Abstract
Background
Obesity is a common comorbidity of patients with chronic thromboembolic pulmonary hypertension referred for pulmonary thromboendarterectomy, yet the effect of obesity on pulmonary thromboendarterectomy outcomes has not been well described.
Methods
We conducted a retrospective cohort study in which 476 consecutive surgeries over a 3.5 year period were examined to determine the effects of obesity on outcomes. Patients were grouped into four categories based on bass mass index (BMI <22 kg/m2, BMI 22-30 kg/m2, 30-40 kg/m2, and BMI >40 kg/m2).
Results
There were important differences in baseline pulmonary hemodynamics with obese patients having significantly lower pulmonary vascular resistances than non-obese patients. All patients achieved a significant reduction in PVR, though the improvement was greatest in the lower BMI groups. The overall in-hospital mortality was 0.8% and there were no differences in risk among BMI groups. Among the BMI groups, there were no differences in incidence of postoperative complications including atrial fibrillation (overall 24.8%), reperfusion lung injury (overall 23.1%), and surgical site infection (overall 4.4%) or median lengths of stay (including ventilator days, ICU days, and postoperative length of stay).
Conclusions
Pulmonary thromboendarterectomy outcomes have continued to improve and this surgery can safely be completed in obese patients, previously deemed to be at high risk for poor outcomes.
Keywords: Outcomes, Obesity, Pulmonary Endarterectomy, Pulmonary Vascular Resistance
Introduction
Obesity is an increasing public health problem in the United States; over one-third of all adults are obese(1). Given that obesity is a risk factor for coronary disease, others have investigated whether obesity adversely affects outcomes after cardiac surgery. Numerous studies examining coronary artery bypass grafting (CABG) have demonstrated that patients with body mass index (BMI) greater than 30 kg/m2 have no increased risk of post-operative mortality compared to normal weight patients(2-6). However, obese patients have been noted to have increased risk for post-operative complications including sternal wound infections(7) and atrial fibrillation after CABG(8).
Another thoracic surgery in which the effect of BMI on outcomes has been evaluated is pulmonary thromboendarterectomy (PTE) for chronic thromboembolic pulmonary hypertension (CTEPH). This procedure is associated with a 30-day mortality rate of 4.7% in a large international registry (9). Historical data demonstrated that a significantly higher BMI was seen in those who did not survive PTE compared to the less obese survivors(10), but this association of obesity and mortality has not been reexamined in more recent PTE registries.
Using an internal quality improvement database which has gathered information on outcomes after pulmonary thromboendarterectomy surgery at the University of California, San Diego, we sought to examine the effects of BMI on our center's outcomes following PTE, including post-operative mortality, pulmonary hemodynamics, lengths of stay, and post-operative complications including surgical site infection, the development of reperfusion lung injury, and atrial fibrillation.
Patients and Methods
Study Design
We conducted a retrospective cohort study to determine the effects of BMI on outcomes after pulmonary thromboendarterectomy (PTE). From January 1, 2010 to June 30, 2013, all patients who underwent PTE at UCSD have been included in a quality improvement database used to follow rates of post-operative complications as well as hemodynamic outcomes. Patients were selected to undergo PTE based on pre-operative testing including ventilation-perfusion scans, right heart catheterization hemodynamics and surgical accessibility of disease as determined by pulmonary angiogram or chest CT angiography. Other than a patient's refusal to undergo surgery, there were no absolute contraindications for PTE and no current absolute BMI thresholds that precluded surgery. This study has been granted an exemption from the UCSD IRB to be conducted using this quality improvement database.
Study Endpoints
Based on the body mass index documented prior to surgery, patients were classified into one of four BMI groups: 1.) BMI <22 kg/m2; 2.) BMI 22-30 kg/m2; 3.) BMI 30-40 kg/m2; and 4.) BMI >40 kg/m2. The primary endpoint in this study was in-hospital mortality which was defined as all deaths within the same hospital stay as the PTE. Intraoperative values for total cardiopulmonary bypass times and circulatory arrest periods, along with the absolute values of and changes in hemodynamics from pre- to post-operative measurements were compared among the four BMI groups. The rates of post-operative complications including atrial fibrillation, reperfusion lung injury and sternal wound infections were compared among the four groups. Atrial fibrillation was determined to be present based on telemetry finding, and recorded as a postoperative complication if it required intervention, such as over-drive pacing, cardioversion, or medication administration. Reperfusion lung injury was defined as a having a PaO2:FiO2 ratio of <300 with radiographic evidence of a new infiltrate in an area of lung that was endarterectomized. The presence of any surgical site infection, including superficial incisional infections and deep sternal infections was determined at the discretion of attending physician based on clinical exam and laboratory findings. Finally, the total ventilator-days, ICU-days and length of post-operative hospitalization were collected for each patient.
Statistical Analyses
All analyses were conducted using the commercially available software program SPSS Version 21 (SPSS IBM, New York, U.S.A). A p-value of <0.05 was deemed significant in all analyses. First, four groups of baseline BMI (i.e <22, 22-30, 30-40, >40) were created. The group demographics, functional classification, and hemodynamics at baseline and post-operatively were compared using an ANOVA. Pairwise comparisons among the subgroups were examined using the Tukey test. The odds of dichotomous outcomes (such as atrial fibrillation, reperfusion lung injury and sternal wound infection) were evaluated using logistic regression. The length of ICU stay, ventilator-days and total hospital length of stay were compared using Kaplan-Meier curves to estimate time to each outcome. Median times-to-event were compared using the log rank test.
Results
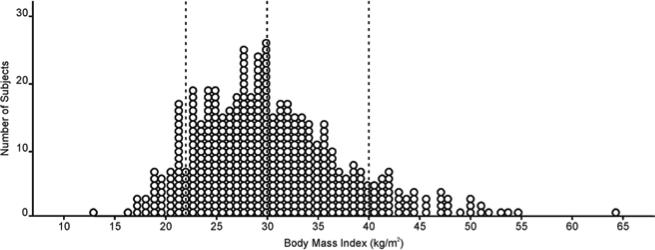
Over the study period, there were 476 patients who underwent PTE surgery at UCSD. The mean BMI was 30.43 kg/m2 with a range from 12.9 to 64.3 kg/m2 (Figure 1). The baseline characteristics of the four BMI groups are described in Table 1. A significantly higher portion of the BMI greater than 40 kg/m2 were female (76.5%) compared to the three lower BMI categories (p<0.001). There were significant differences among the distribution of functional classes in the four BMI groups (p=0.003). While 14.7% of all the cases were referred with a New York Heart Association Functional Classification of I or II, only 1 patient in the BMI >40 kg/m2 group was NYHA class II (p < 0.05). Also, there were more class IV patients in both the BMI <22 kg/m2 and BMI >40 kg/m2 groups compared to the two middle BMI groups (p<0.05).
Figure 1. Scatterplot of the distribution of Body Mass Index among patients undergoing pulmonary thromboendarterectomy at UCSD.
The mean BMI was 30.46 (standard deviation ± 7.38) kg/m2. The largest patient operated on had a BMI of 64.3 kg/m2. Patients were divided into four BMI categories with cut-off values (represented by dashed lines) at BMI of 22 kg/m2, 30 kg/m2, and 40 kg/m2.
Table 1.
Baseline Demographic and Hemodynamic Characteristics of Patients in the Four BMI Subgroups.
| BMI <22 (n=49) | BMI 22-30 (n=201) | BMI 30-40 (n=175) | BMI >40 (n=51) | p-value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 45.71 ± 16.7 | 51.61 ± 15.1 | 53.40 ± 13.2 | 48.10 ± 12.0 | 0.003 |
| Female (%) | 22 (44.9%) | 87 (43.3%) | 87 (49.7%) | 39 (76.5%) | <0.001 |
| BMI (kg/m^2) | 19.94 ± 1.8 | 26.28 ± 2.2 | 33.86 ± 2.8 | 45.31 ± 4.8 | <0.001 |
| NYHA Class (%) | 0.002 | ||||
| I | 0 (0.0%) | 0 (0.0%) | 1 (0.6%) | 0 (0.0%) | |
| II | 10 (20.4%) | 31 (15.6%) | 23 (13.2%) | 1 (2.0%) | |
| III | 32 (65.3%) | 160 (80.4%) | 144 (82.8%) | 43 (84.3%) | |
| IV | 7 (14.3%) | 8 (4.0%) | 6 (3.4%) | 7 (13.7%) | |
| Baseline Hemodynamics | |||||
| Right Atrial Pressure (mmHg) | 10.21 ± 7.3 | 10.44 ± 6.0 | 10.75 ± 5.2 | 12.51 ± 4.9 | 0.134 |
| Mean PA Pressure (mmHg) | 41.40 ± 12.6 | 45.46 ± 11.5 | 43.32 ± 11.7 | 47.83 ± 12.2 | 0.017 |
| Cardiac Output (L/min) | 3.77 ± 0.9 | 4.12 ± 1.3 | 4.79 ± 1.3 | 5.48 ± 1.5 | <0.001 |
| Cardiac Index (L/min/m^2) | 2.21 ± 0.5 | 2.14 ± 0.6 | 2.23 ± 0.6 | 2.45 ± 0.6 | 0.015 |
| PVR (dyn·s/cm5) | 772.21 ± 414.1 | 767.15 ± 390.0 | 580.57 ± 314.0 | 566.22 ± 292.1 | <0.001 |
| PVRi (dyn·s/cm5/m^2) | 445.49 ± 247.5 | 406.26 ± 222.2 | 271.81 ± 160.7 | 253.46 ± 140.2 | <0.001 |
All data is presented as mean ± standard deviation for continuous variables and number and percentage for categorical variables. The pulmonary vascular resistance index in the lower BMI groups (group 1 and 2) was significantly higher than higher BMI groups (group 3 and 4) (p<0.001).
There were important hemodynamic differences seen in the pre-operative right heart catheterization data. Patients with BMI >40 kg/m2 had a significantly higher mean pulmonary artery pressure compared to those with BMI <22 kg/m2, but had similar mean PA pressure compared to those in BMI 22-30 kg/m2 and 30-40 kg/m2 groups. While patients with BMI less than 30 kg/m2 had lower cardiac outputs compared to those with BMI over 30 kg/m2 (p<0.001),even after the data was normalized for body surface area (p=0.015). Both the baseline pulmonary vascular resistance and the pulmonary vascular resistance index were significantly higher in patients with BMI less than 30 compared to those with BMI greater than 30 kg/m2 (p<0.001 for both).
Outcomes including mortality, operative times and pulmonary hemodynamics are presented in Table 2. The overall in-hospital mortality rate after surgery was 0.8%. There were no deaths in either the BMI <22 kg/m2 or >40 kg/m2 groups. Compared to the BMI <22 kg/m2 group, the BMI >30 kg/m2 groups had longer cardiopulmonary bypass times (p=0.012). However, the BMI >40 kg/m2 group did have a significantly lower circulatory arrest time compared to the BMI <22 kg/m2 group (p=0.014). All four groups had similar postoperative mean pulmonary artery pressures, cardiac outputs and cardiac index. All groups achieved reductions in pulmonary vascular resistance and total pulmonary resistance, though the patients with BMI less than 30 kg/m2 achieved a larger reduction in PVR and TPR compared to those with BM greater than 40 kg/m2 (p<0.001 and p=0.001, respectively).
Table 2.
Mortality, Operative Times and Post-Operative Hemodynamics by BMI Group.
| BMI <22 (n=49) | BMI 22-30 (n=201) | BMI 30-40 (n=175) | BMI >40 (n=51) | p-value | |
|---|---|---|---|---|---|
| In-Hospital Mortality, n (%) | 0 (0%) | 3 (1.5%) | 1 (0.6%) | 0 (0%) | |
| Operative Times | |||||
| Circulatory Arrest (minutes) | 48.29 ± 15.9 | 46.97 ± 18.9 | 43.38 ± 16.7 | 40.20 ±15.8 | 0.014 |
| Total cardiopulmonary bypass (minutes) | 250.80 ± 50.9 | 260.42 ± 35.6 | 268.32 ± 35.2 | 268.61 ± 32.2 | 0.012 |
| Post-operative Hemodynamics | |||||
| Right Atrial Pressure (mmHg) | 8.12 ± 4.1 | 8.40 ± 3.7 | 8.89 ± 3.4 | 10.17 ± 3.2 | 0.012 |
| Mean PA Pressure (mmHg) | 24.04 ± 4.1 | 24.51 ± 7.4 | 24.11 ± 6.8 | 25.51 ± 5.1 | 0.664 |
| Cardiac Output (L/min) | 5.01 ± 1.4 | 5.43 ± 1.3 | 5.99 ± 1.3 | 6.14 ± 1.3 | <0.001 |
| Cardiac Index (L/min/m^2) | 2.94 ± 0.7 | 2.81 ± 0.6 | 2.78 ± 0.5 | 2.76 ± 0.6 | 0.379 |
| PVR (dyn·s/cm5) | 290.16 ± 180.8 | 257.76 ± 121.2 | 220.24 ± 109.1 | 218.22 ± 81.1 | <0.001 |
| Delta PVR | 483.26 ± 347.9 | 510.88 ± 355.8 | 360.85 ± 266.9 | 356.29 ± 254.7 | <0.001 |
| PVRi (dyn·s/cm5/m^2) | 170.42 ± 114.4 | 136.05 ± 72.2 | 104.46 ± 59.6 | 96.89 ± 39.4 | <0.001 |
| Total pulmonary resistance | 416.45 ± 211.6 | 383.67 ± 155.3 | 338.23 ± 137.0 | 348.25 ± 107.9 | 0.002 |
| Delta TPR | 566.17 ± 390.1 | 602.19 ± 406.0 | 460.58 ± 324.3 | 429.17 ± 304.2 | 0.001 |
| Jamieson Type III | 18 (36.7%) | 57 (28.4%) | 39 (22.3%) | 10 (19.6%) | 0.119 |
Post-operative Complications
The frequencies and adjusted odds for atrial fibrillation, reperfusion lung injury and sternal wound infection are displayed in table 3. For all comparisons, the BMI groups from 22-30 kg/m2 was established as the reference group. After adjusting for age and sex, there were no significant differences in the odds of developing atrial fibrillation were seen for the three comparator groups. There was also no statistically significant difference in age- , sex-, and baseline PVR-adjusted odds of developing reperfusion lung injury. Despite having the highest pre-operative PVR, the BMI <22 kg/m2 group had the lowest frequency of reperfusion lung injury at 16.3%. The higher BMI groups had higher rates of reperfusion injury despite having the lowest mean baseline PVR, though this increase the adjusted odds ratio was not significant (p=0.293). Finally, the overall frequency of surgical site infections was low in this population and there were no significant differences among the BMI groups. Of the 21 surgical site infections, only 2 represented deep sternal infections. The majority of surgical site infections were mild erythema around the wound that were treated empirically with antibiotics.
Table 3.
Frequencies and Adjusted Odds Ratios for Atrial Fibrillation, Reperfusion Lung Injury and Surgical Site Infection by BMI Group.
| Atrial Fibrillation | Reperfusion Lung Injury | Sternal Wound Infection | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Number (%) | AOR (95°% CI) | p-value | Number (%) | AOR (95% CI) | p-value | Number (%) | AOR (95% CI) | p-value | |
| BMI <22 (n=49) | 7 (14.3%) | 0.59 (0.24-1.51) | 0.28 | 8 (16.3%) | 0.50 (0.21-1.17) | 0.11 | 2 (4.1%) | 0.98 (0.19-4.89) | 0.98 |
| BMI 22-30 (n=201) | 50 (24.9%) | Reference | 55 (27.4%) | Reference | 7 (3.5%) | Reference | |||
| BMI 30-40 (n=175) | 48 (27.4%) | 0.96 (0.58-1.59) | 0.88 | 33 (18.9%) | 0.83 (0.50-1.40) | 0.49 | 7 (4.0%) | 0.89 (0.30-2.65) | 0.85 |
| BMI >40 (n=51) | 13 (25.5%) | 1.73 (0.79-3.78) | 0.17 | 14 (27.5%) | 1.50 (0.70-3.21) | 0.29 | 5 (9.8%) | 2.03 (0.61-6.74) | 0.24 |
| Total (n=476) | 118 (24.8%) | 110 (23.1%) | 21 (4.4%) | ||||||
For all analyses, the BMI 22-30 group was used as the reference category. The odds ratios for the atrial fibrillation and surgical site infection are adjusted for both age and sex. The odds ratios for RPE are adjusted for age, sex and baseline PVR.
Analysis of ventilator-days, ICU days and post-operative length of stay
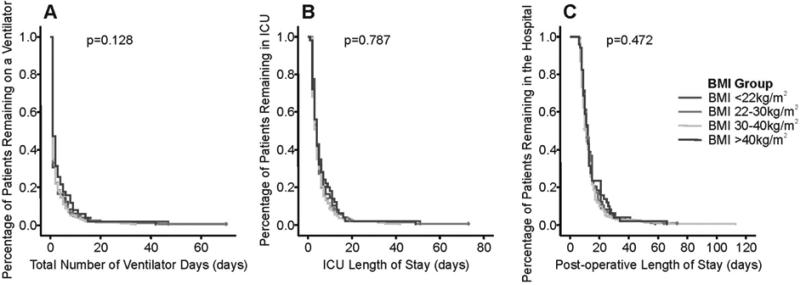
Kaplan-Meier curves for the various time-dependent outcomes for each of the four BMI groups were constructed and are displayed in Figure 2. The overall median number of ventilator-days was 1.0 days and the 75th percentile was 2.0 days. The overall median ICU stay and postoperative length of stay were 4.0 days and 11.0 days, respectively. Among the four BMI groups, there were no significant differences in median number of ventilator days (log rank p=0.128), median number of ICU days (log rank p=0.787) or in median number of total post-operative days (log rank p=0.472).
Figure 2. Kaplan-Meier Time to event curves for Ventilator Days (Panel A), ICU Days (Panel B) and Post-operative Length of Stay (Panel C).
Among the four BMI groups, there were no significant differences in median number of ventilator days (log rank p=0.128), median number of ICU days (log rank p=0.787) or in median number of post-operative days (log rank p=0.472). The four in-hospital deaths were censored and are represented by tick marks on the curve.
Comment
In this large retrospective analysis of three and a half years of consecutive patients undergoing pulmonary thromboendarterectomy, obese patients (BMI 30-40 kg/m2 and >40 kg/m2), compared to non-obese patients (BMI 22-30 kg/m2 and <22 kg/m2) had similar improvements in hemodynamic parameters without significant increase in risk of death or post-operative complications. Among the four BMI groups, there was no difference in median ventilator-days, ICU-day or post-operative length of stay.
Obese patients in this cohort of patients referred for PTE tended to have less severe disease (based on PVR and PVRi) at baseline compared to the non-obese patients. Several possibilities may explain this difference. First, there may be a selection bias in that obese patients with higher PVR may be deemed not to be surgical candidates. Another explanation may be that obese patients suffer dyspnea due to their excessive weight; an association between dyspnea and obesity has been described independent of other conditions such as airflow obstruction (11). This obesity-related dyspnea may prompt earlier evaluation and eventually lead to earlier diagnosis of CTEPH compared to those with a normal or low BMI. Additionally, in patients with pulmonary arterial hypertension, those with near normal functional ability as measured by six minute walk distance of over 450 meters had significantly lower BMI than the more impaired patients(12). A similar association between functional ability and baseline BMI may be present in CTEPH which may delay diagnosis in the non-obese patients. This delay in diagnosis may explain the increased proportion of patients with BMI <22 kg/m2 who presented in New York Heart Association functional class IV compared to the moderate BMI groups (22-30 kg/m2 and 30-40 kg/m2).
This study is notable for the relatively low rates of complications including atrial fibrillation, reperfusion lung injury and surgical site infections. Atrial fibrillation is a prevalent comorbidity seen in all forms of pulmonary hypertension(13) and is a common condition seen in after other cardiac surgeries(14). The rate of post-operative atrial fibrillation in this cohort was 24.4%. Previous studies have shown that increasing BMI is a risk factor for developing atrial fibrillation after CABG(15), but this association was not seen in our population of patients undergoing PTE. Two factors in our technique(16) may account for the lower incidence of atrial fibrillation compared to other cardiac surgeries. First, epicardial wires are placed and patients are frequently atrially paced for the first 24 hours after PTE. This practice has been shown to reduce post-operative atrial fibrillation after other cardiac surgeries (17, 18). Additionally, all patients receive methylprednisolone during rewarming and administration of corticosteroids has demonstrated a reduction in post-operative atrial fibrillation after CABG(19).
Our rates of reperfusion lung injury were also no different among the four BMI groups. Historically, reperfusion lung injury had occurred in about 30% of PTE cases(20). The reduction in incidence to the current overall rate of 23.1% is likely multifactorial, and speculatively related to an improvement in overall surgical technique. There was no association seen with reperfusion lung injury and BMI in this study.
Contrary to previous literature on surgical site infections in obese patients (2, 21), there was no significant increase in the odds of surgical site infections in our obese patients. We do not routinely collect data on the presence of diabetes in our database, but we would expect more cases of diabetes in the obese group which may be contributing to the non-significant increase in infections in the most obese group. The current practice at our center is for patients to start prophylactic antibiotic coverage immediately prior to surgery which are continued until the mediastinal drains are removed. Our overall rate of surgical site infection was 4.4% which would place our center in the top decile (lowest rate) of centers for surgical site infections for CABG based on Medicare claims data(22).
It is also notable that the lower BMI subgroup did well after PTE. Several studies have identified lower BMI as a potential risk factor for adverse outcomes in coronary artery bypass grafting and valvular heart disease(23, 24). A J-shaped or U-shaped curve with higher risk for post-operative complications at the extremes of body mass index is possible but there was no clear signal in this analysis. Even though all of the BMI subgroups had similar outcomes, the mortality and post-operative complication were similar despite the more severe baseline pulmonary hemodynamics in the group with BMI less than 22 kg/m2.
There are two major limitations of this study. First, there is an inherent selection bias in who is referred for PTE and also in who is brought for surgery. It is possible that obese patients with either very high PVR or those with significant comorbidities are never referred for PTE or are turned down for surgery. We do not currently keep records of patients turned down to answer this question. We do not have explicit inclusion criteria for selecting patients for surgery but obesity is considered along with other comorbidities such as renal failure, liver disease, bleeding risk and location of disease. Second, this study only reflects the UCSD experience with this relatively uncommon procedure. While we are able to demonstrate satisfactory outcomes to patients who are deemed to be at high risk for perioperative morbidity and mortality such as the morbidly obese, this data provides little generalizability to newer and less experienced centers. This does, however, provide further evidence of the need for multi-center surgical registry in order to establish centers of excellence for high risk PTE cases.
When the data are viewed as a whole, the low mortality and rates of complications support the belief that PTE at a center experienced in the diagnosis and management of the disease remains the standard of care for patients with CTEPH. Our reported overall in-hospital mortality of 0.8% continues the previously reported trend of decreasing mortality over time with the expansion of clinical experience. (25). Contrary to previously published work(10), increasing BMI does not appear to increase the risk of postoperative mortality. Pulmonary thromboendarterectomy can safely be completed in obese patients with very low in-hospital mortality rates and no difference in perioperative complications or lengths of stay. Obesity, therefore, should not dissuade referral of patients with CTEPH to centers with experienced PTE surgery programs. As new management alternatives for CTEPH such as medical therapy and percutaneous angioplasty are becoming available(26, 27), the gold standard for management of these patients remains pulmonary thromboendarterectomy, even in groups of patients previously deemed to be at high risk for postoperative complications.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among us adults, 1999-2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Birkmeyer NJO, Charlesworth DC, Hernandez F, et al. Obesity and risk of adverse outcomes associated with coronary artery bypass surgery. Circulation. 1998;97:1689–1694. doi: 10.1161/01.cir.97.17.1689. [DOI] [PubMed] [Google Scholar]
- 3.Rockx MAJ, Fox SA, Stitt LW, et al. Is obesity a predictor of mortality, morbidity and readmission after cardiac surgery? Can J Surg. 2004;47:34. [PMC free article] [PubMed] [Google Scholar]
- 4.Yap C, Mohajeri M, Yii M. Obesity and early complications after cardiac surgery. Med J Aust. 2007;186:350. doi: 10.5694/j.1326-5377.2007.tb00935.x. [DOI] [PubMed] [Google Scholar]
- 5.Moulton MJ, Creswell LL, Mackey ME, Cox JL, Rosenbloom M. Obesity is not a risk factor for significant adverse outcomes after cardiac surgery. Circulation. 1996;94:87–92. [PubMed] [Google Scholar]
- 6.Oreopoulos A, Padwal R, Norris CM, Mullen JC, Pretorius V, Kalantar-Zadeh K. Effect of Obesity on Short- and Long-term Mortality Postcoronary Revascularization: A Meta-analysis. Obesity. 2008;16:442–450. doi: 10.1038/oby.2007.36. [DOI] [PubMed] [Google Scholar]
- 7.Lillenfeld DE, Vlahov D, Tenney JH, McLaughlin JS. Obesity and diabetes as risk factors for postoperative wound infections after cardiac surgery. Am J Infect Control. 1988;16:3–6. doi: 10.1016/0196-6553(88)90003-x. [DOI] [PubMed] [Google Scholar]
- 8.Zacharias A, Schwann TA, Riordan CJ, Durham SJ, Shah AS, Habib RH. Obesity and risk of new-onset atrial fibrillation after cardiac surgery. Circulation. 2005;112:3247–3255. doi: 10.1161/CIRCULATIONAHA.105.553743. [DOI] [PubMed] [Google Scholar]
- 9.Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic Thromboembolic Pulmonary Hypertension (CTEPH): Results From an International Prospective Registry. Circulation. 2011;124:1973–1981. doi: 10.1161/CIRCULATIONAHA.110.015008. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert TB, Gaine SP, Rubin LJ, Sequeira AJ. Short-term Outcome and Predictors of Adverse Events following Pulmonary Thromboendarterectomy. World J Surg. 1998;22:1029–1033. doi: 10.1007/s002689900511. [DOI] [PubMed] [Google Scholar]
- 11.Sin DD, Jones RL, Man S. Obesity is a risk factor for dyspnea but not for airflow obstruction. Arch Intern Med. 2002;162:1477–1481. doi: 10.1001/archinte.162.13.1477. [DOI] [PubMed] [Google Scholar]
- 12.Degano B, Sitbon O, Savale L, et al. Characterization of pulmonary arterial hypertension patients walking more than 450 m in 6 min at diagnosis. CHEST. 2010;137:1297–1303. doi: 10.1378/chest.09-2060. [DOI] [PubMed] [Google Scholar]
- 13.Rottlaender D, Motloch LJ, Schmidt D, et al. Clinical impact of atrial fibrillation in patients with pulmonary hypertension. PloS one. 2012;7:e33902. doi: 10.1371/journal.pone.0033902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maisel WH, Rawn JD, Stevenson WG. Atrial Fibrillation after Cardiac Surgery. Ann Intern Med. 2001;135:1061–1073. doi: 10.7326/0003-4819-135-12-200112180-00010. [DOI] [PubMed] [Google Scholar]
- 15.Echahidi N, Mohty D, Pibarot P, et al. Obesity and metabolic syndrome are independent risk factors for atrial fibrillation after coronary artery bypass graft surgery. Circulation. 2007;116:I–213. doi: 10.1161/CIRCULATIONAHA.106.681304. [DOI] [PubMed] [Google Scholar]
- 16.Thistlethwaite PA, Kaneko K, Madani MM, Jamieson SW. Technique and outcomes of pulmonary endarterectomy surgery. Ann Thorac Surg. 2008;14:274–282. [PubMed] [Google Scholar]
- 17.Blommaert D, Gonzalez M, Mucumbitsi J, et al. Effective prevention of atrial fibrillation by continuous atrial overdrive pacing after coronary artery bypass surgery. JACC. 2000;35:1411–1415. doi: 10.1016/s0735-1097(00)00608-2. [DOI] [PubMed] [Google Scholar]
- 18.Fan K, Lee KL, Chiu CSW, et al. Effects of Biatrial Pacing in Prevention of Postoperative Atrial Fibrillation After Coronary Artery Bypass Surgery. Circulation. 2000;102:755–760. doi: 10.1161/01.cir.102.7.755. [DOI] [PubMed] [Google Scholar]
- 19.Halonen J, Halonen P, Järvinen O, et al. Corticosteroids for the prevention of atrial fibrillation after cardiac surgery: A randomized controlled trial. JAMA. 2007;297:1562–1567. doi: 10.1001/jama.297.14.1562. [DOI] [PubMed] [Google Scholar]
- 20.Kerr KM, Auger WR, Marsh JJ, et al. The use of cylexin (CY-1503) in prevention of reperfusion lung injury in patients undergoing pulmonary thromboendarterectomy. Am J Respir Crit Care Med. 2000;162:14–20. doi: 10.1164/ajrccm.162.1.9712142. [DOI] [PubMed] [Google Scholar]
- 21.Olsen MA, Lock-Buckley P, Hopkins D, Polish LB, Sundt TM, Fraser VJ. The risk factors for deep and superficial chest surgical-site infections after coronary artery bypass graft surgery are different. J Thorac Cardiovasc Surg. 2002;124:136–145. doi: 10.1067/mtc.2002.122306. [DOI] [PubMed] [Google Scholar]
- 22.Huang SS, Placzek H, Livingston J, et al. Use of Medicare claims to rank hospitals by surgical site infection risk following coronary artery bypass graft surgery. Infect Control Hosp Epidemiol. 2011;32:775–783. doi: 10.1086/660874. [DOI] [PubMed] [Google Scholar]
- 23.Potapov EV, Loebe M, Anker S, et al. Impact of body mass index on outcome in patients after coronary artery bypass grafting with and without valve surgery. Eur Heart Jl. 2003;24:1933–1941. doi: 10.1016/j.ehj.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Thourani VH, Keeling WB, Kilgo PD, et al. The impact of body mass index on morbidity and short- and long-term mortality in cardiac valvular surgery. J Thorac Cardiovasc Surg. 2011;142:1052–1061. doi: 10.1016/j.jtcvs.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Madani MM, Auger WR, Pretorius V, et al. Pulmonary endarterectomy: recent changes in a single institution's experience of more than 2,700 patients. Ann Thorac Surg. 2012;94:97–103. doi: 10.1016/j.athoracsur.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Ghofrani H-A, D'Armini AM, Grimminger F, et al. Riociguat for the Treatment of Chronic Thromboembolic Pulmonary Hypertension. N Engl J Med. 2013;369:319–329. doi: 10.1056/NEJMoa1209657. [DOI] [PubMed] [Google Scholar]
- 27.Taniguchi Y, Shinke T, Kinutani H, Otake H, Emoto N, Hirata K. Efficacy and safety of balloon pulmonary angioplasty for inoperable patients with peripheral type chronic thromboembolic pulmonary hypertension. Eur Heart J. 2013:34. [Google Scholar]