Abstract
The tolerogenic cytokine IL-9 promotes T regulatory cell function and allergic airway inflammation, but it has not been extensively studied in cancer. In this report, we employed IL-9 deficient mice to investigate the effects of IL-9 in multiple models of breast and colon cancer development. Eliminating endogenous IL-9 enabled sensitization of host T cells to tumors, leading to their early rejection without the requirement of vaccines or immunomodulatory therapies. Notably, IL-9-deficient mice acquired immunologic memory, which actively protected from residual disease and tumor rechallenge, an effect linked to activation of CD8+ T cells. Depletion of either CD8+ or CD4+ T cells abolished the benefits of IL-9 loss to tumor control. Adoptive transfer experiments showed that T cells from tumor-rejecting IL-9-deficient mice retained their effector competency in wild-type animals. Moreover, neutralizing IL-9 antibody phenocopied the effects of IL-9 gene deletion by slowing tumor progression in wild-type animals. Our results show the ability of IL-9 to function as an inhibitor of adaptive immunity that prevents the formation of immunologic memory to a growing tumor, highlighting the potential for IL-9 neutralization as a unique tool for cancer immunotherapy.
Introduction
IL-9 is a paradoxical cytokine, as it mediates both pro-inflammatory events and induction of tolerance. It is secreted by a host of pro-inflammatory immune cells including Th9 cells (1), Th17 cells (2), CD8+ Tc9 cells (3), eosinophils, mast cells, and innate lymphoid cells (1, 4–7). It is also associated with tolerogenic cells such as T regulatory cells (Tregs). In this population IL-9 enhances Treg suppressive potency in an autocrine fashion (8), while promoting T cell tolerance via a paracrine impact upon mast cells (9–11). This wide range of action is followed by an equally wide range of pathologies involving IL-9 secretion.
Most commonly, IL-9 is linked to Th2 responses such as parasite expulsion and allergic airway inflammation, but it is also involved in autoimmunity and graft-versus-host disease (reviewed in (6)). Interestingly, IL-9 can be secreted by cells that promote opposite ends of the immune-spectrum. For example: pro-inflammatory Th17 cells can produce IL-9 and exacerbate experimental autoimmune encephalitis (EAE) (12), whereas IL-9 secreted by Tregs renders them more suppressive and protects against EAE (8). These discrepancies may be explained by the timing of IL-9 secretion in a given pathologic circumstance, and by the range of cells that express the IL-9 receptor (IL9R). These include Tregs, CD4+ T cells, B cells and dendritic cells (expression data from the Immunological Genome Project), as well as CD3+ T cells and CD11b+ Gr1+ cells from tumor-bearing mice.
IL-9 also has seemingly contradictory roles in tumor biology. In many tumors the presence of IL-9 contributes to the establishment of a tolerogenic / immunosuppressive environment, or acts directly to drive tumor growth. For example, IL-9 promotes the proliferation or survival of human lymphoid tumors such as Hodgkin’s lymphoma, acute lymphoblastic leukemia, myeloid leukemia, diffuse large B cell lymphoma and NK T cell lymphoma (13–18). It also promotes the proliferation, migration and adhesion of human lung cancer cells (19). However, IL-9 has the opposite effect on melanoma biology: it slows sub-cutaneous growth of B16F10 as well as reducing B16 seeding in the lungs (20, 21), both groups showed that anti-IL9 opposes this effect. Adoptively transferred IL-9 secreting CD4+ T cells (25% IL-9 positivity) reduce melanoma growth, in a manner that is very similar to the transfer of Th2 polarized T cells (20). In addition in vitro polarized OT-1 CD8+ T cells (Tc9), adoptively transferred to B16-OVA tumor bearing mice, led to tumor clearance (22). However, the authors point out that two weeks after transfer, Tc9 cells loose IL-9 expression and instead, secrete IFNγ, suggesting a repolarization to a Tc1 phenotype, which could explain the efficient tumor clearance. In the B16 tumor model, IL-9 acts on mast cells, and is not T or B cell dependent (20), and also has a direct effect on the lung epithelium, which then recruits dendritic cells (21).
Study of the role of IL-9 in mammary carcinomas is limited to a longitudinal study of soluble factors present in sera of breast cancer patients. Investigators found an increase in serum levels of IL-9 over time in patients that later developed metastatic lesions, suggesting a relationship between IL-9 and tumor progression, or tumor load (23).
In summary, the majority of observations about the role of IL-9 in tumor biology suggest that it has a tolerogenic role. Here we show that IL-9 is a key factor in establishing a permissive growth environment for CT26, a colon carcinoma cell line and two murine breast cancer cells lines: TUBO cells that express Her2/neu (24) and 4T1 cells (25) that resemble aggressive, triple-negative breast cancers.
Material and methods
Mice, cell line and reagents
BALB/c mice were purchased from NCI (Fredrick, MD). IL-9ko mice were originally generated by McKenzie (26). The IL-9ko mice used here are in a BALB/c background (27), and were a gift from Simon P. Hogan Ph.D. (University of Cincinnati College of Medicine). BALB/neuT mice were generated as previously described (24). BALB/neuT/IL-9ko mice were created by breeding heterozygous BALB/neuT mice with IL-9ko mice F1 mice were genotyped for Neu and IL-9ko allele as well as the WT IL-9 allele, using the following primers: (IL-9 genotyping F, gcgattcttcctgaaagcag: IL-9 genotyping R, accggacacgtgatgttctt; NeomycinF, tgtcgatcaggatgatctgg). IL-9+/− /neuT mice were crossed to IL-9ko, and the resulting F2 IL-9ko/neuT mice were crossed to IL-9ko mice to establish the colony. All mice were housed under specific pathogen-free conditions. TUBO cells are derivatives of a spontaneous mammary carcinoma in BALB/neuT mice (24) and were obtained from Dr. Forni (Torino, Italy). 4T1 are a mammary carcinoma line derived from 410.4 mammary tumors. BM 185 cells were derived from bone marrow from an acute lymphoblastic leukemia model, originally provided by D. Kohn (University of Southern California, Los Angeles). CT26 cells were purchase through ATCC. All were maintained in complete RPMI 1640 medium supplemented with 10% FCS, 2 mM glutamine, 5 × 10−5 M 2-ME, and 50 µg/ml gentamicin. Anti-IL-9 mAb (MM9C1) (28) and its isotype control Ab were obtained from Dr. Jacques Van Snick (Ludwig Institute, Brussels, Belgium). Depleting antibodies were obtained as follows: anti-CD4 (clone GK 1.5, BioLegend), anti-CD8α (clone 2.43, LifeSpan BioSciences) antibodies or the corresponding rat IgG isotype control. CD4+ and CD8+ T cells were enriched using negative selection kits (Invitrogen, Carlsbad, CA).
In vivo tumor studies
WT and IL-9ko mice were implanted sub-cutaneously (s.c.) with 1×106 TUBO, 1×106 4T1, or 1×105 CT26 cells. Tumors were measured twice weekly and mice were sacrificed when the tumors reached 1cm2 or showed signs of external necrosis. Tumor volume was calculated from 2 perpendicular measurements using the following formula: [a2×b/2]. For CD4+ and CD8+ T cell depletion experiments, anti-CD4 and –CD8 antibodies and the corresponding isotype control were delivered i.p. at 125ug each. Mice were pretreated 3–4 days before tumor inoculation, and then subsequently once weekly for 4 weeks.
For IL-9 neutralization experiments, WT mice were injected s.c. with 2.5×104 4T1cells and anti-IL-9, isotype control Ab (100 µg each) or left untreated. Abs were delivered to WT and IL-9ko mice through intra-peritoneal (i.p.) injections three times per week for 3 weeks.
ELISpot Assays
Splenocytes or lymph node cells were co-cultured with tumor cells on interferon gamma ELISpot kit plates from Mabtech (cat # 3321-2HW-Plus) for 40 hours following the manufacturers’ instructions. Cell numbers were as follows: 1×105 total splenocytes derived from 4T1 bearing mice with either 5×104 4T1 or TUBO cells, 5×104 total lymph node cells from 4T1 bearing mice with either 2.5×104 4T1 or TUBO cells. CD4+ and CD8+ T cells were enriched from the spleens of naïve and 4T1-bearing WT and IL-9ko mice using a negative isolation kit (Invitrogen, Carlsbad CA). 1×105 T cells were co-cultured with 2.5×104 4T1 cells or with BM185, a non-specific control tumor line as above. Each experimental condition was executed in biological triplicates, which in turn comprised triplicate wells. The plates were imaged and evaluated by ZellNet Consulting, Inc. (Fort Lee New Jersey), and results expressed as average of triplicate spots per condition. Phorbol 12-myristate 13-acetate (PMA) was used as a positive control of cell activation.
Immunohistochemistry
4T1 tumors were harvested from IL-9ko and WT mice, formalin fixed and paraffin embedded. Four micron sections were incubated overnight with or without anti-CD8 alpha antibody (Thermo scientific, MA5-16761) overnight, followed by detection using ImmPRESS™ Reagent Anti Rat IgG, peroxidase (Vector Labs MP-7404) followed by DAB substrate kit (Vector labs SK-4100). Slides were mounted in Permount and visualized with a Leica DMRB microscope. Images were acquired at a magnification of 200× with a numerical aperture of 2, at room temperature with a Olympus DP71 camera using the DPController software (Olympus).
Winn Assays
Total splenocytes were harvested from 4T1 bearing WT, and from IL-9ko mice that had rejected 1×106 4T1 cells 2–3 months prior, and which were rechallenged with 5×105 4T1 cells one week before the start of the assay. Splenocytes were mixed with 2.5×104 4T1 cells and co-injected s.c. into WT mice in the following proportions of splenocytes to 4T1 cells: 100:1, 33:1 and only 4T1. The 100:1 ratio of splenocytes contained the following number of T cells: WT+4T1=3.2 ×105 CD4+ T cells and 1.2 ×105 CD8+ T cells, IL-9ko+4T1= 4.4 ×105 CD4+ T cells and 1.95 ×105 CD8+ T cells. The 33:1 ratio of splenocytes contained the following number of T cells: WT+4T1=1.1 ×105 CD4+ T cells and 4 ×104 CD8+ T cells, IL-9ko+4T1= 1.5 ×105 CD4+ T cells and 6.5 ×104 CD8+ T cells. Mice were monitored twice a week for tumor growth and tumor growth was compared to that of 4T1 cells mixed with splenocytes derived from tumor bearing WT. A repeat experiment was done using negatively enriched CD8+ T from tumor bearing and naïve WT and IL-9ko mice, cells were co-injected with 4T1 cells at a concentration of 25:1 (6.25 ×105 CD8+ T cells: 2.5 ×104 4T1 cells). Each experimental cohort consisted of T cells isolated from three individual mice, and each individual isolate was injected in duplicate, bringing the total per condition to 6.
Tumor seeding in lungs
1×104 4T1 cells in 100uL PBS were injected via the tail vein into WT and IL-9ko mice, and mice were monitored for 18 days. During this time 50ng recombinant IL-9 was injected i.p. 3 times weekly. Mice were sacrificed and lungs inflated with 10% India ink in PBS, and fixed in Feket’s Solution (100mls 70% ethanol, 10mls 10% formalin and 5 mls acetic acid). All tumor foci macroscopically visible through a dissecting microscope were counted.
Statistical Analyses
Statistical significance of data was determined in most cases using the Student’s t-test to evaluate the p value. The log-rank (Mantel-Cox) test was used to evaluate significant differences in survival. For the comparison of differences in growth curves, the tumor size was compared over days 9, 12, and 15 between groups using repeated measures analysis of variance.
Results
TUBO and 4T1 mammary carcinomas are rejected in IL-9ko mice
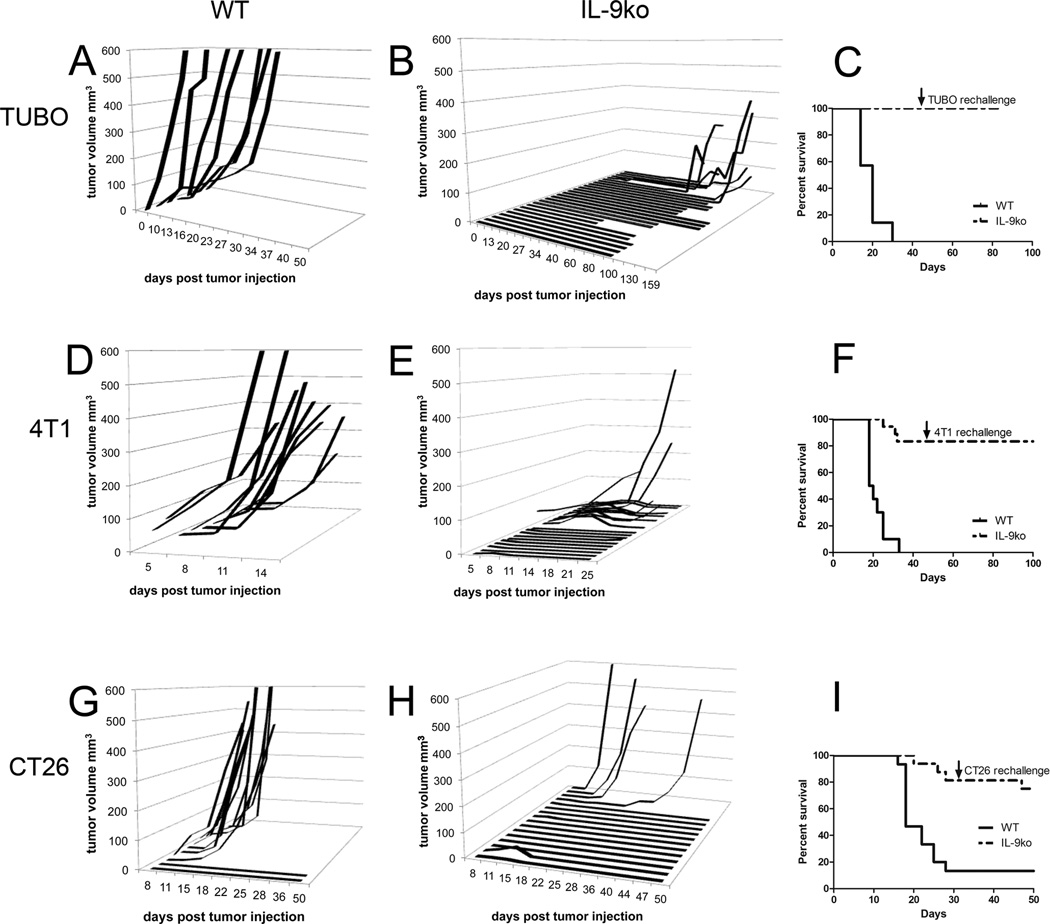
We previously demonstrated that neutralization of IL-9 in conjunction with intra-tumoral CpG-ODN administration led to tumor rejection in two tolerant tumor models (29). We now used IL-9ko mice (26) to further investigate the role of IL-9 in inhibiting anti-tumor immune activity. Injection (s.c.) of TUBO cells in the flank of BALB/c (WT) mice resulted in robust tumor growth within 10 days after tumor delivery (n=7, Figure 1A). Similar s.c. tumor inoculations in IL-9ko mice had a markedly different effect: tumors were rejected in 78% of the IL-9ko mice (21/27 mice) (Figure 1B). In the 6 mice that developed tumors, the average onset of macroscopic tumor growth was delayed to 60 days (p=0.0001, Figure 1B). In addition to significantly delayed tumor onset, we also observed slower growth in IL-9ko mice, resulting in 100% survival 85 days after tumor delivery, compared to 0% survival at day 30 in the WT control group (p<0.0001, Figure 1C). This result was not due to a direct effect of IL-9 on TUBO growth (Supplemental Figure 1A), since TUBO cells do not express the IL-9R or directly secrete IL-9 (supplemental figure 1C and D). To confirm that tumor rejection was based on an immune component and not due to a systemic effect linked to IL-9 deficiency, we repeated the TUBO challenge in 7 of the IL-9ko mice that rejected TUBO two months after the initial challenge (Figure 1C, arrow). All the mice failed to develop tumors, suggesting that these rechallenged mice had developed a memory response to TUBO cells.
Figure 1. TUBO and 4T1 mammary carcinomas are rejected in IL-9ko mice.
Growth of 1×106 TUBO cells implanted s.c. in the flank of (A) WT and (B) IL-9ko mice. (C) Survival plot of TUBO bearing mice, showing 100% of IL-9ko mice surviving after day 85 post tumor injection (p<0.0001). Growth of 1×106 4T1 cells implanted s.c. in the flank of (D) WT and (E) IL-9ko mice. (F) Survival plot of 4T1 bearing mice, showing 75% of IL-9ko mice surviving after day 50 post tumor injection (p<0.0001). Growth of 1×105 CT26 cells implanted s.c. in the flank of (G) WT and (H) IL-9ko mice. (I) Survival plot of CT26 bearing mice, showing 75% of IL-9ko mice surviving after day 50 post tumor injection (p<0.001). Arrows denote the day of rechallenge with 1×106 TUBO or 4T1 cells, or 1×105 CT26 cells (7, 8 and 10 IL-9ko mice respectively). Data for each tumor model are cumulative of a minimum of 2 experiments.
We repeated this experiment with 4T1 cells, and detected palpable tumors 5 days post injection, which grew exponentially in WT mice (n=10, Figure 1D). In contrast, 68% (15/22) of IL-9ko mice failed to develop or rejected tumors (Figure 1E). Of the remaining 32% (7/22) of IL-9ko mice that developed tumors, tumor growth was significantly slower compared to WT mice, and 75% of the IL-9ko mice survived past day 50 post tumor inoculation (p<0.0001, Figure 1F). Again, IL-9 did not promote 4T1 growth (Supplemental Figure 1B), as these cells express very low levels of IL-9R, and do not directly secrete IL-9 (supplemental figure 1C and D). To confirm that 4T1 rejection also elicited a memory response to, we rechallenged 8 of the IL-9ko mice that rejected 4T1 with 4T1 (Figure 1F, arrow). As before, all the mice failed to develop tumors, suggestive of a memory response to 4T1 cells. None of the IL-9ko mice in which original 4T1 tumors progressed showed any evidence of macrometastasis in the lungs, liver or spleen (evaluated 80–100 days post injection), which is routinely observed in WT mice.
To investigate whether the tumor rejection seen in IL-9ko mice was confined to mammary carcinomas, we injected the colon carcinoma cell line CT26 into both WT (n=15) and IL-9ko mice (n=16). CT26 tumors developed in 87% (13/15) of WT mice (Figure 1G), whereas tumors developed in only 25% (4/16) of IL-9ko mice injected (Figure 1H). Once more, the tumors that developed in IL-9ko mice grew slower than those in WT mice, and 75% of IL-9ko mice remained tumor free 30 days after the initial challenge (p=0.001). At this point 10 IL-9ko and 2 WT mice were rechallenged with CT26 (Figure 1I, arrow). No tumor growth was observed in 10 IL-9ko mice and 1 WT mouse (CT26 tumor developed in the second WT mouse), again suggestive of a memory response in IL-9ko mice.
IL-9 deficiency leads to delayed onset of autochthonous mammary tumors in Her2/neu transgenic mice
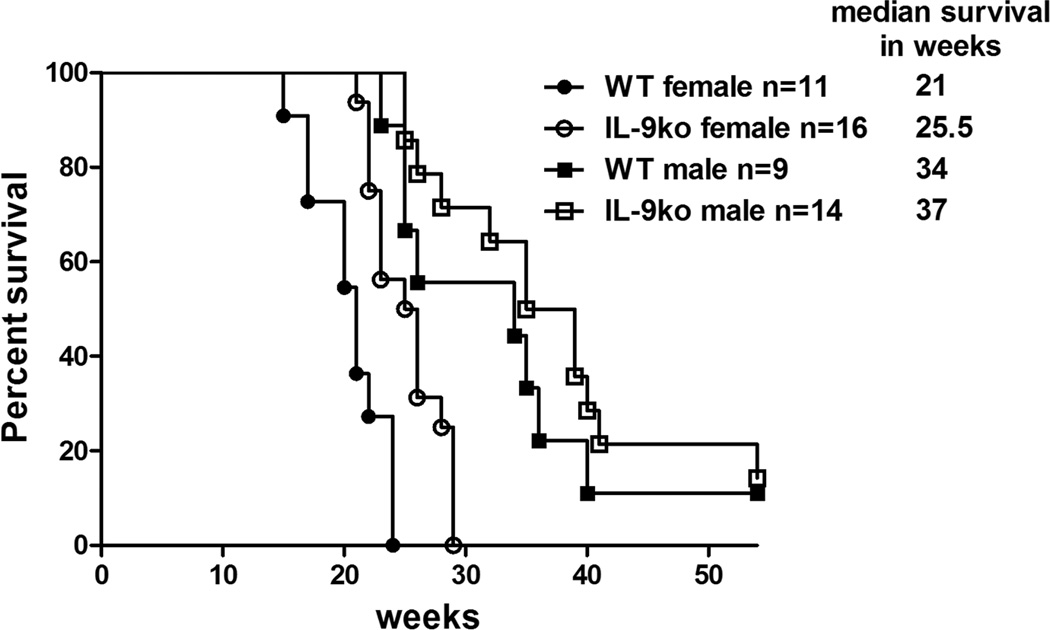
To examine whether IL-9 deficiency also had an effect on growth of autochthonous tumors, we used BALB/neuT mice (24), which develop aggressive, autochthonous mammary tumors in females by 16 weeks of age, and by 24 weeks in males. Double transgenic mice deficient in IL-9 and expressing activated Her2/neu (IL-9ko/Her2/neu) were created by breeding BALB/neuT with IL-9ko mice, and selecting mice that were homozygous IL-9ko and heterozygous for Her2/neu. Both groups of mice were monitored from birth to track tumor onset and growth as compared to that of BALB/neuT mice. Mice were sacrificed when one or more mammary tumors reached 10 mm2, and their life span was recorded in weeks. A survival plot segregating males from females showed a significant increase in survival in Her2/neu transgenic females deficient in IL-9 (p=0.001, Figure 2).
Figure 2. IL-9 deficiency leads to delayed onset of autochthonous mammary tumors in Her2/neu transgenic mice.
Survival plot of WT/Her2-neu (WT) and IL-9ko/Her2-neu mice (IL-9ko) showing that IL-9 deficiency lead to increased survival of female Her2/neu transgenic mice (p=0.001).
T cells are essential for tumor rejection in IL-9ko mice
Evaluation of the immune composition of spleens and lymph nodes of 4T1 bearing WT and IL-9ko mice revealed increased total numbers of CD4+ and CD8+ T cells in IL-9ko mice (Supplemental Figure 2A and B), and a concomitant decrease in numbers of CD11b+Gr1+ cells (p= 0.005, Supplemental Figure 2C). However, closer scrutiny revealed that any difference in total numbers was directly related to tumor size and not to IL-9 status (data not shown). In addition we evaluated Treg cell number, phenotypic characterization and function (Supplementary Figure 3). We found that the percentage of splenic Tregs was significantly higher in 4T1 bearing mice (p<0.001, Supplemental Figure 3A), whereas the percentage of Tregs in tumor draining LN was higher in TUBO bearing IL-9ko mice (p=0.05, Supplemental Figure 3B). This relative increase in Tregs led us to examine Treg–related cellular markers from naïve and 4T1 bearing mice. There were no differences in Treg phenotype between naïve IL-9ko and WT mice. However, fewer Tregs derived from 4T1 bearing IL-9ko mice expressed ITGαE and CTLA-4 and at a lower level of expression than Tregs from WT mice (Supplemental Figure 3C). Moreover, Tregs from IL-9ko mice were less functionally suppressive than their WT counterparts (Supplemental Figure 3D).
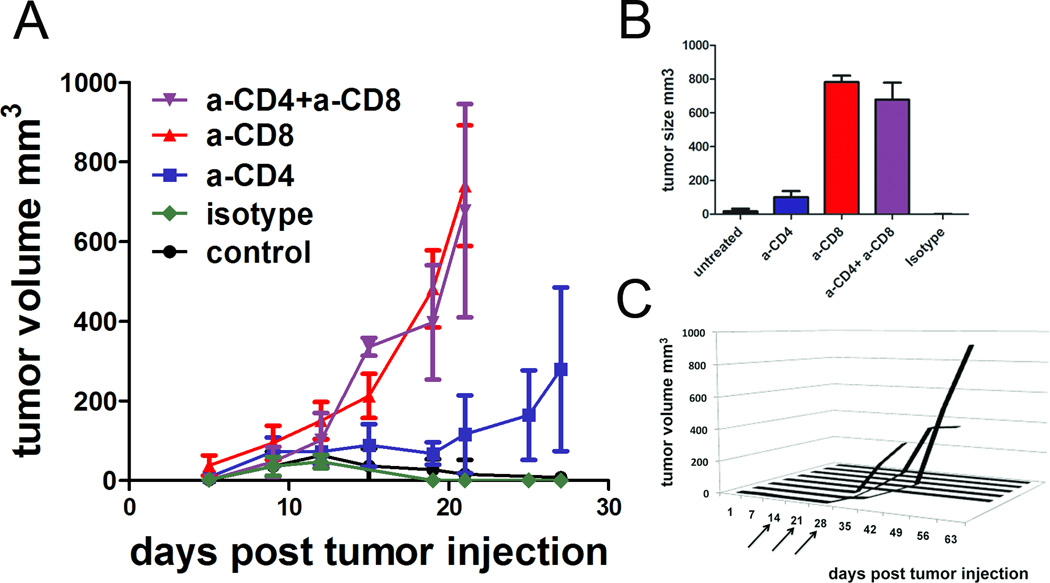
Since we observed that tumor rejection occurred 10–14 days after tumor inoculation (Figure 1E), timing that is reminiscent of an adaptive immune response, and since this resulted in a memory response, we asked whether T cells were involved in tumor rejection in an IL-9 deficient context. To test this, we inoculated IL-9ko mice with 4T1 tumors, and depleted CD4+ and CD8+ T cells with mAb. Cohorts comprised 6–8 mice, and were treated as follows: anti-CD4, anti-CD8, both anti-CD4 and anti-CD8, isotype control antibody or untreated. Growth was monitored over 30 days. IL-9ko mice untreated and treated with isotype control antibody gave evidence of tumor rejection between days 10 and 14 post tumor injection, with rejection completed one week later. In contrast, CD8+ T cell depletion or joint CD4+ and CD8+ T cell depletion resulted in 4T1 tumor growth (Figure 3A) comparable to that observed in WT mice (Figure 1A). Finally, depletion of only CD4+ T cells also prevented 4T1 rejection, but with slower tumor outgrowth than CD8+ depletion. These results demonstrate that in an IL-9 deficient milieu, both CD8+ and CD4+ T cells were involved in tumor eradication, and that neither subset alone was sufficient for cure. Interestingly we found that T cells from tumor bearing mice express high levels of IL9R mRNA (Supplemental Figure 1C). Tumor sizes were tabulated 21 days post tumor injection, and confirmed that CD8+ depletion resulted in large tumors (averaging 700mm3) whereas untreated or isotype treated mice harbored very small tumors (averaging 14 mm3) or no tumors at all (Figure 3B).
Figure 3. CD8+ T cells are essential for tumor rejection in IL-9ko mice.
(A) Growth of 1×106 4T1 cells implanted s.c. in the flank of IL9-ko mice treated with neutralizing antibodies against CD4+, CD8+, CD4+and CD8+ T cells or isotype control. There were 6–8 mice per cohort and data shown are cumulative of two separate experiments. (B) Average tumor sizes in each treatment cohort 21 days post tumor injection. (C) Depletion of CD8+ T cells with anti-CD8 antibody in the IL-9ko mice that rejected 4T1 tumors from 2A. Arrows indicate dosage times once weekly for three weeks.
These results suggest that the presence of IL-9 negatively regulates T cell function within 10–14 days of tumor challenge. The precise source of IL-9 in the tumor microenvironment is yet to be determined. However, both 4T1 and TUBO cells do not transcribe either IL-9 or IL9R mRNA, nor do they secrete IL-9 in vitro, as measured by ELISA using supernatants of tumors generated in IL-9ko mice and cultured for 3 days (Supplemental Figure 1C and D).
To test whether the absence of recurrent tumors in IL-9ko mice challenged with 4T1 reflected active T cell immunosurveillance, we depleted CD8+ T cells in IL-9ko mice (n=8) that had rejected 4T1 cells 21 days prior (Figure 3C, arrows). Tumors grew in 3/8 mice, and no tumor growth was evident in 5/8 mice. These data confirmed that active T cell surveillance was operative in at least a portion of non-recurrences, and raised the possibility that mice without tumor growth following CD8+ T cell depletion might harbor sufficient CD4+ T cell immunologic memory to compensate for the CD8+ T cell depletion, or that the tumor was completely eradicated.
T cells from IL-9ko mice are activated and tumor specific
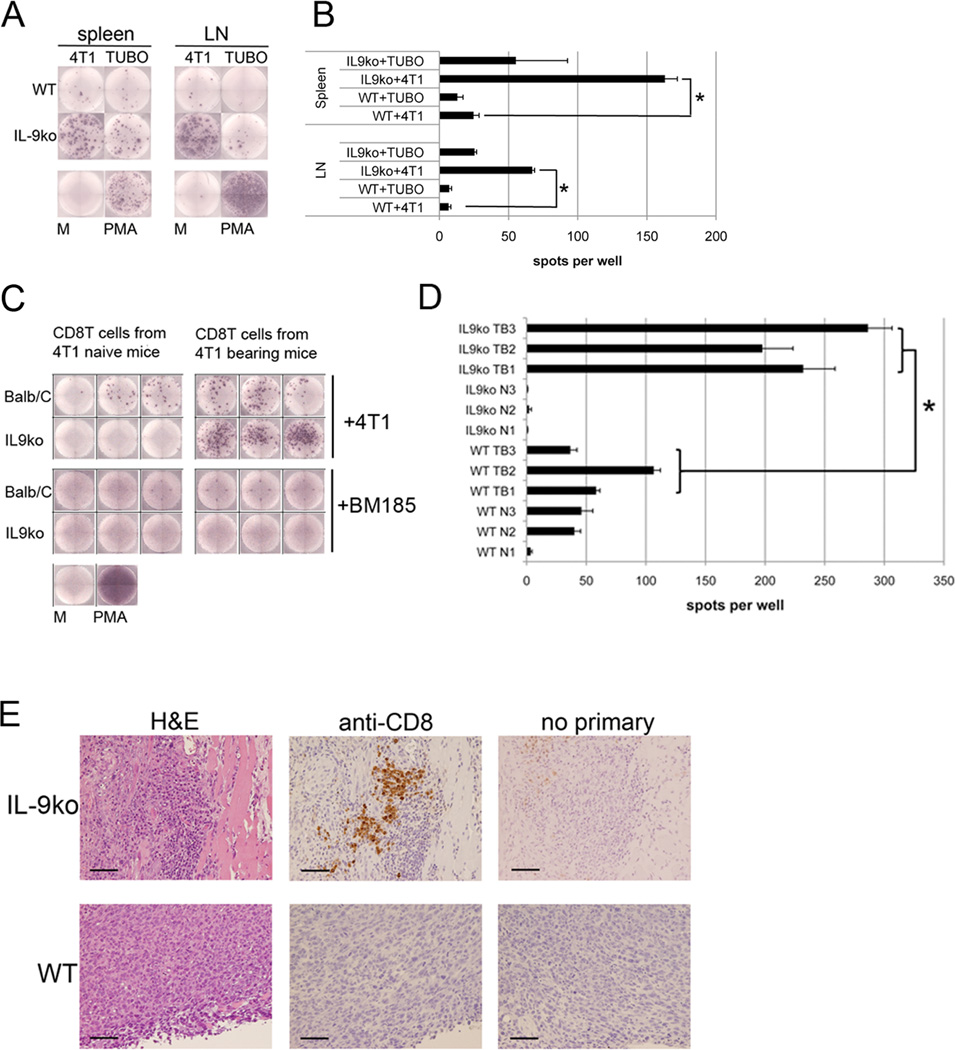
To confirm that tumor rejection was due to the sensitization of a tumor specific immune component, we harvested total splenocytes and lymphocytes (from tumor draining lymph nodes) from 4T1 bearing WT and IL-9ko mice 14 days after tumor injection. These cells were co-cultured with either 4T1 (target tumor) or TUBO (negative control tumor) cells to measure the number of cells that were activated in a tumor specific manner, using the number of IFNγ+ spots as a reporter of activation (Figure 4A). The number of IFNγ+ spots present in WT splenocytes or lymphocytes co-cultured with 4T1 cells was near the levels of negative control (TUBO) tumor. In contrast, IL-9ko derived splenocytes and lymphocytes were activated in a tumor specific manner. Moreover, IL-9ko derived splenocytes (6.5 fold increase) and lymphocytes (9.7 fold increase) showed a significantly higher degree of activation in the presence of 4T1 as compared to WT cells (p=0.001, Figure 4B).
Figure 4. CD8+ T cells are activated in a tumor specific manner and are tumor tropic.
(A) ELISpot analysis measuring IFNγ+ spots derived from total splenocytes (spleen) and lymphocytes (LN) from 4T1 bearing WT and IL-9ko mice. (B) Graph showing the average number of IFNγ+ spots from triplicate wells. *asterisk indicates p=0.001. (C) CD8+ T cells derived from naïve or tumor bearing WT and IL-9ko mice co-cultured with either 4T1 or BM185 cells. Wells shown are representative of triplicate determinations from three biological replicates. (D) Graph showing the average numbers of IFNγ+ spots in each well. Biological replicates are denoted as follows: WT naïve (WT N1-3), WT bearing 4T1 (WT TB1-3), IL-9ko naïve (IL9ko N1-3) and IL-9ko bearing 4T1 (IL9ko TB1-3). The *asterisk represents the significance of the difference in number of spots when comparing CD8+ T cells from tumor bearing WT versus IL-9ko mice (p=0.007). Representative data of duplicate experiments with three mice in each condition. (E) Immunohistochemical evaluation of CD8+ T cells present in 4T1 tumor derived from WT (lower panel) and IL-9ko (upper panel) mice. Each series is comprised of sequential slides to show morphology (H&E), anti-CD8 staining, and a negative control. Images are representative of three tumors from three individual mice in each strain. Bar represents 100 µm. M=media, PMA= Phorbol 12-myristate 13-acetate.
Since we observed that T cell depletion resulted in tumor growth in IL-9ko mice we repeated the ELISpot assay with isolated CD4+ T cells and CD8+ T cells. Total numbers of CD4+ T cells and CD8+ T cells in spleens of naïve and 4T1 tumor bearing mice are shown in Supplemental Figure 2. These cells were co-cultured with 4T1 or BM185 cells, a BALB/c background murine leukemia cell line used here as a negative control (Figure 4C). Again using the number of IFNγ+ spots as a reporter of T cell activation, we found a 3.6 fold increase in the number of activated CD8+ T cells in the population derived from tumor bearing IL-9ko mice as compared to their WT counterparts: an average of 239 of IFNγ+ spots in cells from IL-9ko mice, versus 67 spots in WT cells (p=0.007, Figure 4D). Furthermore, activation of CD8+ T cells was 4T1 specific, since there were no measurable IFNγ+ spots when CD8+ T cells were co-cultured with BM185 cells. Phorbol 12-myristate 13-acetate (PMA) was used as a positive control to confirm that the WT CD8+ T cells were capable of activation. A similar experiment using CD8+ T cells from TUBO bearing mice yielded similar results (data not shown). CD4+ T cells tested under identical conditions produced IFNγ+ spots only if exposed to PMA. The lack of CD4+ T cell activation is consistent with the absence of MHC Class II expressing cells: T cells were cultured with MHC II negative tumor cell lines (verified by FACS, data not shown), in the absence of syngeneic antigen-presenting cells.
CD8+ T cells are found in 4T1 tumors growing in IL-9ko mice, but not in tumors growing in WT mice
Having observed that CD8+ T cells were key effectors in tumor eradication, and that they were activated in a tumor specific manner, we sought to verify the presence of CD8+ T cells in 4T1 tumors that were in the process of being rejected. WT and IL-9ko mice were injected with 4T1 cells, and tumor growth monitored. After 7 days, the tumors in WT mice were robustly growing (an average of 5mm2) (Fig1D), whereas tumors growing in IL-9ko mice were decreasing in size (2–3mm2) (Fig 1E). Tumors were harvested at this point, formalin fixed and paraffin embedded. Staining with anti-CD8 revealed a population of CD8+ cells arranged mostly in groups at the margins of shrinking 4T1 tumors in the IL-9ko mice (Figure 4E). No CD8+ cells were observed in tumors growing in WT mice, even though our ELISpot analyses revealed 4T1-specific CD8+ T cells in the spleens of WT 4T1-bearing mice (Figure 4D).
Splenocytes, or CD8+ T cells from IL-9ko mice that rejected 4T1 tumors, also abrogate 4T1 growth in WT mice
We employed Winn assays to test whether activated splenocytes from IL-9ko mice that had rejected 4T1 tumors were capable of inducing tumor rejection in WT mice. IL-9ko mice that rejected 4T1 tumors were rechallenged with 4T1 cells to boost the levels of memory cells. Total splenocytes were then harvested from the rechallenged mice, and mixed with 4T1 cells prior to injection in the flanks of WT mice. WT mice were segregated into 3 cohorts, which received increasing numbers of splenocytes, holding the number of tumor cells constant: 1) no splenocytes added, 2) 33:1, and 3) 100:1. Splenocytes from 4T1 bearing WT mice were used in the same proportions as a control. Tumor growth was monitored twice weekly, revealing tumor growth in WT mice that received only 4T1, and complete abrogation of tumor growth in all the mice that received IL-9ko activated splenocytes (Figure 5A). Co-injection of 4T1 and splenocytes derived from 4T1 bearing WT mice grew similarly to the mice that received only 4T1. These results show that splenocytes derived from mice that had rejected 4T1 included cells that were capable of eradicating 4T1 tumor cells in a WT context.
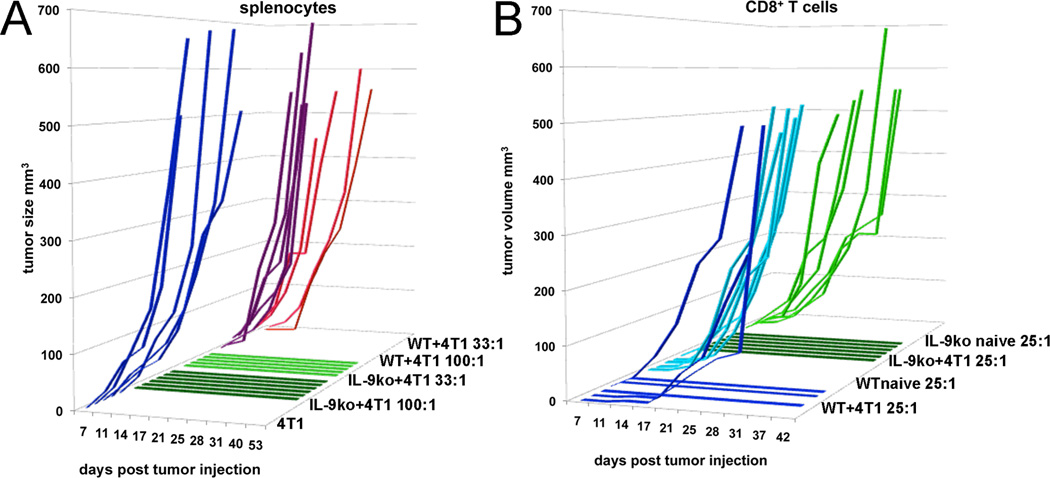
Figure 5. Splenocytes, or CD8+ T cells from IL-9ko mice that rejected 4T1 tumors, also impede 4T1 growth in WT mice.
(A) Splenocytes derived from tumor bearing WT (WT+4T1) or IL-9ko (IL-9ko+4T1) mice were mixed with 4T1 cells and implanted s.c. in the flank of WT mice in the given ratios (100:1 and 33:1), holding the number of 4T1 cells constant at 2.5×104. Each line represents tumor growth in one mouse, and the cohorts are colored as follows: 1) 4T1 only, blue 2) IL-9ko+4T1 100:1, dark green 3) IL-9ko+4T1 33:1, light green 4) WT+4T1 100:1 purple and 5) WT+4T1 33:1, red. Shown is one of two determinations. (B) Enriched CD8+ T cells derived from either naïve or 4T1 tumor bearing, WT or IL-9ko mice were co-injected s.c. into WT mice in a ratio of 25:1. Each cohort contains duplicate mice injected with 4T1 and cells from three individual donor mice. Each line represents tumor growth in one mouse, and the cohort colors define the CD8+ T cell donor sub-sets as follows: 1) WT+4T1, dark blue 2) WT naïve, light blue 3) IL-9ko+4T1, dark green and 4) IL-9ko naïve, light green.
We repeated the experiment with negatively isolated CD8+ T cells to verify our finding that depletion of CD8+ T cells enables tumor growth in IL-9ko mice (Figure 3A). CD8+ T cells were harvested from spleens of the following groups of mice: 1) WT bearing 4T1 tumors, 2) naïve WT, 3) IL-9ko bearing 4T1 tumors, and 4) naïve IL-9ko. CD8+ T cells were mixed with 4T1 cells in a ratio of 25:1 injected into WT mice, and tumor growth was monitored. As observed before (Figure 5A), cells derived from tumor bearing IL-9ko mice prevented 4T1 growth in 6/6 mice. Cells isolated from both IL-9ko and WT naïve mice permitted 4T1 growth. Surprisingly, half of the mice treated with CD8+ T cells from tumor bearing WT mice prevented tumor growth, and half enabled tumor growth. These findings echo our observations from the ELISpot assays (Figure 3C), which show that CD8+ T cells derived from IL-9ko mice have 3.6 fold more tumor reactive cells than their WT equivalents.
Anti-IL-9 treatment results in slowed tumor growth in WT mice
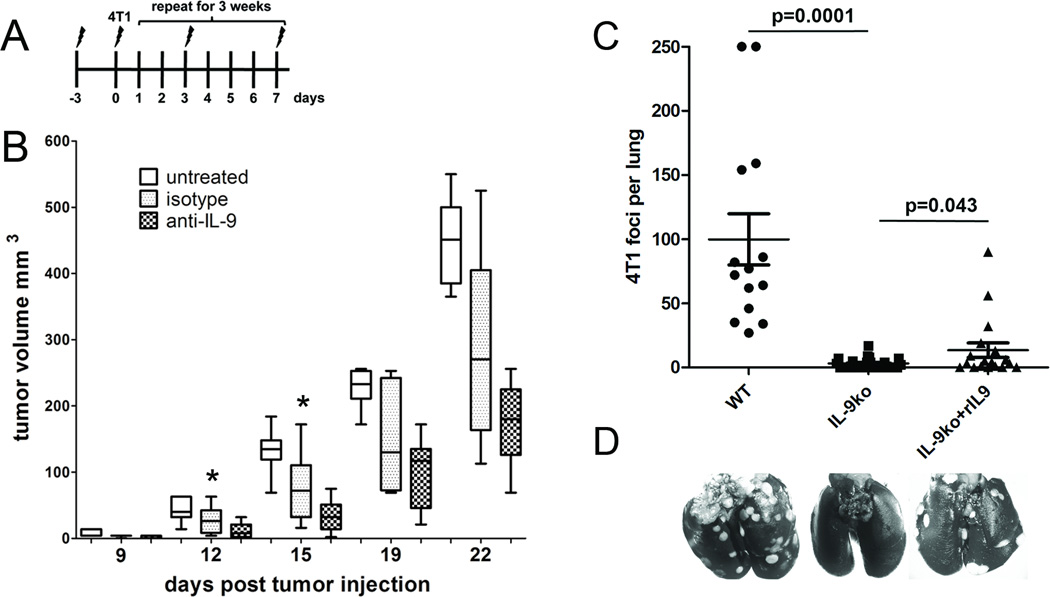
If IL-9 is an important factor in tumor development, then neutralizing IL-9 in WT mice with nascent 4T1 should lead to slowed tumor growth or tumor rejection. We inoculated WT mice with 4T1 cells and separated them into three cohorts: 1) untreated, 2) treated with neutralizing anti-IL-9, and 3) treated with isotype control (Figure 6A) and tumor growth was monitored. Mice treated with anti-IL-9 antibody showed significant delay in tumor growth (days 0–15) as compared to untreated mice (p<0.0001), and also when compared to isotype control antibody (Figure 6B, p=0.03). The difference in growth between anti-IL-9 and untreated remains highly significant throughout the three weeks (p<0.0001). However, due to the high degree of variation in the isotype control cohort there was no measurable significance in 4T1 growth in latter time points.
Figure 6. Anti-IL-9 treatment results in slowed tumor growth in WT mice, whereas addition of recombinant IL-9 increases experimental metastases in IL-9ko mice.
(A) Representation of the treatment schedule to measure the effect of IL-9 depletion on tumor growth. Symbols mark the days of anti-IL-9 injections, twice a week for 3 weeks (3×). 2.5×104 4T1 cells were injected on day 0. (B) Box-whisker plot of tumor growth in 3 cohorts of mice: untreated (white boxes, n=7), isotype control antibody (dotted boxes, n=8) and anti-IL-9 antibody (checkered boxes, n=11). Each box contains a line representing the median, and is bounded by the upper and lower quartiles. Minimum and maximum values are shown as whiskers. The bar frames the period of time (days 0 to 15) during which there is a significant difference in growth between the isotype control and anti-IL-9 treated cohorts (*asterisk, p=0.03). Data are cumulative of two independent experiments. (C) Experimental 4T1 lung metastases in WT and IL-9ko mice. Number of lung metastases per mouse are shown, cross bar indicates the median number of metastases and bounding bars represent upper and lower quartiles. (D) representative images of 4T1 foci in lungs from each treatment group.
IL-9 deficiency prevents the establishment of 4T1 tumor foci in the lung
Since we observed that the few IL-9ko mice that developed 4T1 tumors never had evidence of macro metastases in the lungs, we used an experimental metastasis model to verify whether IL-9 plays a role in 4T1 seeding in the lung. Tail vein injections of 1×105 4T1 cells led to metastatic lesions in WT mice (an average of 87 foci per lung), whereas the majority of IL-9ko mice did not develop visible metastases (an average of 3 foci per lung, p=0.0001) (Figure 6 B and C). Interestingly, addition of recombinant IL-9 led to enhanced 4T1 seeding in the lungs of IL-9ko mice (an average of 13 foci per lung) (p=0.043, Figure 6 B and C).
Discussion
Perhaps the role of IL-9 in tumor biology can be inferred by what is known about its role in Th2 type diseases such as parasitic infections, allergy and asthma. In such cases IL-9 is rapidly, and transiently, expressed by CD4+ T cells (30), CD8+ T cells (31), dendritic cells (32) and innate lymphoid cells (33) in response to activating stimuli. It can therefore be considered a marker of early T cell activation. We hypothesize that upon tumor inoculation, the host immune system is activated and responds by producing IL-9. While several IL-9 secreting cells have been identified in the tumor microenvironment, at present it is unclear which are the first responders. We have observed that in IL-9 deficient mice TUBO, 4T1 and CT26 cells grow and are then resorbed around day 7–10. This leads us to suggest that IL-9 is involved in an immediate, early establishment of a tolerogenic milieu, which hampers the development of an adaptive immune response against a tumor challenge. This is reminiscent of CTLA-4 and PD1, receptors that are expressed on activated T cells, and which, when engaged, act as checkpoints that lead to a dampening of an anti-tumor immune response (34). We hypothesize that the early secretion of IL-9 in the tumor microenvironment may prevent the activation of adaptive immunity, whereas the role of CTLA-4 and PD1 is to curtail an adaptive immune reaction.
The presence of IL-9 does not completely inhibit the formation of tumor specific T cells in WT mice. In our ELISpot assays we observed a modest tumor-specific activation of CD8+ T cells derived from tumor bearing WT mice. In addition, we observed that when total splenocytes derived from tumor bearing WT mice are coinjected with 4T1 cells, all the mice developed tumors, whereas when CD8+ T cells were enriched and coinjected with 4T1, there was a heightened chance of tumor rejection. Therefore the lack of effector function is not due to lack of tumor specific CD8+ T cells in tumor bearing WT mice. Indeed, administration of a higher number of enriched CD8+ T cells leads to tumor rejection even in WT mice. On the other hand, the large proportion of myeloid-derived suppressor cells (MDSCs) present in the spleens of WT 4T1-bearing mice (35) (averaging 40% of MDSCs in a spleen from a 5mm2 tumor) could be the causative factor. MDSCs impede the activation of splenic T cells unless the latter are isolated experimentally. Since IL-9ko mice reject tumors approximately10 days post tumor injection, they do not develop the same immunosuppressive milieu, and therefore tumors are rejected even when total splenocytes are transferred.
As mentioned above, IL-9 and the IL9R are expressed by both innate and adaptive immune cells. We are in the process of identifying the timing and cellular source of IL-9 during a nascent anti-tumor immune reaction, using an IL-9Cre reporter system previously used in the study of airway inflammation (33, 36). However, our work shows that in an IL-9 deficient host, tumor specific CD8+ T cells are generated, and that these are sufficiently activated to be capable of eliminating a tumor challenge in WT mice. Others have shown that in WT hosts, CD8+ T cells can be polarized to secrete IL-9 (31), and that these cells are less cytotoxic in vitro than conventional cytotoxic T cells (CTL) (21, 31), expressing lower levels if IFNγ, granzyme B and perforin. These findings support our observations that more IL-9ko CD8+ T cells express IFNγ and are more cytotoxic that their WT counterparts. However, three discrepancies between our findings and those of others who report that IL-9 promotes anti-tumor activity, remain to be explained. First, addition of recombinant IL-9 increases experimental 4T1 metastasis, whereas Lu et al. show that anti-IL9 increases B16 experimental metastases. Secondly, in our BALB/c based tumor models, tumors are rejected in IL-9 deficient mice; however, Lu et al. found that co-administration of tumor-antigen primed DCs with in vitro polarized Tc9 cells led to tumor shrinkage and cure, which was partially reversed by anti-IL-9 antibody. This anti-tumor activity was exclusively found in tumor models with strong antigen specificity (B16-OVA and MC38-gp100). One explanation was suggested by the authors, who observed that in vivo Tc9 cells re-polarized to a more classical IFNγ expressing CTL profile; these cells were also long lived explaining their enhanced anti-tumor activity (22). Therefore their anti-tumor activity in vivo may not be IL-9 dependent. Most recently Vegran et al. found that TH9 polarized CD4+ T cells, co-administered with IL1β led to slowed tumor growth, reduced tumor foci in the lungs and increased survival in C57BL/6 based tumor models (37). However, in this case also, IL-9 was not the driving factor in tumor rejection, since only IL-21 neutralization reversed the combined anti-tumor effect of TH9 cells + IL1β Taken together, the discrepancy between our findings: who see IL-9 as a tolerizing agent, and those who conclude that IL-9 leads to tumor rejection, may be partly mouse strain dependent. C57BL/6 mice are intrinsically Th1 polarized, whereas BALB/c mice are Th2 polarized (38). Since IL-9 belongs to the overarching Th2 phenotype, its removal may lead to a shift to Th1 profile in BALB/c mice, which could lead to an enhancement of tumor rejection. We are in the process of confirming whether IL-9ko mice in a C57BL/6 also reject tumor challenges
The first step in exploring whether anti-IL-9 treatment can be used as an adjuvant to immunotherapy regimens, is to demonstrate an effect in a WT host. We show here that the administration of anti-IL-9 indeed led to a significant delay in tumor growth of a very aggressive mammary carcinoma. However, we observed that the effect of IL-9 diminished as the tumors increased in size. It is possible that neutralization of IL-9 becomes progressively more difficult as the tumor size increases because there is an increase in IL-9 secreting suppressor cells, as well as poor penetration of the Ab into the tumor. We are actively exploring the pharmacokinetics of anti-IL-9 neutralization as well as other strategies for blocking the IL-9/IL9R signaling axis more efficiently. Interestingly, IL-9 deficiency also plays a role in the development and growth of autochthonous Her2/neu tumors. While the increase in survival is modest, it is significant, especially since this tumor model is extremely aggressive, where female Her2/neu positive mice develop multiple mammary carcinomas by 4 months. Nevertheless we do not know whether this effect is due to the effect of IL-9 on the immune component (for example by reducing the efficiency of immunosurveillance) or the neoplasm itself.
To summarize, we have discovered that IL-9 is involved in the promotion of a tolerogenic environment during the time period that an adaptive immune response against a tumor is primed. IL-9 deficiency leads to a robust tumor rejection, involving a strong sensitization of tumor-specific T cells, which remain operative as effectors rather than tolerized or otherwise subverted. Activated anti-tumor cells can be transferred to WT mice and lead to tumor rejection. Finally, treatment with IL-9 neutralizing Abs significantly slowed the growth of breast cancer cell lines in WT mice. Conversely, addition of recombinant IL-9 increased the number of 4T1 foci in the lungs of IL-9ko mice.
These data demonstrate that among its many other actions, IL-9 inhibits the activation of adaptive anti-tumor immunity. IL-9 ablation enables CD8+ and CD4+ T cells to become promptly sensitized to tumor Ag within the formidable microenvironment of a growing tumor, where only IL-9 is missing, Remarkably, it was unnecessary to perform vaccine or adjunct cytokine maneuvers to achieve this end. Blockade of the IL-9 / IL9R axis is clearly a promising target for potentiation of immunotherapy. Even regional blockade may prove effective for T cell sensitization. Serendipitously, a Phase II clinical trial evaluating humanized IL-9 neutralizing Ab (MEDI–528) for asthma relief has recently ended, revealing no improvement over conventional therapy, but also (and most importantly) no significant side effects (39). We are currently examining the usage of anti-IL-9 as an adjuvant in combinatorial regimens such as anti-IL-9 with chemo- or immunotherapeutic agents.
Supplementary Material
Acknowledgements
This work was supported by the NCI: CA155295-01-03A (SJG), RO1 089846 (PAC) and P50 102701 (PAC, SJG), and the Mayo Foundation. The authors would like to thank Cheryl Myers Ph.D. and Noweeda Mirza Ph.D., for constructive discussions and insight. This work benefited from the technical support provided by Kevin Pollock, the Flow Cytometry Core, the Immunohistochemistry Core, and by Tammy Brehm-Gibson from the Immunology Core. We thank Amylou Dueck for statistical analyses. In addition we thank the Mayo Clinic Natalie Schafer Animal Care attendants for excellent animal care.
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
References
- 1.Stassen M, Schmitt E, Bopp T. From interleukin-9 to T helper 9 cells. Ann N Y Acad Sci. 1247:56–68. doi: 10.1111/j.1749-6632.2011.06351.x. [DOI] [PubMed] [Google Scholar]
- 2.Nowak EC, Noelle RJ. Interleukin-9 as a T helper type 17 cytokine. Immunology. 131:169–173. doi: 10.1111/j.1365-2567.2010.03332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visekruna A, Ritter J, Scholz T, Campos L, Guralnik A, Poncette L, et al. Tc9 cells, a new subset of CD8(+) T cells, support Th2-mediated airway inflammation. Eur J Immunol. 43:606–618. doi: 10.1002/eji.201242825. [DOI] [PubMed] [Google Scholar]
- 4.Goswami R, Kaplan MH. A brief history of IL-9. J Immunol. 186:3283–3288. doi: 10.4049/jimmunol.1003049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jabeen R, Kaplan MH. The symphony of the ninth: the development and function of Th9 cells. Curr Opin Immunol. 24:303–307. doi: 10.1016/j.coi.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noelle RJ, Nowak EC. Cellular sources and immune functions of interleukin-9. Nat Rev Immunol. 10:683–687. doi: 10.1038/nri2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmitt E, Bopp T. Amazing IL-9: revealing a new function for an "old" cytokine. J Clin Invest. 2012;122:3857–3859. doi: 10.1172/JCI65929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elyaman W, Bradshaw EM, Uyttenhove C, Dardalhon V, Awasthi A, Imitola J, et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci U S A. 2009;106:12885–12890. doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eller K, Wolf D, Huber JM, Metz M, Mayer G, McKenzie AN, et al. IL-9 production by regulatory T cells recruits mast cells that are essential for regulatory T cell-induced immune suppression. J Immunol. 186:83–91. doi: 10.4049/jimmunol.1001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 11.Yang Z, Zhang B, Li D, Lv M, Huang C, Shen GX, et al. Mast cells mobilize myeloid-derived suppressor cells and Treg cells in tumor microenvironment via IL-17 pathway in murine hepatocarcinoma model. PLoS ONE. 5:e8922. doi: 10.1371/journal.pone.0008922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nowak EC, Weaver CT, Turner H, Begum-Haque S, Becher B, Schreiner B, et al. IL-9 as a mediator of Th17-driven inflammatory disease. J Exp Med. 2009;206:1653–1660. doi: 10.1084/jem.20090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merz H, Houssiau FA, Orscheschek K, Renauld JC, Fliedner A, Herin M, et al. Interleukin-9 expression in human malignant lymphomas: unique association with Hodgkin's disease and large cell anaplastic lymphoma. Blood. 1991;78:1311–1317. [PubMed] [Google Scholar]
- 14.Lemoli RM, Fortuna A, Tafuri A, Fogli M, Amabile M, Grande A, et al. Interleukin-9 stimulates the proliferation of human myeloid leukemic cells. Blood. 1996;87:3852–3859. [PubMed] [Google Scholar]
- 15.Lemoli RM, Fortuna A, Tafuri A, Grande A, Amabile M, Martinelli G, et al. Interleukin-9 in human myeloid leukemia cells. Leuk Lymphoma. 1997;26:563–573. doi: 10.3109/10428199709050892. [DOI] [PubMed] [Google Scholar]
- 16.Lv X, Feng L, Fang X, Jiang Y, Wang X. Overexpression of IL-9 receptor in diffuse large B-cell lymphoma. Int J Clin Exp Pathol. 2013;6:911–916. [PMC free article] [PubMed] [Google Scholar]
- 17.Lv X, Wang X. The role of interleukin-9 in lymphoma. Leuk Lymphoma. 54:1367–1372. doi: 10.3109/10428194.2012.745072. [DOI] [PubMed] [Google Scholar]
- 18.Nagato T, Kobayashi H, Kishibe K, Takahara M, Ogino T, Ishii H, et al. Expression of interleukin-9 in nasal natural killer/T-cell lymphoma cell lines and patients. Clin Cancer Res. 2005;11:8250–8257. doi: 10.1158/1078-0432.CCR-05-1426. [DOI] [PubMed] [Google Scholar]
- 19.Matsushita K, Arima N, Ohtsubo H, Fujiwara H, Hidaka S, Fukumori J, et al. Frequent expression of interleukin-9 mRNA and infrequent involvement of interleukin-9 in proliferation of primary adult T-cell leukemia cells and HTLV-I infected T-cell lines. Leuk Res. 1997;21:211–216. doi: 10.1016/s0145-2126(96)00109-9. [DOI] [PubMed] [Google Scholar]
- 20.Purwar R, Schlapbach C, Xiao S, Kang HS, Elyaman W, Jiang X, et al. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat Med. doi: 10.1038/nm.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Y, Hong S, Li H, Park J, Hong B, Wang L, et al. Th9 cells promote antitumor immune responses in vivo. J Clin Invest. 2012;122:4160–4171. doi: 10.1172/JCI65459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu Y, Hong B, Li H, Zheng Y, Zhang M, Wang S, et al. Tumor-specific IL-9-producing CD8+ Tc9 cells are superior effector than type-I cytotoxic Tc1 cells for adoptive immunotherapy of cancers. Proc Natl Acad Sci U S A. 2014;111:2265–2270. doi: 10.1073/pnas.1317431111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlsson A, Wingren C, Kristensson M, Rose C, Ferno M, Olsson H, et al. Molecular serum portraits in patients with primary breast cancer predict the development of distant metastases. Proc Natl Acad Sci U S A. 2011;108:14252–14257. doi: 10.1073/pnas.1103125108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rovero S, Amici A, Di Carlo E, Bei R, Nanni P, Quaglino E, et al. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. J Immunol. 2000;165:5133–5142. doi: 10.4049/jimmunol.165.9.5133. [DOI] [PubMed] [Google Scholar]
- 25.Pulaski BA, Ostrand-Rosenberg S. Mouse 4T1 breast tumor model. Curr Protoc Immunol. 2001;Chapter 20(Unit 20):2. doi: 10.1002/0471142735.im2002s39. [DOI] [PubMed] [Google Scholar]
- 26.Townsend JM, Fallon GP, Matthews JD, Smith P, Jolin EH, McKenzie NA. IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity. 2000;13:573–583. doi: 10.1016/s1074-7613(00)00056-x. [DOI] [PubMed] [Google Scholar]
- 27.Forbes EE, Groschwitz K, Abonia JP, Brandt EB, Cohen E, Blanchard C, et al. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J Exp Med. 2008;205:897–913. doi: 10.1084/jem.20071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan WI, Richard M, Akiho H, Blennerhasset PA, Humphreys NE, Grencis RK, et al. Modulation of intestinal muscle contraction by interleukin-9 (IL-9) or IL-9 neutralization: correlation with worm expulsion in murine nematode infections. Infect Immun. 2003;71:2430–2438. doi: 10.1128/IAI.71.5.2430-2438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith SE, Hoelzinger DB, Dominguez AL, Van Snick J, Lustgarten J. Signals through 4-1BB inhibit T regulatory cells by blocking IL-9 production enhancing antitumor responses. Cancer Immunol Immunother. 60:1775–1787. doi: 10.1007/s00262-011-1075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan C, Aziz MK, Lovaas JD, Vistica BP, Shi G, Wawrousek EF, et al. Antigen-specific Th9 cells exhibit uniqueness in their kinetics of cytokine production and short retention at the inflammatory site. J Immunol. 2010;185:6795–6801. doi: 10.4049/jimmunol.1001676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Visekruna A, Ritter J, Scholz T, Campos L, Guralnik A, Poncette L, et al. Tc9 cells, a new subset of CD8(+) T cells, support Th2-mediated airway inflammation. Eur J Immunol. 2013;43:606–618. doi: 10.1002/eji.201242825. [DOI] [PubMed] [Google Scholar]
- 32.Leech MD, Grencis RK. Induction of enhanced immunity to intestinal nematodes using IL-9-producing dendritic cells. J Immunol. 2006;176:2505–2511. doi: 10.4049/jimmunol.176.4.2505. [DOI] [PubMed] [Google Scholar]
- 33.Turner JE, Morrison PJ, Wilhelm C, Wilson M, Ahlfors H, Renauld JC, et al. IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. J Exp Med. 2013;210:2951–2965. doi: 10.1084/jem.20130071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ko JS, Rayman P, Ireland J, Swaidani S, Li G, Bunting KD, et al. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res. 2010;70:3526–3536. doi: 10.1158/0008-5472.CAN-09-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilhelm C, Hirota K, Stieglitz B, Van Snick J, Tolaini M, Lahl K, et al. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol. 2011;12:1071–1077. doi: 10.1038/ni.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vegran F, Berger H, Boidot R, Mignot G, Bruchard M, Dosset M, et al. The transcription factor IRF1 dictates the IL-21-dependent anticancer functions of TH9 cells. Nat Immunol. 2014;15:758–766. doi: 10.1038/ni.2925. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe H, Numata K, Ito T, Takagi K, Matsukawa A. Innate immune response in Th1- and Th2-dominant mouse strains. Shock. 2004;22:460–466. doi: 10.1097/01.shk.0000142249.08135.e9. [DOI] [PubMed] [Google Scholar]
- 39.Oh CK, Leigh R, McLaurin KK, Kim K, Hultquist M, Molfino NA. A randomized, controlled trial to evaluate the effect of an anti-interleukin-9 monoclonal antibody in adults with uncontrolled asthma. Respir Res. 14:93. doi: 10.1186/1465-9921-14-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.