Highlights
-
•
Event-related potential measure of reactivity to social feedback in youth.
-
•
Youth were more likely to reject peers who previously rejected them.
-
•
Feedback negativity (FN) was sensitive to social rejection vs. acceptance feedback.
-
•
Symptoms of depression and social anxiety related to behavioral responses and FN.
Keywords: Event-related potentials, Peer relationships, Social rejection, Feedback negativity, Depression, Anxiety
Abstract
Peer relationships become a major concern in adolescence, yet event-related potential (ERP) measures of reactivity to social feedback in adolescence are limited. In this pilot study, we tested a novel task to elicit reactivity to social feedback in youth. Participants (10–15 years old; 57.9% male; N = 19) played a game that involved exchanging personal information with peers, voting to remove players from the game, and receiving rejection and acceptance feedback from peers. Results indicated that participants modified their voting behavior in response to peer feedback, and rejection feedback was associated with a negativity in the ERP wave compared to acceptance (i.e., the feedback negativity, FN). The FN predicted behavioral patterns, such that participants who showed greater neural reactivity to social feedback were less likely to reject co-players. Preliminary analyses suggest that the task may be a useful measure of individual differences: adolescents higher in social anxiety symptoms were less likely to reject peers and showed an enhanced FN to rejection vs. acceptance feedback, and higher depressive symptoms predicted an increased FN to rejection specifically. Results suggest that the FN elicited by social feedback may be a useful, economical neural measure of social processing across development and in clinical research.
1. Introduction
1.1. Reactivity to social feedback in adolescence
Peer relationships assume increasing importance in adolescence and shape adolescent behavior in both positive and negative ways (Allen et al., 2005, Brown, 2004, Steinberg and Morris, 2001). There has been growing interest in evaluating the neural correlates underlying the response to social feedback in order to understand normative developmental changes, as well as mechanisms underlying internalizing disorders (Bolling et al., 2011, Crowley et al., 2010, Gunther et al., 2010, Guyer et al., 2012, Sebastian et al., 2010, Sebastian et al., 2011, Silk et al., 2013, Somerville, 2013). A major challenge in this work is developing realistic computerized social interaction tasks. Though several paradigms have been created to evaluate the development of neural reactivity to social feedback using functional magnetic resonance imaging (fMRI), very little work has focused on event-related potential (ERP) measures of reactivity to social feedback across development.
1.2. fMRI paradigms for measuring reactivity to social feedback
Previous fMRI work in youth has evaluated reactivity to social exclusion as well as peer feedback indicating rejection and acceptance. For example, Cyberball, a virtual ball-tossing paradigm in which participants are eventually excluded from a game (Williams et al., 2000), is a task used to measure reactivity to social exclusion in youth. In adolescents, activation in the subgenual anterior cingulate cortex (subACC) and insula related to greater distress during exclusion, while activation in the ventrolateral prefrontal cortex (vlPFC) negatively related to distress (Masten et al., 2009). In addition, there is evidence of developmental changes in these networks, with exclusion associated with increasing activation in the vlPFC from childhood to adulthood, and greater reactivity in the ventral anterior cingulate cortex (ACC) in adolescence compared to childhood (Bolling et al., 2011, Sebastian et al., 2011).
Although social exclusion implies rejection, it is likely that ostracism in Cyberball elicits complex negative emotional responses that may include frustration, anger, and jealousy (Harmon-Jones et al., 2009, Peterson et al., 2011). In order to more directly examine neural activity linked to explicit feedback regarding peer acceptance and rejection, there has been growing interest in the development of social paradigms that include the pretense of more direct and mutual communication with peers. For example, in the Chat Room task, participants rate how interested they are in participating in an online chat with other youth based on their photographs, and then receive feedback regarding how interested the other people are in chatting with them (Guyer et al., 2008). Compared to rejection, receipt of acceptance feedback activated social affiliation and reward regions, including the ACC, striatum, superior temporal gyrus, insula, and thalamus, and increasing age across late childhood and adolescence was associated with greater neural responses to social acceptance feedback, particularly for females (Guyer et al., 2012). Relatedly, Silk et al. (2012) developed a Chat Room Interact task, in which participants and computerized co-players make decisions regarding with whom to discuss specific topics, and participants receive rigged acceptance and rejection feedback. This task is among the first to include biographical profiles of the confederates and to measure reactivity to simulated live interaction using both eye tracking (Silk et al., 2012) and fMRI (Silk et al., 2013). In one fMRI study, pubertal maturation in adolescence predicted greater neural reactivity to rejection feedback in the amygdala, parahippocampal gyrus, caudate, and subACC (Silk et al., 2013).
Taken together, previous fMRI work has begun to identify neural networks involved in social feedback processing (Guyer et al., 2012, Sebastian et al., 2011, Silk et al., 2013), and provide evidence that adolescents may be particularly emotionally reactive to peer feedback, with systems to regulate these emotional responses continuing to develop into adulthood (Bolling et al., 2011, Guyer et al., 2012, Sebastian et al., 2011, Silk et al., 2013).
1.3. Utility of event-related potential measures
Compared to fMRI research, little work has evaluated event-related potential (ERP) measures of reactivity to social feedback across development. ERPs have excellent temporal resolution, providing neural measures of very early stages of processing that can be applied across a range of development and may be particularly useful for clinical applications, given their relative cost-effectiveness compared to other neural measures (Banaschewski and Brandeis, 2007, Luck, 2005, Nelson and McCleery, 2008).
The feedback negativity (FN) is an ERP component that could be used to measure reactivity to social feedback. The FN is a relative negativity in the ERP wave following receipt of negative feedback compared to positive feedback that peaks approximately 250–300 ms after feedback over frontocentral recording sites (Foti et al., 2011, Gehring and Willoughby, 2002). That is, negative feedback (e.g., monetary loss or negative performance feedback) appears as a more negative deflection in the FN wave, whereas positive feedback (e.g., monetary reward or positive performance feedback) appears as a relative positivity (Gehring and Willoughby, 2002, Hajcak et al., 2006, Luu et al., 2003, Nieuwenhuis et al., 2004). It is plausible that the FN would also be modulated by social rejection and acceptance feedback; however, additional work is needed to evaluate this possibility.
The FN is thought to be generated as part of a reinforcement learning signal used to modify behaviors with negative outcomes and reinforce behaviors with positive outcomes (Holroyd and Coles, 2002). In monetary reward tasks, the FN correlates with activation in reward-related brain regions, including ventral striatum and medial prefrontal cortex (Becker et al., 2014, Carlson et al., 2011). Importantly, the FN has also demonstrated excellent psychometric properties across development (Bress et al., in press) and contributed to understanding of developmental changes in the processing of feedback. For example, compared to adults and adolescents, children show enhanced ERPs to feedback overall but less differentiation in the FN response to positive vs. negative feedback (Ferdinand and Kray, 2014, Hämmerer et al., 2011). That is, although children may react more strongly to external feedback, they appear to be less efficient in discriminating between positive and negative outcomes. As the FN provides a very early measure of reactivity to feedback and can be easily assessed across development, it has the potential to provide insight into developmental changes in social processing and may be particularly useful given the importance of peer relationships in adolescence (Allen et al., 2005, Brown, 2004, Steinberg and Morris, 2001).
Despite the potential contributions of ERP measures of social processing to research on neural development, to our knowledge no previous study has evaluated the FN to social feedback in youth. In one Cyberball study, Crowley et al. (2010) found some evidence that social exclusion modulates a component similar to the FN; however, the effect did not reach significance, which could be attributed to the lack of explicit acceptance and rejection feedback in the Cyberball task. Given the potential utility of the FN to studying social feedback in developmental research, additional work is needed to create a paradigm with direct, personally relevant feedback that may modulate the FN.
1.4. Implications for the development of internalizing symptoms
Recent fMRI work has also begun to evaluate associations between internalizing disorders and neural reactivity to social feedback. Compared to controls, youth with major depressive disorder (MDD) exhibited heightened amygdala, subACC, insula and nucleus acumbens reactivity to rejection in the Chat Room Interact task (Silk et al., 2013). Relatedly, controlling for initial depressive symptoms in adolescence, greater subACC activation to exclusion in Cyberball prospectively predicted an increase in depressive symptoms 1 year later (Masten et al., 2011). With regards to anxiety, abnormalities in neural networks involved in social processing may be particularly apparent among adolescents with social anxiety, which is especially common in adolescence and marked by excessive concern about social rejection and humiliation (Weems and Silverman, 2013). Consistent with this, one fMRI study found that youth with anxiety disorders and clinically significant social fears exhibited heightened amygdala-hippocampal activation following rejection feedback compared to controls (Lau et al., 2012). With growing interest in evaluating psychopathology using dimensional measures across multiple levels of analysis (e.g., Sanislow et al., 2010), ERPs furnish an additional approach to assessing neural indicators of clinically relevant constructs, can provide converging evidence along with fMRI, and may aid in clarifying pathways in the development of internalizing symptoms.
1.5. Goals and hypotheses
The primary goal of the current study was to create a novel, realistic ERP paradigm (“Island Getaway”) to elicit electrocortical and behavioral reactivity to social acceptance and rejection feedback. During the Island Getaway task, participants ostensibly play a game with peers, in which participants vote to reject and accept co-players, while also receiving a combination of rejection and acceptance feedback from their co-players. To closely mimic real life interactions, participants and co-players gradually exchange personal information (e.g., age, photograph, likes, dislikes, interests) throughout the task, and participants are led to believe that rejection and acceptance feedback may be based on these responses. We examined behavioral (i.e., proportion of votes to reject vs. accept co-players) and ERP responses to rejection and acceptance feedback. We hypothesized that participants would be more likely to reject co-players who had previously rejected them, and that a relative negativity would be observed in the ERP for social rejection compared to social acceptance feedback (i.e., the FN). We also evaluated whether the FN to social feedback related to the tendency to reject peers. Lastly, we computed preliminary correlations to explore whether ERP and behavioral measures of reactivity to social feedback relate to individual differences, including sex, age, and symptoms of depression and social anxiety.
2. Materials and methods
2.1. Participants
Participants were recruited from the Long Island, NY community using a commercial mailing list. A total of 20 children and adolescents participated in this pilot study, and data from one participant were excluded due to an FN that was a significant outlier according to Grubb's test (Grubbs, 1969), a statistical test for detecting outliers by comparing the Z score to a critical value adjusted for the sample size. The final sample included 19 participants (eight female; 10–15 years old; mean age = 12.68 (SD = 1.64); all Caucasian). One participant responded to only half of the items on the self-report measure of depressive symptoms and was not included in correlations between self-reported depression and the FN.
2.2. Social feedback task
Island Getaway is a computerized social feedback task based on the television show “Survivor” and a behavioral peer rejection task (Reijntjes et al., 2006a, Reijntjes et al., 2006b). The code for the task is available at: http://arfer.net/projects/survivor.1 At the start of the game, participants were presented with the story line, in which they are traveling along the Hawaiian Islands and trying to avoid being voted off the islands by the other players. Participants were introduced to 11 computerized co-players with preset appearances and behavior who were ostensibly other youth playing the game with the participant over the Internet.
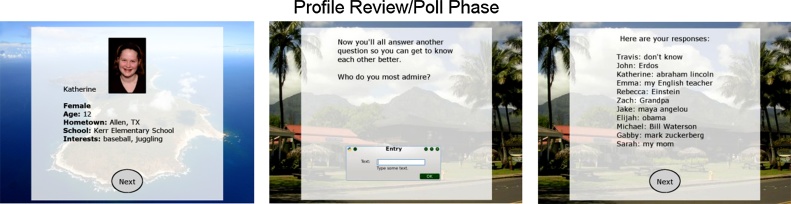
The task began with a description of the goal of the game. Next, participants were asked to enter basic demographic information (first name, age, gender, hometown, school, and interests) and were shown their profiles, which included their own photograph taken by the experimenter at the start of the laboratory visit. Participants were then presented with each of the co-player's profiles (Fig. 1). The gender distribution of the co-players varied depending on the participant's gender, so that including the participant, there were six male and six female players in each game. Ages of the co-players ranged from 10 to 13, and locations were distributed throughout the United States. Co-player photographs were taken from stock photographs available online as well as images from the NIMH Child Emotional Faces Picture Set (Egger et al., 2011) and were edited to appear as if they had been taken in a laboratory. Co-player profile information and photographs were randomly assembled during each run of the task, though male and female images were always matched with a male or female first name.
Fig. 1.
Screen shots from the Island Getaway task, including a profile from one of the co-players and an example poll question and list of poll responses.
After viewing co-player profiles, participants were told that they would vote on whether each co-player should stay in the game and were instructed to vote off at least one other player. Participants were again presented with each co-player profile in a random order, with the question “Should we keep [co-player's name] or kick [him or her] out?” and buttons marked “Kick Out” and “Keep”.
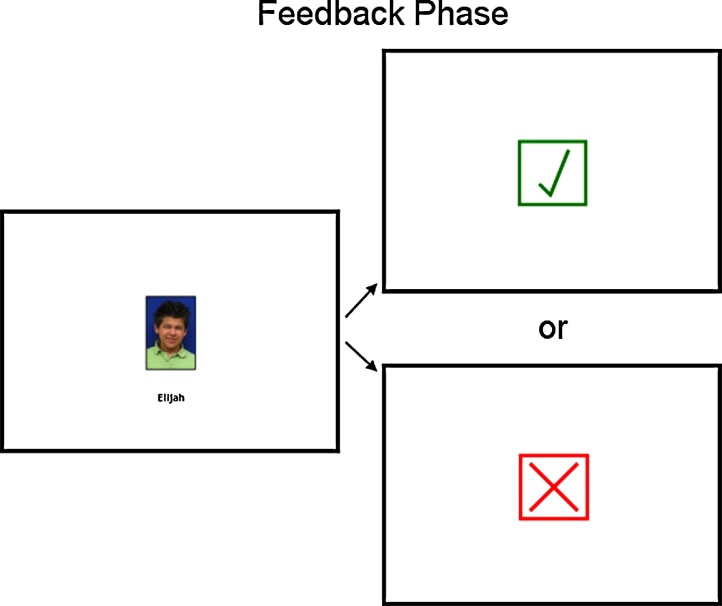
Finally, participants were presented with feedback regarding whether each co-player voted for the participant to stay in the game. First a fixation “+” was presented for 2000–3000 ms, followed by the co-player's name and photograph for 2000 ms and another fixation “+” for 1000 ms. Last, feedback was presented for 1500 ms. A green check mark indicated that the co-player voted to keep the participant and a red X indicated that the co-player voted for the participant to leave the game (Fig. 2). After participants received feedback from all co-players, they were told that one of the co-players was sent home and the next round began. The remaining rounds began with a free-response poll question, such as “Who do you most admire?” or “If you could bring only one thing to a desert island, what would it be?”. Participants were shown each co-player's response (Fig. 1), and during the voting stage, poll responses were added to each co-player's profile.
Fig. 2.
Screen shots from the feedback stage of the Island Getaway task. First, participants viewed the name and photograph of the co-player voting, and then received feedback regarding whether that co-player voted to keep or kick out the participant.
The task consisted of six rounds total and took approximately 25 min to complete. Because participants received feedback from each co-player's votes, there were a total of 51 feedback trials evenly split into rejection or acceptance trials with the last trial randomly determined. At the end of the task, participants were informed that they had won the game by making it to the Big Island along with the remaining five co-players. Lastly, participants completed a series of questions about the task, including the task disengagement items described below.
2.3. EEG data acquisition and processing
Continuous EEG was recorded using a 34-electrode cap and the ActiveTwo BioSemi System (BioSemi, Amsterdam, the Netherlands). The electrooculogram was collected from electrodes placed approximately 1 cm from the outer corners of the eyes and 1 cm above and below the right eye. Recordings were digitized at a 24-bit resolution with a sampling rate of 1024 Hz using a low-pass fifth-order sinc filter with a half-power cutoff of 204 Hz. Offline analysis was completed using BrainVision Analyzer (Brain Products, Munich, Germany). Data were referenced to the average of the recordings from left and right mastoid electrodes and band-pass filtered with cutoffs of .1 and 30 Hz. Ocular artifacts were corrected using the procedure developed by Gratton et al. (1983). Artifacts were removed using a semi-automated procedure with a maximum allowed voltage step of 50 μV between sampling points, a maximal voltage difference of 300 μV in a given trial and a lowest allowed activity of .5 μV within an interval of 100 ms. Visual inspection was used to reject remaining artifacts.
The continuous EEG was segmented 200 ms before the onset of feedback and continuing for 500 ms after feedback, averaged separately for rejection and acceptance trials, and baseline-corrected using the window 200 ms before feedback onset. The FN was scored as the mean amplitude 200–300 ms after feedback for rejection and acceptance trials at a pooling of frontocentral sites where the ERP difference wave was maximal (i.e., Fz, FCz). The FN was examined as the mean amplitude on acceptance and rejection trials individually, as well as the rejection minus acceptance difference score. As negative feedback presents as a relative negativity in the FN compared to positive feedback, more negative values for the rejection FN correspond to greater reactivity to rejection feedback, while more negative values for the acceptance FN correspond to reduced reactivity to acceptance feedback. The loss minus gain difference score (ΔFN) is often used in monetary reward tasks (e.g., Bress and Hajcak, 2013, Carlson et al., 2011), and more negative values in the difference score reflect greater differentiation between acceptance and rejection – that is, heightened reactivity to social rejection vs. acceptance.
2.4. Questionnaires
After completing the Island Getaway task, participants completed four questionnaire items to assess task disengagement. The items were rated on a five point scale (1 = not at all, 5 = extremely) and read: “I kept hoping I would not be kicked off,” “After a while I lost interest in staying in the game,” “How much would you want to play this game again?” and “I thought ‘this game is dumb.”’ Items 1 and 3 were reverse-scored and responses to these four scores were averaged to measure disengagement.
Participants completed the short version of the Children's Depression Inventory self-report (CDI:SR; Kovacs, 1992) to assess depressive symptoms. The CDI:SR is a symptom-focused measure designed for youth between the ages of 7–17. The short version contains 10 items with responses rated on a scale from 0 to 2 and generally yields comparable results to the long form version (Kovacs, 1992). Parents also completed an informant version of the CDI (CDI:P), which consists of 17 questions scored from 0 to 3. Internal consistency (Cronbach's alpha) was acceptable for both CDI scales (.60 and .89 for self-report and parent versions, respectively).
In addition, participants completed the social anxiety scale of the Screen for Child Anxiety Related Emotional Disorders (SCARED; Birmaher et al., 1997). The self-report version of the SCARED social anxiety scale (SCARED-SA:SR) includes seven items rated on a 0–2 scale that focus on shyness and feeling nervous in social situations. Parents completed an informant version of the SCARED social anxiety scale (SCARED-SA:P), which also includes seven items. Internal consistency (Cronbach's alpha) was good for both SCARED scales (.85 and .88 for self-report and parent versions, respectively).
2.5. Procedure
At the start of the visit, informed consent was obtained from all parents and assent was obtained from all youth. Parents were informed about the deception associated with the Island Getaway task (i.e., believing they are playing a game with real peers) during the consent process. Next, EEG sensors were attached and participants completed a series of 3–4 counterbalanced tasks, including Island Getaway, while continuous EEG was recorded. During the EEG assessment, parents completed the questionnaire measures, and following completion of the EEG assessment, participants completed self-report measures. At the end of the visit, participants were fully debriefed about the task and permitted to ask questions about the purpose and design of the study.
3. Results
3.1. Behavioral measures
The mean rating on the self-reported task disengagement scale was 2.34 (SD = .94) out of a total possible score of 5, indicating that on average participants were fairly engaged in the game, despite the length of the task.
On average, participants voted co-players out of the game 31.9% of the time (SD = 14.7%). Participants showed a greater propensity to reject co-players who had voted the participant off in the previous round (36.6% rejection rate; SD = 19.2%) compared to co-players who had voted to keep the participant in the previous round (28.7% rejection rate; SD = 18.7%; t(18) = 2.49, p < .05).
3.2. Feedback negativity to rejection and acceptance
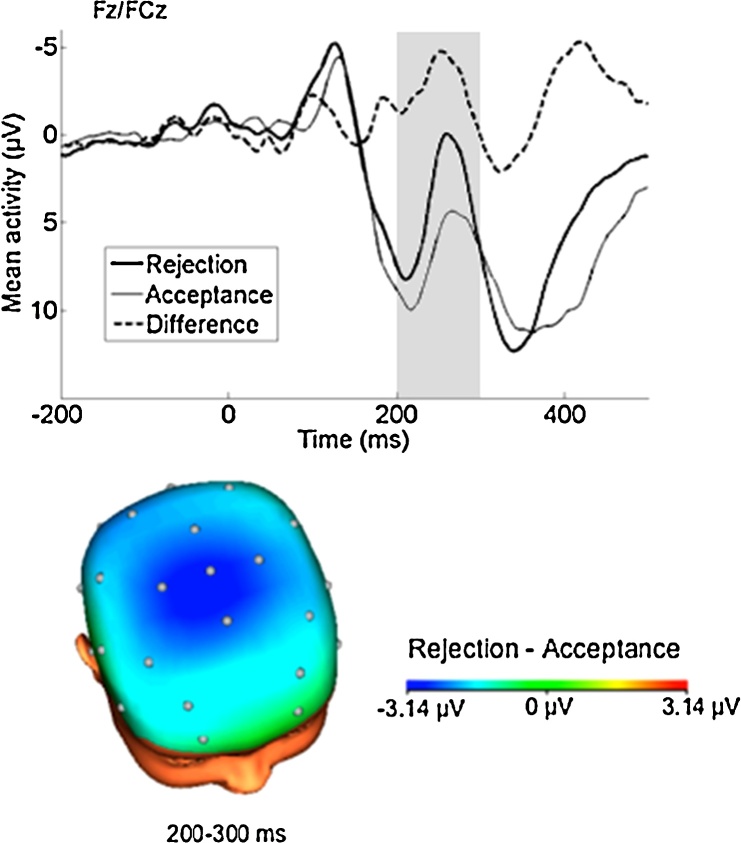
Rejection feedback was associated with an enhanced (i.e., more negative) FN (M = 4.00, SD = 5.74) compared to acceptance feedback (M = 6.60, SD = 6.45), t(18) = 2.59, p < .05, and scalp distributions confirmed that this difference was maximal over frontocentral sites, consistent with the FN to monetary or performance feedback (Fig. 3). The mean rejection minus acceptance FN difference score (ΔFN) was −2.61 (SD = 4.38).
Fig. 3.
ERPs (negative up) at a pooling of Fz/FCz following rejection and acceptance feedback and the rejection minus acceptance difference wave. Scalp distribution depicting the rejection minus acceptance difference 200–300 ms after feedback onset.
Next, bivariate correlations were calculated to evaluate whether behavioral measures of decisions to reject or accept peers (i.e., rejection rate) related to electrocortical measures of sensitivity to rejection and acceptance feedback (i.e., FN). Rejection rate was significantly related to ΔFN, r(17) = .49, p < .05, such that youth who exhibited greater neural reactivity to rejection relative to acceptance feedback were less likely to reject peers. This effect appeared to be primarily driven by the relationship between ΔFN and lower rejection rates for co-players who had previously voted for the participant to stay in the game, r(17) = .48, p < .05. The association between rejection rate for co-players who had previously voted the participant out of the game and the FN did not reach significance, r(17) = .33, p > .05. The difference between these two correlations was not significant, however.
3.3. Individual differences in sensitivity to social feedback
Lastly, preliminary analyses were computed to evaluate whether behavioral voting patterns and/or the FN to social feedback were related to individual differences. Bivariate correlations were computed to evaluate whether overall rejection rate was related to sex, age, CDI, or SCARED-SA scores. Mean symptom scores were relatively low for CDI self- and parent-report measures (Ms = 1.73, 8.59; SDs = 1.87, 7.09, respectively), as well as SCARED-SA self- and parent-report measures (Ms = 5.00, 3.37; SDs = 2.85, 3.61, respectively),
SCARED-SA:SR, r(17) = −.49, p < .05, and SCARED-SA:P, r(17) = −.45, p = .05, were significantly correlated with rejection rates, such that youth higher in social anxiety rejected co-players less frequently than youth lower in social anxiety. Sex, age, CDI:SR, and CDI:P did not significantly predict voting patterns.
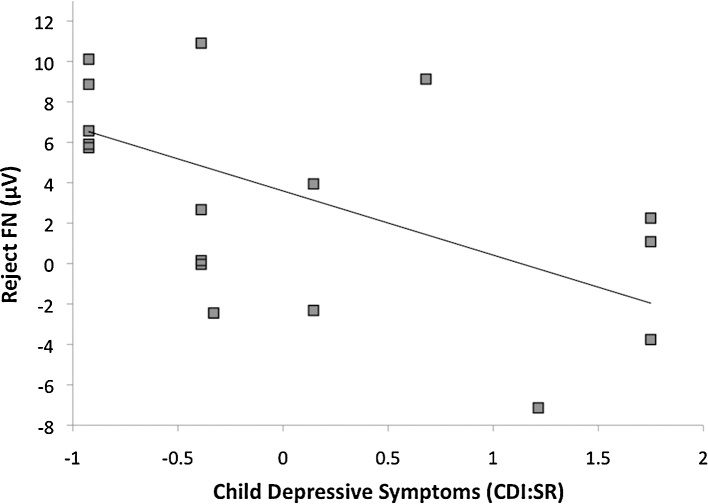
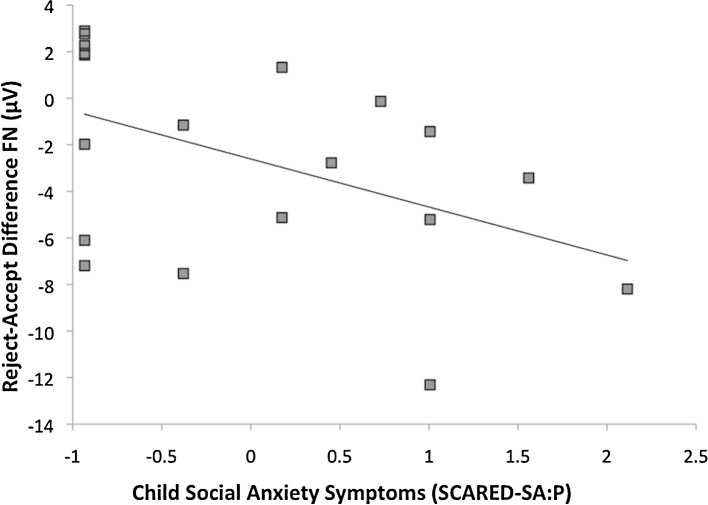
Finally, bivariate correlations were computed to examine whether the FN on acceptance trials, FN on rejection trials, or ΔFN were related to sex, age, CDI, or SCARED-SA scores. Greater CDI:SR scores were related to a more negative FN (i.e., greater reactivity) to rejection feedback, r(16) = −.56, p < .05 (Fig. 4), and a trend toward a less positive FN (i.e., reduced reactivity) to acceptance feedback, r(16) = −.45, p = .06. In addition, SCARED-SA:P scores related to ΔFN, r(17) = −.47, p < .05, such that higher symptoms of social anxiety were related to greater differentiation between rejection and acceptance feedback (Fig. 5). None of the associations between sex or age and the FN variables were significant.
Fig. 4.
Scatter plot depicting the association between the FN on rejection trials and self-report depressive symptoms. Note. Z-scored symptom measures were used for presentation purposes.
Fig. 5.
Scatter plot depicting the association between ΔFN and parent-report social anxiety symptoms. Note. Z-scored symptom measures were used for presentation purposes.
4. Discussion
In this pilot study, we evaluated electrocortical and behavioral measures during the Island Getaway task, a novel paradigm developed to elicit reactivity to direct social feedback in youth in the context of interactions that involve the exchange of personal information. Rejection compared to acceptance feedback in the Island Getaway task was associated with a relative negativity in the ERP wave that was maximal 200–300 ms after feedback at frontocentral sites, consistent with the feedback negativity (FN). Though the FN has previously been shown to be modulated by performance feedback (Gehring and Willoughby, 2002, Hajcak et al., 2006, Luu et al., 2003, Nieuwenhuis et al., 2004), this study is among the first to find that the FN is significantly modulated by feedback indicating acceptance and rejection by peers. The FN is thought to reflect a system that is involved in shaping and reinforcing adaptive behavior (Holroyd and Coles, 2002), and the current results suggest that it may also be involved in monitoring behavior that relates to social interactions. Compared to fMRI measures, ERPs provide greater temporal resolution, are relatively inexpensive and are easy to administer across development (Banaschewski and Brandeis, 2007, Luck, 2005, Nelson and McCleery, 2008); thus, the Island Getaway task may be useful for ERP research on social processing that can complement existing fMRI work.
The Island Getaway task also provides behavioral measures of participants’ reactivity to acceptance and rejection. The current results suggest that participants modify their behavior in response to feedback from co-players, such that they are more likely to reject co-players who voted against them in the previous round. This finding suggests that participants are engaged in the task and concerned about co-players’ voting patterns. Moreover, behavioral responses were related to the FN, such that youth who showed enhanced electrocortical reactivity to social rejection compared to acceptance were less likely to reject peers, particularly if those peers had not previously rejected them. This suggests that behavioral and electrocortical measures from the Island Getaway task may provide insight into distinct, but related, aspects of social feedback processing.
Lastly, preliminary analyses indicated that the Island Getaway task may be useful in studying individual differences in reactivity to social feedback. Symptoms of social anxiety were related to lower rates of rejection for peers and enhanced electrocortical reactivity to rejection compared to acceptance feedback; moreover, symptoms of depression related to heightened neural response to rejection and a trend for reduced neural response to acceptance. In fMRI studies of youth, anxiety has been linked to increased amygdala reactivity to rejection feedback (Lau et al., 2012), while depression has been associated with heightened subACC activation during rejection and social exclusion (Masten et al., 2011, Silk et al., 2013). In addition, a reduced FN to monetary rewards has been linked to depression symptoms and risk in youth (Bress et al., 2013, Kujawa et al., 2014), suggesting the FN may be particularly useful for examining the contributions of both social and monetary reward processing to the development of internalizing symptoms. Importantly, the current results also indicate that individual differences in neural reactivity to social feedback may be evident at a very early stage of processing (200–300 ms after feedback).
Several limitations of the current study should be noted. First, the sample size is small, as this study was designed to provide an initial pilot test of the Island Getaway task. Nonetheless, the results are promising, and the task is freely available to other researchers, which will allow for tests of the reproducibility of results as well as extensions to larger and more diverse samples. In particular, associations with symptoms of depression and social anxiety must be interpreted cautiously given the small sample size and limited range of scores. Moreover, some of these associations were observed only for child or parent report, rather than for both informants. Though these findings suggest that the Island Getaway task may be a useful measure of individual differences in sensitivity to social feedback, additional research is needed to evaluate the implications of the FN in social feedback tasks for understanding the development of internalizing symptoms. In addition, in the current study, we did not collect data regarding whether participants believed that the co-players in the task were real peers. As a result, we were unable to evaluate the extent to which beliefs about the deception may influence the FN or voting patterns, and this is another important question for future research. Lastly, additional research is needed to evaluate the extent to which the FN in social feedback tasks correlates with activation in neural structures previously identified in fMRI work (e.g., Guyer et al., 2012, Lau et al., 2012, Silk et al., 2013).
5. Conclusions
The current results suggest that social feedback in the Island Getaway task elicits a very early ERP measure of reactivity to social rejection and acceptance (i.e., the FN) and behavioral changes in decisions to reject peers, both of which appear to relate to one another and to individual differences in internalizing symptoms within a non-clinical sample. Given the importance of social relationships in adolescence and growing interest in neural measures of social processing (Somerville, 2013), Island Getaway may be a useful task for evaluating neural reactivity to social feedback in developmental and clinical studies.
Conflict of interest
The authors have no conflicts of interest to disclose.
Funding
This work was supported by National Institute of Mental Health Grants RO1 MH069942 to Daniel N. Klein, RO3 MH094518 to Greg Hajcak Proudfit, and F31 MH09530701 to Autumn Kujawa.
Acknowledgments
We would like to thank Michael J. Crowley, Ph.D. for his helpful comments on the initial idea for the task, and Emily Hale-Rude for help with data collection.
Footnotes
Available online 27 August 2014
Following this initial pilot study, we modified the task to further increase engagement and believability. The code for the most recent version of the task, as well as the version used in the current paper are available online.
References
- Allen J.P., Porter M.R., McFarland F.C., Marsh P., McElhaney K.B. The two faces of adolescents’ success with peers: adolescent popularity, social adaptation, and deviant behavior. Child Dev. 2005;76(3):747–760. doi: 10.1111/j.1467-8624.2005.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaschewski T., Brandeis D. Annotation: what electrical brain activity tells us about brain function that other techniques cannot tell us – a child psychiatric perspective. J. Child Psychol. Psychiatry. 2007;48(5):415–435. doi: 10.1111/j.1469-7610.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- Becker M.P., Nitsch A.M., Miltner W.H., Straube T. A single-trial estimation of the feedback-related negativity and its relation to BOLD responses in a time-estimation task. J. Neurosci. 2014;34(8):3005–3012. doi: 10.1523/JNEUROSCI.3684-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B., Khetarpal S., Brent D., Cully M., Balach L., Kaufman J., Neer S.M. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(4):545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Bolling D.Z., Pitskel N.B., Deen B., Crowley M.J., Mayes L.C., Pelphrey K.A. Development of neural systems for processing social exclusion from childhood to adolescence. Dev. Sci. 2011;14(6):1431–1444. doi: 10.1111/j.1467-7687.2011.01087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bress J.N., Foti D., Kotov R., Klein D.N., Hajcak G. Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology. 2013;50(1):74–81. doi: 10.1111/j.1469-8986.2012.01485.x. [DOI] [PubMed] [Google Scholar]
- Bress J.N., Hajcak G. Self-report and behavioral measures of reward sensitivity predict the feedback negativity. Psychophysiology. 2013;50(7):610–616. doi: 10.1111/psyp.12053. [DOI] [PubMed] [Google Scholar]
- Bress J.N., Meyer A., Proudfit G.H. The stability of the feedback negativity and its relationship with depression during childhood and adolescence. Child Dev. 2014 doi: 10.1017/S0954579414001400. (in press) [DOI] [PubMed] [Google Scholar]
- Brown B.B. Adolescents’ relationships with peers. In: Lerner R.M., Steinberg L., editors. vol. 2. John Wiley & Sons, Inc.; Hoboken, NJ: 2004. pp. 363–394. (Handbook of Adolescent Psychology). [Google Scholar]
- Carlson J.M., Foti D., Mujica-Parodi L.R., Harmon-Jones E., Hajcak G. Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: a combined ERP and fMRI study. Neuroimage. 2011;57(4):1608–1616. doi: 10.1016/j.neuroimage.2011.05.037. [DOI] [PubMed] [Google Scholar]
- Crowley M.J., Wu J., Molfese P.J., Mayes L.C. Social exclusion in middle childhood: rejection events, slow-wave neural activity, and ostracism distress. Soc. Neurosci. 2010;5(5–6):483–495. doi: 10.1080/17470919.2010.500169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger H.L., Pine D.S., Nelson E., Leibenluft E., Ernst M., Towbin K.E., Angold A. The NIMH Child Emotional Faces Picture Set (NIMH-ChEFS): a new set of children's facial emotion stimuli. Int. J. Methods Psychiatr. Res. 2011;20(3):145–156. doi: 10.1002/mpr.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdinand N.K., Kray J. Developmental changes in performance monitoring: how electrophysiological data can enhance our understanding of error and feedback processing in childhood and adolescence. Behav. Brain Res. 2014;263:122–132. doi: 10.1016/j.bbr.2014.01.029. [DOI] [PubMed] [Google Scholar]
- Foti D., Weinberg A., Dien J., Hajcak G. Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: temporospatial principal components analysis and source localization of the feedback negativity. Hum. Brain Mapp. 2011;32(12):2207–2216. doi: 10.1002/hbm.21182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring W.J., Willoughby A.R. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295(5563):2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Gratton G., Coles M.G., Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Grubbs F.E. Procedures for detecting outlying observations in samples. Technometrics. 1969;11(1):1–21. [Google Scholar]
- Gunther M.B., van Leijenhorst L., Rombouts S.A.R.B., Crone E.A., Van der Molen M.W. Do you like me? Neural correlates of social evaluation and developmental trajectories. Soc. Neurosci. 2010;5(5–6):461–482. doi: 10.1080/17470910903526155. [DOI] [PubMed] [Google Scholar]
- Guyer A.E., Choate V.R., Pine D.S., Nelson E.E. Neural circuitry underlying affective response to peer feedback in adolescence. Soc. Cogn. Affect. Neurosci. 2012;7(1):81–92. doi: 10.1093/scan/nsr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., Lau J.Y., McClure-Tone E.B., Parrish J., Shiffrin N.D., Reynolds R.C., Fox N.A. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch. Gen. Psychiatry. 2008;65(11):1303. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G., Moser J.S., Holroyd C.B., Simons R.F. The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biol. Psychol. 2006;71(2):148–154. doi: 10.1016/j.biopsycho.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Hämmerer D., Li S.-C., Müller V., Lindenberger U. Life span differences in electrophysiological correlates of monitoring gains and losses during probabilistic reinforcement learning. J. Cogn. Neurosci. 2011;23(3):579–592. doi: 10.1162/jocn.2010.21475. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E., Peterson C.K., Harris C.R. Jealousy: novel methods and neural correlates. Emotion. 2009;9(1):113. doi: 10.1037/a0014117. [DOI] [PubMed] [Google Scholar]
- Holroyd C.B., Coles M.G. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol. Rev. 2002;109(4):679. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Kovacs M. Multi-Health Systems, Inc.; Toronto, ON: 1992. Children's Depression Inventory. [Google Scholar]
- Kujawa A., Proudfit G.H., Klein D.N. Neural reactivity to rewards and losses in offspring of mothers and fathers with histories of depressive and anxiety disorders. J. Abnorm. Psychol. 2014;123(2):287–297. doi: 10.1037/a0036285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau J.Y.F., Guyer A.E., Tone E.B., Jenness J., Parrish J.M., Pine D.S., Nelson E.E. Neural responses to peer rejection in anxious adolescents: contributions from the amygdala-hippocampal complex. Int. J. Behav. Dev. 2012;36(1):36–44. doi: 10.1177/0165025411406854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck S.J. The MIT Press; Cambridge, MA: 2005. An Introduction to the Event-Related Potential Technique. [Google Scholar]
- Luu P., Tucker D.M., Derryberry D., Reed M., Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychol. Sci. 2003;14(1):47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- Masten C.L., Eisenberger N.I., Borofsky L.A., McNealy K., Pfeifer J.H., Dapretto M. Subgenual anterior cingulate responses to peer rejection: a marker of adolescents’ risk for depression. Dev. Psychopathol. 2011;23(01):283–292. doi: 10.1017/S0954579410000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten C.L., Eisenberger N.I., Borofsky L.A., Pfeifer J.H., McNealy K., Mazziotta J.C., Dapretto M. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Soc. Cogn. Affect. Neurosci. 2009;4(2):143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C.A., McCleery J.P. Use of event-related potentials in the study of typical and atypical development. J. Am. Acad. Child Adolesc. Psychiatry. 2008;47(11):1252–1261. doi: 10.1097/CHI.0b013e318185a6d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S., Yeung N., Holroyd C.B., Schurger A., Cohen J.D. Sensitivity of electrophysiological activity from medial frontal cortex to utilitarian and performance feedback. Cereb. Cortex. 2004;14(7):741–747. doi: 10.1093/cercor/bhh034. [DOI] [PubMed] [Google Scholar]
- Peterson C.K., Gravens L.C., Harmon-Jones E. Asymmetric frontal cortical activity and negative affective responses to ostracism. Soc. Cogn. Affect. Neurosci. 2011;6(3):277–285. doi: 10.1093/scan/nsq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijntjes A., Stegge H., Terwogt M.M., Kamphuis J.H., Telch M.J. Children's coping with in vivo peer rejection: an experimental investigation. J. Abnorm. Child Psychol. 2006;34(6):873–885. doi: 10.1007/s10802-006-9061-8. [DOI] [PubMed] [Google Scholar]
- Reijntjes A., Stegge H., Terwogt M.M., Kamphuis J.H., Telch M.J. Emotion regulation and its effects on mood improvement in response to an in vivo peer rejection challenge. Emotion. 2006;6(4):543. doi: 10.1037/1528-3542.6.4.543. [DOI] [PubMed] [Google Scholar]
- Sanislow C.A., Pine D.S., Quinn K.J., Kozak M.J., Garvey M.A., Heinssen R.K., Cuthbert B.N. Developing constructs for psychopathology research: research domain criteria. J. Abnorm. Psychol. 2010;119(4):631–639. doi: 10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
- Sebastian C., Tan G.C.Y., Roiser J.P., Viding E., Dumontheil I., Blakemore S.-J. Developmental influences on the neural bases of responses to social rejection: implications of social neuroscience for education. Neuroimage. 2011;57(3):686–694. doi: 10.1016/j.neuroimage.2010.09.063. [DOI] [PubMed] [Google Scholar]
- Sebastian C., Viding E., Williams K.D., Blakemore S.-J. Social brain development and the affective consequences of ostracism in adolescence. Brain Cogn. 2010;72(1):134–145. doi: 10.1016/j.bandc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Silk J.S., Siegle G.J., Lee K.H., Nelson E.E., Stroud L.R., Dahl R.E. Increased neural response to peer rejection associated with adolescent depression and pubertal development. Soc. Cogn. Affect. Neurosci. 2013 doi: 10.1093/scan/nst175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk J.S., Stroud L.R., Siegle G.J., Dahl R.E., Lee K.H., Nelson E.E. Peer acceptance and rejection through the eyes of youth: pupillary, eyetracking and ecological data from the Chatroom Interact task. Soc. Cogn. Affect. Neurosci. 2012;7(1):93–105. doi: 10.1093/scan/nsr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H. The teenage brain: sensitivity to social evaluation. Curr. Dir. Psychol. Sci. 2013;22(2):121–127. doi: 10.1177/0963721413476512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L., Morris A.S. Adolescent development. J. Cogn. Educ. Psychol. 2001;2(1):55–87. [Google Scholar]
- Weems C.F., Silverman W.K. Anxiety disorders. In: Beauchaine T.P., Hinshaw S.P., editors. Child and Adolescent Psychopathology. John Wiley & Sons; Hoboken, NJ: 2013. [Google Scholar]
- Williams K.D., Cheung C.K.T., Choi W. Cyberostracism: effects of being ignored over the Internet. J. Pers. Soc. Psychol. 2000;79(5):748–762. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]