Abstract
Type 2 innate lymphoid cells (ILC2s) have recently been identified in human nasal polyps, but whether numbers of ILC2s differ by polyp endotype or are influenced by corticosteroid use is unknown. Here, we show that eosinophilic nasal polyps contained double the number of ILC2s vs. non-eosinophilic polyps. Polyp ILC2s were also reduced by 50% in patients treated with systemic corticosteroids. Further, using a fungal allergen challenge mouse model, we detected greatly reduced Th2 cytokine-producing and Ki-67+ proliferating lung ILC2s in mice receiving dexamethasone. Finally, ILC2 Annexin V staining revealed extensive apoptosis after corticosteroid treatment in vivo and in vitro. Thus, ILC2s are elevated in the eosinophilic nasal polyp endotype and systemic corticosteroid treatment correlated with reduced polyp ILC2s. Finally, allergen-challenged mice showed reduced ILC2s and increased ILC2 apoptosis after corticosteroid treatment suggesting that ILC2 may be responsive to corticosteroids in eosinophilic respiratory disease.
Keywords: Alternaria, Chronic rhinosinusitis, Nasal polyps, ILC2s, Type 2 innate lymphoid cells
1. Introduction
Type 2 innate lymphoid cells (ILC2s) are a recently identified population of lineage-negative cells (lacking cell surface markers for T cell, B cell or NK cell lineages) that produce large amounts of Th2 cytokines [1–4]. In humans and mice, several cytokines and inflammatory mediators have been shown to induce ILC2 Th2 cytokine production and/or proliferation including TSLP, IL-25, IL-33, LTD4, and PGD2 [2–10]. While a great deal has been learned about the activation of ILC2s in murine models of disease, very few studies have shown connections between tissue ILC2s and human disease. Further, there are limited reports demonstrating negative regulation or pharmacologic inhibition of ILC2s.
ILC2s have been reported as enriched in human nasal polyps from patients with chronic rhinosinusitis (CRS), largely considered to be type 2 inflammatory disease [6]. Epithelial cytokines TSLP and IL-33, as well as leukotrienes, have been detected at higher levels in patients with CRS and are thus available for potent ILC2 stimulation [11–13]. A recent CRS consensus report suggests the importance of “endotypes” within CRSwNP as defined by histopathologic features, cytokine profiles and presence of different cell types [14]. Interestingly, emerging evidence has shown that eosinophilic and non-eosinophilic nasal polyp endotypes have different inflammatory profiles and contrasting responses to corticosteroid treatment [15, 16]. However, whether ILC2s are selectively enriched in distinct polyp endotypes or whether use of systemic corticosteroids may affect polyp ILC numbers is unknown.
Given the clinical and pathological distinctions between eosinophilic and non-eosinophilic polyposis, our first aim was to determine whether ILC2s, a novel Th2 cytokine producing cell population, are selectively enriched in eosinophilic nasal polyps as compared to non-eosinophilic polyps. Secondly, we sought to determine whether the numbers of ILC2s in human nasal polyps are affected by treatment with systemic corticosteroids. Corticosteroids reduce tissue eosinophilia through induction of cellular apoptosis, inhibition of type 2 cytokine production, and reduction in T-lymphocytes [17–19]. While corticosteroids are the generally first line therapy for rhinosinusitis and asthma, some patients have corticosteroid refractory disease with persistent eosinophilia [20]. A very recent report has shown that TSLP may induce partial corticosteroid resistance in mouse ILC2 in vitro as well as during administration of dexamethasone in OVA/alum sensitized and OVA/IL-33 challenged mice [21]. However, whether corticosteroids have an effect on ILC2 in vivo during exposure to a naturally encountered aeroallergen is unknown. Thus, our final aim was to determine the effect of corticosteroid treatment on mouse respiratory tissue ILC2 after in vivo natural allergen challenge with Alternaria alternata.
2. Materials and methods
2.1. Human nasal polyps
Nasal polyps and sinus mucosa samples were collected from 25 human subjects with chronic rhinosinusitis (CRS) undergoing endoscopic sinus surgery after Institutional Review Board approval at UCSD and Scripps Green Hospital. Subjects were consented to have their nasal polyp tissue used for research purposes. The demographic and clinical characteristics of patients were obtained by retrospective chart review and included age, gender, ethnicity, use of topical steroids, antibiotics or systemic steroids at the time of surgery. Physician diagnosis of cystic fibrosis and asthma were obtained by chart review. Aspirin exacerbated respiratory disease (AERD) diagnosis was defined by compelling history involving hypersensitivity reaction within 2–3 hours of ingestion of aspirin or NSAID, and confirmed by aspirin challenge in 2 of 3 patients.
Fresh tissue samples were transported in T cell media consisting of 10% FBS, 100 ug/ml Pen/Strep, 2mM L-glutamine and 50 uM 2-Mercaptoethanol in RPMI and processed for flow cytometry the same day. Polyps were divided into eosinophilic (n=8) or non-eosinophilic (n=10) groups based on eosinophil levels determined by FACS. Eosinophilic polyposis was defined as CCR3+FcεR1- granulocytes >10% of CD45+ cells or > 40% of granulocytes. Cytospun samples were stained with Giemsa-Wright and independent blinded pathological analysis of hematoxylin and eosin (H&E) stained paraffin sections of polyps was performed to confirm FACS results. Sinus mucosa (n=7) was collected from patients with chronic sinusitis without nasal polyps (CRSsNP). Finally, ILC2 levels were analyzed from patients treated with a course of systemic steroids (prednisone dose greater than 30 mg daily for a minimum of 5 days, or equivalent methylprednisolone daily dose) within 2 weeks prior to surgery.
2.2. Identification of human nasal polyp ILC2
Nasal polyp tissue obtained at the time of surgery was digested with Collagenase D (500 μg/ml) (Sigma-Aldrich) or Liberase TM (125 μg/ml) (Roche) in combination with DNase I (100μg/ml) (Roche), and then filtered into a single cell suspension. To detect ILC2 in human polyps, cells were stained with a FITC lineage cocktail (CD3, CD14, CD16, CD19, CD20, CD56; BD, Franklin Lakes NJ), and FITC conjugated TCRγδ (BD, Franklin Lakes NJ, USA), CD4, CD11b, CD235a, FcεR1, (eBioscience San Diego, CA), PerCP conjugated human Anti-CD45 (eBioscience), and PE-conjugated CRTH2 (CD294) (Miltenyi Biotec). The % of ILC2s are reported as the % ILC2 of CD45+ polyp cells. Flow cytometry was performed using a BD Accuri FACS machine and analyzed with FlowJo software (Tree Star, Inc).
2.3. Mice
Studies of ILC2s in mice require sufficient amounts of tissue for FACS analysis, as ILC2s have no unique cell surface markers for immunohistochemistry studies. As there are currently no reported mouse models of nasal polyposis in which sufficient numbers of ILC2s for functional studies are available, we used mouse lung rather than the small amount of mucous membrane in mouse sinuses to investigate the ILC2 responses to corticosteroids in vivo and in vitro. Female wild-type C57BL/6 mice 6–10 weeks of age (Jackson Laboratories) were intranasal challenged with 25μg Alternaria alternata (Greer, NC) on days 0, 3, and 6, and given oral gavage of either dexamethasone (Abcam) at 3 mg/kg or PBS on days 0, 2, 4, 6, and 8. On day 10, mice were euthanized and bronchoalveolar lavage and lungs were collected. All studies were approved by the University of California San Diego Institutional Animal Care and Use Committee.
2.4. Mouse BAL and lung processing and flow cytometry
BAL was performed as previously described [22, 23]. Lungs were processed into single cell suspensions using a tissue dissociator (Miltenyi Biotec). BAL and lung cells were incubated with a monoclonal antibody to CD16/CD32 (24G.2) for 10 min to block Fc receptors. Cells were stained with PE-conjugated Siglec-F and FITC-conjugated CD11c (eBioscience) and eosinophils were identified as Siglec F-positive CD11c-negative cells [24]. BAL and lung Th2 cells were identified as T1/ST2-positive CD4-positive cells after staining with biotin-T1/ST2 (MD biosciences) and CD4 (eBioscience) followed by streptavidin-APC (eBioscience). To identify mouse lung ILC2s, cells were stained with PerCP-conjugated CD45.2 (eBioscience), APC-conjugated Thy1.2 (CD90.2) (eBioscience), and FITC-conjugated Lineage cocktail as previously reported [7]. To assess the production of ILC2 IL-5 and IL-13, cells were permeabilized following surface staining using the BD intracellular staining kit (BD Biosciences) and stained with PE-conjugated IL-5 or IL-13 (eBioscience). To assess proliferation of ILC2s, cells were permeabilized using the FoxP3 Staining Buffer Set (eBioscience) following surface staining and stained with eFluor 660-conjugated anti-mouse Ki-67 or isotype control (eBioscience). To detect apoptotic ILC2s, cells were stained with the Annexin V Apoptosis detection kit (eBioscience). All samples were analyzed using an Accuri C6 Flow Cytometer (BD Biosciences) and data was further analyzed using FlowJo software (Tree Star).
2.5. Lung cell culture
ILC2 were first expanded in vivo after intranasal challenges of 25μg of Alternaria and lung cells (1×106 cells/well) were cultured in a 96-well, flat-bottom plate (Corning) with either IL-2 alone (10ng/ml) or IL-2 with dexamethasone at 10 μM or 1 mM concentrations. Cells were harvested at 1 day and 3 days and total numbers of ILC2s were assessed followed by annexin V analysis performed by flow cytometry.
2.6. ELISA
Supernatants were collected following centrifugation of BAL samples at 1400 RPM for 4 minutes at 4°C (Beckman Coulter). ELISA for IL-4, IL-5, and IL-13 (R&D Systems) were performed according to the manufacturer’s protocol.
2.7. Statistical analysis
Statistical analysis was performed using Prism software (Graphpad). Comparison of %ILC2 between eosinophilic, non-eosinophilic and sinus mucosa samples was performed using one-way ANOVA calculation. Direct comparison analysis of steroid vs. non-steroid groups was performed with unpaired t-test with Welch’s correction. Mann-whitney U test or t-test used for mouse studies as indicated.
3. Results
3.1. Patient baseline characteristics
Baseline patient age, gender, ethnicity, use of topical steroids, antibiotics and systemic steroids at the time of surgery were similar between eosinophilic versus non-eosinophilic endotypes (Table 1). As compared to the non-eosinophilic group, subjects with eosinophilic polyps had a greater likelihood of asthma. In the non-eosinophilic polyp group, 5 patients had cystic fibrosis and none of the patients had prior environmental allergy testing performed. Topical nasal steroids were used in fifteen out of eighteen patients and three patients had unknown use at the time of surgery.
Table 1.
Demographic data in human subjects with CRSwNP
| Eosinophilic (n=8) | Non-eosinophilic (n=10) | P value | |
|---|---|---|---|
| Mean Age | 43 | 49 | 0.38 |
| SD | 11.6 | 14.8 | |
| Gender | |||
| Male (%) | 62.5 | 70 | 0.75 |
| Female (%) | 37.5 | 30 | 0.92 |
| Ethnicity | |||
| Caucasian (%) | 88.9 | 70 | 0.34 |
| Hispanic (%) | 11.1 | 10 | 1.0 |
| Asian (%) | 0 | 10.0 | 0.36 |
| Environmental Allergies (%) | 87.5 | Not tested | N/A |
| Diagnosis of Asthma (%) | 44.4 | 11.1 | <0.05 |
| Cystic Fibrosis (%) | 0.0 | 50 | <0.05 |
| AERD (%) | 33.3 | 0 | <0.05 |
| Systemic steroid use (%) | 50.0 | 40 | 0.69 |
| Topical steroid use (%) | 89.9 | 80 | 0.62 |
| Antibiotics (%) | 50.0 | 60 | 0.69 |
3.2. ILC2s are selectively enriched in the eosinophilic nasal polyp endotype
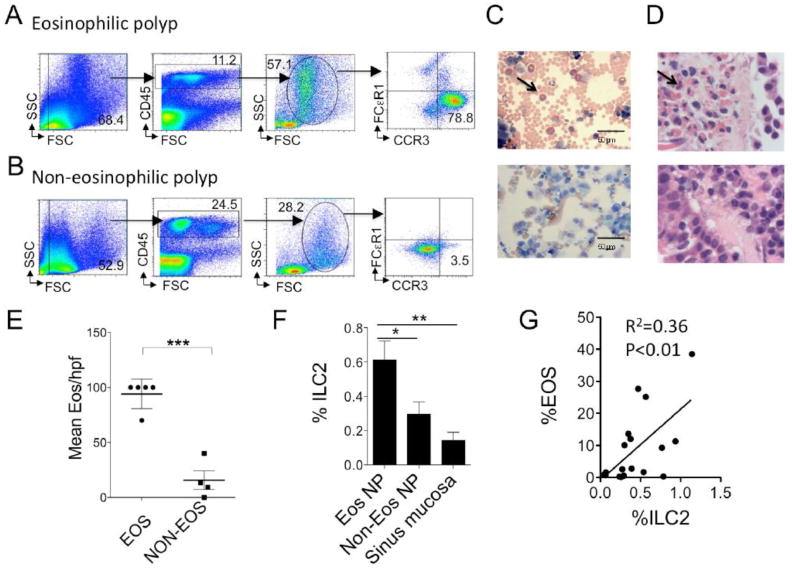
To determine levels of eosinophils in nasal polyps, single cell suspensions were stained for CD45, CCR3, and FcεR1. Eosinophilic polyposis was defined by the presence of CCR3+FcεR1- granulocytes that were >10% of all CD45+ cells or > 40% of the granulocyte population (Fig 1A). In contrast, non-eosinophilic polyps can be easily identified as having very low numbers (< 10%) that are CCR3+FcεR1- (Fig 1B). Wright-Giemsa staining of cytospin samples confirmed the presence of eosinophils in the eosinophilic polyp samples (Fig 1C). Further, independent pathological analysis of 9 polyp tissue specimens revealed that the mean eosinophil numbers in the eosinophilic polyp group were 72.3 per high power field (HPF) compared to a mean of 15.7 per HPF in the non-eosinophilic group and confirmed that our method of eosinophil detection by flow cytometry was valid (Fig 1D & E). Thus, we successfully stratified polyps into eosinophilic or non-eosinophilic endotypes.
Figure 1. ILC2s are selectively enriched in the eosinophilic nasal polyp endotype.
Single cell polyp suspensions were stained for eosinophils and identified as CD45+ granulocytes expressing CCR3 and negative for FcεR1 (A & B). Representative examples shown from (A) eosinophilic polyp and (B) non-eosinophilic polyp. (C) Giemsa-Wright stained cytospun nasal polyp cells (scale bar 50μm). Representative eosinophilic polyp (top) and non-eosinophilic polyp (bottom) shown. (D) H&E stained section of nasal polyp tissue examined at 100x. Eosinophilic (top) and non-eosinophilic shown (bottom). (E) Mean +/− SEM eosinophil counts per hpf (t-test, ***P<0.001). Arrows pointed at one of many eosinophils (C & D). (F) %ILC2 in eosinophilic NP (n=8) compared to non-eosinophilic NP (n=10) and sinus mucosa control tissue from patients with CRSsNP (n=7) (One-way ANOVA, *P<0.05, **P<0.01). %ILC2 defined as %CRTH2+ Lineage-negative lymphocyte lymphocytes of CD45+ cells. (G) Correlation plot of %ILC2 and eosinophil levels (Pearson correlation coefficient, two-tailed r2=0.36, P<0.01).
We identified nasal polyp ILC2s as CD45+ lineage-negative lymphocytes that express CRTH2+ cells as described in the methods and as previously reported [6, 25]. ILC2s lack CD3, CD14, CD16, CD19, CD20, CD56, TCRγδ, CD4, CD11b, CD235a, and FcεRI, and thus are not B, T, NK, NKT, and mast cells, or basophils. We assessed for levels of ILC2s (%ILC2 of CD45+ cells) in eosinophilic compared with non-eosinophilic polyps. Eosinophilic polyps contained approximately two times more ILC2s compared to non-eosinophilic polyps (Fig 1F). There was a further significant increase in %ILC2s in eosinophilic nasal polyps as compared to sinus mucosa from patients with CRSsNP and a positive correlation between the percentages of ILC2s and eosinophilia in polyp tissue (Fig 1G). In contrast, there was no correlation between the percentage of ILC2s and FcεR1+ staining cells, which may represent basophils or mast cell populations (r2 =0.00, p=0.96). Thus, ILC2s were selectively enriched in the eosinophilic nasal polyps we assessed compared with non-eosinophilic polyps and sinus mucosa.
3.3. Systemic corticosteroid therapy is associated with reduced nasal polyp ILC2s
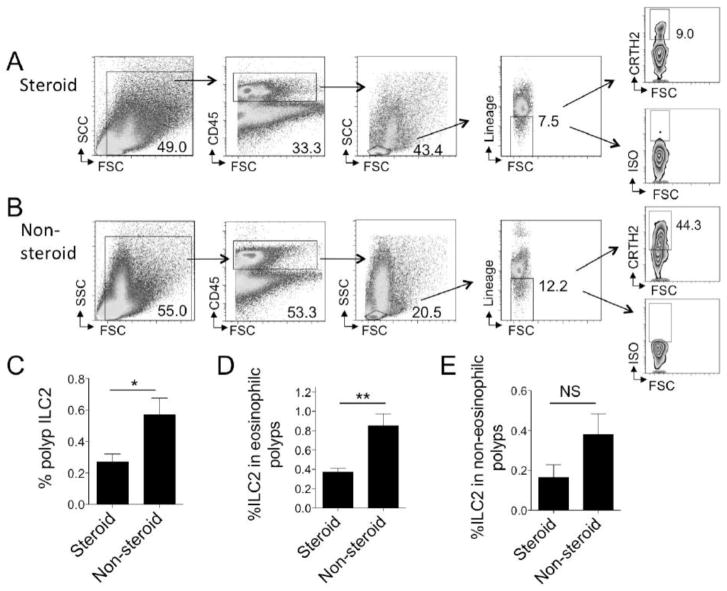
ILC2s are a recently discovered population of cells and whether corticosteroids might have an effect on human tissue ILC2s has not been reported. Thus, we further stratified our polyp samples based on treatment with systemic corticosteroids. We detected about half the number of ILC2s in nasal polyp tissue in steroid treated compared to patients who did not receive systemic corticosteroids (Fig 2A–C). Within the eosinophilic endotype, patients treated with systemic steroids had significantly reduced ILC2s as compared to patients who did not receive steroid treatment (Fig 2D). Interestingly, there was a trend toward reduced ILC2s in steroid treated patients with non-eosinophilic polyps as compared to non-steroid treated patients, but this did not meet statistical significance (p=0.14)(Fig 2E). Topical corticosteroid use was not correlated with changes in ILC2 levels (not shown). Thus, systemic steroid use in the eosinophilic polyp endotype was associated with reduced nasal polyp ILC2s compared with polyp ILC2 levels in non-steroid treated individuals.
Figure 2. Systemic corticosteroid therapy correlates with reduced nasal polyp ILC2s.
Polyp ILC2s were identified as CD45-positive lymphocytes that are lineage-negative and express CRTH2. Representative ILC2 gating strategy for eosinophilic nasal polyp from patient treated with corticosteroids prior to surgery (A) as compared to eosinophilic nasal polyp without corticosteroid treatment (B). (C) Mean +/− SEM %ILC2 in all CRSwNP patients treated with preoperative systemic corticosteroids (n=8) as compared to CRSwNP without corticosteroid treatment (n=10) (left, t-test, *p<0.05). Mean +/− SEM of %ILC2 in patients within the CRSwNP eosinophilic nasal polyp endotype pre-treated with systemic corticosteroids (n=4) as compared to patients who did not receive prednisone or equivalent within the eosinophilic endotype (n=4) (middle, t-test, **p<0.01). Mean +/− SEM of %ILC2 in patients with CRSwNP with the non-eosinophilic nasal polyp endotype pre-treated with systemic corticosteroids (n=4) as compared to those who did not receive prednisone or equivalent within non-eosinophilic group (n=6) (right, p=0.14, t-test).
3.4. Corticosteroid treatment reduces fungal allergen-induced eosinophilic inflammation
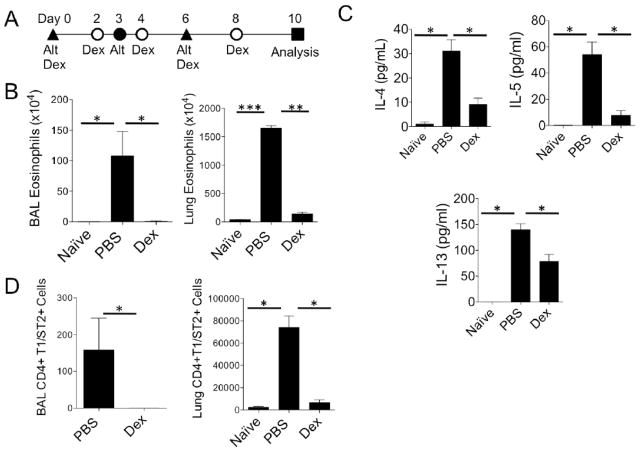
Eosinophilic chronic rhinosinusitis has been associated with fungal allergen sensitivity, including to the allergen Alternaria alternata [26]. We investigated the effects of corticosteroids on mouse lung ILC2s instead of mouse sinus ILC2s, as there is a very limited amount of sinus mucosa compared to lung available for FACS studies. In addition, immunohistochemistry cannot be used to identify ILC2 in tissue [27, 28]. Therefore, to determine whether respiratory tract ILC2s are responsive to corticosteroids in vivo as suggested by our human polyp results, we employed a mouse model of Alternaria-induced eosinophilic lung inflammation that expands ILC2s and induces robust eosinophilic infiltration, airway Th2 cytokine production and accumulation of Th2 cells as we have previously reported [7, 23]. Mice were challenged with Alternaria intranasally on days 0, 3, and 6 and orally administered dexamethasone or PBS every other day (Fig 3A). Dexamethasone treated mice showed a complete abrogation of airway and lung eosinophilia compared with Alternaria-challenged mice not treated with corticosteroid (Fig. 3B). BAL Th2 cytokines were also reduced and accumulation of CD4+T1/ST2+ Th2 cells was prevented following steroid treatment (Fig. 3C & D). Thus, the development of a type 2 eosinophilic lung response induced by the fungal allergen Alternaria was severely impaired by corticosteroid treatment.
Figure 3.
Dexamethasone inhibits Alternaria-induced type 2 lung inflammation. (A) Protocol for Alternaria-induced lung inflammation. Alt = intranasal Alternaria; Dex = gavage Dexamethasone. (B) Total BAL and lung eosinophils enumerated. (C) BAL IL-4, IL-5, and IL-13 were analyzed by ELISA. (D) BAL and lung CD4+T1/ST2+ cells enumerated. Data shown is representative of 2 independent experiments with 4 mice per group Alternaria challenged and 2 naïve mice. *P < 0.05, **P < 0.005, ***P < 0.001, Mann-Whitney test.
3.5. Dexamethasone impairs lung ILC2 accumulation and induces ILC2 apoptosis in vivo
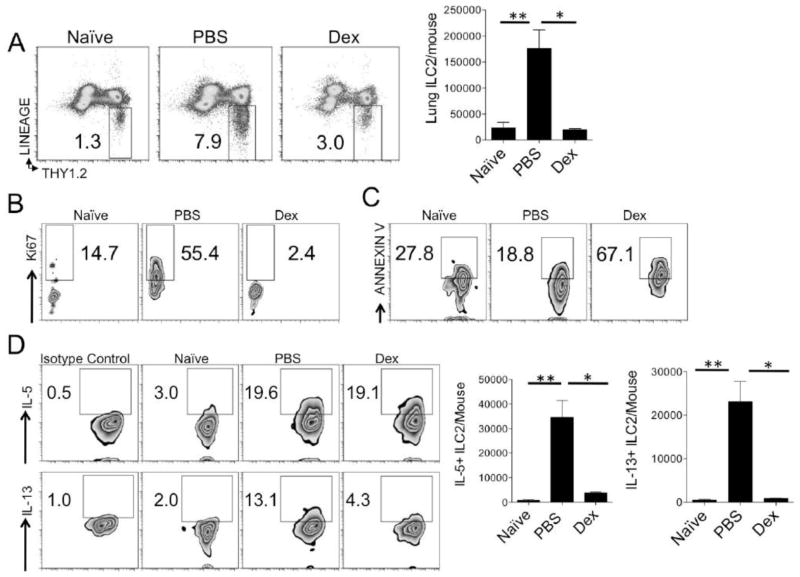
We have previously shown the number of lung ILC2s increases by 10–20 fold utilizing the protocol in Fig 3A [7, 23]. Thus, this model allowed for testing the effects of corticosteroids on ILC2 accumulation, apoptosis, proliferation and cytokine production. Similar to the Th2 cell reduction, the increase in total number and percentage of ILC2s in Alternaria-challenged mice was severely impaired and similar to levels found in naïve mice (Fig. 4A). We next assessed whether corticosteroids reduced ILC2 proliferation (Ki-67+ ILC2s) and/or increased apoptosis of ILC2s (Annexin V ILC2+) that may account for the reduction in total ILC2s. ILC2s markedly proliferated after the Alternaria challenge protocol in Fig 3A (Fig 4B). In contrast, lung ILC2s from dexamethasone treated mice had very low levels of Ki-67+ proliferation. Thus, dexamethasone treatment reduced ILC2 accumulation and proliferation in-vivo.
Figure 4.
Dexamethasone impairs lung ILC2 proliferation and induces ILC2 apoptosis in vivo. Mice undergoing the protocol in figure 3A were assessed for percent and total numbers of lung ILC2s (A). Proliferating ILC2s were measured by analysis of Ki67+ cells (B). Apoptotic ILC2s were measured by Annexin V expression (C). Intracellular staining was performed for ILC2 IL-5 and IL-13 production (D). Gated on lineage-negative Thy1.2+ lymphocytes (B–D). Data shown is representative of 2 independent experiments. *P < 0.05, **P < 0.005; Mann-Whitney test (A–C), unpaired t test (D).
Corticosteroid treatment inhibits eosinophil survival and induces apoptosis [29]. We hypothesized that corticosteroid treatment may also induce ILC2 apoptosis and performed staining with annexin V, a marker of early apoptosis. Allergen challenge without dexamethasone treatment led to minimally reduced ILC2 apoptosis beyond what is found naïve mice (Fig. 4C). However, dexamethasone treatment significantly increased annexin V+ ILC2s suggesting that corticosteroid steroid treatment increases ILC2 apoptosis in vivo.
3.6. Dexamethasone reduces ILC2 Th2 cytokine production in vivo
Next we sought to determine whether dexamethasone influenced ILC2 cytokine production in vivo as analyzed by intracellular staining. We have previously shown that Alternaria induces lung ILC2 IL-5 and IL-13 production [23]. In non-steroid treated mice, ILC2 IL-5 and IL-13 levels increased as expected (Fig 4D). Interestingly, dexamethasone treatment did not alter the % of IL-5+ ILC2s, but the total number of IL-5+ ILC2s were reduced due to a reduction in total ILC2s. In contrast, corticosteroid treatment reduced both the percent and total number of IL-13+ ILC2s. Overall, dexamethasone treatment reduced the number of IL-5 and IL-13 producing ILC2s in vivo.
3.7. Dexamethasone induces ILC2 apoptosis in vitro
Our in vivo results suggest that ILC2s are susceptible to corticosteroid-induced death and we investigated whether similar results were found in vitro. We cultured murine lung cells from WT mice that were challenged with Alternaria in presence of IL-2 alone or IL-2 and dexamethasone at 10 μM or 1 mM concentrations. IL-2 is a homeostatic survival factor for ILC2s and a previous report suggested that IL-2 may render ILC2s steroid resistant [21]. We next assessed the number of total ILC2s on days 1 and 3 after culture and performed annexin V staining to measure levels of ILC2 apoptosis. ILC2 numbers were not different one day after culture, but were significantly reduced in the presence of dexamethasone after three days of culture (Fig 5A). The % of Annexin V+ cells was increased by approximately 20% on day one in both concentrations of dexamethasone (Fig 5B). On day 3, the Annexin V+ ILC2s were further increased in a dose dependent manner in the dexamethasone treated wells. Thus, ILC2s from fungal allergen-challenged mice are sensitive to corticosteroid-induced apoptosis in vitro and thus support our in vivo findings.
Figure 5.
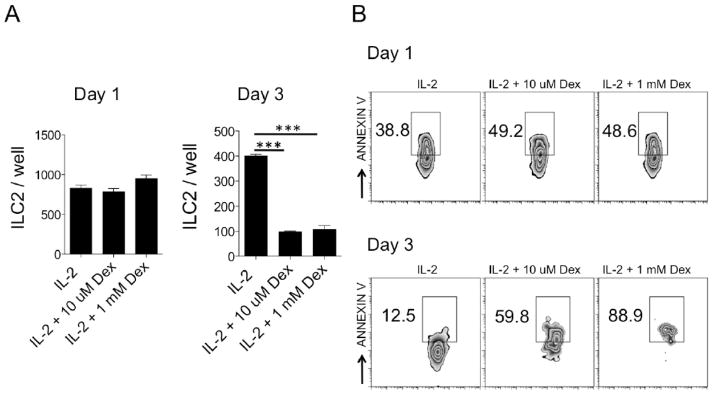
Dexamethasone induces apoptosis of ILC2s in vitro. Lung ILC2s were expanded in vivo after Alternaria challenges and cultured with IL-2 or dexamethasone (10 μM or 1 mM). (A) Total numbers of ILC2s one and three days after culture. (B) Annexin V+ ILC2s, shown, gated on lineage-negative Thy1.2+ cells. Data shown is representative of 2 independent experiments, ***P < 0.001; Mann-Whitney test.
4. Discussion
Our studies have demonstrated several novel findings. First, we have identified that ILC2s are selectively enriched in eosinophilic nasal polyps compared with non-eosinophilic polyps. Secondly, we detected lower levels of ILC2s in eosinophilic nasal polyps from patients treated with systemic corticosteroid compared with eosinophilic polyps from patients that were not treated with systemic corticosteroids. Further, we demonstrate that mouse lung ILC2s undergo extensive apoptosis in vivo after treatment with corticosteroids during exposure to the fungal allergen Alternaria. Finally, we show that corticosteroid treatment in vitro induces apoptosis of lung ILC2s that were activated and expanded in vivo.
Chronic rhinosinusitis is a heterogeneous collection of diseases afflicting millions of people each year [30]. A recent CRS consensus report suggests the importance of CRS endotypes as defined by histopathologic features, cytokine profiles and presence of different cell types [14]. Given the clinical and pathological distinctions between eosinophilic and non-eosinophilic polyposis, our findings that ILC2s (a novel Th2 cytokine producing cell population) are selectively enriched in the eosinophilic polyp endotype have important potential implications for ILC2s in polyp pathogenesis.
ILC2s have only recently been discovered and are emerging as a potentially important innate immune effector cell in human type 2 inflammatory diseases [31]. ILC2s do not express cell surface markers for T cell, B cell or NK cell lineages and produce Th2 cytokines in response to epithelial derived cytokines IL-25, TSLP and IL-33, as well as CysLTs and Prostaglandin D2 [2–4, 6, 7, 23, 32]. Importantly, TSLP, IL-33, and CysLTs are increased in CRS and thus may be available to potently stimulate ILC2s [11–13, 33]. The presence of ILC2s in nasal polyps from human subjects that were not stratified by endotype has previously been reported [6, 25]. A subsequent recent report demonstrated that a greater number of ILC2 were present in the ethmoid sinus mucosa of 6 patients with CRSwNP compared to 4 patients with CRSsNP [11]. Thus, there is mounting evidence that ILC2s may contribute to nasal polyp pathogenesis and our work further supports the role of ILC2 in the eosinophilic polyp endotype.
Our studies also demonstrate the novel finding that nasal polyp tissue from human subjects treated with systemic corticosteroids showed reduced levels of ILC2s. These findings have important implications, as there is limited information on ways to inhibit ILC2 proliferation and activation. Recently, Lipoxin A4 was shown to decrease IL-13 production from peripheral blood ILC2s and represents one potential ILC2 inhibitor [32]. Interestingly, all but three patients in our studies were reportedly using topical corticosteroids chronically and there was no correlation between topical steroid use and polyp ILC2 levels. This suggests that ILC2s may be refractory to topical corticosteroid administration and may require higher dose systemic corticosteroid to reduce ILC2 levels. Topical corticosteroids are a mainstay of treatment for CRS and our work suggests that further treatments are needed to reduce ILC2 levels without the known side effects of systemic corticosteroid treatment. However, future controlled human trials with CRS patients would need to be performed to validate our findings.
The limitations of the study include the absence of in vitro studies of the effect of corticosteroids on human nasal polyp ILC2s, as well as the use of lung rather than sinus ILC2 in our mouse studies. ILC2s represent < 0.5% of all polyp cells and the low numbers of ILC2 preclude appropriate in vitro investigation. We therefore tested the effect of corticosteroids on mouse lung ILC2s. As ILC2s have no unique cell surface marker, immunohistochemistry of mouse nasal mucosa cannot accurately detect ILC2s. In addition, the very limited amount of tissue obtained from the sinus mucosa lining the mouse sinuses (compared to the much larger amount of tissue in mouse lung) precludes having sufficient numbers of ILC2 for analysis. Nevertheless, there is evidence that lung and sinus ILC2s may be functionally similar. For example, mediators that activate ILC2s including TSLP, IL-33, PGD2 and CysLTs are increased in both CRS as well as asthma, and are also found to play important roles in mouse models of disease [7, 11–13, 32–39]. Further, immune cell recruitment to the nose after allergen challenge likely utilizes similar mechanisms that occur in the lung [40]. Overall, these points suggest that the study of mouse lung ILC2 responsiveness to corticosteroids may be relevant to other respiratory disease including nasal and sinus disease.
We found that lung ILC2s from mice challenged with a clinically relevant fungal allergen underwent extensive apoptosis in response to corticosteroid both in vivo and in vitro. A recent study suggested that mouse lung ILC2s may be corticosteroid resistant in the presence of TSLP [21]. There are several important differences between the previous study and our work. First, we utilized Alternaria, a natural aeroallergen that is associated with severe asthma and chronic rhinosinusitis, to examine the effect of dexamethasone treatment on ILC2 numbers and function [26, 41]. In contrast, the previous study utilized OVA/alum intraperitoneal sensitization followed by airway IL-33 and OVA challenges and showed that dexamethasone did not reduce ILC2s in this model. Further, absence or blockade of TSLP led to a partial increase in steroid sensitivity of lung ILC2s suggesting that under some conditions TSLP may promote ILC2 steroid resistance. The Alternaria protocol we utilized is highly dependent on IL-33 [23, 37] and the previous finding that lung ILC2s were sensitive to dexamethasone after administration of IL-33 alone is consistent with our results. Additionally, we administered dexamethasone throughout the Alternaria protocol and the previous study treated with dexamethasone during recall challenges with OVA and IL-33 after sensitization. Finally, our in vitro studies showed that lung ILC2s that were activated and expanded by in vivo Alternaria challenges were sensitive to dexamethasone in the presence of IL-2. In contrast, the previous report showed that TSLP stimulation of naïve lung ILC2s in vitro led to partial dexamethasone resistance. Thus, differences in the in vivo models and in vitro culture systems may account for differences in conclusions between our work and the previous study. Overall, our findings that mouse ILC2s were sensitive to corticosteroids is consistent with our human nasal polyp data showing that systemic steroid treated individuals had fewer polyp ILC2s.
In summary, we have identified that ILC2s are enriched in the eosinophilic nasal polyp endotype and we detected fewer ILC2s in eosinophilic nasal polyps from patients treated with systemic corticosteroid compared with polyps from patients not treated with systemic corticosteroids. We also demonstrated that mouse lung ILC2s undergo extensive apoptosis after exposure to corticosteroids both in vivo and in vitro. Future studies are needed to identify whether ILC2s may become corticosteroid refractory in certain conditions or may vary between individuals.
Highlights.
ILC2s are selectively elevated in the eosinophilic nasal polyp endotype.
Systemic corticosteroid treatment was associated with reduced polyp ILC2s.
Corticosteroids induce mouse lung ILC2 apoptosis in vivo and in vitro.
Acknowledgments
This work was supported by the National Institutes of Health; AI 38425, AI 70535, AI 107779, and AI 72115 to DB and NIH KO8 AI 080938 to TD. Also, TD is supported by the ALA/AAAAI Allergic Respiratory Diseases Award and ALA Biomedical Research Award.
Abbreviations
- Dex
Dexamethasone
- IL-5
Interleukin-5
- IL-13
Interleukin-13
- IL-25
Interleukin-25
- IL-33
Interleukin-33
- ILC2
Type 2 innate lymphoid cell
- LTD4
Leukotriene D4
- PBS
Phosphate buffered saline
- PGD2
Prostaglandin D2
- TSLP
Thymic stromal lymphopoietin
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Scanlon ST, McKenzie A. Type 2 innate lymphoid cells: new players in asthma and allergy. Curr Opin Immunol. 2012;24:707–712. doi: 10.1016/j.coi.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 5.Xue L, Salimi M, Panse I, Mjosberg JM, McKenzie AN, Spits H, Klenerman P, Ogg G. Prostaglandin D activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on T2 cells. J Allergy Clin Immunol. 2014;133:1184–1194. doi: 10.1016/j.jaci.2013.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 7.Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol. 2013;132:205–213. doi: 10.1016/j.jaci.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barlow JL, Peel S, Fox J, Panova V, Hardman CS, Camelo A, Bucks C, Wu X, Kane CM, Neill DR, Flynn RJ, Sayers I, Hall IP, McKenzie AN. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J Allergy Clin Immunol. 2013;132:933–941. doi: 10.1016/j.jaci.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 10.Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, Hepworth MR, Van Voorhees AS, Comeau MR, Artis D. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5:170ra116. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw JL, Fakhri S, Citardi MJ, Porter PC, Corry DB, Kheradmand F, Liu YJ, Luong A. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2013;188:432–439. doi: 10.1164/rccm.201212-2227OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagarkar DR, Poposki JA, Tan BK, Comeau MR, Peters AT, Hulse KE, Suh LA, Norton J, Harris KE, Grammer LC, Chandra RK, Conley DB, Kern RC, Schleimer RP, Kato A. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;132:593–600. e512. doi: 10.1016/j.jaci.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinke JW, Bradley D, Arango P, Crouse CD, Frierson H, Kountakis SE, Kraft M, Borish L. Cysteinyl leukotriene expression in chronic hyperplastic sinusitis-nasal polyposis: importance to eosinophilia and asthma. J Allergy Clin Immunol. 2003;111:342–349. doi: 10.1067/mai.2003.67. [DOI] [PubMed] [Google Scholar]
- 14.Akdis CA, Bachert C, Cingi C, Dykewicz MS, Hellings PW, Naclerio RM, Schleimer RP, Ledford D. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2013;131:1479–1490. doi: 10.1016/j.jaci.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen W, Liu W, Zhang L, Bai J, Fan Y, Xia W, Luo Q, Zheng J, Wang H, Li Z, Xia J, Jiang H, Liu Z, Shi J, Li H, Xu G. Increased neutrophilia in nasal polyps reduces the response to oral corticosteroid therapy. J Allergy Clin Immunol. 2012;129:1522–1528. e1525. doi: 10.1016/j.jaci.2012.01.079. [DOI] [PubMed] [Google Scholar]
- 16.Payne SC, Early SB, Huyett P, Han JK, Borish L, Steinke JW. Evidence for distinct histologic profile of nasal polyps with and without eosinophilia. Laryngoscope. 2011;121:2262–2267. doi: 10.1002/lary.21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bentley AM, Hamid Q, Robinson DS, Schotman E, Meng Q, Assoufi B, Kay AB, Durham SR. Prednisolone treatment in asthma. Reduction in the numbers of eosinophils, T cells, tryptase-only positive mast cells, and modulation of IL-4, IL-5, and interferon-gamma cytokine gene expression within the bronchial mucosa. Am J Respir Crit Care Med. 1996;153:551–556. doi: 10.1164/ajrccm.153.2.8564096. [DOI] [PubMed] [Google Scholar]
- 18.Miyamasu M, Misaki Y, Izumi S, Takaishi T, Morita Y, Nakamura H, Matsushima K, Kasahara T, Hirai K. Glucocorticoids inhibit chemokine generation by human eosinophils. J Allergy Clin Immunol. 1998;101:75–83. doi: 10.1016/S0091-6749(98)70196-4. [DOI] [PubMed] [Google Scholar]
- 19.Meagher LC, Cousin JM, Seckl JR, Haslett C. Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes. J Immunol. 1996;156:4422–4428. [PubMed] [Google Scholar]
- 20.Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. 2004;113:101–108. doi: 10.1016/j.jaci.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 21.Kabata H, Moro K, Fukunaga K, Suzuki Y, Miyata J, Masaki K, Betsuyaku T, Koyasu S, Asano K. Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat Commun. 2013;4:2675. doi: 10.1038/ncomms3675. [DOI] [PubMed] [Google Scholar]
- 22.Doherty TA, Khorram N, Sugimoto K, Sheppard D, Rosenthal P, Cho JY, Pham A, Miller M, Croft M, Broide DH. Alternaria Induces STAT6-Dependent Acute Airway Eosinophilia and Epithelial FIZZ1 Expression That Promotes Airway Fibrosis and Epithelial Thickness. J Immunol. 2012;188:2622–2629. doi: 10.4049/jimmunol.1101632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doherty TA, Khorram N, Chang JE, Kim HK, Rosenthal P, Croft M, Broide DH. STAT6 regulates natural helper cell proliferation during lung inflammation initiated by Alternaria. Am J Physiol Lung Cell Mol Physiol. 2012;303:L577–588. doi: 10.1152/ajplung.00174.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens WW, Kim TS, Pujanauski LM, Hao X, Braciale TJ. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J Immunol Methods. 2007;327:63–74. doi: 10.1016/j.jim.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mjosberg J, Bernink J, Golebski K, Karrich JJ, Peters CP, Blom B, Te Velde AA, Fokkens WJ, van Drunen CM, Spits H. The Transcription Factor GATA3 Is Essential for the Function of Human Type 2 Innate Lymphoid Cells. Immunity. 2012;37:649–659. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Shin SH, Ponikau JU, Sherris DA, Congdon D, Frigas E, Homburger HA, Swanson MC, Gleich GJ, Kita H. Chronic rhinosinusitis: an enhanced immune response to ubiquitous airborne fungi. J Allergy Clin Immunol. 2004;114:1369–1375. doi: 10.1016/j.jaci.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Lindsay R, Slaughter T, Britton-Webb J, Mog SR, Conran R, Tadros M, Earl N, Fox D, Roberts J, Bolger WE. Development of a murine model of chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2006;134:724–730. doi: 10.1016/j.otohns.2005.11.048. discussion 731–722. [DOI] [PubMed] [Google Scholar]
- 28.Cho SH, Oh SY, Zhu Z, Lee J, Lane AP. Spontaneous eosinophilic nasal inflammation in a genetically-mutant mouse: comparative study with an allergic inflammation model. PLoS One. 2012;7:e35114. doi: 10.1371/journal.pone.0035114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallen N, Kita H, Weiler D, Gleich GJ. Glucocorticoids inhibit cytokine-mediated eosinophil survival. J Immunol. 1991;147:3490–3495. [PubMed] [Google Scholar]
- 30.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P, Georgalas C, Goossens H, Harvey R, Hellings P, Hopkins C, Jones N, Joos G, Kalogjera L, Kern B, Kowalski M, Price D, Riechelmann H, Schlosser R, Senior B, Thomas M, Toskala E, Voegels R, de Wang Y, Wormald PJ. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50:1–12. doi: 10.4193/Rhino12.000. [DOI] [PubMed] [Google Scholar]
- 31.Scanlon ST, McKenzie AN. Type 2 innate lymphoid cells: new players in asthma and allergy. Curr Opin Immunol. 2012;24:707–712. doi: 10.1016/j.coi.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Barnig C, Cernadas M, Dutile S, Liu X, Perrella MA, Kazani S, Wechsler ME, Israel E, Levy BD. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med. 2013;5:174ra126. doi: 10.1126/scitranslmed.3004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baba S, Kondo K, Kanaya K, Suzukawa K, Ushio M, Urata S, Asakage T, Kakigi A, Suzukawa M, Ohta K, Yamasoba T. Expression of IL-33 and its receptor ST2 in chronic rhinosinusitis with nasal polyps. Laryngoscope. 2014;124:E115–122. doi: 10.1002/lary.24462. [DOI] [PubMed] [Google Scholar]
- 34.Ying S, O’Connor B, Ratoff J, Meng Q, Fang C, Cousins D, Zhang G, Gu S, Gao Z, Shamji B, Edwards MJ, Lee TH, Corrigan CJ. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181:2790–2798. doi: 10.4049/jimmunol.181.4.2790. [DOI] [PubMed] [Google Scholar]
- 35.Ying S, O’Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, Robinson D, Zhang G, Zhao J, Lee TH, Corrigan C. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 36.Prefontaine D, Lajoie-Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, Lemiere C, Martin JG, Hamid Q. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol. 2009;183:5094–5103. doi: 10.4049/jimmunol.0802387. [DOI] [PubMed] [Google Scholar]
- 37.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188:1503–1513. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wenzel SE, Larsen GL, Johnston K, Voelkel NF, Westcott JY. Elevated levels of leukotriene C4 in bronchoalveolar lavage fluid from atopic asthmatics after endobronchial allergen challenge. Am Rev Respir Dis. 1990;142:112–119. doi: 10.1164/ajrccm/142.1.112. [DOI] [PubMed] [Google Scholar]
- 39.Fajt ML, Gelhaus SL, Freeman B, Uvalle CE, Trudeau JB, Holguin F, Wenzel SE. Prostaglandin D(2) pathway upregulation: relation to asthma severity, control, and TH2 inflammation. J Allergy Clin Immunol. 2013;131:1504–1512. doi: 10.1016/j.jaci.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Passalacqua G, Ciprandi G, Canonica GW. The nose-lung interaction in allergic rhinitis and asthma: united airways disease. Curr Opin Allergy Clin Immunol. 2001;1:7–13. doi: 10.1097/01.all.0000010978.62527.4e. [DOI] [PubMed] [Google Scholar]
- 41.Bush RK, Prochnau JJ. Alternaria-induced asthma. J Allergy Clin Immunol. 2004;113:227–234. doi: 10.1016/j.jaci.2003.11.023. [DOI] [PubMed] [Google Scholar]