Abstract
Purpose
To evaluate the benefits and complications of periocular depot corticosteroid injections in patients with ocular inflammatory disorders.
Design
Multicenter retrospective cohort study.
Participants
A total of 914 patients (1192 eyes) who had received at least one periocular corticosteroid injection at 5 tertiary uveitis clinics in the United States.
Methods
Patients were identified from the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study. Demographic and clinical characteristics were obtained at every visit via medical record review by trained reviewers.
Main Outcome Measures
Control of inflammation, improvement of visual acuity to 20/40 or better, improvement of visual acuity loss attributed to macular edema, incident cataract affecting visual acuity, cataract surgery, ocular hypertension and glaucoma surgery.
Results
Among 914 patients (1192 eyes) who received at least one periocular injection during follow-up, 286 (31.3%) were classified as having anterior uveitis, 303 (33.3%) as intermediate uveitis, 324 (35.4%) as posterior or panuveitis. Cumulatively by ≤6 months, 72.7% [95% confidence interval (95%CI): 69.1-76.3] of the eyes achieved complete control of inflammation and 49.7% [95%CI:45.5-54.1] showed an improvement in visual acuity (VA) from worse than 20/40 to 20/40 or better. Among the subset with VA worse than 20/40 attributed to macular edema, 33.1% [95%CI: 25.2-42.7] improved to 20/40 or better. By 12 months, the cumulative incidence of one or more visits with an intraocular pressure≥24 mmHg and ≥30 mmHg was 34.0% [95%CI: 24.8-45.4] and 15.0% [95%CI: 11.8-19.1] respectively; glaucoma surgery was performed in 2.4% [95%CI: 1.4-3.9] of eyes. Within 12 months, among phakic eyes initially 20/40 or better, the incidence of a reduction in VA to worse than 20/40 attributed to cataract was 20.2% [95%CI: 15.9-25.6]; cataract surgery was performed within 12 months in 13.8 % [95%CI: 11.1-17.2] of the initially phakic eyes.
Conclusion
Periocular injections were effective in treating active intraocular inflammation and in improving reduced visual acuity attributed to macular edema in a majority of patients. The response pattern was similar across anatomic locations of uveitis. Overall, visual acuity improved in in half of the patients at some point within six months. However, cataract and ocular hypertension occurred in a substantial minority.
Corticosteroids, a mainstay of therapy in ocular inflammation since their initial use in the 1950s, can be administered topically, regionally, or systemically.1 Regional administration of corticosteroids typically is used to include two approaches: periocular injections and intravitreal injections. Periocular injections can be performed using different injection techniques: into the subconjunctival space, into the sub-Tenon's space, into the orbital floor alongside the globe— usually inferiorly—via a transcutaneous or transconjunctival injection, or into the retrobulbar space.2 Sub-Tenon's or orbital floor injections are widely used techniques in the treatment of ocular inflammatory disorders. Regional administration has the advantage of minimizing systemic adverse effects, while maximizing drug delivery to the target tissue. Furthermore, regional corticosteroids injections often are a useful adjunct to systemic treatment for uveitis, when there is persistent or refractory macular edema.3 The use of regional corticosteroids for the treatment of macular edema (ME) or active intraocular inflammation in uveitis is well established and widespread.4 Sub-Tenon's injections can be given repeatedly; typically the effect on active inflammation is observed within days to weeks and improvement in visual acuity or macular edema occurs within weeks to months.4-6 However, corticosteroid-induced elevation of intraocular pressure/glaucoma and cataract are potential complications and can cause significant ocular morbidity.7-9 Although presumed to be less frequent than with intravitreal injections, elevation in IOP with periocular injection has been reported to occur with rates as high as 0.35/eye-year (EY) within 6 months following injection and in rare cases to be persistent8,10. Although regional administration of corticosteroids has been utilized widely since it was described by Nozik in 1972,7 our knowledge of its effects largely has been drawn from case series and cohorts of 125 patients or less.9,10
Here we assess the effectiveness and ocular side effects of periocular corticosteroid injections, and risk factors associated with favorable and unfavorable outcomes, in a large cohort.
METHODS
STUDY POPULATION
Patients who had received at least one periocular corticosteroid injection were identified from an institutional review board (IRB) approved parent study, the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study, the methods of which have been reported previously.11 Briefly, all patients with a diagnosis of non-infectious ocular inflammatory diseases were identified at five ocular immunology and uveitis clinics. Data from each eye with ocular inflammation were abstracted from all clinic visits. The cases reported in this study include all patients with noninfectious uveitis who had received at least one periocular corticosteroid injection at any time during their follow-up, which was between 1979 and 2007. All routes of periocular injection and all forms of corticosteroids were included, although the centers involved typically used the Sub-Tenon's or orbital floor approach to administer triamcinolone acetonide 40 mg during the study period. Of note, 156 eyes of 126 patients treated with periocular corticosteroid injections at a single center that are included in this series have been reported previously.10,12
DATA COLLECTION
During the parent study, trained reviewers reviewed the medical records of all patients and entered information into a custom Microsoft Access database (Microsoft Corporation, Redmond, Washington, USA). Demographic information obtained during the initial visit and details of all medications in use at every clinic visit were recorded. Details of ocular inflammation activity status (based on clinical evaluation using external, slit-lamp, and dilated fundus examination) also were recorded. Sequelae of ocular inflammation were noted when documented in the records.
MAIN OUTCOME MEASURES
All visits, beginning from the 1st periocular injection onward, were included in analyses. Among the available data, both favorable (improvement in visual acuity, evidence of improvement of ME, decrease in ocular inflammation activity status) and adverse (cataract affecting visual acuity, cataract surgery, ocular hypertension, or glaucoma surgery) outcomes were of interest. Concomitant ocular morbidity, intraocular pressure (IOP), and visual acuity were recorded. If the visual acuity was worse than 20/40, the primary reason for this decrease was noted at each visit (e.g., macular edema, cataract). At each visit intraocular inflammation was categorized as “active,” “slightly active,” or “inactive” as described elsewhere previously.13,14 For example, eyes were considered to have “active” inflammation if terms such as “active,” “uncontrolled,” or “worsening inflammation” were used in the medical records.” If terms such as “mild,” “few,” “trace cells,” or “trace activity” were used, inflammation was considered “slightly active”. Inflammation was defined as “inactive” when descriptors such as “quiet,” “quiescent,” “no cells” were used. If the uveitis activity could not be ascertained from the notes, activity was entered as missing. Systemic anti-inflammatory medications in use also were recorded. Because most favorable effects associated with periocular corticosteroid injections occur within 1-6 months before the treatment wears off but ocular side effects occurring later than that were of interest,3 we assessed the cumulative proportion of eyes experiencing favorable and unfavorable outcomes by 6 months and 12 months, respectively, from the time of the first injection.
When visual acuity was worse than 20/40, the SITE protocol required entry of the primary cause of reduced visual acuity. For the purposes of this analysis, macular edema affecting visual acuity was defined as eyes with visual acuity worse than 20/40 where macular edema was recorded as the primary cause of vision loss. Improvement of macular edema in these cases was defined as at least 0.2 logMAR of improvement in visual acuity following periocular corticosteroid injection. Incident macular edema affecting visual acuity was defined, among eyes with visual acuity of 20/40 or better and no macular edema at the time of the first injection, as incidence of visual acuity worse than 20/40 attributed to macular edema. Visually important cataract was defined, among phakic eyes, as incidence of visual acuity worse than 20/40 where cataract was recorded as the cause of vision loss.
STATISTICAL METHODS
SAS version 9.2 (SAS Corporation, Cary, North Carolina, USA) was used for all analyses. The time of first injection is considered “baseline”. The frequencies of demographic and clinical characteristics at the time of the first periocular injection were tabulated. For each outcome of interest, risk calculations only considered eyes that had received a periocular corticosteroid injection and were at risk of that particular outcome. For example, for the outcome “improvement to a visual acuity of 20/40 or better,” only eyes that were worse than 20/40 at baseline were included in analyses, similarly, for the outcome of complete resolution of inflammation eyes at risk were those with any inflammation at baseline. Eyes were censored if participants stopped attending a study clinic or if the end of the data collection period was reached without an observed event. A time-to-event approach was used to quantify the incidence of each favorable or unfavorable outcome using a Kaplan-Meier (product-limit) method. Estimates of cumulative risk for each stratum (by 6 months from baseline or by 12 months from baseline) were based on person-level analyses (only evaluating eyes at risk and taking time-tothe first event if both eyes were at risk) and were tabulated using SAS Proc LIFETEST with the product-limit method. Multivariable comparisons of risk (hazard ratios, confidence intervals, and p-values) adjusted for covariates were done based on all eyes at risk of the outcome of interest for that model, adjusting for clustering of eyes within a person, using SAS Proc PHREG (Cox proportional hazards model). All p-values were 2-sided and nominal.
RESULTS
Nine hundred fourteen patients were identified who received one or more periocular injections in 1,192 eyes with uveitis during follow-up. Their disease characteristics at the time of first injection are given in Table 1. The median age was 37.4 years (range, 0– 84 years); 67.3% were female and 70.6% were white. The mean duration of inflammation prior to the first observed injection was 4.8 years (range, 0-36.3 years). Bilateral uveitis was present in 80% of patients; 11.1% of patients were on systemic corticosteroids or immunosuppressive drugs at the time of the initial injection. The primary site of ocular inflammation was anterior in 286 (31.3%), intermediate in 304 (33.3%), and 324 (35.4%) had posterior or panuveitis. Among injected eyes, 64.4% of eyes had one or more structural complications of uveitis at the time of first injection, macular edema being the most common (46.2%), followed by prior cataract surgery (23.9%).
Table 1.
Patient- and eye-specific characteristics at the time of the first periocular depot corticosteroid injection
| Person-specific characteristics | Anterior uveitis | Intermediate uveitis | Posterior or Panuveitis | Total |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | |
| Number of patients | 286 (31.3%) | 304 (33.3%) | 324 (35.4%) | 914 |
| Age at diagnosis, years (range) | 37.9 (0-84) | 32.4 (4.6-80.5) | 41.6 (2.9-82.7) | 37.4 (0-84) |
| Sex (% male) | 92 (32.2%) | 104 (34.2%) | 103 (31.8%) | 299 (32.7%) |
| Race/ethnicity | ||||
| White | 191 (66.8%) | 241 (79.3%) | 213 (65.7%) | 645 (70.6%) |
| Black | 68 (23.8%) | 31 (10.2%) | 71 (21.9%) | 170 (18.6%) |
| Hispanic/Native American | 7 (2.4%) | 10 (3.3%) | 14 (4.3) | 31 (3.4%) |
| Other | 20 (7.0%) | 22 (7.2%) | 26 (8.0%) | 68 (7.4%) |
| Duration, years (range) | 5.4 (0-33.3) | 4.2 (0-27.3) | 4.9 (0-36.3) | 4.8 (0-36.3) |
| Bilateral uveitis | 199 (69.6%) | 251 (82.6%) | 281 (86.7%) | 731 (80.0%) |
| Systemic Therapy* | ||||
| Systemic corticosteroids | 12 (4.2%) | 25 (9.1%) | 28 (8.9%) | 65 (7.5%) |
| Systemic immunosuppressives | 7 (2.5%) | 10 (3.6%) | 14 (4.5%) | 31 (3.6%) |
| Neither | 263 (93.3%) | 239 (87.2%) | 271 (86.6%) | 773 (89.0%) |
| Eye-specific characteristics | ||||
| Eyes that received ≥1 periocular corticosteroid injection | 359 | 397 | 436 | 1192 |
| Eyes that received ≥2 injections | 154/354 (43.5%) | 211/397 (53.1%) | 224/435 (51.5%) | 589/1186 (49.7%) |
| Presence of any ocular complication** | 225/354 (63.6%) | 269/396 (67.9%) | 267/431 (61.9%) | 761/1181 (64.4%) |
| Ocular hypertension | ||||
| ≥ 24 mm Hg | 28/342 (8.2%) | 24/380 (6.3%) | 33/411 (8.0%) | 85/1133 (7.5%) |
| ≥ 30 mm Hg | 3/342 (0.9%) | 1/380 (0.3%) | 4/411 (1.0%) | 8/1133 (0.7%) |
| IOP≤ 7mmHg | 8/334 (2.4%) | 2/379 (0.5%) | 17/408 (4.2%) | 27/1121 (2.4%) |
| Prior glaucoma surgery | 19/354 (5.4%) | 5/397 (1.2%) | 19/435 (4.4%) | 43/1186 (3.6%) |
| Prior cataract surgery | 118/348 (33.9%) | 45/392 (11.4%) | 115/422 (27.2%) | 278/1162 (23.9%) |
| Cataract causing VA worse than 20/40 | 14/354 (4.0%) | 16/397 (4.0%) | 28/434 (6.4%) | 58/1185 (4.9%) |
| Macular edema | 116/289 (40.1%) | 209/369 (56.6%) | 151/372 (40.6%) | 476/1030 (46.2%) |
| Macular edema causing VA worse than 20/40*** | 68/289 (23.5%) | 103/369 (27.9%) | 80/372 (21.5%) | 251/1030 (24.4%) |
| Inflammatory activity | ||||
| Non-missing total | 353 | 392 | 421 | 1166 |
| Inactive | 71 (20.1%) | 53 (13.5%) | 78 (18.5%) | 202 (17.3%) |
| Slightly active | 30 (8.5%) | 38 (9.7%) | 46 (10.9%) | 114 (9.8%) |
| Active | 252 (71.4%) | 301 (76.8%) | 297 (70.5%) | 850 (72.9%) |
| Visual acuity | ||||
| Non-missing total | 354 | 397 | 434 | 1185 |
| 20/40 or better | 136 (38.4%) | 144 (36.3%) | 119 (27.4%) | 399 (33.7%) |
| 20/50 to 20/200 | 120 (33.9%) | 167 (42.1%) | 169 (38.9%) | 456 (38.5%) |
| 20/200 or worse | 98 (27.7%) | 86 (21.7%) | 146 (33.6%) | 330 (27.8%) |
Systemic therapy at the time of first periocular injection. Systemic immunosuppressives include those using systemic immunosuppressives in combination with systemic corticosteroids (there were only 4 such cases: 2 each in the anterior and intermediate uveitis groups).
In addition to the complications listed subsequently in the table, “any ocular complication” includes band keratopathy, peripheral synechiae, posterior synechiae, hypotony, ocular hypertension or glaucoma, history of cataract or glaucoma surgery, retinal vasculitis, macular edema, epiretinal membrane, exudative retinal detachment, pre-retinal or choroidal neovascularization.
Eyes with visual acuity worse than 20/40 where macular edema was recorded as the primary cause of visual acuity loss at the time of first periocular corticosteroid injection.
Among eyes with macular edema, 50.7% also had visual acuity worse than 20/40 which was primarily a consequence of macular edema (24.4% of all eyes treated with periocular injection). As of the first injection, 2.7% of the eyes previously had undergone glaucoma surgery, 7.5% had ocular hypertension (≥24 mmHg), whereas a low IOP (≤7 mmHg) was present in 2.4%. At the time of first injection, 72.9% of the eyes had active and 9.8% had slightly active inflammation; 66.3% had a visual acuity worse than 20/40. Almost half of the eyes received multiple injections (49.7%) during follow-up.
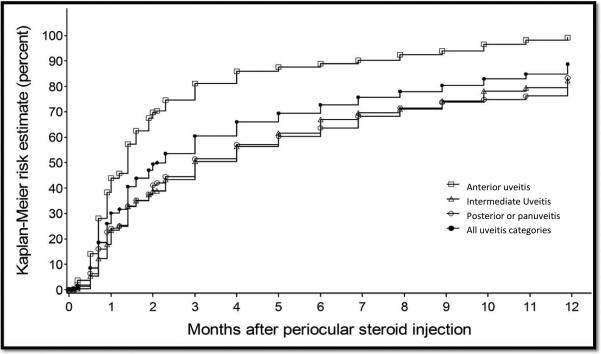
Within 6 months after the first injection, the Kaplan-Meier estimate of the proportion of eyes at risk obtaining complete resolution of inflammation ( “no activity”) was 72.7% [95% CI: 69.1-76.3; see Table 2]. Eyes with anterior uveitis were significantly more likely to gain “no activity” within six months [88.9%, 95% CI: 83.9-92.9], than eyes with intermediate uveitis [66.9%, 95%CI: 60.2-73.5] and eyes with posterior or panuveitis [63.6%, 95% CI: 57.0-70.2]. Considering a lower standard of improvement to either “no activity” or “slightly active” as a success, the estimated proportion controlled within six months was 82.8% overall [95% CI: 79.7-85.7]—95.7% [95% CI: 92.1-98.0] for anterior uveitis, 78.1% [95%CI: 72.2-83.6] for intermediate uveitis, and 75.9% [95%CI: 70.0-81.3] for posterior or panuveitis.
Table 2.
Outcomes of periocular corticosteroid injections*
| Anterior uveitis | Intermediate uveitis | Posterior or Panuveitis | Total | |
|---|---|---|---|---|
| Treatment outcomes | Events per eye-year [95% CI] Cumulative risk % [95% CI] |
Events per eye-year [95% CI] Cumulative risk % [95% CI] |
Events per eye-year [95% CI] Cumulative risk % [95% CI] |
Events per eye-year [95% CI] Cumulative risk % [95% CI] |
| Inflammation (within ≤6 months) | ||||
| Resolution of inflammation (“no activity”) | 1.36 [1.21-1.56] 88.9 [83.9-92.9] |
0.96 [0.83-1.11] 66.9 [60.2-73.5] |
0.90 [0.80-1.05] 63.6 [57.0-70.2] |
1.06 [0.97-1.14] 72.7 [69.1-76.3] |
| Improvement to “no activity” or slightly active” | 1.43[1.25-1.61] 95.7 [92.1-98.0] |
1.14 [0.97-1.27] 78.1 [72.2-83.6] |
1.05 [0.92-1.19] 75.9 [70.0-81.3] |
1.19 [1.11-1.29] 82.8 [79.7-85.7] |
| Visual acuity (within ≤6 months) | ||||
| Improvement to 20/40 or better | 0.86 [0.71-1.02] 50.4 [42.8-58.4] |
0.77 [0.64-0.92] 55.7[48.1-63.7] |
0.59 [0.49-0.70] 44.6 [38.1-51.7] |
0.72 [0.64-0.78] 49.7 [45.5-54.1] |
| Macular edema affecting VA** (within ≤6 months) | ||||
| Improved | 0.53 [0.28-0.93] 29.4 [15.4-51.5] |
0.60 [0.38-0.89] 34.7 [23.2-49.8] |
0.57 [0.33-0.91] 34.3 [21.6-51.7] |
0.57 [0.43-0.75] 33.1 [25.2-42.7] |
| Incident | 0.19 [0.10-0.33] 14.8 [10.1-21.5] |
0.28 [0.18-0.42] 20.7 [15.1-28.0] |
0.28 [0.19-0.40] 17.0 [12.3-23.2] |
0.26 [0.20-0.33] 17.6 [14.5-21.3] |
| IOP elevation (within ≤12 months)* | ||||
| Increase to ≥24 mmHg | 0.29 [0.22-0.38] 67.9 [28.2-97.9] |
0.25 [0.18-0.32] 25.6 [19.2-33.6] |
0.26 [0.20-0.33] 27.3 [20.8-35.4] |
0.26 [0.23-0.31] 34.0 [24.8-45.4] |
| Increase to ≥30 mmHg | 0.14 [0.09-0.20] 16.4[10.4-25.2] |
0.12 [0.08-0.17] 14.5 [9.6-21.5] |
0.13 [0.09-0.18] 14.5 [9.7-21.3] |
0.13 [0.10-0.16] 15.0 [11.8-19.1] |
| Incident glaucoma surgery (within ≤12 months) | 0.03 [0.01-0.06] 3.1 [1.4-6.9] |
0.03 [0.01-0.06] 2.9 [1.3-6.3] |
0.01 [0.002-0.03] 1.3 [0.4-4.0] |
0.02 [0.01-0.04] 2.4 [1.4-3.9] |
| Incident cataract causing VA worse than 20/40 (within ≤12 months) | 0.14 [0.09-0.20] 21.4 [13.6-32.7] |
0.14 [0.09-0.19] 21.2 [13.7-32.2] |
0.14 [0.10-0.19] 18.6 [12.5-27.0] |
0.14 [0.11-0.17] 20.2 [15.9-25.6 |
| Incident cataract surgery (within ≤12 months) | 0.18 [0.12-0.26] 18.8 [13.2-26.3] |
0.07 [0.04-0.11] 7.2 [4.3-11.9] |
0.15 [0.11-0.22] 17.3[12.2-24.1] |
0.13 [0.10-0.16] 13.8[11.1-17.2] |
Cumulative incidences for efficacy related outcomes, such as resolution of inflammation, improvement in macular edema affecting VA or improvement in visual acuity, are evaluated at 6 months; cumulative incidences for adverse effect related outcomes such as IOP elevation, glaucoma, cataract and cataract surgery are evaluated at 12 months. Results are calculated among all eligible eyes (with at least 1 periocular injection) that are at risk of each respective outcome. For example, for the outcome improvement to visual acuity equal or better than 20/40, only eyes that were worse than 20/40 at baseline were included in analyses for this outcome. Events per eye-year are calculated by taking the number of eyes with the event in the defined time period and dividing by the total eye-years at risk for that time period.
Macular edema affecting VA is defined as eyes with macular edema and VA worse than 20/40 where the vision loss was attributed to macular edema. Resolution in such cases is defined as ≥0.2 logMAR improvement in VA. Incident macular edema is defined as eyes with VA of 20/40 or better and no macular edema at the time of first injection that developed visual acuity worse than 20/40 with macular edema recorded as the cause of vision loss.
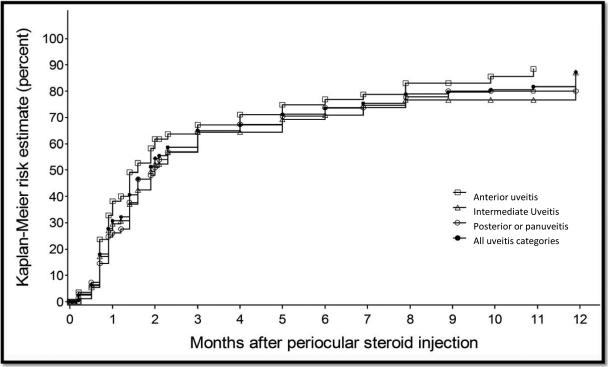
Visual acuity improved to 20/40 or better in 49.7% [95% CI: 45.5-54.1] of all eyes initially worse than 20/40. The visual outcome within six months was similar across sites of uveitis (50.4% [95% CI: 42.8-58.4] for anterior uveitis, 55.7% [95%CI: 48.1-63.7] for intermediate uveitis, and 44.6% [95%CI: 38.1-51.7] for posterior or panuveitis). In the subset of eyes with reduced visual acuity attributed to macular edema, 33.1% [95%CI: 25.2-42.7] experienced at least 0.2 logMAR improvement (“improved macular edema affecting visual acuity”). In contrast, among eyes initially free of macular edema and visual acuity 20/40 or better, incident macular edema (macular edema causing visual acuity worse than 20/40) within the first 6 months was noted in 17.6% of eyes at risk [95% CI:14.5-21.3].
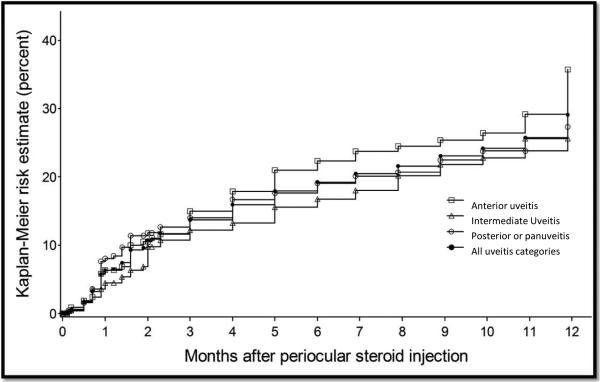
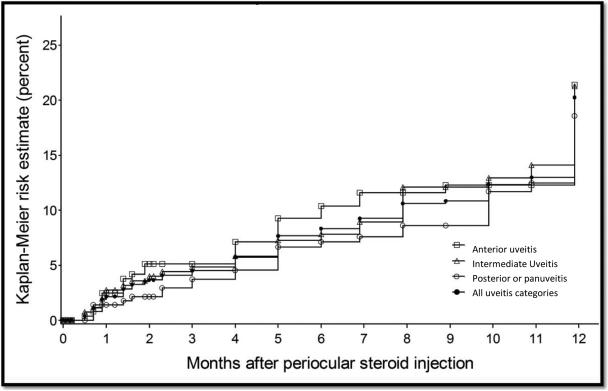
Within 12 months following the initial injection, intraocular pressure elevation to 24 mmHg or above and to 30 mmHg or above had been observed during at least one visit in 34.0% [95% CI: 24.8-45.4] and in 15.0% [95% CI: 11.8-19.1] of eyes at risk, respectively. Glaucoma surgery occurred in 2.4% [95% CI: 1.4-3.9] of eyes (Table 2) within 12 months. An incident reduction in visual acuity to worse than 20/40 attributed to cataract occurred in 20.2% [95% CI: 15.9-25.6], and cataract surgery occurred in 13.8% of eyes at risk [95% CI: 11.1-17.2] within the first 12 months (Table 2).
Cox proportional hazards models were constructed to evaluate the beneficial and adverse outcomes of interest, adjusting for race/ethnicity, sex, age at diagnosis, site of uveitis, duration of uveitis at first periocular injection, number of periocular injections, and the use of systemic anti-inflammatory medications (see Tables 3 and 4). Adjusting for other factors, eyes with intermediate uveitis and posterior or panuveitis were significantly less likely to show resolution of inflammation (improvement to “no activity”) than eyes with anterior uveitis (aHR=0.44, 95% CI, 0.35-0.55 for intermediate uveitis; aHR=0.44, 95% CI, 0.35-0.54 for posterior and panuveitis). Longer duration of uveitis also was associated with a lower likelihood of improvement in inflammation, with a 3% decrease in odds for improvement per each additional year since uveitis diagnosis (aHR: 0.97; 95% CI, 0.95-0.99; p=0.005). Other factors, including the number of injections received, were not associated with differences in time-to-resolution of inflammation.
Table 3.
Factors associated with favorable outcomes following periocular corticosteroid injection*
| Outcome* | Resolution of Inflammation | Improvement in VA (20/40 or better) | Improvement in ME affecting VA | ||||
|---|---|---|---|---|---|---|---|
| Covariate | Description | Adjusted HR (95% CI) | p | Adjusted HR (95% CI) | p | Adjusted HR (95% CI) | p |
| Site of uveitis | Anterior | 1.0 | - | 1.0 | - | 1.0 | |
| Intermediate | 0.44 (0.35-0.55) | <.0001 | 0.74 (0.53-1.04) | 0.08 | 0.70 (0.40-1.24) | 0.23 | |
| Posterior or panuveitis | 0.44 (0.35-0.54) | <.0001 | 0.60 (0.44-0.81) | 0.001 | 0.57 (0.30-1.08) | 0.08 | |
| Race | White | 1.0 | - | 1.0 | - | 1.0 | - |
| African-American | 1.07 (0.85-1.34) | 0.58 | 0.77 (0.54-1.09) | 0.14 | 0.98 (0.54-1.80) | 0.96 | |
| Hispanic/Native American | 1.01 (0.58-1.76) | 0.97 | 0.52 (0.28-0.98) | 0.04 | 1.32 (0.68-2.58) | 0.41 | |
| Other | 0.69 (0.44-1.07) | 0.10 | 0.60 (0.33-1.09) | 0.10 | 1.88 (1.10-3.20) | 0.02 | |
| Unknown | 0.70 (0.37-1.30) | 0.26 | 0.14 (0.03-0.70) | 0.02 | 0.53 (0.20-1.41) | 0.20 | |
| Sex | Male | 1.0 | - | 1.0 | - | 1.0 | - |
| Female | 0.94 (0.77-1.14) | 0.51 | 0.80 (0.61-1.05) | 0.11 | 0.75 (0.45-1.24) | 0.26 | |
| Age, years | <20 | 1.0 | - | 1.0 | - | 1.0 | - |
| 20 - <50 | 0.96 (0.76-1.21) | 0.73 | 0.86 (0.61-1.21) | 0.39 | 0.95 (0.48-1.90) | 0.88 | |
| 50+ | 0.94 (0.71-1.25) | 0.67 | 0.50 (0.32-0.78) | 0.002 | 1.09 (0.46-2.57) | 0.85 | |
| Uveitis duration** | Per year | 0.97 (0.95-0.99) | 0.005 | 0.95 (0.92-0.98) | 0.0005 | 0.98 (0.94-1.02) | 0.39 |
| Systemic therapy*** | Neither | 1.0 | - | 1.0 | - | 1.0 | - |
| Systemic corticosteroids only | 0.90 (0.65-1.24) | 0.50 | 0.77 (0.41-1.44) | 0.41 | 0.82 (0.37-1.83) | 0.64 | |
| Immunosuppressives +/− systemic corticosteroids | 0.72 (0.43-1.21) | 0.21 | 0.35 (0.14-0.84) | 0.02 | 0.63 (0.20-1.97) | 0.43 | |
| Number of injections | 1 | 1.0 | - | 1.0 | - | 1.0 | - |
| 2 or 3 | 0.91 (0.76-1.10) | 0.33 | 0.91(0.69-1.20) | 0.52 | 1.33 (0.76-2.32) | 0.32 | |
| 4 or more | 0.94 (0.74-1.18) | 0.59 | 0.89 (0.64-1.24) | 0.50 | 1.60 (0.84-3.07) | 0.16 | |
Multivariable survival analyses (Cox proportional hazards model) for favorable outcomes. For all categorical variables, the first row represents the reference category.
Outcome definitions: Resolution of inflammation= improvement from “active” or “slightly active” inflammation to “no activity.” Improvement in visual acuity (VA)= Eyes that showed improvement from worse than 20/40 VA to 20/40 or better VA. Improvement of macular edema (ME) affecting VA= ME affecting VA was defined as eyes with VA worse than 20/40 where ME was recorded as the primary cause of vision loss at the time of first periocular corticosteroid injection. Improvement in ME in these cases was defined as at least 0.2 logMAR of improvement in VA following periocular corticosteroid injection.
Uveitis Duration of uveitis reflects duration at the time of first periocular injection.
Systemic Therapy: Systemic therapy reflects the use of systemic corticosteroids or immunosuppressive drugs at the time of or prior to the first injection.
Systemic corticosteroids includes any systemic corticosteroid use with no concomitant immunosuppressive therapy use (n=215); immunosuppressive drugs include all immunosuppressive drugs listed in table 1 as well as cases where immunosuppressives were used in combination with oral corticosteroids (n=263).
HR=hazard ratio; CI=confidence interval.
Table 4.
Factors associated with unfavorable outcomes following periocular corticosteroid injection*
| Outcome | IOP increase to ≥30mmHg | Glaucoma surgery | Cataract surgery | Incident cataract affecting VA | Incident ME affecting VA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Covariate | Description | Adjusted HR (95% CI) | p | Adjusted HR (95% CI) | p | Adjusted HR (95% CI) | p | Adjusted HR (95% CI) | p | Adjusted HR (95% CI) | p |
| Site of uveitis | Anterior | 1.0 | - | 1.0 | - | 1.0 | - | 1.0 | - | 1.0 | - |
| Intermediate | 0.84 (0.44-15.8) | 0.58 | 0.68 (0.29-1.62) | 0.39 | 0.54 (0.34-0.86) | 0.009 | 0.95 (0.53-1.72) | 0.87 | 1.33 (0.63-2.82) | 0.45 | |
| Posterior or panuveitis | 0.87 (0.47-1.61) | 0.66 | 0.58 (0.25-1.34) | 0.20 | 0.95 (0.63-1.45) | 0.82 | 0.82 (0.49-1.37) | 0.46 | 1.23 (0.60-2.50) | 0.58 | |
| Race | White | 1.0 | - | 1.0 | - | 1.0 | - | 1.0 | - | 1.0 | - |
| African-American | 1.28 (0.71-2.33) | 0.41 | 1.30 (0.54-3.16) | 0.56 | 1.74 (1.10-2.76) | 0.02 | 1.40 (0.82-2.39) | 0.22 | 1.03 (0.55-1.94) | 0.92 | |
| Hispanic or Native American | 0.42 (0.06-3.08) | 0.39 | 1.64 (0.38-7.05) | 0.51 | 0.79 (0.31-1.99) | 0.61 | 3.12 (1.36-7.13) | .007 | 1.40 (0.42-4.73) | 0.59 | |
| Other | n/a | -- | n/a | -- | 1.90 (0.95-3.80) | 0.07 | 1.04 (0.30-3.53) | 0.95 | 0.81 (0.16-4.18) | 0.80 | |
| Unknown | 1.00 (1.16-6.21) | 0.99 | 1.85 (0.24-14.10) | 0.55 | 1.50 (0.38-5.94) | 0.56 | n/a | n/a | 5.29 (1.74-16.05) | 0.003 | |
| Sex | Male | 1.0 | - | 1.0 | - | 1.0 | - | 1.0 | - | 1.0 | - |
| Female | 0.90 (0.52-1.55) | 0.71 | 0.91 (0.45-1.83) | 0.78 | 0.89 (0.62-1.27) | 0.52 | 0.66 (0.42-1.04) | 0.08 | 0.89 (0.52-1.50) | 0.65 | |
| Age, years | <20 | 1.0 | - | 1.0 | - | 1.0 | - | 1.0 | - | 1.0 | - |
| 20 - <50 | 0.56 (0.29-1.07) | 0.08 | 0.76 (0.31-1.89) | 0.56 | 1.96 (1.10-3.51) | 0.02 | 0.66 (0.35-1.24) | 0.20 | 1.50 (0.67-3.35) | 0.33 | |
| 50+ | 0.59 (0.26-1.32) | 0.20 | 0.61 (0.18-2.07) | 0.43 | 2.77 (1.45-5.29) | 0.002 | 1.48 (0.75-2.90) | 0.26 | 2.28 (0.88-5.92) | 0.09 | |
| Uveitis duration** | Per year | 0.94 (0.89-0.99) | 0.02 | 1.00 (0.95-1.04) | 0.89 | 1.01 (0.98-1.05) | 0.52 | 0.96 (0.92-1.00) | 0.08 | 0.97 (0.91-1.03) | 0.34 |
| Systemic therapy*** | Neither | 1.0 | - | 1.0 | - | 1.0 | - | 1.0 | - | 1.0 | - |
| Systemic corticosteroids only | 0.82 (0.29-2.31) | 0.71 | 1.96 (0.60-6.36) | 0.26 | 1.70 (0.89-3.23) | 0.10 | 1.52 (0.79-2.94) | 0.21 | 0.71 (0.27-1.89) | 0.49 | |
| Immunosuppressives +/− systemic corticosteroids | 0.37 (0.05-2.68) | 0.33 | 0.87 (0.14-5.37) | 0.88 | 1.81 (1.01-3.22) | 0.04 | 1.70 (0.73-3.97) | 0.22 | 0.58 (0.15-2.28) | 0.44 | |
| Number of injections | 1 | 1.0 | - | 1.0 | - | 1.0 | - | 1.0 | - | 1.0 | - |
| 2 or 3 | 0.70 (0.40-1.24) | 0.22 | 1.24 (0.58-2.64) | 0.59 | 1.66 (1.05-2.62) | 0.03 | 1.61 (0.93-2.78) | 0.09 | 1.44 (0.77-2.70) | 0.26 | |
| 4 or more | 0.53 (0.28-1.00) | 0.05 | 0.77 (0.31-1.91) | 0.57 | 2.05 (1.32-3.17) | 0.001 | 2.12 (1.22-3.68) | 0.008 | 2.77 (1.50-5.09) | 0.001 | |
Multivariable survival analyses (Cox proportional hazards model) for unfavorable outcomes. For all categorical variables, the first row represents the reference category.
Outcome definitions: IOP increase to ≥30mmHg= Eyes that had less than 30 mmHg at the time of first periocular corticosteroid injection and developed IOP elevation to≥30mmHg following periocular corticosteroid injection. Glaucoma surgery= Incident glaucoma surgery. Cataract surgery= incident cataract surgery excluding the first 30 days following the first periocular corticosteroid injection (where prevention of post-cataract surgery inflammation likely was the indication for treatment). Incident cataract affecting VA= eyes with visual acuity worse than 20/40 where cataract was recorded as the cause of vision loss. Incident ME affecting vision= eyes with visual acuity 20/40 or better and no macular edema at the time of first injection that developed visual acuity less than 20/40 and macular edema recorded as the cause of vision loss.
Uveitis Duration of uveitis reflects duration at the time of first periocular injection.
Systemic Therapy: Systemic therapy reflects the use of systemic corticosteroids or immunosuppressive drugs at the time of or prior to the first injection.
Systemic corticosteroids includes any systemic corticosteroid use with no concomitant immunosuppressive therapy use (n=215); immunosuppressive drugs include all immunosuppressive drugs listed in table 1 as well as cases where immunosuppressives were used in combination with oral corticosteroids (n=263).
HR=hazard ratio; CI=confidence interval.
Compared to eyes with anterior uveitis, improvement in visual acuity to 20/40 or better was less likely to occur in eyes with posterior or panuveitis (aHR: 0.60; 95%CI, 0.44-0.81). Eyes with a longer duration of uveitis (aHR: 0.95 (per year); 95% CI, 0.92-0.98), eyes of patients ages 50 years or older compared to <20 years (aHR: 0.50; 95% CI, 0.32-0.78) and eyes of Hispanic or Native American patients (aHR: 0.52; 95% CI, 0.28-0.98), or an unknown race/ethnicity (aHR=0.14, 95%CI, 0.03-0.70) compared to eyes of white patients had a lower incidence of improvement in visual acuity to 20/40 or better. Eyes of patients who received systemic immunosuppressives during follow-up (aHR: 0.35; 95% CI: 0.14-0.84) also had less improvement in visual acuity, but use of systemic corticosteroids during follow-up without immunosuppressives was not significantly associated with greater or less incidence of visual improvement than use of neither (aHR: 0.77; 95% CI: 0.41-1.44).
Macular edema affecting VA improved to a similar degree independent of the site of ocular inflammation. Macular edema affecting VA was more likely to improve among eyes of patients of other race compared to White (aHR: 1.88; 95% CI, 1.10-3.20; p=0.02) but otherwise the incidence of improvement did not differ significantly across the variables studied. Incident macular edema responsible for decreased visual acuity to a level worse than 20/40 was more likely to occur in eyes of patients of unknown race compared to whites (aHR: 5.29; 95% CI: 1.74-16.05; p=0.003) and in eyes receiving multiple injections than in eyes receiving a single injection (aHR: 2.77; 95% CI, 1.50-5.09; p=0.001 for ≥4 injections vs. 1 injection; aHR: 1.44, 95% CI: 0.77-2.70; p=0.26 for 2-3 vs 1 injection). None of the other factors studied were associated with incident macular edema affecting vision.
Regarding potential adverse effects of depot corticosteroid injection therapy (see Table 4), an increase in IOP to either ≥24 mmHg (aHR: 0.95/year, 95% CI, 0.92-0.99) or ≥30mmHg (aHR: 0.94/year; 95% CI, 0.89-0.99; p=0.02) was less likely to occur when the duration of uveitis prior to injection was longer. Additionally, an increase in IOP to ≥30mmHg was marginally less likely to occur in eyes receiving 4 or more with respect to 1 injection (aHR: 0.53; 95% CI, 0.28-1.00), whereas eyes receiving 2 or 3 injections (aHR: 0.70, 95% CI, 0.40-1.24) did not have a significantly different risk of this event. The risk of IOP elevation did not differ substantially by site of inflammation or other factors. With the available statistical power for this rare event, the incidence of glaucoma surgery was not significantly associated with any of variables studied. Eyes of Hispanic or Native American patients compared to eyes of white patients (aHR: 3.12; 95% CI: 1.36-7.13) and eyes receiving 4 or more injections with respect to eyes receiving 1 injection (aHR: 2.12; 95% CI: 1.22-3.68) were more likely to develop a visual acuity worse than 20/40 that was attributed to cataract; however eyes receiving 2 or 3 injections did not show a significantly different risk (aHR: 1.61, 95% CI: 0.93-2.78). Eyes with intermediate uveitis (aHR: 0.54; 95%CI, 0.34-0.86) were less likely to have cataract surgery during follow-up than eyes with anterior uveitis (aHR=0.95; 95% CI: 0.63-1.45). Eyes of African-American compared with White patients (aHR: 1.74; 95% CI, 1.10-2.76), eyes of older patients (ages 20-49 vs <20 years, aHR: 1.96; 95%CI, 1.10-3.51; ages 50 or more vs <20 years, aHR: 2.77; 95% CI, 1.45-5.29) , and of patients on systemic immunosuppressives (aHR: 1.81; 95% CI, 1.01-3.22; p=0.04) were more likely to undergo cataract surgery within 12 months. Eyes that received multiple injections were also more likely to undergo cataract surgery, following a dose-response pattern (aHR: 1.66; 95% CI, 1.05-2.62; p=0.03 for 2-3 vs 1 injection; aHR: 2.05, 95% CI: 1.32-3.17 for ≥4 vs 1 injection).
DISCUSSION
Our results strongly suggest that periocular depot corticosteroid injections are useful in controlling inflammation and treating macular edema in most patients, and result in improved visual acuity in about one-half of the patients, confirming clinical impressions and the results of previous smaller studies. 5,6,10,12,15-22 Although direct comparison is difficult, due to variable follow-up and definitions used in other studies, the magnitude of improvement in inflammation and in macular edema affecting visual acuity in this cohort appears comparable to most previous reports.6,9,10,12,15-22
Prior smaller studies had correspondingly limited ability to assess factors potentially predictive of beneficial or adverse outcomes of periocular corticosteroid injections. In this large cohort, we found that anterior uveitis was associated with more favorable outcomes in terms of resolution of inflammation and visual acuity improvement to ≥20/40 than intermediate or posterior/panuveitis, perhaps reflecting less severe disease and/or disease with an intrinsically greater incidence of remission. However, macular edema outcomes did not differ substantially by the site of inflammation, perhaps because the site of involvement of macular edema is the same regardless of the site of inflammation. Shorter disease duration also consistently was associated with better outcomes, perhaps because the probability of irreversible damage to the eye may have been lower in newer cases, providing additional evidence that early referral for aggressive treatment of uveitis is valuable.23 Younger age was a favorable factor for visual acuity improvement, perhaps because younger eyes tend to be fundamentally healthier, with a lower risk of comorbidities such as cataract. Helm et al also found younger age to be a favorable prognostic factor for visual acuity improvement following periocular injections.19
The observation that treatment with immunosuppressive drugs was associated with less improvement in visual acuity in all likelihood reflects an indication-for-treatment bias; immunosuppressive drugs are prescribed for the more severe types of uveitis, ones less likely to respond to treatments. Previous studies of adjunctive regional corticosteroid injections have not evaluated the relative response rates but have suggested a much lower rate of relapse after the injection “wears off” when it is given in conjunction with systemic therapy.3,24 Evaluation of the benefits of multiple injections also probably is contaminated by indication-for-treatment bias, as cases successfully managed by the first injection may have been less likely to receive subsequent injections, with the result that repeated injections’ benefits likely are underestimated. Similarly, the association between multiple injections (4 or more) and incident macular edema likely simply reflects an indication for treatment in that cases with recurrent inflammation or macular edema may have been more likely to receive repeat treatments. Observations regarding less benefit from therapy in eyes of Hispanic or Native American persons are harder to explain, but could reflect a different or more severe spectrum of uveitides among these patients, or different patterns of accessing/utilizing healthcare. For example, Vogt-Koyanagi-Harada disease, a panuveitis, is more common among Hispanic and Native American patients than among non-Hispanic Whites in the United States.25
In prior reports, the most commonly reported complications following periocular corticosteroid injections were increased IOP and cataract progression. These complications have been addressed only in a few previous studies and ranged in general between 11% and 44% for IOP elevation, and up to 27% for cataract progression26-29 using various event definitions and follow-up times. In a large study of 115 patients that received periocular triamcinolone injections for various indications other than uveitis IOP elevation to ≥24 mmHg was found in 22.5%, cataract progression was observed in 15%, cataract surgery occurred in 6.7% and glaucoma surgery occurred in 0.9% within 1 year.26 Our cohort of uveitic eyes showed higher cumulative incidences of these unfavorable outcomes (IOP≥24 in 34%, vision-reducing cataract in 20.2%, cataract surgery in 13.8% and glaucoma surgery in 2.4%) within the same time frame, possibly reflecting the adverse impact of intraocular inflammation itself on these outcomes. Although definitions were slightly different for some outcomes, our results are similar to those found in the smaller but overlapping cohort reported by Leder et al and Salek et al.10,12 Salek et al reported 36% and 48% resolution of inflammation at 1 and 3 months, respectively, compared to 30% and 60% in the current cohort (results not shown in Table-2). Leder et al reported resolution of macular edema at one and 3 months of 53% and 58%, respectively with a single periocular corticosteroid injection and resolution of macular edema in 78% at one month after the 3rd periocular corticosteroid injection, suggesting benefit for multiple injections, when a single one is insufficient. We found no significant association between sites of uveitis and ocular complications, with the exception that intermediate uveitis cases were less likely to undergo cataract surgery. The latter observation may reflect a lesser intensity of inflammation in the vicinity of the lens and/or a greater tolerance of clinicians for low-grade vitreous inflammation and/or less use of other corticosteroids for inflammation in this site. Rates of IOP elevation to ≥30mm Hg were similar between our cohort (0.13/EY) and the reports from Salek et al (0.18/EY) and Leder et al (0.14/EY). Similarly, the rates for glaucoma surgery (0.02/EY, 0.01/EY, 0.02/EY, respectively) and cataract surgery (0.13/EY, 0.22/EY, 0.10/EY, respectively) were similar across these three studies.10,12
The exact mechanism for corticosteroid-induced IOP elevation is uncertain, but is hypothesized to arise from adverse effects of corticosteroids on aqueous outflow, with genetic differences and variations in corticosteroid receptors perhaps contributing to susceptibility.27,28 In uveitic eyes it often is difficult to attribute IOP elevation or glaucoma to a single cause, as multiple mechanisms are at play. Although younger age is one of the proposed risk factors for corticosteroid-induced ocular hypertension or glaucoma,20,26,29-33 we did not find any association between ocular hypertension and age. Neither did multiple injections appear to be a risk factor for IOP elevation in our cohort, perhaps because clinicians tended to avoid repeat injections in patients who initially experienced IOP elevation. Indeed, receipt of 4 or more injections was marginally associated with less incidence of an IOP≥30 mmHg, which might additionally reflect more severe disease in which the uveitis adversely affected the eye's capacity for aqueous secretion (e.g., through ciliary body injury or cyclitic bands) in cases for which so many injections were thought to be indicated. We observed longer disease duration to be a protective factor for IOP elevation, perhaps for the same reason. These risk factors may help identify cases where IOP-elevation in response to periocular corticosteroid injection is more or less likely. However, the risk of IOP elevation is not a universal contraindication to use of periocular corticosteroid therapy, because IOP elevations can be controlled without glaucoma surgery in the large majority of cases. In our study, fewer than 3% required glaucoma surgery within one year. In this retrospective cohort study, ideal data regarding the extent of cataract were not available. Therefore, we evaluated cases where a reduction of visual acuity to a level worse than 20/40 had been attributed primarily to cataract and cataract surgery as surrogate outcomes. Intraocular inflammation itself contributes to cataract progression,34 consistent with our observation that longer disease duration was a significant risk factor for visual impairment due to cataract. In addition, corticosteroids increase the risk of cataract, 35, 36 with increasing dose and duration of systemic corticosteroid therapy,37 as well as with repeated periocular injections.38,39 Our results confirm the latter point. Given that multiple injections were not significantly associated with an increase in favorable outcomes but tended to be associated with increased risk of cataract and cataract surgery, clinical use of multiple injections should be considered carefully in phakic eyes. Although the cataract incidence in this study was comparable to other reports,20,22 it is unclear what proportion of these patients would have had cataract surgery even in the absence of periocular corticosteroid injections. Because the indications for periocular corticosteroid injection in this study are unknown, it is possible that some cases underwent periocular injection therapy in an effort to quiet the eye prior to cataract surgery, which would result in an overestimation of the risk of cataract surgery (which was nevertheless lower than the incidence of visual acuity-reducing cataract). Because development of cataract and scheduling of cataract surgery takes time, it is possible that the risk of cataract surgery would further increase beyond 12 months. African-American race was also a risk factor for cataract surgery, which could reflect differences at the point in the disease course at which patients accessed care, genetic factors and/or a more severe disease course in this racial/ethnic subset of patients. A meta-analysis of population-based samples did not find a notably higher prevalence of cataract or cataract surgery among black with respect to white race.40 Interestingly, even though eyes with intermediate uveitis were significantly less likely to undergo cataract surgery, incident cataract was comparable across all anatomic locations of uveitis. Additionally, rate of cataract surgery in this cohort was comparable to those found among Multicenter Uveitis Steroid Treatment (MUST) trial patients who received standard systemic therapy (that included systemic corticosteroids and immunosuppressives) (0.15/EY) and lower than those who received flucinolone acetonide implant (0.40/EY).41
As with every retrospective cohort study relying on clinical record abstraction, our results should be interpreted carefully. Patients did not visit clinics following a set protocol, and ascertainment of the outcomes was non-standardized. We were unable to take into account the type of periocular injection used, the dose, or the type of depot corticosteroid used (although the participating centers habitually use triamcinolone acetonide for depot injections). However, the effectiveness with different routes of application appears to be comparable in previous reports.6,20 Other adverse effects associated with periocular corticosteroid injections such as hypopigmentation, ptosis and inadvertent globe perforation42 could not be evaluated in this retrospective study. We also could not adjust for the severity of macular edema or the presence of various degrees of cataract at baseline, or a history of corticosteroid-responsive IOP elevation events (or a lack thereof) prior to baseline. Neither was it known whether patients had previously received corticosteroid injections. In addition, the sites are tertiary referral clinics and their population likely reflects a greater disease severity than might be encountered in non-tertiary settings, although similar to other tertiary settings. Strengths of the study include a much larger sample size than in previous reports (which were also retrospective, tertiary reports), allowing for substantially more precise risk estimation than was previously possible, and for assessment of factors associated with the incidence of benefits and side effects of the therapy. Data were collected following a common protocol with quality control mechanisms to ensure as much standardization as possible within the constraints of the overall retrospective cohort study design.
In summary, our results suggest that periocular injections are highly effective for quelling acute inflammation or macular edema across a broad array of forms of uveitis, but incur risks, most notably the modest risk of cataract in phakic eyes. The results indicate that periocular corticosteroid injections are highly effective in treating both active intraocular inflammation and macular edema-induced vision loss in the majority of patients, and frequently (~50%) results in improved visual acuity at least in the short run. Factors predictive of better effectiveness included anterior uveitis (for visual acuity and inflammatory response), younger age (visual acuity), and shorter duration of uveitis (all outcomes). Ocular side effects such as ocular hypertension, glaucoma and need for cataract surgery were much less common than has been reported with intravitreal injections of corticosteroids, but were non-trivial, and cataract and cataract surgery occurred in a minority, especially those undergoing repeated injections. Taking these findings into account, periocular corticosteroid injections are likely to be especially useful in severe presentations or flare-ups of remitting forms of ocular inflammation or for non-chronic uveitic macular edema, and likely are safest in already pseudophakic eyes in which intraocular pressure spikes would be unlikely to cause irreversible glaucoma damage. Randomized clinical trials would be ideal to compare the outcomes of periocular depot corticosteroid injections to the various alternatives now available.
PRECIS.
In the Systemic Immunosuppression Therapy for Eye Diseases (SITE) Cohort Study periocular corticosteroid injections were effective in treating active intraocular inflammation and macular edema and resulted in improved vision in approximately half of the patients.
Figure 1.
Kaplan-Meier estimation of the incidence of resolution of inflammation (improvement in inflammation to no activity) by uveitis site.
Figure 2.
Kaplan-Meier estimation of the incidence of resolution of macular edema causing vision loss to worse than 20/40 by uveitis site.
Figure 3.
Kaplan-Meier estimation of the incident IOP elevation to >=24mmHg by uveitis site.
Figure 4.
Kaplan-Meier estimation of the incidence of cataract affecting visual acuity (cataract causing visual acuity worse than 20/40) by uveitis site.
Figure 5.
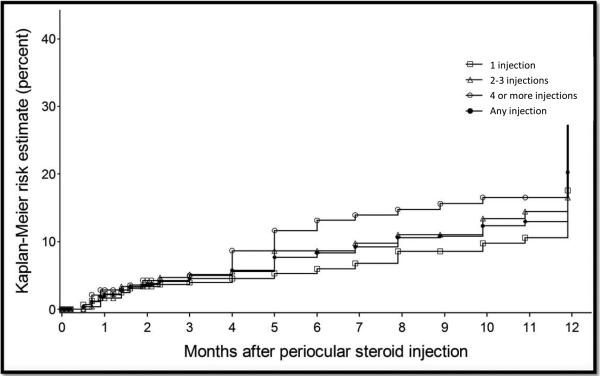
Kaplan-Meier estimation of the incidence of cataract affecting visual acuity (cataract causing visual acuity worse than 20/40) by number of periocular corticosteroid injections.
Acknowledgments
Financial Support: This study was supported primarily by National Eye Institute Grant EY014943 (Dr. Kempen). Additional support was provided by Research to Prevent Blindness (RPB), the Paul and Evanina Mackall Foundation, and the Lois Pope Life Foundation. Dr Kempen was an RPB James S. Adams Special Scholar Award recipient and Dr. Thorne was an RPB Harrington Special Scholar Award recipient during the conduct of the study. Drs. Jabs and Rosenbaum were RPB Senior Scientific Investigator Award recipients during the conduct of the study. Dr. Suhler is supported in part by the Department of Veterans Affairs. Dr. Levy-Clarke was previously supported by and Drs Sen and Nussenblatt continue to be supported by intramural research program of the National Eye Institute. None of the sponsors had any role in the design and conduct of the report; collection, management, analysis, and interpretation of the data; nor in the preparation, review, and approval of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting presentation: This manuscript has been presented at the American Academy of Ophthalmology Annual Meeting, October, 2010 (Best paper award)
Conflict of Interest/Financial Disclosure(s):
The author(s) have made the following disclosure(s):
C. Stephen Foster: (equity owner) Eyegate, (consultant, lecturer) Allergan; (consultant, lecturer) Bausch & Lomb; (consultant) Sirion; (lecturer) Alcon; (lecturer) Inspire; (lecturer) Ista; (lecturer) Centocor; Douglas A. Jabs: (consultant) Roche; (consultant) Genzyme Corporation; (consultant) Novartis; (consultant) Allergan; (consultant) Glaxo Smith Kline; (consultant) Applied Genetic Technologies Corporation; (consultant) The Emmes Corporation; (consultant) The Johns Hopkins Dana Center for Preventive Ophthalmology; John H. Kempen: (consultant) Lux Biosciences; (consultant) Alcon; (consultant) Allergan; (consultant) Can-Fite; (consultant) Clearside; (consultant) Xoma; (consultant) NIAID/NIH; (consultant) Sanofi-Pasteur; (grant recipient) Food and Drug Administration; (grant recipient) EyeGate; (grant recipient) Lions Club International Foundation; (grant recipient) National Eye Institute; James Rosenbaum: (consultant) Abbott; (consultant), Allergan, (consultant) Lux Biosciences, (consultant) Centocor, (consultant) Genentech, (consultant) Novartis.
Contributions Design and conduct of the study (HNS, SV, JHK); acquisition, management, analysis, and interpretation of the data (all authors) drafting the article, revising it critically for important intellectual content and final approval of the version to be published (all authors)
References
- 1.Gordon DM. Prednisone and prednisolone in ocular disease. Am J Ophthalmol. 1956;41:593–600. [PubMed] [Google Scholar]
- 2.Gaudio PA. A review of evidence guiding the use of corticosteroids in the treatment of intraocular inflammation. Ocul Immunol Inflamm. 2004;12:169–92. doi: 10.1080/092739490500192. [DOI] [PubMed] [Google Scholar]
- 3.Sallam A, Taylor SR, Habot-Wilner Z, et al. Repeat intravitreal triamcinolone acetonide injections in uveitic macular oedema [letter online]. Acta Ophthalmol. 2012;90:e323–5. doi: 10.1111/j.1755-3768.2011.02247.x. [DOI] [PubMed] [Google Scholar]
- 4.Jermak CM, Dellacroce JT, Heffez J, Peyman GA. Triamcinolone acetonide in ocular therapeutics. Surv Ophthalmol. 2007;52:503–22. doi: 10.1016/j.survophthal.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Tanner V, Kanski JJ, Frith PA. Posterior sub-Tenon's triamcinolone injections in the treatment of uveitis. Eye (Lond) 1998;12:679–85. doi: 10.1038/eye.1998.168. [DOI] [PubMed] [Google Scholar]
- 6.Ferrante P, Ramsey A, Bunce C, Lightman S. Clinical trial to compare efficacy and side-effects of injection of posterior sub-Tenon triamcinolone versus orbital floor methylprednisolone in the management of posterior uveitis. Clin Experiment Ophthalmol. 2004;32:563–8. doi: 10.1111/j.1442-9071.2004.00902.x. [DOI] [PubMed] [Google Scholar]
- 7.Nozik RA. Periocular injection of steroids. Trans Am Acad Ophthalmol Otolaryngol. 1972;76:695–705. [PubMed] [Google Scholar]
- 8.Jea SY, Byon IS, Oum BS. Triamcinolone-induced intraocular pressure elevation: intravitreal injection for macular edema and posterior subtenon injection for uveitis. Korean J Ophthalmol. 2006;20:99–103. doi: 10.3341/kjo.2006.20.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshikawa K, Kotake S, Ichiishi A, et al. Posterior sub-Tenon injections of repository corticosteroids in uveitis patients with cystoid macular edema. Jpn J Ophthalmol. 1995;39:71–6. [PubMed] [Google Scholar]
- 10.Leder HA, Jabs DA, Galor A, et al. Periocular triamcinolone acetonide injections for cystoid macular edema complicating noninfectious uveitis. Am J Ophthalmol. 2011;152:441–8. doi: 10.1016/j.ajo.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Kempen JH, Daniel E, Gangaputra S, et al. Methods for identifying long-term adverse effects of treatment in patients with eye diseases: the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study. Ophthalmic Epidemiol. 2008;15:47–55. doi: 10.1080/09286580701585892. [DOI] [PubMed] [Google Scholar]
- 12.Salek SS, Leder HA, Butler NJ, et al. Periocular triamcinolone acetonide injections for control of intraocular inflammation associated with uveitis. Ocul Immunol Inflamm. 2013;21:257–63. doi: 10.3109/09273948.2013.767353. [DOI] [PubMed] [Google Scholar]
- 13.Gangaputra S, Newcomb CW, Liesegang TL, et al. Systemic Immunosuppressive Therapy for Eye Diseases Cohort Study. Methotrexate for ocular inflammatory diseases. Ophthalmology. 2009;116:2188–98. doi: 10.1016/j.ophtha.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasadhika S, Kempen JH, Newcomb C, et al. Azathioprine for ocular inflammatory diseases. Am J Ophthalmol. 2009;148:500–9. doi: 10.1016/j.ajo.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jennings T, Rusin MM, Tessler HH, Cunha-Vaz JG. Posterior sub-Tenon's injections of corticosteroids in uveitis patients with cystoid macular edema. Jpn J Ophthalmol. 1988;32:385–91. [PubMed] [Google Scholar]
- 16.Bakri SJ, Kaiser PK. Posterior subtenon triamcinolone acetonide for refractory diabetic macular edema. Am J Ophthalmol. 2005;139:290–4. doi: 10.1016/j.ajo.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 17.Tsujikawa A, Fujihara M, Iwawaki T, et al. Triamcinolone acetonide with vitrectomy for treatment of macular edema associated with branch retinal vein occlusion. Retina. 2005;25:861–7. doi: 10.1097/00006982-200510000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Lin JM, Chiu YT, Hung PT, Tsai YY. Early treatment of severe cystoid macular edema in central retinal vein occlusion with posterior sub-Tenon triamcinolone acetonide. Retina. 2007;27:180–9. doi: 10.1097/01.iae.0000237584.56552.1c. [DOI] [PubMed] [Google Scholar]
- 19.Helm CJ, Holland GN. The effects of posterior subtenon injection of triamcinolone acetonide in patients with intermediate uveitis. Am J Ophthalmol. 1995;120:55–64. doi: 10.1016/s0002-9394(14)73759-6. [DOI] [PubMed] [Google Scholar]
- 20.Lafranco Dafflon M, Tran VT, Guex-Crosier Y, Herbort CP. Posterior sub-Tenon's steroid injections for the treatment of posterior ocular inflammation: indications, efficacy and side effects. Graefes Arch Clin Exp Ophthalmol. 1999;237:289–95. doi: 10.1007/s004170050235. [DOI] [PubMed] [Google Scholar]
- 21.Roesel M, Gutfleisch M, Heinz C, et al. Intravitreal and orbital floor triamcinolone acetonide injections in noninfectious uveitis: a comparative study. Ophthalmic Res. 2009;42:81–6. doi: 10.1159/000220600. [DOI] [PubMed] [Google Scholar]
- 22.Okada AA, Wakabayashi T, Morimura Y, et al. Trans-Tenon's retrobulbar triamcinolone infusion for the treatment of uveitis. Br J Ophthalmol. 2003;87:968–71. doi: 10.1136/bjo.87.8.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dana MR, Merayo-Lloves J, Schaumberg DA, Foster CS. Prognosticators for visual outcome in sarcoid uveitis. Ophthalmology. 1996;103:1846–53. doi: 10.1016/s0161-6420(96)30417-x. [DOI] [PubMed] [Google Scholar]
- 24.Habot-Wilner Z, Sallam A, Pacheco PA, et al. Intravitreal triamcinolone acetonide as adjunctive treatment with systemic therapy for uveitic macular edema. Eur J Ophthalmol. 2011;21(suppl):S56–61. doi: 10.5301/EJO.2010.6062. [DOI] [PubMed] [Google Scholar]
- 25.Read RW, Rao NA, Cunningham ET. Vogt-Koyanagi-Harada disease. Curr Opin Ophthalmol. 2000;11:437–42. doi: 10.1097/00055735-200012000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Iwao K, Inatani M, Kawaji T, et al. Frequency and risk factors for intraocular pressure elevation after posterior sub-Tenon capsule triamcinolone acetonide injection. J Glaucoma. 2007;16:251–6. doi: 10.1097/IJG.0b013e31802d696f. [DOI] [PubMed] [Google Scholar]
- 27.Akduman L, Kolker AE, Black DL, et al. Treatment of persistent glaucoma secondary to periocular corticosteroids. Am J Ophthalmol. 1996;122:275–7. doi: 10.1016/s0002-9394(14)72027-6. [DOI] [PubMed] [Google Scholar]
- 28.Armaly MF, Becker B. Intraocular pressure response to topical corticosteroids. Fed Proc. 1965;24:1274–8. [PubMed] [Google Scholar]
- 29.Levy J, Tessler Z, Klemperer I, Lifshitz T. Acute intractable glaucoma after a single low-dose sub-Tenon's corticosteroid injection for macular edema. Can J Ophthalmol. 2004;39:672–3. doi: 10.1016/s0008-4182(04)80035-8. [DOI] [PubMed] [Google Scholar]
- 30.Levin DS, Han DP, Dev S, et al. Subtenon's depot corticosteroid injections in patients with a history of corticosteroid-induced intraocular pressure elevation. Am J Ophthalmol. 2002;133:196–202. doi: 10.1016/s0002-9394(01)01372-1. [DOI] [PubMed] [Google Scholar]
- 31.Hirooka K, Shiraga F, Tanaka S, et al. Risk factors for elevated intraocular pressure after trans-Tenon retrobulbar injections of triamcinolone. Jpn J Ophthalmol. 2006;50:235–8. doi: 10.1007/s10384-005-0306-9. [DOI] [PubMed] [Google Scholar]
- 32.Sallam A, Sheth HG, Habot-Wilner Z, Lightman S. Outcome of raised intraocular pressure in uveitic eyes with and without a corticosteroid-induced hypertensive response. Am J Ophthalmol. 2009;148:207–13. doi: 10.1016/j.ajo.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 33.Ng JS, Fan DS, Young AL, et al. Ocular hypertensive response to topical dexamethasone in children: a dose-dependent phenomenon. Ophthalmology. 2000;107:2097–100. doi: 10.1016/s0161-6420(00)00357-2. [DOI] [PubMed] [Google Scholar]
- 34.Smith RE, Godfrey WA, Kimura SJ. Complications of chronic cyclitis. Am J Ophthalmol. 1976;82:277–82. doi: 10.1016/0002-9394(76)90434-7. [DOI] [PubMed] [Google Scholar]
- 35.Kalina PH, Erie JC, Rosenbaum L. Biochemical quantification of triamcinolone in subconjunctival depots. Arch Ophthalmol. 1995;113:867–9. doi: 10.1001/archopht.1995.01100070041022. [DOI] [PubMed] [Google Scholar]
- 36.Aydin A, Akin T, Bilge AH. Management of persistent glaucoma secondary to depot methylprednisolone. Ophthalmic Surg Lasers Imaging. 2007;38:399–401. doi: 10.3928/15428877-20070901-07. [DOI] [PubMed] [Google Scholar]
- 37.Oglesby RB, Black RL, von Sallmann L, Bunim JJ. Cataracts in patients with rheumatic diseases treated with corticosteroids. Further observations. Arch Ophthalmol. 1961;66:625–30. doi: 10.1001/archopht.1961.00960010627005. [DOI] [PubMed] [Google Scholar]
- 38.Urban RC, Jr, Cotlier E. Corticosteroid-induced cataracts. Surv Ophthalmol. 1986;31:102–10. doi: 10.1016/0039-6257(86)90077-9. [DOI] [PubMed] [Google Scholar]
- 39.Castellarin A, Pieramici DJ. Anterior segment complications following periocular and intraocular injections. Ophthalmol Clin North Am. 2004;17:583–90. doi: 10.1016/j.ohc.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 40.Eye Diseases Prevalence Research Group Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004;122:487–94. doi: 10.1001/archopht.122.4.487. [DOI] [PubMed] [Google Scholar]
- 41.Multicenter Uveitis Steroid Treatment (MUST) Trial Research Group. Kempen JH, Altaweel MM, Holbrook JT, et al. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the Multicenter Uveitis Steroid Treatment Trial. Ophthalmology. 2011;118:1916–26. doi: 10.1016/j.ophtha.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith JR, George RK, Rosenbaum JT. Lower eyelid herniation of orbital fat may complicate periocular corticosteroid injection. Am J Ophthalmol. 2002;133:845–7. doi: 10.1016/s0002-9394(02)01412-5. [DOI] [PubMed] [Google Scholar]