Abstract
Background
Studies have suggested that when intravenous (IV) soybean oil (SO) is replaced with fish oil (FO), direct hyperbilirubinemia is more likely to resolve. The necessary duration of FO has not been established. This study seeks to determine if 24 weeks of FO is an effective and safe therapy for intestinal failure associated liver disease (IFALD).
Materials and Methods
This is a clinical trial using patients with IFALD between the ages of 2 weeks and 18 years. SO was replaced with FO (1 g/kg/day) in 10 subjects who were receiving the majority of their calories from parenteral (PN). Subjects were compared to 20 historical controls receiving SO. SO for both groups was prescribed by the primary medical team at variable doses. The primary outcome was time to reversal of cholestasis. Secondary outcomes were death, transplant, and full enteral feeds. Safety measurements included growth, essential fatty acid deficiency, and laboratory markers to assess bleeding risk.
Results
The Kaplan-Meier method estimates that 75% in the FO group will experience resolution of cholestasis by 17 weeks vs. 6% in the SO group (p < 0.0001). When compared to the SO group, the FO group had decreased serum direct bilirubin concentrations at weeks 8 (p=0.03), 12, 16, 20 and 24 (p< 0.0001). While length Z-score at the end of the study increased in the FO group compared to baseline (p=0.03), there were no significant differences in other outcomes.
Conclusions
A limited duration of FO appears to be safe and effective in reversing IFALD.
Keywords: cholestasis, short bowel syndrome, fish oil, soybean oil, prematurity
Introduction
Parenteral nutrition (PN) is a major contributing factor to the long-term survival of children with intestinal failure (IF)1. IF results in prolonged PN dependence secondary to intestinal malabsorption. With an incidence of 3–5 cases per 100,000 births, IF in children is commonly caused by shortened and/or dysfunctional bowel due to necrotizing enterocolitis and congenital gastrointestinal (GI) disorders such as gastroschisis, intestinal atresias and volvulus1–4. For children with IF, PN serves as a bridge to bowel adaptation, a process by which the intestine recovers and improves its absorptive capacity. During this time, PN provides necessary fluids and micro- and macronutrients to prevent dehydration and promote growth and neurodevelopment.
The process of bowel adaptation can take weeks to years and can be complicated by intestinal failure associated liver disease (IFALD), which in turn is associated with a high morbidity and mortality1,3,4–7. Pediatric IFALD is hallmarked by cholestasis exhibited biochemically by direct hyperbilirubinemia, elevated serum liver transaminases, and histological findings on liver biopsy including cholestasis, steatosis, lipidosis, and eventually fibrosis and cirrhosis. As IFALD progresses, patients can develop end stage liver disease exhibited by ascites, coagulopathy, portal hypertension, and hepatic encephalopathy. While an isolated intestine or combined liver-intestine transplantation may be life-saving for children with IF, these may not be feasible options for some children because of their small size, co-morbidities, and lack of timely organ availability8. The wait-list mortality rate for children awaiting a combined liver-intestine transplantation has historically been much higher than those awaiting transplantation of other solid organs8. For those who receive a transplant, the five-year survival is 50–70% and can be complicated by graft rejection, infection, and malignancy1,8.
The safest and most effective treatment for IFALD remains successful rehabilitation to full enteral nutrition (EN). However, enteral autonomy in children with IF may take time, or may not be feasible at all due to GI dysmotility, malabsorption, and/or insufficient GI length1,4,5. Moreover, IFALD has been shown to develop as early as a few weeks to months after PN initiation, particularly in low birth weight infants5,9–11.
Intravenous (IV) fatty acid emulsions are prescribed with PN to provide additional calories, prevent essential fatty acid deficiencies, promote growth and neurodevelopment, and improve metabolic efficiency. Available fatty acid emulsions differ in composition and recommended dose, which complicate direct comparisons of efficacy. While the US Food and Drug and Administration (FDA)-approved soybean oil-based emulsions (SO) have traditionally been prescribed at a maximum dose of 3–4 g/kg/d, a non-FDA approved fish oil-based emulsion (FO) used in Europe and Asia is prescribed at 1 g/kg/d and is available off-label in the US under compassionate use and research protocols.
The development of IFALD in children has been associated with the composition and dose of fatty acid emulsions9–15. Animal studies, case reports and cohort studies have provided encouraging evidence that when 1g/kg/d of exclusive FO is substituted for SO, biochemical IFALD is more likely to resolve9–13,16–19. Despite these reports and increased experience with FO at several US centers, there remains significant barriers for administering FO to IF patients due to a much higher unit cost, lack of FDA approval, and uncertainty of long-term safety and efficacy. Because FO is considered an experimental therapy, third party payers did not always provide reimbursement. Consequently, hospitals and investigators shoulder the majority of the cost of this therapy.
The required duration of FO monotherapy is unknown because prior studies usually prescribe FO until PN discontinuation9–11. From the available studies in the literature, biochemical reversal of IFALD with FO monotherapy has been shown to occur between 35 days and 24 weeks9–12. We sought to determine whether a finite period of FO monotherapy followed by resumption of SO and continued PN was safe and efficacious in children with IF. This study reports on the preliminary results of a prospective clinical trial in which 24 weeks of FO was substituted for SO in 10 subjects with IFALD. Subjects were compared to 20 historical controls who received SO.
A FO treatment course of 24 weeks was selected based off available evidence from studies at the design of this study, our experience with FO under compassionate use, and to minimize FO cost and potential adverse events9,12,20,21. Considering the number of children who would satisfy the study’s inclusion criteria and that some subjects may require PN for years, the cost of prolonged FO treatment would have been prohibitive. In addition, resuming SO at a dose ≥ 1 g/kg/d, once the liver has matured, IFALD has biochemically resolved, and EN has increased, may be safe and satisfy the nutritional requirements for growth.
Methods
Patient Population
Ten interventional subjects were recruited from the University of California, Los Angeles (UCLA) Mattel Children’s Hospital. Eligibility criteria included clinical evidence of IFALD, a direct bilirubin 2 mg/dL on two consecutive measurements separated by at least 1 week, anticipated PN course > 30 days, an acquired or congenital GI disorder, > two weeks of age but < 18 years of age, > 60% of total calories from PN, and failure of standard therapies used to treat IFALD. Subjects with a primary liver disease, inborn error of metabolism, seafood, egg or FO allergy, hemorrhagic disorder, hemodynamic instability or shock, comatose state, stroke, pulmonary embolism, myocardial infarction, diabetes, or fatal chromosomal disorder, or on extracorporeal membrane oxygenation were excluded.
Twenty historical controls were selected from an institutional review board (IRB) approved home PN and neonatal intensive care database from 2004 to 2009. Controls satisfied applicable inclusion and exclusion criteria.
IF was defined as PN dependence for > 60 days, but was not required for study entry. PN dependence was defined as receiving > 60% of one’s calories from PN. The number of GI surgeries, not including gastrostomy tube placements or rectal biopsies, and bloodstream infections (a positive blood culture, signs and symptoms suggestive of sepsis, and need for at least five days of IV antibiotics) were compared between the two groups.
Written informed consent was obtained from interventional subjects and the study was approved by the UCLA IRB and FDA (IND 105,326) beginning in August 2009. A waiver of consent was approved by the IRB for historical controls. The study is registered at www.clinicaltrials.gov (NCT00969332).
Study Procedures and Methods
Once enrolled, each of the 10 interventional subjects received 0.5 g/kg/d IV for the first two days, then 1 g/kg/d IV of FO (Omegaven, Fresenius Kabi, Bad Homberg Germany) over 8–24 hours in the inpatient or outpatient setting for an intended period of 24 weeks. FO was to be discontinued prior to 24 weeks if the subject no longer required PN, underwent liver and/or intestinal transplantation, or developed serious adverse complications from FO. In order to observe for tolerance, the first two FO doses were administered in the inpatient setting or UCLA’s Clinical and Translational Science Institute. If a subject demonstrated persistent or recurrent cholestasis after 24 weeks of FO and continued to satisfy inclusion and exclusion criteria, he or she could receive two more courses of FO monotherapy for a maximum of 48 additional weeks. The 20 historical controls received SO (Intralipid, Fresenius Kabi,Uppsala, Sweden) at variable doses selected by their primary caregivers. Data collection for all 30 subjects began at the time the subject enrolled in the trial or the control subject satisfied inclusion and exclusion criteria. Data collection then continued for 6 months or until death, transplant or PN discontinuation, whichever came first. FO subjects were followed post intervention for another 1.5 years.
Study Outcomes
Outcomes were measured after 24 weeks of FO or SO treatment or death, transplant, or PN discontinuation, whichever came first. The primary outcome was time to resolution of cholestasis (defined as a direct bilirubin < 2 mg/dL on consecutive measurements separated by one week). Secondary outcomes included death, transplant, and full enteral feeds. Tertiary outcomes included number of hospital readmissions, number of inpatient days, and normalization of serum liver transaminases on consecutive measurements separated by one week. Measures of safety included growth (weight, length, and head circumference) and the development of an essential fatty acid deficiency in the FO group only, and platelet counts and International Normalized Ratios (INR) in both groups. An essential fatty acid deficiency was defined as a triene:tetraene ratio (mead acid:arachidonic acid) ≥ 0.5.
Statistical Methods
Continuous variables were compared between groups using the Wilcoxon rank sum test. Categorical variables were compared between the groups using the Fisher’s exact test. Laboratory values were transformed using a log base 10 scale since these values are normally distributed on the log scale. Median profiles were compared over time using a repeated measure analysis of variance model. Since not all laboratory values were measured exactly at 4, 8, 12, 16, 20 and 24 weeks for a given subject, some of the subjects’ values were aligned to these times using local linear interpolation. Median platelet, albumin, triglyceride, and INR values were compared at baseline, 8, 16, and 24 weeks. There was no extrapolation beyond times where values were not observed.
The probabilities of experiencing cholestasis resolution across time were estimated using the Kaplan-Meier method. The Kaplan-Meier curves were compared between the groups using the log rank test.
Growth for FO subjects was assessed by z scores. All anthropometric measurements were corrected for prematurity if the subject was less than two years of age. Mean and standard deviations for weight, length, and head circumference were obtained from the Center for Disease Control and Prevention (CDC).
A p-value < 0.05 was considered statistically significant.
Results
Baseline characteristics between the FO and SO groups were similar (Table 1). Both the study and control populations were premature with a median gestational age of 34 weeks. The primary GI diagnoses for the two groups were similar. Both the groups’ median number of GI surgeries and mean small bowel length, if an intestinal resection occurred and small bowel length was recorded by a surgeon, were comparable. FO and SO’s median small bowel length was not significantly different with a median (range) of 23 cm (0–51 cm) and 27 cm (0–55 cm) in 7 and 13 subjects, respectively. The appendix provides specific diagnoses for each subject and the length of small bowel remaining if bowel was resected and this information was recorded. As defined, IF was present in 80% of the FO group and 55% of the SO group, which was not statistically significant.
Table 1.
Characteristics of subjects in the fish oil (FO) and soybean oil (SO) group at 3 baseline. Results are depicted either as a median with the corresponding range or 4 percentage (n).
| Variable | FO (n=10) | SO (n=20) | p value |
|---|---|---|---|
| Age at Start of Study, days | 136 (33–334) | 76 (27–866) | 0.29 |
| Age at End of Study, days | 259 (81–456) | 241 (96–1035) | 0.66 |
| Sex – Male % (n) | 30 (3) | 65 (13) | 0.12 |
| Gestational Age (weeks) | 34 (25–39) | 34 (25–39) | 0.89 |
| Birth Weight (kg) | 2.3 (0.9–3.1)† | 2.3 (0.9–3.3)‡ | 0.85 |
| Primary Gastrointestinal Diagnosis | |||
| -gastroschisis % (n) | 40 (4) | 40 (8) | 0.37 |
| -intestinal atresia % (n) | 20 (2) | 20 (4) | |
| -necrotizing enterocolitis % (n) | 20 (2) | 25 (5) | |
| -volvulus % (n) | 0 (0) | 15 (3) | |
| -malabsorption syndrome % (n) | 10 (1) | 0 (0) | |
| -MMIHS % (n) | 10 (1) | 0 (0) | |
| No. of Prior Gastrointestinal Surgeries | 1 (0–5) | 2 (1–8) | 0.65 |
| Intestinal Failure - Yes % (n) | 80 (8) | 55 (11) | 0.25 |
| Small Bowel Length (cm) | 23(0–51)§ | 27(0–55)¶ | 0.78 |
| Presence of an Ileocecal Valve – Yes % (n) | 60 (6) | 55 (11) | 0.13 |
| 100% of Colon Present – Yes % (n) | 70 (7) | 55 (11) | 0.69 |
| Small Bowel Connected to Colon – Yes % (n) | 70 (7) | 55 (11) | 0.69 |
| Ursodeoxycholic acid – Yes % (n) | 30 (3) | 25 (5) | 0.99 |
Data was only available for 8,
19,
7, and
13 subjects. MMIHS, megacystis microcolon intestinal hypoperistalsis syndrome.
PN at the beginning and end of the study was comparable between the two groups with respect to duration, total calories, glucose delivery rate, and amino acid dose (Table 2). However, at the start and end of the study, the mean intravenous fat dose (SD) was less in the FO group in comparison to the SO group (1.50.9 vs. 2.60.7 g/kg/d, p < 0.006 and 0.90.3 vs. 1.71.1 g/kg/d, p=0.02). While not statistically significant, at the beginning of the study, the FO group received more enteral calories than the SO group with a median intake of 23 kcal/kg/day (range 0–43 kcal/kg/day) compared 4 kcal/kg/day (range 0–39 kcal/kg/day) (p=0.05). During the study, the number of bloodstream infections did not differ between the FO and SO groups. The FO group had a median of 1.5 infections (range 0–4) and the SO group had a median of 0.5 infections (range 0–3) (p=0.14). There was no difference in the use of ursodeoxycholic acid between the FO and SO group (30 vs. 25%, respectively, p=0.99). The SO group, however, had more GI surgeries during the study period when compared to the FO group with a median of 1 (range 0–2) vs. 0 (range 0–1) (p=0.02).
Table 2.
Nutritional characteristics of the fish oil (FO) and soybean oil (SO) group. Results are depicted either as a median with the corresponding range, mean ± standard deviation (*), or percentage (n). PN, parenteral.
| Variable | FO (n=10) | SO (n=20) | p value |
|---|---|---|---|
| At the Beginning of the Study | |||
| PN duration (days) | 137 (33–334) | 72 (27–710) | 0.09 |
| GDR (g/kg/min)* | 16.0±4.4 | 15.2±3.8† | 0.96 |
| Amino Acids (g/kg/day)* | 2.8±0.8 | 2.7±0.7‡ | 0.94 |
| Soybean Oil (g/kg/day)* | 1.5±0.9 | 2.6±0.7§ | 0.006 |
| PN kcal/kg/day* | 98±17 | 95±20§ | 0.83 |
| Enteral kcal/kg/day | 23 (0–43) | 4 (0–39)§ | 0.05 |
| Solid Foods–Yes% (n) | 0(0) | 5(1) | 0.99 |
| At the End of the Study | |||
| GDR (g/kg/min)* | 17.0±6 | 15.0±4¶ | 0.66 |
| Amino Acids (g/kg/day)* | 1.9±0.5 | 1.8±0.8§§ | 0.68 |
| FishorSoybeanOil (g/kg/day)* | 0.9±0.3 | 1.7±1.l†† | 0.02 |
| PN kcal/kg/day* | 74±20 | 80±24§§ | 0.85 |
| Enteral kcal/kg/day | 32 (0–109) | 0.6 (070)* | 0.21 |
| Solid Foods–Yes% (n) | 40(4) | 25(5) | 0.43 |
Data was only available for 15,
17,
18,
13,
14,
12, and
16 subjects.
At baseline, the FO and SO group’s median (SEM) total bilirubin were comparable (10.21.1 vs. 70.5 mg/dL, p=0.2). The direct bilirubin was also comparable for the FO and SO group (6.10.8 vs. 4.10.4 mg/dL, p = 0.3). Baseline median aspartate aminotransferase (AST), alanine transferase (ALT), gamma-glutamyl transpeptidase (GGT) (data not shown), and triglyceride concentrations were similar between the two groups (Figure 1). The FO group’s baseline median albumin was higher than the SO group’s albumin (3.60.09 vs. 3.10.05 mg/dL, p = 0.04).
Figure 1.
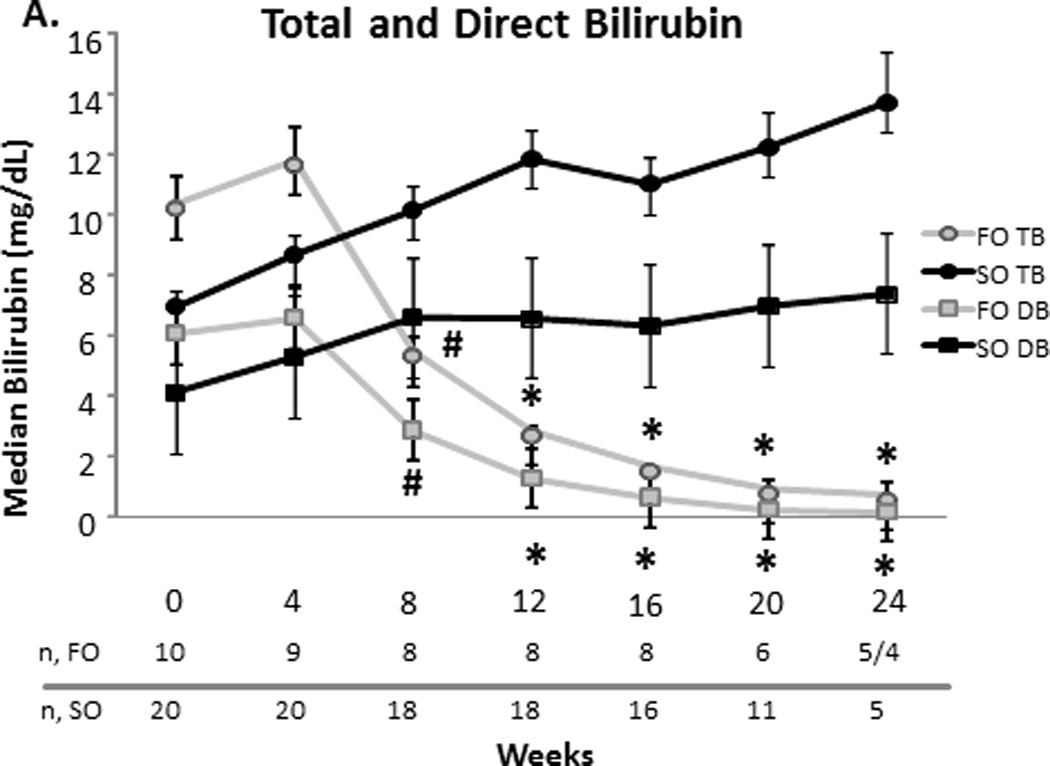
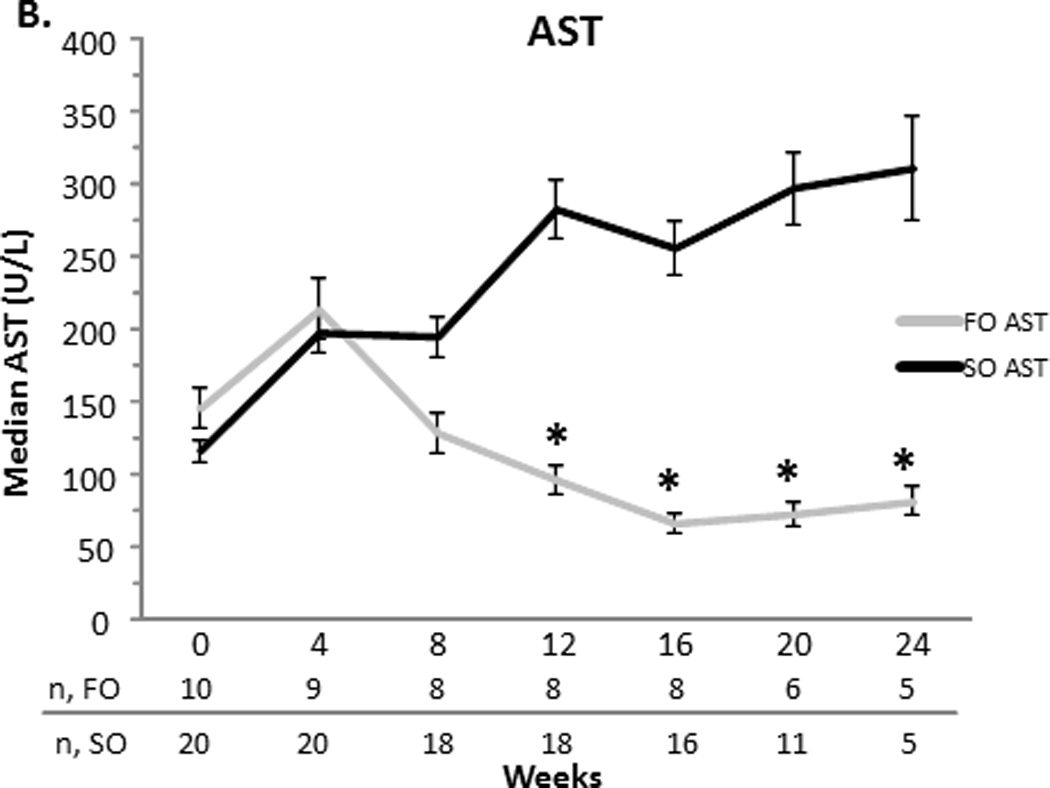
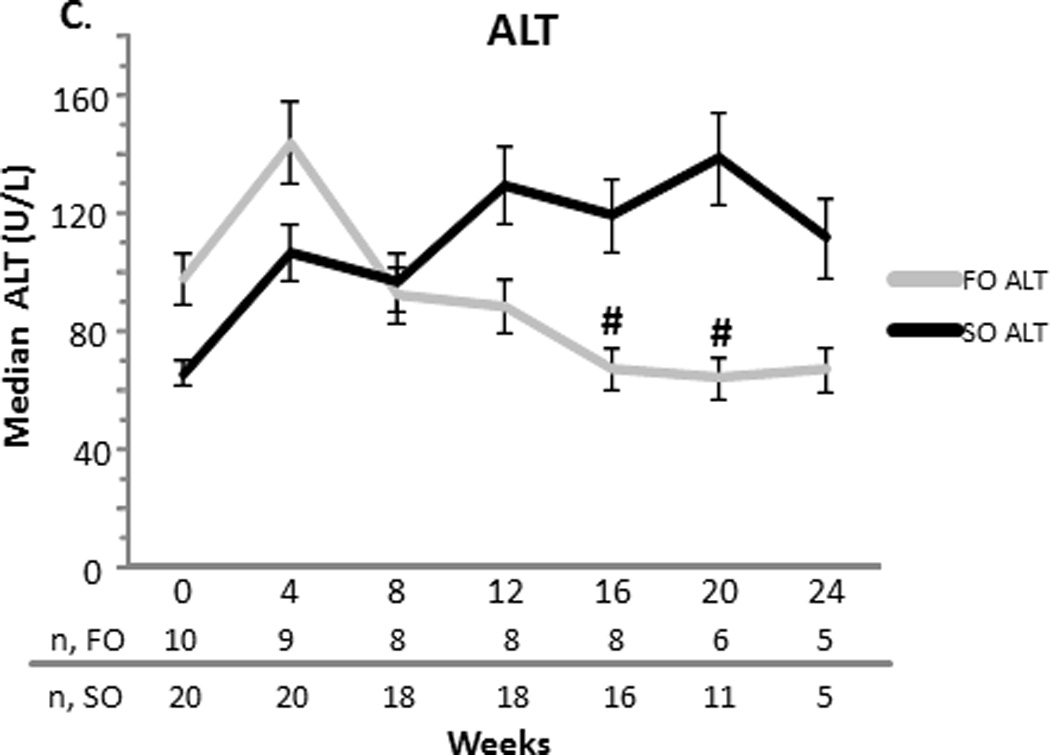
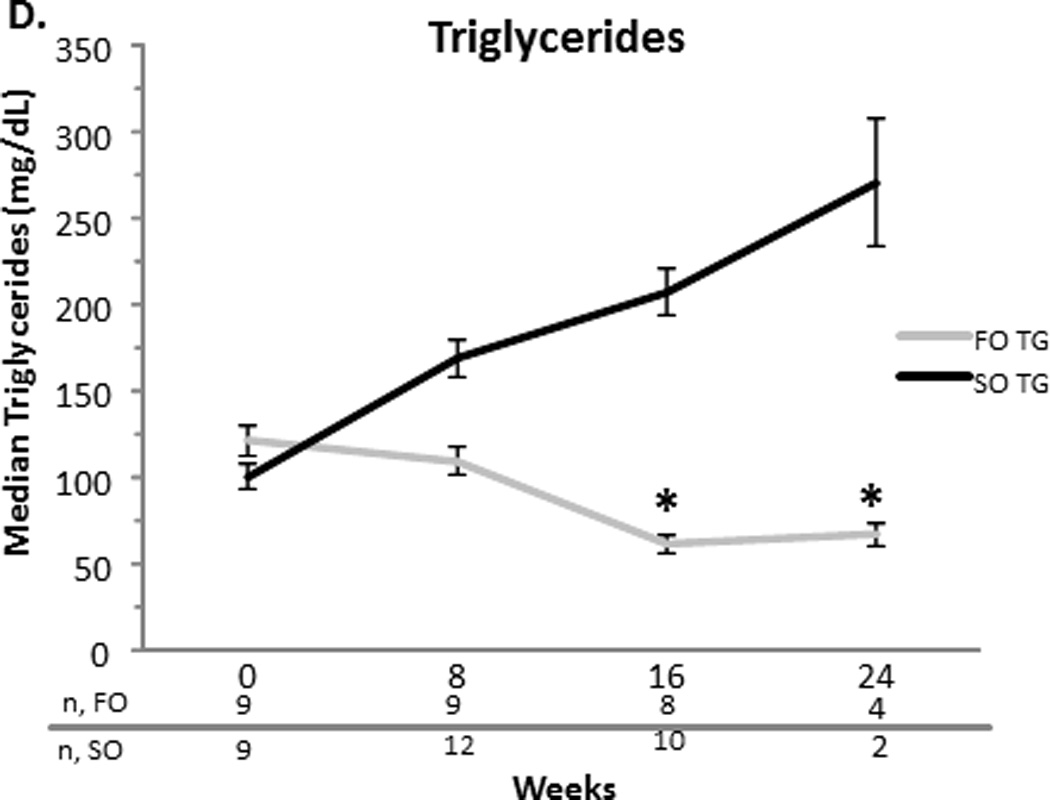
A–D. Median serum total bilirubin (TB) and direct bilirubin (DB), aspartate aminotransferase (AST), alanine transferase (ALT), and triglyceride concentrations with the standard error of the mean for the fish oil (FO) and soybean oil (SO) group. Sample sizes (n) are depicted for FO and SO by week beneath the graph. * p < 0.001. # p < 0.05.
Compared to the SO group, the FO group’s direct and total bilirubin decreased significantly over time. When compared to the SO group at specific time points, FO’s direct bilirubin was less at weeks 8 (2.9 ± 0.4 vs. 6.6 ± 0.6 mg/dL, p = 0.03), 12 (1.3 ± 0.2 vs. 6.6 ± 0.5 mg/dL, p < 0.0001), 16 (0.7 ± 0.1 vs. 6.3 ± 0.6 mg/dL, p <0.0001), 20 (0.3 ± 0.04 vs. 7 ± 0.7 mg/dL, p <0.0001) and 24 weeks (0.2 ± 0.03 vs. 7.4 ± 1.1 mg/dL, p < 0.0001). Total bilirubin followed a similar trend. Compared to the SO group, the FO group’s AST significantly decreased at weeks 12, 16, 20 and 24 (p<0.001 for each week). Compared to the SO group, the FO group’s ALT decreased later beginning at week 16 (p<0.05). This decrease remained significant at week 20 (p<0.05), but was not significant at week 24. At week 16 only, albumin was increased significantly in the FO group when compared to the SO group (3.60.1 vs. 3.00.1 mg/dL, p=0.02) (data not shown). At weeks 16 and 24, the FO group’s triglycerides were significantly less than that of the SO group (p<0.001 for each week) (Figure 1).
Primary Outcome
Median follow-up time for the primary endpoint, time to resolution of cholestasis, was 11.5 weeks (range 2.4 – 18 weeks) and 24 weeks (range 5.4 – 24 weeks) for the FO and SO group, respectively. As shown in Figure 2, the Kaplan-Meier method estimates (±SEM) that 75% (±16%) in the FO group will experience resolution of cholestasis by 17 weeks vs. 6% (±6%) in the SO group (p < 0.0001). After 11.5 weeks of FO monotherapy, 50% of subjects will biochemically reverse their IFALD.
Figure 2.
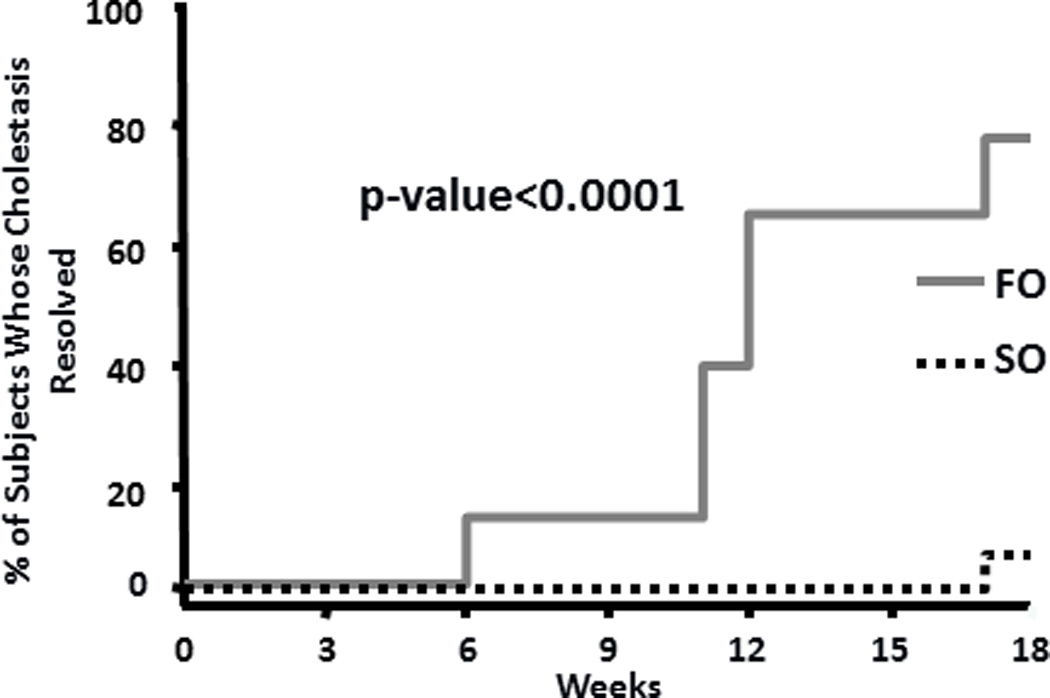
Kaplan-Meier curves for resolution of cholestasis.
Secondary and Tertiary Outcomes
There was no difference in death, transplant, percentage of patients on full enteral feeds, number of readmissions, number of inpatients days, and percentage of subjects with normalization of liver function tests (Table 3). Twenty percent of FO subjects expired, while 10% of SO subjects died (2 vs. 2). Ten percent of subjects in the FO and SO group received a transplant (1 vs. 2). Both FO subjects who died were diagnosed with septic shock and medical care was withdrawn because continuation of care was considered futile. While one FO subject who died demonstrated hepatic deterioration, the other subject’s cholestasis had resolved. The two deaths in the SO group were attributed to liver failure.
Table 3.
Secondary and tertiary outcomes for the fish oil (FO) and soybean oil (SO) group. Results are either as medians with the corresponding range, or a percentage (n)
| Outcome | FO (n=10) | SO (n=20) | p value |
|---|---|---|---|
| Death – Yes % (n) | 20 (2) | 10 (2) | 0.58 |
| Transplant – Yes % (n) | 10 (1) | 10 (2) | 0.99 |
| Full Enteral Feeds – Yes % (n) | 10 (1) | 15 (3) | 0.99 |
| Number of Readmits | 2 (0–4) | 1 (0–4) | 0.38 |
| Number of Inpatient Days | 39 (17–59) | 87 (16–170) | 0.08 |
Safety
There was no difference in platelet concentrations and INR at baseline and weeks 8, 16, and 24 between the two groups (Figure 3). Mean z-scores for weight and head circumference at baseline and the end of the study were comparable for the FO group. The FO group’s mean length Z-score (SEM) at the end of the study increased when compared to baseline (−0.90.3 vs. −1.80.4, p= 0.03) (Figure 4). None of the FO subjects developed an essential fatty acid deficiency and the range for mean triene:tetraene ratios during the study was 0.01–0.03.
Figure 3.
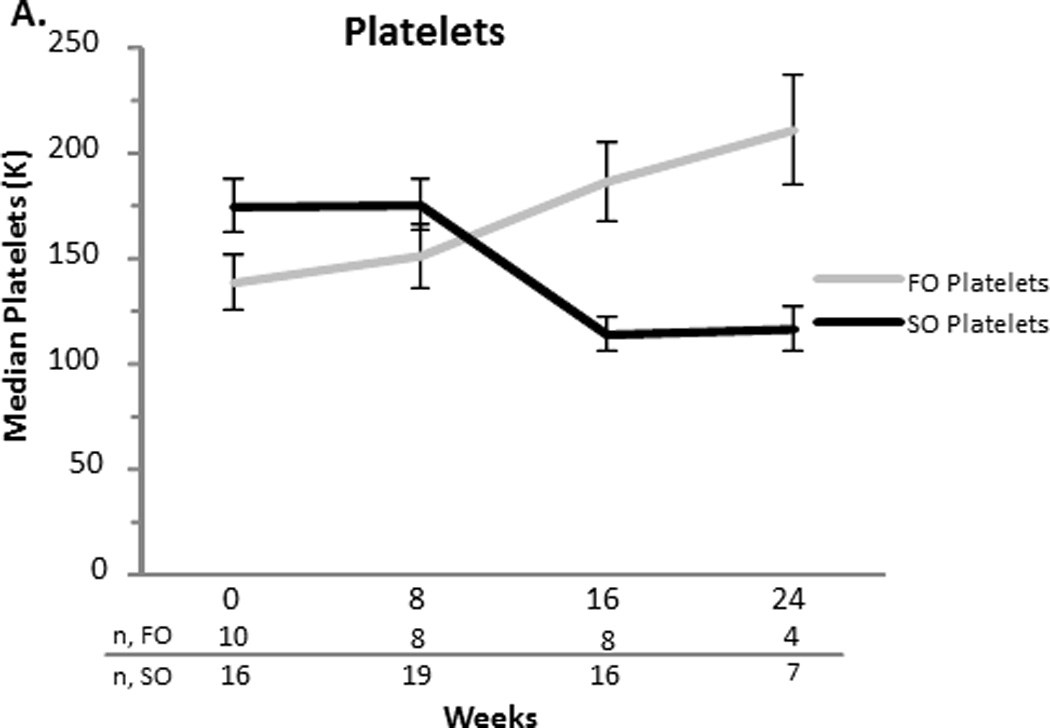
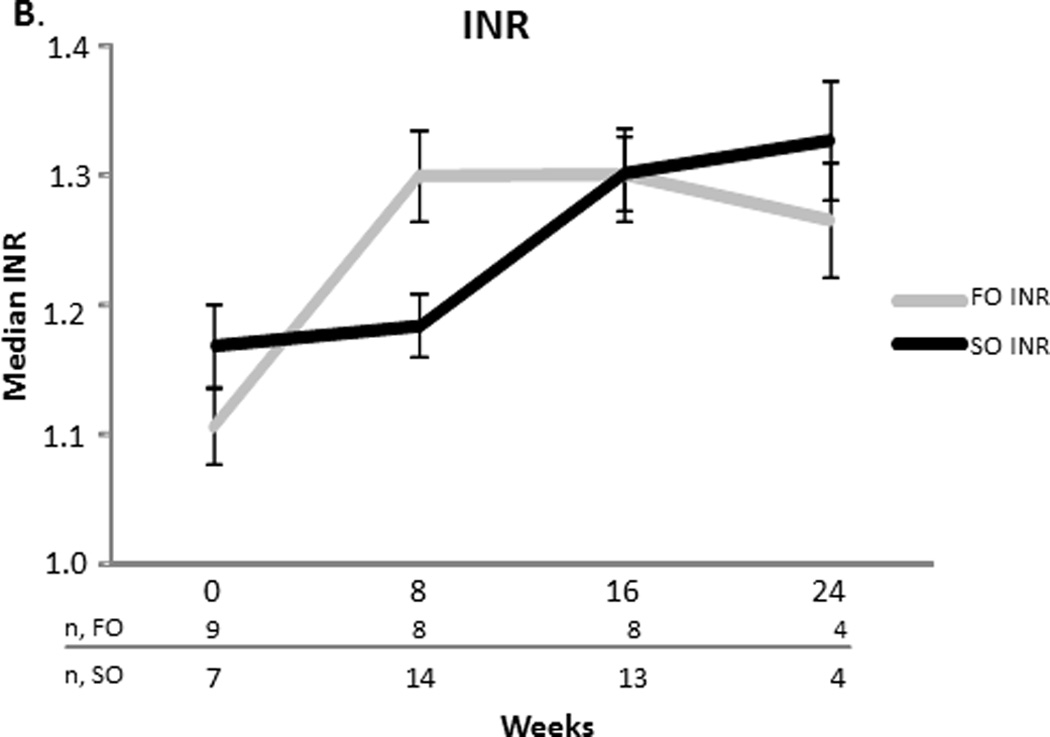
A and B. Median platelets and INR values with the standard error of the mean for the fish oil (FO) and soybean oil (SO) group. Sample sizes (n) are depicted for FO and SO by week beneath the graph.
Figure 4.
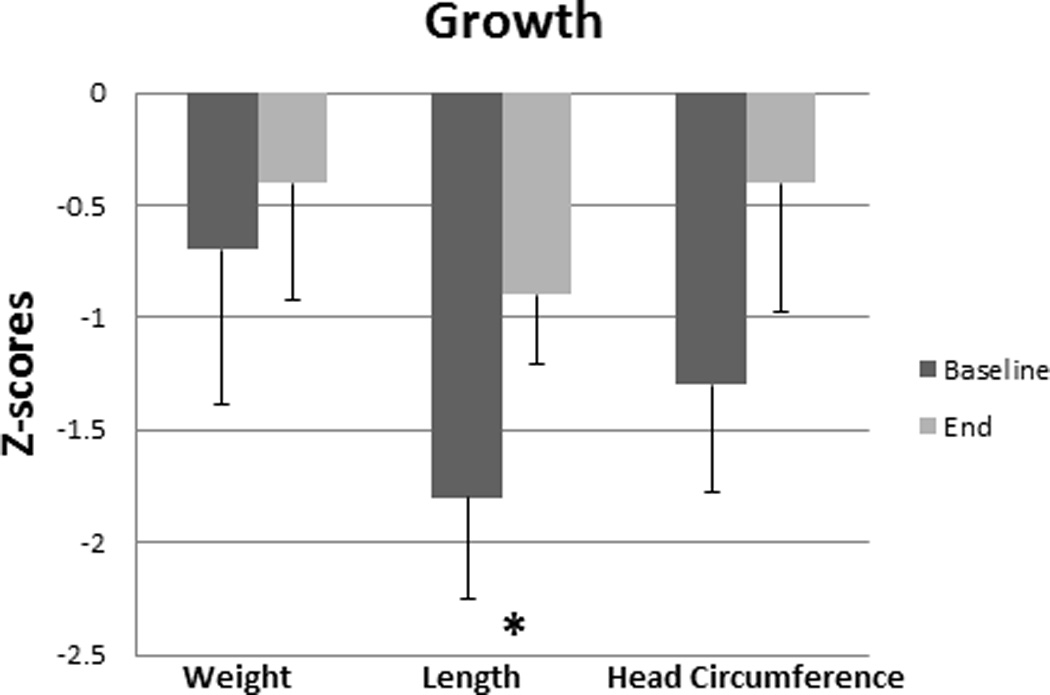
Median z scores for weight, length, and head circumference with the corresponding standard error of the mean for the fish oil (FO) group at baseline and the end of the study. *p < 0.05.
Follow-up
Eight subjects including the one subject who received a transplant were alive at the end of FO intervention. When FO was discontinued, 6 of the 8 subjects (75%) remained PN dependent and were transitioned to SO with a mean dose of 0.9 g/kg/d (range 0.6–1 g/kg/d). The decision to restart SO and SO dose was at the discretion of the primary medical team. One subject was not available for long-term follow-up after FO discontinuation. Subjects were followed for a median 1.9 years (range 1 - 2 years). During follow-up, one subject remained free of cholestasis and experienced intestinal adaptation after 12 additional months of PN and SO. Another PN-dependent subject received a multi-visceral transplant and redeveloped cholestasis prior to transplant.
At the end of follow-up, 3 of the 7 living children who received FO and were available for follow-up continued to receive PN with a mean SO dose of 1 g/kg/d (range 0.5–1.3 g/kg/d) and PN kcal/kg/d of 63.5 kcal/kg/d (range 44–75 kcal/kg/d). These subjects continued to have normal direct bilirubin concentrations. Median serum ALT and AST concentrations at the end of the follow-up period decreased when compared to measurements at FO termination (32 and 33 U/L vs. 67 and 81 U/L, respectively).
Discussion
The substitution of 1 g/kg/d of FO monotherapy for 24 weeks in 10 IFALD subjects was associated with biochemical reversal of cholestasis and improved liver function at the end of FO intervention when compared to 20 historical controls who received SO at variable doses. Of the FO group, 75% demonstrated resolution of their cholestasis by 17 weeks of FO monotherapy. After a median of 1.9 years of follow-up, all surviving subjects who were available for follow-up were free of cholestasis including those who received a transplant or continued to receive PN with SO.
In this study, the FO and SO group were at high risk for complications from IF based on their prematurity, low volumes of EN, unfavorable GI anatomy, and baseline serum direct bilirubin concentrations and liver function tests. Neonates born premature and with congenital or acquired GI disorders are at high risk for IFALD because they are exposed to long PN courses due to intestinal dysfunction3,5,6. Predisposing factors for these populations include an immature biliary system, small for gestational age, necrotizing enterocolitis, abdominal surgeries, intestinal resections, and sepsis3,4,22,23. Most of these factors result in insufficient enteral intake.
Children with congenital GI disorders are predisposed to develop IFALD5,14. As serum bilirubin concentrations climb, so does mortality7,11. In fact, 38% of patients with a conjugated bilirubin ≥ 10 mg/dL will die or require a transplant referral7. Premature neonates and those with ultra-short gut, intestinal discontinuity, without distal ileum and/or an ileocecal valve, and repeated episodes of sepsis are at highest risk1,6,5,23.
PN-specific contributors to IFALD include PN duration and an excess, deficiency, or imbalance of PN macro- or micronutrients5,24. In this study, glucose delivery rate and amino acid dose were comparable, and baseline median PN duration was 137 and 72 days in the FO and SO group, respectively. Reflecting changes in medical practice, the FO group was receiving less SO (g/kg/d) at baseline than historical controls14–15. After FO termination, subjects were prescribed PN with a low dose of SO (mean 0.9 g/kg/d). During follow-up, lipid-sparing was continued, and one subject redeveloped IFALD requiring a transplant. Prior observations have demonstrated that SO dose reduction can result in resolution of cholestasis or a decreased incidence of cholestasis in children and adults15,24–26. When compared to a historical cohort who received the standard SO dose, surgical neonates with cholestasis who received 1 g/kg of SO twice a week had an increased incidence of IFALD resolution (42 vs. 10%). However, this cohort also demonstrated an increased risk for a mild fatty acid deficiency, defined as a triene:tetraene ratio > 0.05, and had abnormal concentrations of specific polyunsaturated fatty acids15.
Concerns with lipid minimization include the risk for essential fatty acid deficiency and decreased caloric intake from fat resulting in adverse effects on growth and long-term neurodevelopment. Appropriate provisions of fat, specifically during critical periods of development, are important for myelination and brain growth. Children born premature and with short bowel syndrome children have high rates of growth failure and developmental delays3,27–30. This study, along with other SO lipid minimization and FO studies, have not reported a change in short-term growth9,15. While there was improvement in the Z-score for length, overall growth in our study population was suboptimal. Long-term studies are lacking and it remains unknown if lipid restriction adversely affects cognitive and behavioral development. In order to make-up for decreased fat calories, clinicians may increase glucose delivery rates, which may promote lipid deposition in the liver and peripheral tissues, IFALD, hyperinsulinemia, and carbon dioxide retention24.
Our results lend further support to case reports and recent studies regarding FO’s efficacy and safety as a therapy for IFALD9–13,16–20. Puder et al and Premkumar et al published the results of the largest cohorts to date10,11. One study compared 42 IFALD subjects to 49 historical controls while the other study prospectively followed 57 subjects10,11. While our study’s sample size is much smaller than these studies, our FO cohort would be predicted to be at high risk for IFALD complications, including death. Compared to the Premkumar et al and Puder et al study, our median age is 4.5 vs. 39 weeks post-menstrual age vs. 3 months and baseline direct bilirubin is 6.1 vs. 7.5 vs. 5.5 mg/dL9,10. The median small bowel length in our study for the FO group was 23 cm resulting in a median enteral tolerance of 23 kcal/kg/d at the start of the study and a meager 9 kcal/kg/day increase by the end of the study. After 6 months of FO therapy and a median follow-up of 1.9 years, 3 of 7 children who were alive and available for follow-up remained PN-dependent and were without cholestasis.
Of the FO cohort, 70% achieved biochemical reversal of their cholestasis during FO treatment. Our data predicts that approximately 50% of children with IFALD will reach this goal by study week 11.5. In a cohort described by Premkumar et al, 83% of surviving subjects achieved reversal with a median time of 35 days11. Approximately 45 and 60% of the populations described by Gura et al and Puder et al, respectively, achieved this goal with a median time of 9–12 weeks9,10. In addition to gestational age and severity of IFALD, co-morbidities, EN at baseline, the intestine’s ability to adapt, and the number of septic episodes and abdominal surgeries during treatment may serve as predictors of FO non-responders and slow responders11,31.
A major confounding factor of all FO studies, including our study, has been the dose of the lipid product9–14. It remains unclear if dose alone, composition, or dose and composition are factors that aid in the biochemical resolution of IFALD. Interestingly, in a study by Diamond et al, four of the nine subjects experienced resolution of their cholestasis with a combination of FO and SO, each dosed at 1 g/kg/d, while five subjects were switched to FO monotherapy prior to reaching their endpoint12. This brings into question if an emulsion composed entirely of FO at 1 g/kg/d is required for the treatment of IFALD. SMOFlipid (Fresenius Kabi, Bad Homberg, Germany), an emulsion comprised of some FO and SO, along with medium chain triglycerides in the form of coconut oil and monounsaturated fatty acids in the form of olive oil, can be dosed > 1 g/kg/d. While a mixed emulsion may appear to be more “balanced,” results regarding IFALD prevention and treatment have been conflicting32–39.
FO’s mechanism is likely multifactorial. SO is predominately made up of omega-6 fatty acids and contains the only 2 essential polyunsaturated fatty acids in humans, linoleic and ∝-linolenic acid. Linoleic acid is converted to arachidonic acid, which generates inflammatory prostaglandins, leukotrienes, and thromboxanes. ∝-linolenic acid is metabolized to eicosapentaenoic and docosahexaenoic acid, which are known for their anti-inflammatory properties and role in visual and cognitive development40. FO, on the other hand, is mainly comprised of the omega-3 fatty acids, eicosapentaenoic and docosahexaenoic acid, which are absent in SO, but can be synthesized from ∝-linolenic acid. By the “traditional” definition, essential fatty acids must be consumed in the diet and cannot be synthesized de novo. However, in premature neonates and children with short bowel syndrome, specifically those with cholestasis, provision and absorption of endogenous fatty acids may be limited41–43. At the same time, production of downstream products may be insufficient because of an increased demand or deficiencies of desaturase enzymes41. Placental transfer of polyunsaturated fatty acids mainly occurs in the third trimester, thereby increasing the risk for deficiencies in preterm babies43. As a result, PN dependent neonates whose lipid source is SO have increased omega-6:omega-3 fatty acid ratios, thereby promoting inflammation and hepatic injury44.
Polyunsaturated fatty acids promote lipid peroxidation. In order to minimize oxidative stress, PN is protected from light and Vitamin E is added. The amount of Vitamin E in SO may be inadequate and is in the form, γ-tocopherol, which is not as effective in preventing free radical generation when compared to α-tocopherol, which FO contains and is provided in a much larger concentration40,45. Moreover, preterm children and children with short bowel syndrome children are deficient in Vitamin E because they lack exogenous sources and have decreased adiposity46.
While SO contains a high amount of phytosterols, FO lacks phytosterols. Phytosterols reduce biliary flow by antagonizing the farnesoid X receptor, a bile acid nuclear receptor that regulates the multi-drug resistant genes 1 and 2 (mdr 1 and 2). These genes encode P-glycoproteins that are responsible for promoting bile acid secretion47–50. Animal studies, in general, have also demonstrated that FO increases biliary flow, while SO impairs flow51. As a result, it is not surprising that bilirubin profiles improve prior to other hepatocellular indices as described in this study and other studies9–12.
There are concerns that FO may cause an essential fatty acid deficiency. However, this study supports previous findings, which suggest that FO contains sufficient omega-6 fatty acids to prevent the development of an essential fatty acid deficiency as measured by a triene:tetraene ratio. While we used a liberal cut-off, a triene:tetraene ratio ≥ 0.5, the mean triene:tetraene ratio was between 0.01–0.039. Triene to tetraene ratios, however, should be used with caution for detecting omega-6 and -3 fatty acid deficiencies. In order to develop an essential fatty acid deficiency by means of a triene:tetraene ratio, there must be an increase in mead acid (an omega-9 fatty acid), and decrease in arachidonic acid (an omega-6 fatty acid). Because desaturase enzymes have a higher affinity for omega-3 fatty acids, mead acid production may not increase with FO, which has adequate provisions of ∝-linolenic acid and high concentrations of eicosapentaenoic and docosahexaenoic acid. When absolute concentrations of specific polyunsaturated fatty acids are measured, there could be fatty acid deficiencies and toxicities with FO and SO15,52. Long-term consequences of lipid restriction, specifically < 1 g/kg/d, are unknown and could have adverse effects on organogenesis52.
Last, because FO is antithrombotic, there is a theoretical concern that FO can increase the risk of bleeding in a population whose baseline risk is already increased. Similar to previous studies, there was no increase in bleeding, and INR and platelet counts remained unchanged or improved in our study9–11.
Data on FO’s long-term effect on liver histology in human subjects is lacking, and while cholestasis may reverse, it is unclear if portal hypertension or fibrosis can be reversed39,53–55. In the setting of IFALD, animal and human studies have demonstrated that biochemical parameters may be imprecise54–56. Moreover, while some studies have associated parenteral omega-3 fatty acids with hepatic collagen deposition, others have demonstrated improved histology on liver biopsy39,55. In one study, rodents given tail injections with FO had normal liver histology compared to animals who received SO or SMOFlipid® (Fresenius Kabi, Bad Homberg, Germany) who developed hepatic steatosis and fatty liver39.
Although FO appears to be associated with a biochemical reversal of IFALD, it is unclear if transplant-free survival is altered. Liver and intestine transplants are life-saving and mortality rates continue to improve with modern medicine and surgery. Intestinal transplant with or without a liver inclusive graft may be considered in a variety of circumstances such as critical loss of venous access, history of multiple catheter related infections, severe fluid and metabolic derangements, inability to achieve enteral autonomy, and/or advanced IFALD as manifested by laboratory evaluation, physical exam, clinical course, and histology57. In our study, transplant represented an important for two FO subjects to achieve a PN-free state without cholestasis. One subject failed to respond to FO, developed end-stage liver failure, and received a transplant during FO intervention. The other subject received a transplant during follow-up. Despite 24 weeks of FO and biochemical resolution of her IFALD, she continued to exhibit advanced fibrosis on liver biopsy and redeveloped cholestasis. She also had a history of multiple bloodstream infections, and was predicted to be PN dependent indefinitely because of extreme short bowel syndrome. For these reasons, the family and primary medical team opted to proceed with transplant.
Limitations of this analysis include the use of historical controls, missing data in the historical cohort, and interpolation for laboratory data. In an attempt to overcome problems associated with historical controls, the sample size for the control cohort was doubled. While subjects were not matched on specific confounding variables, they were relatively similar with regards to most baseline characteristics except for EN, entry SO dose, and lipid dose at the end of the study. These factors would positively favor the primary outcome for the FO group and cannot be ignored. Moreover, in the past 10 years, a multi-disciplinary approach to IF has been utilized at our institution, which has been associated with an improved prognosis for IF patients58,59.
In addition, considering the number of comparisons made between the two cohorts, there is an increased risk of a type I error. A priori, we opted not to adjust for multiple comparisons. Our goal was to clearly define the cohorts’ risk factors for IFALD and associated complications.
Although the study remains open and data continues to be collected, follow-up post-24 weeks of FO will be essential to determine if patients who continue on PN and restart SO remain free of cholestasis and if transplant-free survival is decreased.
Conclusion
A 24-week course of FO appears to be safe and effective in reversing IFALD in most pediatric patients. While FO appears to be promising as a treatment for IFALD, there still remains a need to optimize PN therapy to promote the well-being of all children and, at the same time, minimize toxicities and side effects associated with PN. In order to reduce the morbidity and mortality associated with IFALD, attention must be given to all factors that drive the development and progression of IFALD. Further studies regarding IFALD prevention and long-term follow-up on growth and neurodevelopment are needed.
Supplementary Material
Clinical Relevancy Statement.
Prevention and treatment of intestinal failure associated liver disease (IFALD) is essential for reducing the morbidity and mortality in the intestinal failure (IF) population. Preliminary results from this study support that fish oil (FO) monotherapy at 1 g/kg/day for 24 weeks is a safe and effective modality for biochemical resolution of cholestasis.
Acknowledgements
The authors would like to acknowledge Mark Puder, MD, PhD and Kathleen Gura, PharmD, BCNSP from Boston Children’s Hospital for their assistance and Jeffrey Gornbein, PhD, Statistical Biomathematics Consulting Clinic, University of California, Los Angeles for his statistical analytical assistance.
Funding
The authors have received funding from NIH/NCRR M01-RR00865. Kara Calkins, MD has received funding from NIH K12HD00140 and T32G075776. Stephen Shew, MD has received funding from NIH K08HD052885. Kara Calkins, MD and Sherin Devaskar, MD are supported by the National Center for Advancing Translational Sciences through UCLA CTSI Grant UL1TR000124. Robert Venick, MD has received funding from the Today’s and Tomorrow’s Children Fund, Mattel Children’s Hospital, University of California, Los Angeles. Intravenous fish oil was purchased with funds from Department of Pediatric Surgery; Woman’s Auxiliary Club, and James Yoo, MD, Department of Surgery, UCLA.
Abbreviations
- PN
parentreal nutrition
- IF
intestinal failure
- IFALD
intestinal failure associated liver disease
- FO
fish oil
- SO
soybean oil
- IV
intravenous
- EN
enteral nutrition
- GI
gastrointestinal
Appendix
Diagnosis, gestational age (GA) and small bowel (SB) length (cm) for each subject who received fish oil (FO) or soybean oil (SO). Small bowel length (cm) was recorded from the medical chart if an intestinal resection was performed and a surgeon measured and recorded the length of the small bowel. NEC, necrotizing enterocolitis. MMIHS, megacystis microcolon intestinal hypoperistalsis syndrome. -- indicates the length was not recorded or an intestinal resection was not performed.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to disclose.
Contributor’s Statement
All of the above authors have contributed substantially to this research and article.
References
- 1.Squires RH, Duggan C, Teitelbaum DH, et al. Natural history of pediatric intestinal failure: initial report from the Pediatric Intestinal Failure Consortium. J Pediatr. 2012;161:723–728. doi: 10.1016/j.jpeds.2012.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sigalet D, Boctor D, Robertson M, et al. Improved outcomes in paediatric intestinal failure with aggressive prevention of liver disease. Eur J Pediatr Surg. 2009;19:348–353. doi: 10.1055/s-0029-1241865. [DOI] [PubMed] [Google Scholar]
- 3.Cole CR, Hansen NI, Higgins RD, Ziegler TR, Stoll BJ. Very low birth weight preterm infants with surgical short bowel syndrome: incidence, morbidity, and mortality, and growth outcomes at 18–22 months. Pediatrics. 2008;122:e573–e582. doi: 10.1542/peds.2007-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wales PW, de Siliva N, Kim JH, Leece L, Sandhu A, Moore AM. Neonatal short bowel syndrome: a cohort study. J Pediatr Surg. 2005;40:755–762. doi: 10.1016/j.jpedsurg.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 5.Javid PJ, Malone FR, Dick AA, et al. A contemporary analysis of parenteral-associated liver disease in surgical infants. J Pediatr Surg. 2011;46:1913–1917. doi: 10.1016/j.jpedsurg.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Christenson RD, Henry E, Wiedmeier SE, Burnett J, Lambert DK. Identifying patients, on the first day of life, at high risk of developing parenteral-associated liver disease. J Perinatol. 2007;27:284–290. doi: 10.1038/sj.jp.7211686. [DOI] [PubMed] [Google Scholar]
- 7.Willis TC, Barter BA, Rogers SP, Hawthorne KM, Hicks PD, Abrams SA. High rates of mortality and morbidity occur in infants with parenteral-associated cholestasis. JPEN J Parenter Enteral Nutr. 2010;34:32–37. doi: 10.1177/0148607109332772. [DOI] [PubMed] [Google Scholar]
- 8.Farmer DG, Venick RS. Morbidity and mortality associated with chronic intestinal failure. Transplantation. 2008;27:1385–1386. doi: 10.1097/TP.0b013e31817cf8aa. [DOI] [PubMed] [Google Scholar]
- 9.Gura KM, Lee S, Valim C, et al. Safety and efficacy of a fish-oil based fat emulsion in the treatment of parenteral associated liver disease. Pediatrics. 2008;121:e678–e686. doi: 10.1542/peds.2007-2248. [DOI] [PubMed] [Google Scholar]
- 10.Puder M, Valim C, Meisel JA, et al. Parenteral fish oil improves outcomes inpatients with parenteral-associated liver injury. Ann Surg. 2009;250:395–402. doi: 10.1097/SLA.0b013e3181b36657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Premkumar MH, Carter BA, Hawthorne KM, King K, Abrams SA. High rates of resolution of cholestasis in parenteral-associated liver disease with fish oil-based emulsion monotherapy. J Pediatr. 2012 doi: 10.1016/j.jpeds.2012.10.019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 12.Diamond IR, Sterescu A, Pencharz PB, Kim JH, Wales PW. Changing the paradigm: Omegaven for the treatment of liver failure in pediatric short bowel. J Pediatr Gastroenterol Nutr. 2009;48:209–215. doi: 10.1097/MPG.0b013e318182c8f6. [DOI] [PubMed] [Google Scholar]
- 13.Lee SI, Valim C, Johnston P, et al. Impact of fish oil-based lipid emulsion on serum triglyceride, bilirubin, and albumin levels in children with parenteral associated liver disase. Pediatr Res. 2009;66:698–703. doi: 10.1203/PDR.0b013e3181bbdf2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nehra D, Fallon EM, Carlson SJ, et al. Provision of a soy-based intravenous lipid emulsion at 1 g/kg/day does not prevent cholestasis in neonates. JPEN J Parenter Enteral Nutr. 2012 doi: 10.1177/0148607112453072. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15.Cober MP, Killu G, Brattain A, Welch KB, Kunisaki SM, Teitelbaum DH. Intravenous fat emulsions reduction for patients with parenteral-associated liver disease. J Pediatr. 2012;160:421–427. doi: 10.1016/j.jpeds.2011.08.047. [DOI] [PubMed] [Google Scholar]
- 16.Chung PH, Wong KK, Wong RM, Tsoi NS, Chan KL, Tam PK. Clinical experience in managing pediatric patients with ultra-short bowel syndrome using omega-3 fatty acid. Eur J Pediatr Surg. 2010;20:139–142. doi: 10.1055/s-0029-1238283. [DOI] [PubMed] [Google Scholar]
- 17.Calhoun AW, Kullivan JE. Omegaven for the treatment of parenteral associated liver disease: a case study. J Ky Med Assoc. 2009;107:55–57. [PubMed] [Google Scholar]
- 18.Fuchs J, Fallon EM, Gura KM, Puder M. Use of omega-3 fatty acid emulsion in the treatment of parenteral-induced cholestasis in patients with microvillus inclusion disease. J Pediatr Surg. 2011;46:2376–2382. doi: 10.1016/j.jpedsurg.2011.09.061. [DOI] [PubMed] [Google Scholar]
- 19.Gura K, Strijobsch R, Arnold S, McPherson C, Puder M. The role of an intravenous fat emulsion composed of fish oil in a parenteral-dependent patient with hypertriglyceridemia. Nutr Clin Pract. 2007;22:664–672. doi: 10.1177/0115426507022006664. [DOI] [PubMed] [Google Scholar]
- 20.Calkins K, Lowe A, Shew SB, et al. Short-term intravenous fish oil and pediatric intestinal failure associated liver disease: 3-year follow-up on liver function and nutrition. J Pediatr Surg. 2013 doi: 10.1016/j.jpedsurg.2012.10.044. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohl M, Wedel T, Entenmann S, et al. Influence of different intravenous lipid emulsions on hepatobiliary dysfunction in a rabbit model. J Pediatr Gastroenterol Nutr. 2007;44:237–404. doi: 10.1097/01.mpg.0000252193.99331.03. [DOI] [PubMed] [Google Scholar]
- 22.Robinson DT, Ehrekranz RA. Parenteral-associated cholestasis in small for gestational age infants. J Pediatr. 2008;152:59–62. doi: 10.1016/j.jpeds.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Sondheimer JM, Asturias E, Cadnapaphornchai M. Infection and cholestasis in neonates with intestinal resections and long-term parenteral. J Pediatr Gastroenterol Nutr. 1998;27:131–137. doi: 10.1097/00005176-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Shin JI, Namgung R, Park MS, Lee C. Could lipid infusion be a risk for parenteral-associated cholestasis in low birth weight neonates? Eur J Pediatr. 2008;167:197–202. doi: 10.1007/s00431-007-0454-7. [DOI] [PubMed] [Google Scholar]
- 25.Colomb V, Jobert-Giraud A, Lacaille F, Goulet O, Fournet JC, Ricour C. Role of lipid emulsions in cholestasis associated with long-term parenteral in children. JPEN J Parenter Enteral Nutr. 2000;24:345–350. doi: 10.1177/0148607100024006345. [DOI] [PubMed] [Google Scholar]
- 26.Allardyce DB. Cholestasis caused by lipid emulsions. Surg Gynecol Obstet. 1982;154:641–647. [PubMed] [Google Scholar]
- 27.Ehrenkranz RA, Dusick AM, Vohr BR, et al. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight neonates. Pediatrics. 2006;117:1253–1261. doi: 10.1542/peds.2005-1368. [DOI] [PubMed] [Google Scholar]
- 28.Stephens BE, Walden RV, Gargus RA, et al. First-week protein and energy intakes are associated with 18-month developmental outcomes in extremely low birth weight infants. Pediatrics. 2009;123:1337–1343. doi: 10.1542/peds.2008-0211. [DOI] [PubMed] [Google Scholar]
- 29.Hack M, Wilson-Costello D, Friedman H, Taylor GH, Scluchter M, Fanaroff A. Neurodevelopment and predictors of outcomes of children with birth weights less than 1000 g. Arch Pediatr Adoles Med. 2000;154:725–731. doi: 10.1001/archpedi.154.7.725. [DOI] [PubMed] [Google Scholar]
- 30.Thevenin DM, Baker A, Kato T, Tzakis A, Fernandez M, Dowling M. Neurodevelopmental outcomes for children transplanted under the age of 3 years. Transplant Proc. 2006;38:1692–1693. doi: 10.1016/j.transproceed.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 31.Arsenault DA, Potemkin AK, Robinson EM, et al. Surgical intervention in the setting of parenteral-associated cholestasis may exacerbate liver injury. J Pediatr Surg. 2011;46:122–127. doi: 10.1016/j.jpedsurg.2010.09.072. [DOI] [PubMed] [Google Scholar]
- 32.Angsten G, Finkel Y, Lucas S, Kassa AM, Paulsson M, Lilja HE. Improved outcome in neonatal short bowel syndrome using parenteral fish oil in combination with-ω-6/9 lipid emulsions. JPEN J Parenter Enteral Nutr. 2012;36:587–595. doi: 10.1177/0148607111430507. [DOI] [PubMed] [Google Scholar]
- 33.Tomsits E, Pataki M, Tölgyesi A, Fekete G, Rischak K, Szollár L. Safety and efficacy of a lipid emulsion containing a mixture of soybean oil, medium-chain triglycerides, olive oil, fish oil: a randomised, double-blind clinical trial in premature infants requiring parental nutrition. J Pediatr Gastroenterol Nutr. 2010;51:514–521. doi: 10.1097/MPG.0b013e3181de210c. [DOI] [PubMed] [Google Scholar]
- 34.Muhammed R, Bremner R, Prtheroe S, Johnson T, Holden C, Murphy MS. Resolution of parenteral-associated jaundice on changing from soybean oil emulsion to a complex mixed-lipid emulsion. J Pediatr Gastroenterol Nutr. 2012;54:797–802. doi: 10.1097/MPG.0b013e3182447daf. [DOI] [PubMed] [Google Scholar]
- 35.Lilja HE, Finkel Y, Paulsson M, Lucas S. Prevention and reversal of intestinal failure associated liver disease in premature infants with short bowel syndrome using intravenous fish oil in combination with omega-6-9 lipid emulsions. J Pediatr Surg. 2010;46:1361–1367. doi: 10.1016/j.jpedsurg.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 36.Skouroliakou M, Konstantinou D, Koutri K, et al. A double-blind, randomized clinical trial of the effect of omega-3 fatty acids on the oxidative stress of preterm neonates fed through parenteral. Eur J Clin Nutr. 2010;64:940–947. doi: 10.1038/ejcn.2010.98. [DOI] [PubMed] [Google Scholar]
- 37.Göbel Y, Koletzko B, Böhles H, et al. Parenteral fat emulsion based on olive oil and soybean oils: a randomized clinical trial in preterm infants. J Pediatr Gastroenterol Nutr. 2003;37:161–167. doi: 10.1097/00005176-200308000-00015. [DOI] [PubMed] [Google Scholar]
- 38.Goulet O, Antébi H, Wolf C, et al. A new fat emulsion containing soybean oil, medium-chain triglycerides, olive oil, fish oil: a single-center, double-blind randomized study on efficacy and safety in pediatric patients receiving home parenteral. JPEN J Parenter Enteral Nutr. 2010;34:485–495. doi: 10.1177/0148607110363614. [DOI] [PubMed] [Google Scholar]
- 39.Meisel JA, Le HD, de Meijer VE, et al. Comparison of 5 intravenous lipid emulsions and their effects on hepatic steatosis in a murine model. J Pediatr Surg. 2011;46:666–673. doi: 10.1016/j.jpedsurg.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 40.Koletzko B, Goulet O. Fish oil containing intravenous lipid emulsions in parenteral-associated cholestatic liver disease. Curr Opin Clin Nutr Metab Care. 2010;12:321–326. doi: 10.1097/MCO.0b013e3283385407. [DOI] [PubMed] [Google Scholar]
- 41.Robinson DT, Carslon SE, Murthy K, Frost B, Li S, Caplan M. Docosahexaenoic and arachidonic acid levels in extremely low birth weight infants with prolonged exposure to parenteral lipids. J Pediatr. 2013;162:56–61. doi: 10.1016/j.jpeds.2012.06.045. [DOI] [PubMed] [Google Scholar]
- 42.Socha P, Koletzko B, Pawlowska J, Socha J. Essential fatty acid status in children with cholestasis, in relation to serum bilirubin concentrations. J Pediatr. 1997;131:700–706. doi: 10.1016/s0022-3476(97)70096-9. [DOI] [PubMed] [Google Scholar]
- 43.Fleith M, Clandinin MT. Dietary PUFA for preterm and term infants: review of clinical trials. Crit Rev Food Sci Nutr. 2005;45:205–229. doi: 10.1080/10408690590956378. [DOI] [PubMed] [Google Scholar]
- 44.Le HD, de Meijer VE, Robinson EM, et al. Parenteral fish oil improves fatty acid profiles ad lipids in parenteral-dependent children. Am J Clin Nutr. 2011;94:749–758. doi: 10.3945/ajcn.110.008557. [DOI] [PubMed] [Google Scholar]
- 45.de Meijer, Gura KM, Le HD, Meisel JA, Puder M. Fish oil-based lipid emulsions prevent and reverse parenteral-associated liver disease: the Boston experience. JPEN J Parenter Enteral Nutr. 2009;33:541–547. doi: 10.1177/0148607109332773. [DOI] [PubMed] [Google Scholar]
- 46.Kositamongkol S, Suthutvoravut U, Chongviriyaphan N, Feungpean B, Nuntnarumit P. Vitamin A and E status in very low birth weight infants. J Perinatol. 2011;31:471–476. doi: 10.1038/jp.2010.155. [DOI] [PubMed] [Google Scholar]
- 47.Carter BA, Taylor OA, Prendergast DR, et al. Stigmasterol, a soy lipid-derived phytosterol, is an antagonist of the bile acid nuclear receptor FXR. Pediatr Res. 2007;62:301–306. doi: 10.1203/PDR.0b013e3181256492. [DOI] [PubMed] [Google Scholar]
- 48.Donner MG, Schumacher S, Warskulat U, Heinemann J, Häussinger D. Obstructive cholestasis induces TNF-alpha- and IL-1–mediated periportal downregulation of Bsep and zonal regulation of Ntcp, Oatp 1a4, and Oatp1b2. Am J Physiol Gastrointest Liver Physiol. 2007;293:1134–1146. doi: 10.1152/ajpgi.00079.2007. [DOI] [PubMed] [Google Scholar]
- 49.Clayton PT, Bowron A, Mills KA, Massoud A, Castells M, Milla PJ. Phytosterolemia in children with parenteral-associated cholestatic liver disease. Gastroenterology. 1993;105:1806–1813. doi: 10.1016/0016-5085(93)91079-w. [DOI] [PubMed] [Google Scholar]
- 50.Kurvinen A, Nissinen MJ, Andersson S, et al. Parenteral plant sterols and intestinal failure-associated liver disease in neonates. J Pediatr Gastroenterol Nutr. 2012;54:803–811. doi: 10.1097/MPG.0b013e3182474118. [DOI] [PubMed] [Google Scholar]
- 51.Van Aerde JE, Duerksen DR, Gramlick L, et al. Intravenous fish oil emulsion attenuates total parenteral-induced cholestasis in newborn piglets. Pediatr Res. 1999;45:202–208. doi: 10.1203/00006450-199902000-00008. [DOI] [PubMed] [Google Scholar]
- 52.Abrams SA. Step forward, backward, and sideways: intravenous lipid emulsions for critically ill neonates. JPEN J Parenter Enteral Nutr. 2013 doi: 10.1177/0148607112454300. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 53.Fitzgibbons SC, Jones BA, Hull MA, et al. Relationship between biopsy proven-parenteral associated liver fibrosis and biochemical cholestasis in children with short bowel syndrome. J Pediatr Surg. 2010;45:95–99. doi: 10.1016/j.jpedsurg.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mercer DF, Hobson BD, Fisher RT, et al. Hepatic fibrosis persists and progresses despite biochemical improvement in children treated with intravenous fish emulsion. J Pediatr Gastroenterol Nutr. 2013;56:364–349. doi: 10.1097/MPG.0b013e31827e208c. [DOI] [PubMed] [Google Scholar]
- 55.Soden JS, Lovell MA, Brown K, Partrick DA, Sokol RJ. Failure of resolution of portal fibrosis during omega-3 fatty acid lipid emulsion therapy in two patients with irreversible intestinal failure. J Pediatr. 2010;156:327–331. doi: 10.1016/j.jpeds.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 56.Moss RL, Das JB, Raffensperger JG. Total parenteral nutrotion-associated cholestasis: clinical and histopathological correlation. J Pediatr Surg. 1993;28:1270–1274. doi: 10.1016/s0022-3468(05)80311-2. [DOI] [PubMed] [Google Scholar]
- 57.Beath S, Pironi L, Gabe S, et al. Collaborative strategies to reduce mortality and morbidity in patients with chronic intestinal failure including those who are referred for small bowel transplant. Transplantation. 2008;85:13758–1384. doi: 10.1097/TP.0b013e31816dd513. [DOI] [PubMed] [Google Scholar]
- 58.Hess RA, Welch KB, Brown PI, Teitelbaum DH. Survival outcomes of pediatric intestinal failure patients: analysis of factors contributing to improved survival over the past two years. J Surg Res. 2011;170:27–31. doi: 10.1016/j.jss.2011.03.037. [DOI] [PubMed] [Google Scholar]
- 59.Pichler J, Horn V, Macdonald S, Hill S. Intestinal failure-associated liver disease in hospitalized children. Arch Dis Child. 2012;97:211–214. doi: 10.1136/archdischild-2011-300274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


