung, pancreas, pituitary gland, prostate, testis, thyroid gland, tongue and a lymph node. Besides the spleen, only the lung specimen was positive by IHC for KVP1 with strong staining throughout the tissue as represented in Figure 5d-f. Closer inspection of the lung showed KVP1 staining of some alveolar surfaces, raising the possibility that the infected cells might be pneumocytes. In addition, other cells (Figure 5f, arrows) of unknown identity that did not border the alveolar spaces were also positive, suggesting that KIPyV infects multiple cell types present in the lung. Definitive identification of the specific cell types staining positive in this tissue was not possible due to the lack of available tissue. According to the autopsy report, the lungs showed bronchopneumonia, but no granulomas or acid fast bacilli were identified. The lung weights and pathological findings did not suggest an acute interstitial pneumonia.
Discussion
We detected KIPyV VP1 (KVP1) antigen in lung and splenic tissues for the first time using a newly developed IHC assay. In one patient, we identified a subset of the KVP1-positive lung cells as being CD68-positive. Together with morphologic data, these results suggest that alveolar macrophages harbor KVP1. The majority of prior KIPyV studies focused on respiratory secretions and relied on PCR of bulk-extracted DNA, making it impossible to determine specific cell types. Nonetheless, the simplest hypotheses regarding KIPyV tropism have focused on respiratory epithelial cells, the cell types infected by common respiratory viruses such as RSV (12) and influenza (13). While the IHC results alone do not prove that KIPyV can productively infect the CD68-positive cells, they do demonstrate expression of capsid protein from the late region of the genome, a step in the polyomavirus life cycle that is generally thought to occur concomitantly with DNA replication (14). These results also suggest that efforts to develop in vitro culture systems for KIPyV should explore the possibility that macrophage and monocyte cell lines may be permissive for productive infection.
Alveolar macrophages are long-lived, terminally-differentiated cells that permanently reside in the lung. They are thought to be one of the first cell types to respond to pathogens (15). The identification of alveolar macrophages as a potential site of KIPyV infection greatly expands our understanding of KIPyV tropism and provides new insights into the biology of KIPyV. The infection of macrophages and monocytes by BK polyomavirus (16), early hematopoietic progenitor cells by JC polyomavirus (17) and detection of Merkel cell polyomavirus (18) in monocytes have been reported. Alveolar macrophages display α-2,3- and α-2,6-linked sialic acid (19), both of which are known receptors for other polyomaviruses. BK polyomavirus and murine polyomavirus both bind to α-2,3-linked sialic acid, and JC polyomavirus binds to α-2,6-linked sialic acid (20). It is currently unknown what receptors are utilized by KIPyV, but it is possible that KIPyV might use similar receptors to BK and JC polyomaviruses.
The detection of KVP1 in CD68-positive cells could arise from one or more possible scenarios. First, alveolar macrophages may be susceptible to productive infection. Alternatively, it is also conceivable that circulating monocytes become infected in the bloodstream and remained infected after differentiation into alveolar macrophages in the lung. Third, other respiratory viruses, such as type A influenza virus, are known to abortively infect alveolar macrophages (21), where infection and subsequent steps of the viral life cycle occur but no virions are produced. Finally, we cannot rule out the formal possibility that the presence of KVP1 merely reflects phagocytosis of other virus infected cells. However, given the observation that KVP1 staining is nuclear, this seems like an unlikely option. Bona fide infection versus abortive infection of macrophages by KIPyV would likely lead to different outcomes, as is seen during influenza A infection (21). For example, an abortive infection would make the macrophage a dead end host and might allow the human host to mount a more effective immune response and delay viral spread. Conversely, a productive infection, whether initiated in the blood or lung, could incite a pro-inflammatory response from the infected macrophages, leading to greater immunopathology and increased viral spread.
In both of the cases presented here, the patients were immunocompromised and had lung tissues with detectable KVP1, supporting the hypothesis that the lung is an important site for KIPyV infection. However, the two cases presented with unique clinical features and disease pathologies. Patient #3001 was a pediatric patient who underwent a bone marrow transplant with multiple complications, including severe graft-versus-host disease, and died from acute respiratory failure and extensive pulmonary hemorrhage. In contrast, the other patient was an HIV-positive adult with multiple AIDS-defining illnesses at the time of death. The detection of KVP1 in the spleen of the adult HIV case and its absence in patient 3001 may reflect a distinct modality of KIPyV infection or possibly a dependence on HIV-mediated immunosuppression. Regardless, the IHC detection of KVP1 in the spleen of this patient corroborates the published PCR results (9), and provides evidence that additional specific cell types harbor KIPyV. Collectively, these results demonstrate that KVP1 can be detected in multiple distinct cell types in more than one tissue type in the human body and suggest the possibility that additional parameters, such as host immune status or age, may be important factors affecting viral tropism. While the role of KIPyV in human disease remains to be determined, it is interesting to speculate about a potential role in respiratory illness given the high titers of KIPyV in patient 3001.
In conclusion, we have established an immunohistochemical assay to detect KIPyV antigen in human tissue. We also demonstrated KVP1-positive staining in two lungs and one spleen from two separate patient cases. Further analysis revealed a subset of the positive cells in one lung was likely to be alveolar macrophages. This discovery furthers our understanding of KIPyV biology. Moreover, the knowledge that KIPyV can be detected in CD68-positive cells will benefit future efforts to develop cell culture systems for propagation of this virus.
Supplementary Material
Highlight.
Development of an immunohistochemical assay for KI polyomavirus.
Detection of KI polyomavirus in the spleen and lung of immunocompromised patients.
Detection of KI polyomavirus in CD68-positive cells.
Acknowledgments
Experimental support was provided by the Hybridoma Facility of the Rheumatic Diseases Core Center. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under Award Number P30AR048335. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors also wish to thank: Chris-Anne McKenzie for help with procurement and preparation of the tissue slides; the University of Edinburgh Medical Research Council HIV Brain & Tissue Bank; the Washington University AMP Core Labs for sample preparation; Dr. Erika Crouch for help with interpretation of the pathology and manuscript preparation; and Michelle Sabo and Adam Zuiani in the laboratory of Michael Diamond at Washington University for the HCV antibody. This study was funded in part by R21AI095922 to DW and by the Children's Discovery Institute at Washington University in St. Louis. EAS was supported by the Department of Defense through the National Defense Science & Engineering Graduate Fellowship Program. The funding sources did not have any involvement in the research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allander T, Andreasson K, Gupta S, Bjerkner A, Bogdanovic G, Persson MA, Dalianis T, Ramqvist T, Andersson B. Identification of a third human polyomavirus. J Virol. 2007;81:4130–4136. doi: 10.1128/JVI.00028-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babakir-Mina M, Ciccozzi M, Perno CF, Ciotti M. The novel KI, WU, MC polyomaviruses: possible human pathogens? New Microbiol. 2011;34:1–8. [PubMed] [Google Scholar]
- 3.Nguyen NL, Le BM, Wang D. Serologic evidence of frequent human infection with WU and KI polyomaviruses. Emerg Infect Dis. 2009;15:1199–1205. doi: 10.3201/eid1508.090270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kean JM, Rao S, Wang M, Garcea RL. Seroepidemiology of human polyomaviruses. PLoS Pathog. 2009;5:e1000363. doi: 10.1371/journal.ppat.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knowles WA. Discovery and epidemiology of the human polyomaviruses BK virus (BKV) and JC virus (JCV) Adv Exp Med Biol. 2006;577:19–45. doi: 10.1007/0-387-32957-9_2. [DOI] [PubMed] [Google Scholar]
- 6.Kazem S, van der Meijden E, Kooijman S, Rosenberg AS, Hughey LC, Browning JC, Sadler G, Busam K, Pope E, Benoit T, Fleckman P, de Vries E, Eekhof JA, Feltkamp MC. Trichodysplasia spinulosa is characterized by active polyomavirus infection. J Clin Virol. 2012;53:225–230. doi: 10.1016/j.jcv.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Gjoerup O, Chang Y. Update on human polyomaviruses and cancer. Adv Cancer Res. 2010;106:1–51. doi: 10.1016/S0065-230X(10)06001-X. [DOI] [PubMed] [Google Scholar]
- 8.Siebrasse EA, Bauer I, Holtz LR, Le BM, Lassa-Claxton S, Canter C, Hmiel P, Shenoy S, Sweet S, Turmelle Y, Shepherd R, Wang D. Human polyomaviruses in children undergoing transplantation, United States, 2008-2010. Emerg Infect Dis. 2012;18:1676–1679. doi: 10.3201/eid1810.120359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharp CP, Norja P, Anthony I, Bell JE, Simmonds P. Reactivation and mutation of newly discovered WU, KI, and Merkel cell carcinoma polyomaviruses in immunosuppressed individuals. J Infect Dis. 2009;199:398–404. doi: 10.1086/596062. [DOI] [PubMed] [Google Scholar]
- 10.Russell DG, Cardona PJ, Kim MJ, Allain S, Altare F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat Immunol. 2009;10:943–948. doi: 10.1038/ni.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabo MC, Luca VC, Prentoe J, Hopcraft SE, Blight KJ, Yi M, Lemon SM, Ball JK, Bukh J, Evans MJ, Fremont DH, Diamond MS. Neutralizing monoclonal antibodies against hepatitis C virus E2 protein bind discontinuous epitopes and inhibit infection at a postattachment step. J Virol. 2011;85:7005–7019. doi: 10.1128/JVI.00586-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hacking D, Hull J. Respiratory syncytial virus--viral biology and the host response. J Infect. 2002;45:18–24. doi: 10.1053/jinf.2002.1015. [DOI] [PubMed] [Google Scholar]
- 13.Kuiken T, Taubenberger JK. Pathology of human influenza revisited. Vaccine. 2008;26 Suppl 4:D59–66. doi: 10.1016/j.vaccine.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White MK, Safak M, Khalili K. Regulation of gene expression in primate polyomaviruses. J Virol. 2009;83:10846–10856. doi: 10.1128/JVI.00542-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneberger D, Aharonson-Raz K, Singh B. Monocyte and macrophage heterogeneity and Toll-like receptors in the lung. Cell Tissue Res. 2011;343:97–106. doi: 10.1007/s00441-010-1032-2. [DOI] [PubMed] [Google Scholar]
- 16.Traavik T, Uhlin-Hansen L, Flaegstad T, Christie KE. Antibody-mediated enhancement of BK virus infection in human monocytes and a human macrophage-like cell line. J Med Virol. 1988;24:283–297. doi: 10.1002/jmv.1890240306. [DOI] [PubMed] [Google Scholar]
- 17.Monaco MC, Atwood WJ, Gravell M, Tornatore CS, Major EO. JC virus infection of hematopoietic progenitor cells, primary B lymphocytes, and tonsillar stromal cells: implications for viral latency. J Virol. 1996;70:7004–7012. doi: 10.1128/jvi.70.10.7004-7012.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mertz KD, Junt T, Schmid M, Pfaltz M, Kempf W. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus. J Invest Dermatol. 2010;130:1146–1151. doi: 10.1038/jid.2009.392. [DOI] [PubMed] [Google Scholar]
- 19.Yu WC, Chan RW, Wang J, Travanty EA, Nicholls JM, Peiris JS, Mason RJ, Chan MC. Viral replication and innate host responses in primary human alveolar epithelial cells and alveolar macrophages infected with influenza H5N1 and H1N1 viruses. J Virol. 2011;85:6844–6855. doi: 10.1128/JVI.02200-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neu U, Stehle T, Atwood WJ. The Polyomaviridae: Contributions of virus structure to our understanding of virus receptors and infectious entry. Virology. 2009;384:389–399. doi: 10.1016/j.virol.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Short KR, Brooks AG, Reading PC, Londrigan SL. The fate of influenza A virus after infection of human macrophages and dendritic cells. J Gen Virol. 2012;93:2315–2325. doi: 10.1099/vir.0.045021-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


