Abstract
The glycocalyx layer on the vascular endothelium is known to have an important role as a transport barrier and in the mechanotransduction of fluid shear stress. The detailed structure and distribution of the glycocalyx in the bovine and human aqueous humor outflow pathways has not yet been reported. The purpose of this study was to determine whether this layer exists in the bovine and human aqueous outflow pathways and to compare the distribution and thickness therein. Enucleated bovine (N=4) and human (N=4) eyes were fixed using Alcian Blue to preserve the glycocalyx. The glycocalyx distribution and thickness (in regions where it was seen) were measured on the trabecular beams (TM), Schlemm’s canal (SC)/aqueous plexus (AP), and collector channels (CC). The glycocalyx, which appears as a layer of hair-like brushes, coats the surface of the endothelium non-uniformly in the bovine and human aqueous outflow pathways with a thickness in bovine eyes of 68–122 nm and in human eyes of 52–166 nm (25th to 75th percentiles). The distribution of the glycocalyx in different regions of the outflow pathway is not the same between bovine and human eyes. In both species, the glycocalyx was most uniform in the CCs. Less coverage of glycocalyx was found in the AP than the TM in bovine eyes, while more coverage was found in SC than the TM in human eyes. Most interestingly, glycocalyx was also found filling most pores of the endothelium of AP/SC in both bovine and human eyes. Glycocalyx was usually not found coating the inner membranes of the giant vacuoles (GVs); however, in GVs with a visible pore, glycocalyx was frequently observed on the inner membranes of the GVs. Based on our findings and those from the vascular endothelium, it is likely that the glycocalyx in SC plays a role in transduction of shear stress and perhaps regulation of outflow resistance.
Keywords: Alcian blue staining, endothelium, Schlemm’s canal, giant vacuole, pore, trabecular meshwork, collector channel, electron microscopy
1. Introduction
It is well-known that flow-induced shear stress acting on vascular endothelial cells leads to cell elongation and cell alignment in the direction of flow (Dewey 1983, Dewey 1984, Remuzzi et al. 1984). Surprisingly, even the relatively low shear stress in Schlemm’s canal (SC) leads their endothelial cells to align with the flow (Ethier, Read, and Chan 2004). This alignment is mediated by the glycocalyx and “the presence of the glycocalyx is necessary for the endothelial cells to respond to fluid shear,” (Yao, Rabodzey, and Dewey 2007).
The glycocalyx also plays an important role in the mechanotransduction of fluid shear stress (Tarbell and Ebong 2008, Tarbell 2010, Weinbaum, Tarbell, and Damiano 2007, Pries, Secomb, and Gaehtgens 2000), particularly as related to activation of endothelial nitric oxide synthase (eNOS) and subsequent nitric oxide (NO) release. The latter mediates flow-induced vasodilation in vascular endothelium. Modifications to the glycocalyx including removal of heparan sulphate, hyaluronic acid, or sialic acid abolishes the release of nitric oxide and consequent flow induced vasodilation (Tarbell and Ebong 2008). In addition, transgenic mice overexpressing eNOS have lower IOP and aqueous outflow resistance (Stamer et al. 2011).
It is thus of interest to characterize the morphological structure of the glycocalyx in the aqueous humor outflow pathway. In this study, we preserved this labile layer using Alcian Blue 8GX, and then examined its thickness and distribution in the aqueous outflow pathway of both bovine and human eyes. We found this layer to be irregular in both the bovine and human outflow pathways with significant differences between the two species.
2. Materials and Methods
Enucleated bovine eyes (N=4) were obtained from a local abattoir and delivered on ice within 6 hours post-mortem. Enucleated human eyes (N=4) from anonymous donors without any known history of ocular diseases were obtained from National Disease Research Interchange (Philadelphia, PA) within 24 hours post-mortem. The bovine eyes were either perfusion-fixed (N=2) or immersion-fixed (N=2), while the human eyes were perfusion-fixed. Perfusion-fixed eyes were perfused at 15 mmHg with Dulbecco’s phosphate-buffered saline (DPBS) with 5.5 mM of D-glucose for at least 30 minutes at 34°C, the anterior chambers exchanged with 1% glutaraldehyde and 4% paraformaldehyde in DPBS containing 30 mmol/L MgCl2 and 0.05% (w/v) Alcian Blue 8GX, and then perfused with the same solution for an additional hour. The eye was then hemisected and immersion-fixed in the same solution overnight. Immersion-fixed eyes were dissected to remove the lens and vitreous humor before immersing in the same fixation solution as perfusion-fixed eyes for at least 12 hours. All eyes were dissected into small tissue wedges and post-fixed in 1% aqueous osmium tetroxide and 1% lanthanum nitrate hexahydrate for 2 hours at room temperature, followed by en-bloc staining with uranyl acetate, dehydration in ascending series of ethanol, then replaced with propylene oxide and finally embedded in 100% Epon-Araldite. Ultra-thin sections (85 nm) of the trabecular outflow pathway were cut and examined with a transmission electron microscope (JOEL JEM-1011, Tokyo, JAPAN).
Electron micrographs [bovine: ≥ 12 images/eye; human: ≥ 18 images/eye] were taken randomly at different regions (trabecular meshwork: TM; Schlemm’s canal: SC; aqueous plexus: AP; collector channels: CC) of the aqueous outflow pathway and pooled for each region from all eyes to calculate the percent distribution and thickness of the glycocalyx. Percent distribution of glycocalyx on the endothelial surface (length of glycocalyx covering endothelial surface / total length of endothelial surface) was determined by the intensity plot profile of glycocalyx close to and parallel to the luminal membrane to include all the observable glycocalyx (including collapsed glycocalyx) using ImageJ (NIH) (bovine: ≥16 measurements/region; human: ≥48 measurements/region). A preset intensity was applied across all images to separate the glycocalyx from the background. In those regions where the glycocalyx was seen, its thickness was measured at randomly chosen regions of the TM, in the AP/SC, and CCs. Episcleral veins were used as positive controls. The results given in box-plots represent the 25th and 75th percentile, while the center line represents the median, with whiskers extending to a maximum of 1.5 interquartile range. The statistical analysis was done using R version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria.). Because the datasets were not normally distributed as determined using the Shapiro-Wilk test, the Mann-Whitney U nonparametric test was used to assess the statistical significance of difference seen in the distribution and thickness of glycocalyx in different regions.
3. Results
The glycocalyx, which appears as a layer of hair-like brushes similar to the endothelial glycocalyx reported previously (van den Berg, Vink, and Spaan 2003) (Figure 1), validated our modified Alcian Blue staining method adapted for the aqueous outflow pathway. Figure 2 showed our positive controls, the glycocalyx in the episcleral veins of the bovine and human aqueous outflow pathways. We did not find any significant differences in the glycocalyx distribution in different regions of the outflow pathway when comparing immersion- and perfusion-fixed bovine eyes (glycocalyx distribution between immersion vs. perfusion: AP: 3–20 % vs. 3–65% p=0.49; TM: 21–43 % vs. 28–43 %, p=0.52, respectively), and thus, for bovine eyes, immersion- and perfusion-fixed data were pooled for the analysis.
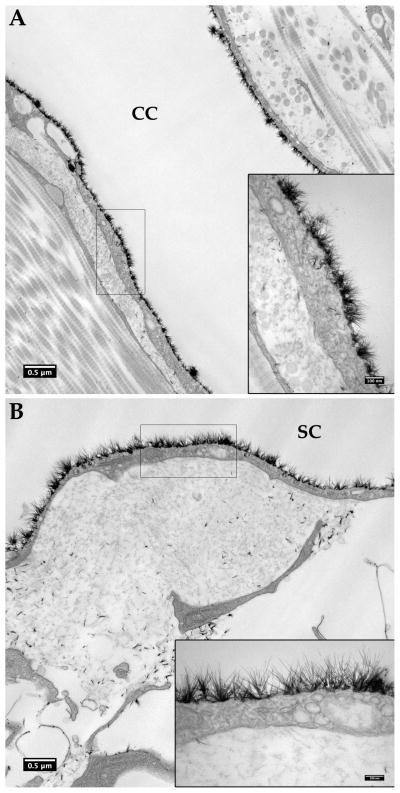
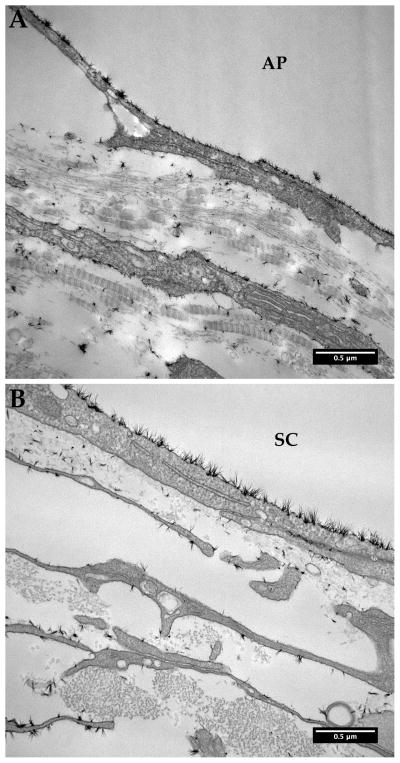
Figure 1.
Endothelial glycocalyx layer (glycocalyx) in bovine and human aqueous outflow pathways. Glycocalyx appears as a layer of hair-like structures in bovine collector channel (A) and human Schlemm’s canal (B) of the aqueous outflow pathway. Insets show the glycocalyx at a higher magnification.
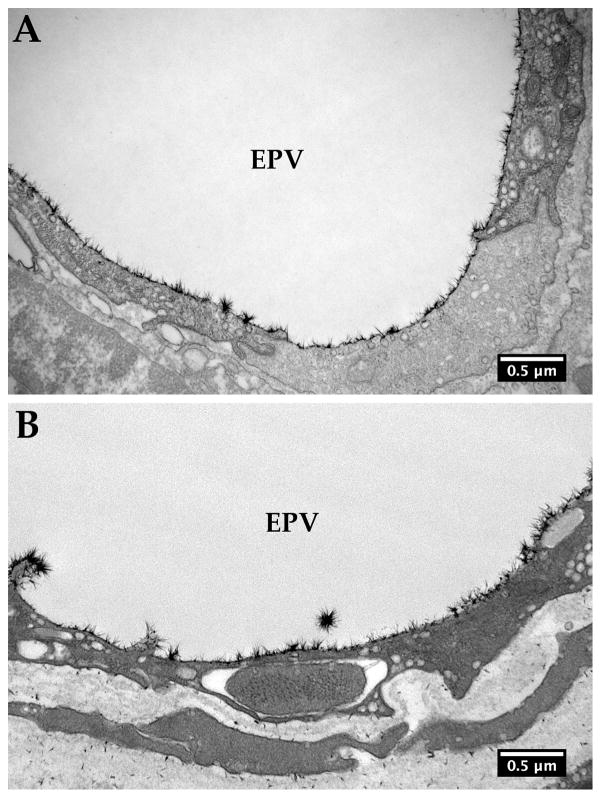
Figure 2.
Glycocalyx in the bovine and human episceral veins. Epithelial veins (EPV) served as a control. The glycocalyx was relatively uniform in both bovine (A) and human (B) episceral veins.
The distribution of the glycocalyx was non-uniform in both the bovine (Figure 3A–C) and human (Figure 3D–F) aqueous outflow pathways. In both species, the glycocalyx was most uniform in the CCs (Figure 3A, D). The distribution of glycocalyx was more variable in the proximal aspects of the outflow pathway, but differed somewhat between the two species. In the bovine eyes, the glycocalyx distribution was most variable on the wall of the AP, with some regions showing little or no coverage (Figure 3B); the TM showed somewhat more uniform coverage than the AP but was less uniform than that seen in the CCs (Figure 3C). The human eyes showed a different pattern, with the most variable coverage in the TM, and a somewhat more uniform coverage of SC (Figure 3E, F).
Figure 3.
Glycocalyx in the bovine (A–C) and human (D–E) aqueous outflow pathways. A, D: In the bovine and human collector channels (CC), the glycocalyx (black arrows) was relatively uniform. B, E: In the bovine aqueous plexus (AP), the glycocalyx (black arrows) was variable and less uniform than that in the CC, with some regions showing little or no glycocalyx (arrowheads); in human Schlemm’s canal (SC), the glycocalyx was relatively uniform but less so than in the CC with some regions showing little or no glycocalyx (arrowheads). C, F: On the bovine trabecular beams, the glycocalyx (black arrows) was also less uniform than that in the CC with some regions showing little or no glycocalyx (arrowhead); on the human trabecular beams, the glycocalyx was less uniform than that in the CC and SC with some regions showing little or no glycocalyx (arrowheads). ITS: Intertrabecular space.
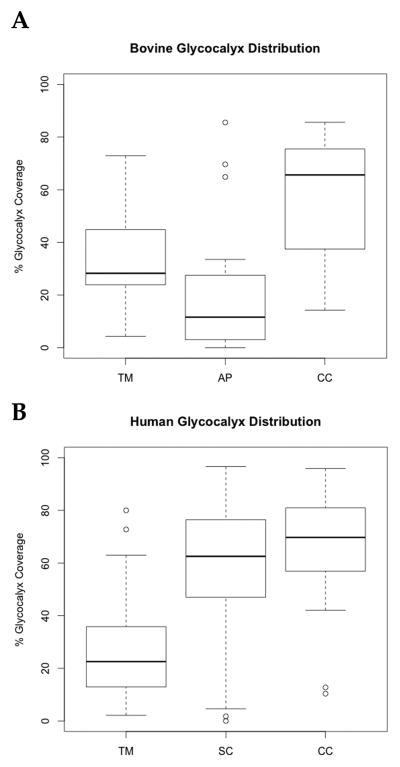
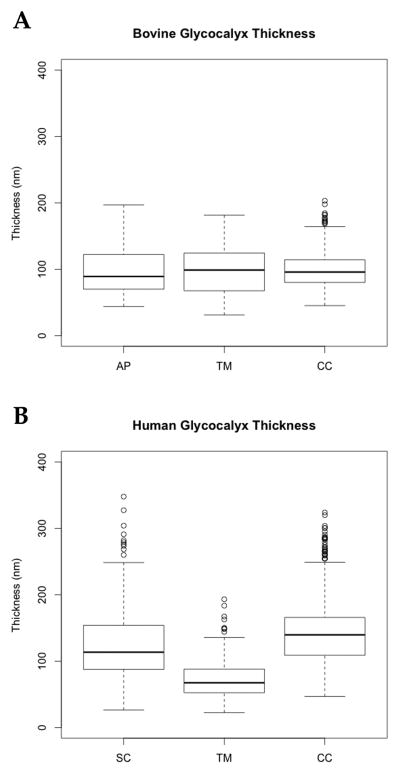
Quantitative studies confirmed these differences in glycocalyx distribution between the two species. In both bovine and human eyes, the surface coverage by glycocalyx was most complete in the CC (bovine: 37–76 %, n=23 images; human: 57–81 %, n=52 images); this was followed in the bovine by the TM at 27–43 % (n=16), and then AP at 3–27 % (n=26) (p < 0.01) (Figure 4A); in the human, this order was reversed with the coverage in SC at 47–76 % (n=110) and the TM at 13–36 % (n=49) (p < 0.01) (Figure 4B). In bovine eyes, the thickness of the glycocalyx was very uniform in those regions where it was found: AP (70–122 nm, n=158: 25th to 75th percentiles), TM (68–124 nm, n=199), and CC (80–114 nm, n=404) (p > 0.05) (Figure 5A). In human eyes, the glycocalyx was thickest in the CC (109–166 nm, n= 521) followed by SC (88–154 nm, n=1043), and then the TM (52–88 nm, n=288) (p < 0.001) (Figure 5B).
Figure 4.
Glycocalyx distribution. In bovine eyes (A), the ordering of decreasing percentage of the endothelial surface covered by glycocalyx was CC > TM > AP (p < 0.01). In humans, more uniform glycocalyx was seen in SC than in AP of bovines. This ordering was different: CC > SC > TM (p < 0.01)
Figure 5.
Glycocalyx thickness. In bovine eyes, the glycocalyx thickness was not significantly different on the surfaces of the AP, TM, or CC (p > 0.05), whereas in human eyes, the thickness in these three areas was different (CC > SC > TM, p < 0.001).
Ultrastructural observations revealed that the glycocalyx covered the plasma membranes of the TM, SC/AP, and CC endothelial cells (Figure 3). Alcian Blue stained the glycocalyx coated on the luminal surfaces of the SC/AP, while very sparse coating was seen on the basal surfaces of the endothelial cells of SC/AP. Additionally, the plasma membranes of some juxtacanalicular tissue (JCT) cells also showed sparse distribution of glycocalyx in both human and bovine eyes (Figure 6). Also very noteworthy was the Alcian Blue staining pattern associated with the giant vacuoles (GVs) of the endothelium of SC. In both bovine and human eyes, glycocalyx was usually seen only coating the outer membrane and not inner membrane of the GVs of the AP (Figure 7A, B) or SC (Figure 7C, D) whether the sections were passing through a basal opening or not. No Alcian Blue staining was found filling the lumens of either GVs or SC/AP, consistent with their being empty spaces. Lastly, glycocalyx distributions appeared similar on the outer surfaces of GVs in SC/AP as compared to the surrounding endothelium.
Figure 6.
Glycocalyx in the juxtacanalicular tissue (JCT) Region. Glycocalyx was found sparsely distributed on the plasma membranes of some JCT cells in both bovine (A) and human (B) eyes, which was much less than the respective endothelial cells of aqueous plexus (AP)/ Schlemm’s canal (SC) in the same image.
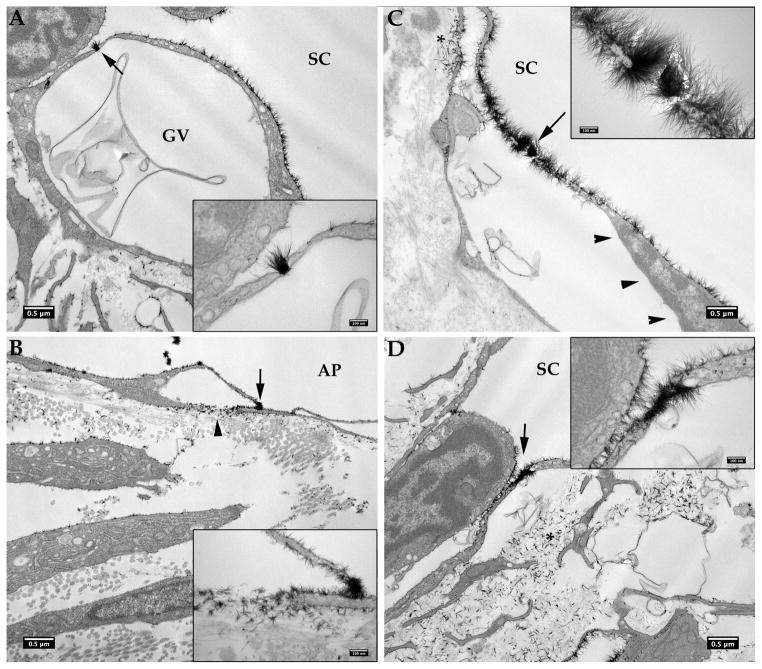
Figure 7.
Glycocalyx on the giant vacuoles.
In bovine eyes, glycocalyx only sparsely coated the outer membrane, not the inner membrane of a giant vacuole (GV) of aqueous plexus (AP) endothelium whether a basal opening was not present (A) or present (B, arrowhead). The same is true in human eyes; glycocalyx is usually seen only coating the outer membrane but not inner membrane of a GV of Schlemm’s canal (SC) endothelium whether a basal opening is not observed (C) or is observed (arrowhead, D). More uniform glycocalyx was seen on the outer membrane of GVs in human eyes than in bovine eyes.
A most interesting finding in our study was that most transcellular pores passing through the endothelium of SC/AP were filled with glycocalyx, including both GV associated and non-GV associated pores (Figure 8). This was true for 10 of the 12 pores seen in human eyes and 8 of 9 pores seen in bovine eyes. Interestingly, in those GVs with a visible pore, particularly those in which a basal opening to the GV was also apparent, glycocalyx was found not only coating the outer membrane of the GVs but also sparsely coating the inner membrane of the GVs closest to the basal opening and closest to the pore (Figure 8B,C). Glycocalyx was also seen in the extracellular matrix nearest the basal openings of the pore-associated GVs (Figure 8B,C).
Figure 8.
Glycocalyx in the pores. A: A pore in a GV (arrow, insert) of a human eye is filled with glycocalyx, which also coats the outer membrane, but not the inner membrane of the GV; note the membranous material apparently in the passage through the GV. B: Glycocalyx is seen filling a pore (arrow, inset) in a GV, coating the outer membrane, and sparsely coating the inner membrane of the GV close to the basal opening and the pore, as well as the extracellular matrix near the basal opening (arrowhead) of the GV in a bovine eye. C: Glycocalyx was seen within a pore of a giant vacuole (arrow), and coating the outer surface and left side of the inner membrane of the giant vacuole, which is close to a basal opening (*), but not the right side of the inner membrane of the giant vacuole (arrowheads) in a human eye. D: Glycocalyx was seen filling a pore not associated with the giant vacuole (arrow). Insets show the pore filling with glycocalyx at a higher magnification. Significant Alcian Blue staining was also seen in the basal side of extracellular matrix (*).
4. Discussion
The glycocalyx is comprised of glycoproteins bearing acidic oligosaccharides and terminal sialic acids, proteoglycans, and glycosaminoglycans (GAGs) (Reitsma et al. 2007). Previous studies have used electron microscopy to examine the structure of glycocalyx in blood vessels, including rat myocardial capillaries (van den Berg, Vink, and Spaan 2003), rat aorta (Devaraj et al. 2009), and human umbilical vein (Chappell et al. 2009). Richardson (Richardson 1982) previously examined the presence of GAGs on the surface of the aqueous outflow pathway in feline using ruthenium red, but that label is known to collapse the glycocalyx (Tarbell and Ebong 2010), and thus the existence, detailed structure, and distribution of glycocalyx in the outflow pathway have remained unclear. To better preserve the labile glycocalyx, we applied the modified Alcian blue protocol published by van den Berg and colleagues in this study (van den Berg, Vink, and Spaan 2003). Alcian blue, a copper-containing phthalocyanine, is a polyvalent basic dye that, when used in the fixative at physiological pH, can bind to glycocalyx (extracellular glycoproteins and glycosaminoglycans) forming insoluble precipitates to preserve the labile glycocalyx during dehydration and embedding processes (Hayat 1993). While it is possible that the Alcian blue did not assess all aspects of the outflow pathway (and thus absence of staining does not necessarily imply absence of glycocalyx), our study has attempted to minimize that possibility by cutting tissue into small segments prior to Alcian blue-fixative immersion overnight.
We found that the glycocalyx in the aqueous outflow pathway was less thick than that reported for vascular endothelium and also less uniform. The thickness of the glycocalyx, in regions where it was found, ranged from 68 to 122 nm in the bovine outflow pathway and 52 to 166 nm in the human (25th to 75th percentiles). This is less than the range reported for vascular glycocalyx (200–500 nm), measured using comparable methods (Tarbell and Ebong 2010).
Glycocalyx is reported to be uniformly distributed in rat and mouse aortas but not in the microvessels, with perhaps half either not covered, or covered with a much thinner glycocalyx (Yen et al. 2012). Some arterioles have been reported to be without glycocalyx. These findings are, in general, consistent with our findings in the episceral veins, CCs and SC; however, in the TM of human eyes and in the AP of bovine eyes, glycocalyx was found to be even more sparse and variable than in most other microvessels. The variability in the distribution of the glycocalyx in the outflow pathway that we found may relate to non-uniform flow of aqueous humor through these tissues (Overby, Stamer, and Johnson 2009, Keller et al. 2011, Battista et al. 2008, Yang et al. 2013). This non-uniform flow in the aqueous outflow pathways results in heterogeneously distributed shear forces similar to variable glycocalyx distribution in the vascular system resulting from heterogeneous blood flow distribution (van den Berg, Vink, and Spaan 2003, van den Berg, Spaan, and Vink 2009).
The finding of a glycocalyx in SC/AP allows for speculation as to its role as a possible regulator of aqueous outflow resistance, and thereby, IOP. Changes in shear stress levels in SC/AP would be expected to be transduced by the glycocalyx and modulate NO release (Tarbell and Ebong 2010). In SC/AP, this autocrine response would then be expected to relax these cells, a response that has been associated with decreased outflow resistance (Zhou et al. 2012). Thus, for example, if IOP were to increase, this would cause narrowing of SC/AP (Johnstone and Grant 1973, Van Buskirk 1982, Battista et al. 2008, Zhang et al. 2009), thus increasing the shear stress level in SC/AP (shear stress is inversely proportional to the size of SC/AP), and thereby initiating this regulatory response to lower the IOP.
Overall, our observation of glycocalyx staining pattern in the aqueous outflow pathway delineated by Alcian Blue appeared similar to previous observation using cationic ferritin (de Kater, Melamed, and Epstein 1989, Ethier and Chan 2001, Tripathi, Tripathi, and Spaeth 1987), ruthenium red(Richardson 1982, Grierson, Lee, and Abraham 1977), and colloidal iron (Grierson and Lee 1975, Armaly and Wang 1975, Tripathi, Tripathi, and Spaeth 1987) but with much better ultrastructure. The outer membrane of giant vacuoles had significantly more continuous glycocalyx coverage compared to that of the inner membrane, which is likely due to the differences in the sialic acid residues between the luminal and basal lining of giant vacuoles (Tripathi, Tripathi, and Spaeth 1987). Furthermore, we found that most inner wall pores were filled with glycocalyx. Although a previous study had observed the accumulation of cationic ferritin at possible pore locations, that study used the scanning electron microscope with which the authors were only able to identify the surface but not inside of the pores (Ethier and Chan 2001). Since the inner wall pores are thought to be passages for aqueous humor to enter SC (Johnson 2006), glycocalyx filling the pores may play a role in regulating aqueous outflow resistance.
Weinbaum et al. (2003) estimated the specific hydraulic conductivity of glycocalyx to be 3.2 nm2. Using Darcy’s law, we can estimate the flow resistance of a glycocalyx-filled pore of diameter 1 μm and thickness 0.1 μm and compare this to the resistance of empty pore of the same dimensions. We found that a glycocalyx-filled pore has potentially a far higher flow resistance than an empty pore, confirming that the glycocalyx filling the pores could potentially play a role in regulating aqueous outflow resistance1. If so, the bulk of aqueous flow might pass through pores that are not filled with glycocalyx. However, Ethier and Chan showed that removal of sialic acid (a component of glycocalyx) with neuraminidase does not alter aqueous outflow resistance (Ethier and Chan 2001). Further studies are warranted to understand the role of glycocalyx in the aqueous outflow pathway.
In summary, this study has demonstrated the detailed structure and distribution of the glycocalyx in both bovine and human aqueous outflow pathways, which is species-dependent. The implications of a non-uniform glycocalyx in the aqueous outflow pathway remain to be clarified. More importantly, based on our findings that showed the glycocalyx lines the wall of SC and fills most of the pores entering SC and findings from vascular endothelium, it is likely that the glycocalyx in SC plays a role in transduction of shear stress and perhaps regulation of outflow resistance.
Highlights.
We examined glycocalyx distribution in bovine and human aqueous outflow pathways.
Glycocalyx unevenly coats the surface of the endothelium of aqueous outflow pathways.
Glycocalyx distribution and thickness were different between bovine and human eyes.
Glycocalyx lines most outer but not inner membrane of the giant vacuoles.
Glycocalyx fills most pores of the endothelium of Schlemm’s canal and aqueous plexus.
Acknowledgments
Grant support:
NIH Grant EY019696, EY022634, Sigma Xi Grant-in-Aid, and The Massachusetts Lions Eye Research Fund.
We thank Rui Jin, MS for technical assistance.
Footnotes
For a glycocalyx-filled pore, Darcy’s law gives a flow resistance of μL/(KA) with aqueous humor viscosity μ, pore length L, permeability of the glycocalyx K, and A=πR2 with pore radius R; for an empty pore, Sampson’s law give a resistance of 3μ/R3 and thus the ratio of these two resistances is LR/(3πK). With K=3.2 nm2 (Weinbaum et al., 2003), assuming L= 200 nm (a typical thickness of the wall of a giant vacuole), this ratio is 3300 for a 1 μm diameter pore.
Commercial relationship:
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armaly MF, Wang Y. Demonstration of acid mucopolysaccharides in the trabecular meshwork of the Rhesus monkey. Invest Ophthalmol. 1975;14(7):507–16. [PubMed] [Google Scholar]
- Battista SA, Lu Z, Hofmann S, Freddo T, Overby DR, Gong H. Reduction of the available area for aqueous humor outflow and increase in meshwork herniations into collector channels following acute IOP elevation in bovine eyes. Invest Ophthalmol Vis Sci. 2008;49(12):5346–52. doi: 10.1167/iovs.08-1707. iovs.08-1707 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell D, Jacob M, Paul O, Rehm M, Welsch U, Stoeckelhuber M, Conzen P, Becker BF. The glycocalyx of the human umbilical vein endothelial cell: an impressive structure ex vivo but not in culture. Circ Res. 2009;104(11):1313–7. doi: 10.1161/CIRCRESAHA.108.187831. [DOI] [PubMed] [Google Scholar]
- de Kater AW, Melamed S, Epstein DL. Patterns of aqueous humor outflow in glaucomatous and nonglaucomatous human eyes. A tracer study using cationized ferritin. Arch Ophthalmol. 1989;107(4):572–6. doi: 10.1001/archopht.1989.01070010586035. [DOI] [PubMed] [Google Scholar]
- Devaraj S, Yun JM, Adamson G, Galvez J, Jialal I. C-reactive protein impairs the endothelial glycocalyx resulting in endothelial dysfunction. Cardiovasc Res. 2009;84(3):479–84. doi: 10.1093/cvr/cvp249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey CF., Jr Effects of fluid flow on living vascular cells. J Biomech Eng. 1984;106(1):31–5. doi: 10.1115/1.3138453. [DOI] [PubMed] [Google Scholar]
- Dewey CF. Orientation of endothelial cells in shear fields in vitro. Clinical Hemorheology. 1983;3:224. doi: 10.3233/bir-1984-21419. [DOI] [PubMed] [Google Scholar]
- Ethier CR, Chan DW. Cationic ferritin changes outflow facility in human eyes whereas anionic ferritin does not. Invest Ophthalmol Vis Sci. 2001;42(8):1795–802. [PubMed] [Google Scholar]
- Ethier CR, Read AT, Chan D. Biomechanics of Schlemm’s canal endothelial cells: Influence on F-actin architecture. Biophysical J. 2004;87:2828–2837. doi: 10.1529/biophysj.103.038133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grierson I, Lee WR. Acid mucopolysaccharides in the outflow apparatus. Exp Eye Res. 1975;21(5):417–31. doi: 10.1016/0014-4835(75)90124-4. [DOI] [PubMed] [Google Scholar]
- Grierson I, Lee WR, Abraham S. The appearance of the outflow apparatus of the eye after staining with ruthenium red. Acta Ophthalmol (Copenh) 1977;55(5):827–36. doi: 10.1111/j.1755-3768.1977.tb08282.x. [DOI] [PubMed] [Google Scholar]
- Hayat MA. The Language of science. New York: Plenum Press; 1993. Stains and cytochemical methods. [Google Scholar]
- Johnson M. What controls aqueous humour outflow resistance? Experimental Eye Research. 2006;82(4):545–57. doi: 10.1016/j.exer.2005.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone MA, Grant WM. Pressure dependent changes in the structures of the aqueous outflow system of human and monkey eyes. American Journal of Ophthalmology. 1973;75:365. doi: 10.1016/0002-9394(73)91145-8. [DOI] [PubMed] [Google Scholar]
- Keller KE, Bradley JM, Vranka JA, Acott TS. Segmental versican expression in the trabecular meshwork and involvement in outflow facility. Invest Ophthalmol Vis Sci. 2011;52(8):5049–57. doi: 10.1167/iovs.10-6948. iovs.10-6948 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overby D, Stamer DW, Johnson M. The changing paradigm of outflow resistance generation: towards synergistics models of the JCT and inner wall endothelium. Experimental Eye Research. 2009;88(4):656–670. doi: 10.1016/j.exer.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch. 2000;440(5):653–66. doi: 10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]
- Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454(3):345–59. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remuzzi A, Dewey CF, Jr, Davies PF, Gimbrone MA., Jr Orientation of endothelial cells in shear fields in vitro. Biorheology. 1984;21(4):617–30. doi: 10.3233/bir-1984-21419. [DOI] [PubMed] [Google Scholar]
- Richardson TM. Distribution of glycosaminoglycans in the aqueous outflow system of the cat. Invest Ophthalmol Vis Sci. 1982;22(3):319–29. [PubMed] [Google Scholar]
- Stamer WD, Lei Y, Boussommier-Calleja A, Overby DR, Ethier CR. eNOS, a pressure-dependent regulator of intraocular pressure. Invest Ophthalmol Vis Sci. 2011;52(13):9438–44. doi: 10.1167/iovs.11-7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbell JM. Shear stress and the endothelial transport barrier. Cardiovasc Res. 2010;87(2):320–30. doi: 10.1093/cvr/cvq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbell JM, Ebong EE. The endothelial glycocalyx: a mechano-sensor and -transducer. Sci Signal. 2008;1(40):pt8. doi: 10.1126/scisignal.140pt8. [DOI] [PubMed] [Google Scholar]
- Tarbell JM, Ebong EE. Endothelial glycocalyx structure and role in mechanotransduction. In: Hsiai TK, Blackman B, Jo H, editors. Hemodynamics and Mechanobiology of Endothelium. Singpore: World Scientific Publishing Co. Pte. Ltd; 2010. pp. 69–96. [Google Scholar]
- Tripathi RC, Tripathi BJ, Spaeth GL. Localization of sialic acid moieties in the endothelial lining of Schlemm’s canal in normal and glaucomatous eyes. Exp Eye Res. 1987;44(2):293–306. doi: 10.1016/s0014-4835(87)80013-1. [DOI] [PubMed] [Google Scholar]
- Van Buskirk EM. Anatomic correlates of changing aqueous outflow facility in excised human eyes. Investigative Ophthalmology and Visual Science. 1982;22:625–632. [PubMed] [Google Scholar]
- van den Berg BM, Spaan JA, Vink H. Impaired glycocalyx barrier properties contribute to enhanced intimal low-density lipoprotein accumulation at the carotid artery bifurcation in mice. Pflugers Arch. 2009;457(6):1199–206. doi: 10.1007/s00424-008-0590-6. [DOI] [PubMed] [Google Scholar]
- van den Berg BM, Vink H, Spaan JA. The endothelial glycocalyx protects against myocardial edema. Circ Res. 2003;92(6):592–4. doi: 10.1161/01.RES.0000065917.53950.75. [DOI] [PubMed] [Google Scholar]
- Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–67. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- Weinbaum S, Zhang X, Han Y, Vink H, Cowin SC. Mechanotransduction and flow across the endothelial glycocalyx. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(13):7988–95. doi: 10.1073/pnas.1332808100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CY, Liu Y, Lu Z, Ren R, Gong H. Effects of y27632 on aqueous humor outflow facility with changes in hydrodynamic pattern and morphology in human eyes. Invest Ophthalmol Vis Sci. 2013;54(8):5859–70. doi: 10.1167/iovs.12-10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Rabodzey A, Dewey CF., Jr Glycocalyx modulates the motility and proliferative response of vascular endothelium to fluid shear stress. Am J Physiol Heart Circ Physiol. 2007;293(2):H1023–30. doi: 10.1152/ajpheart.00162.2007. [DOI] [PubMed] [Google Scholar]
- Yen WY, Cai B, Zeng M, Tarbell JM, Fu BM. Quantification of the endothelial surface glycocalyx on rat and mouse blood vessels. Microvasc Res. 2012;83(3):337–46. doi: 10.1016/j.mvr.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Toris CB, Liu Y, Ye W, Gong H. Morphological and hydrodynamic correlates in monkey eyes with laser induced glaucoma. Exp Eye Res. 2009;89(5):748–56. doi: 10.1016/j.exer.2009.06.015. S0014-4835(09)00188-2 [pii] [DOI] [PubMed] [Google Scholar]
- Zhou EN, Krishnan R, Stamer WD, Perkuman K, Rejendran K, Nabhan JF, Lu Q, Fredberg JJ, Johnson M. Mechanical responses of the endothelial cell of Schlemm’s canal: Scope, variability and their potential role in controlling aqueous humor outflow. Journal of the Royal Society Interface. 2012;(9):1144–1155. doi: 10.1098/rsif.2011.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]