Abstract
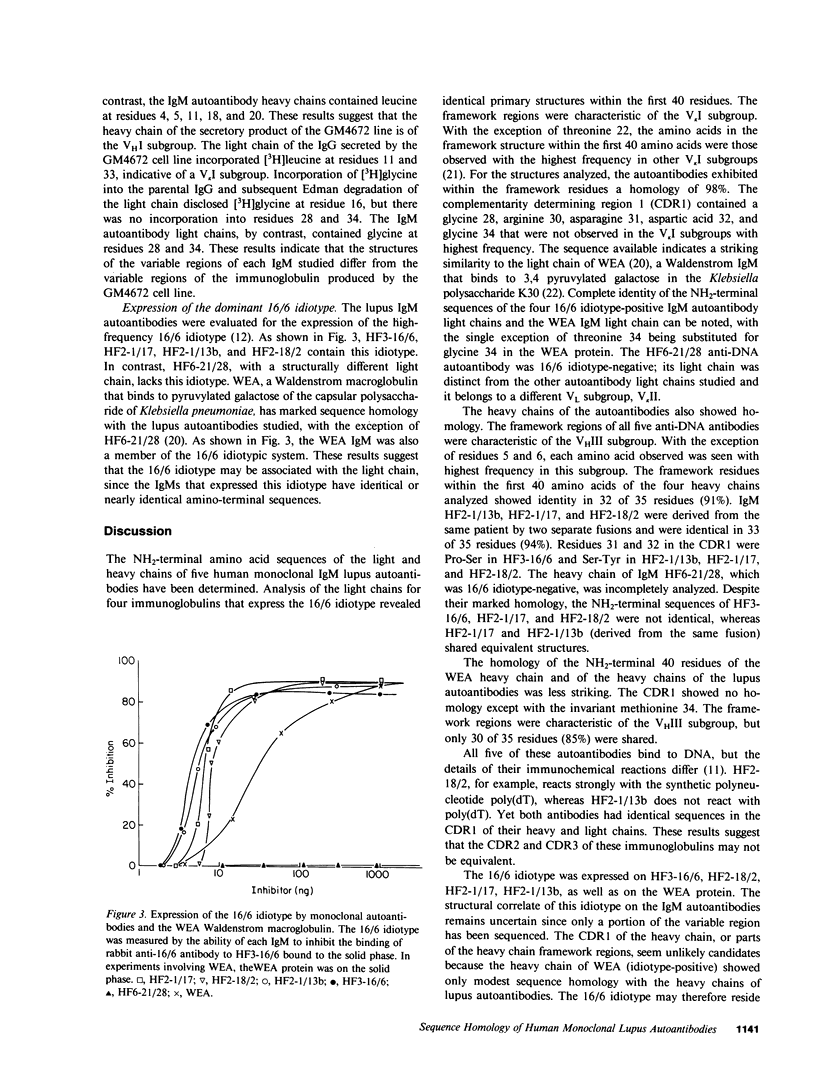
The NH2-terminal amino acid sequences have been determined by automated Edman degradation for the heavy and light chains of five monoclonal IgM anti-DNA autoantibodies that were produced by human-human hybridomas derived from lymphocytes of two patients with systemic lupus erythematosus. Four of the antibodies were closely related to the idiotype system 16/6, whereas the fifth antibody was unrelated idiotypically. The light chains of the 16/6 idiotype-positive autoantibodies (HF2-1/13b, HF2-1/17, HF2-18/2, and HF3-16/6) had identical amino acid sequences from residues 1 to 40. Their framework structures were characteristic of VKI light chains. The light chain of the 16/6 idiotype-negative autoantibody HF6-21/28 was characteristic of the VKII subgroup. The heavy chains of the 16/6 idiotype-positive autoantibodies had nearly identical amino acid sequences from residues 1 to 40. The framework structures were characteristic of the VHIII subgroup. In contrast, the GM4672 fusion partner of the hybridoma produced small quantities of an IgG with a VHI heavy chain and a VKI light chain. The heavy chains of the lupus autoantibodies and the light chains of those autoantibodies that were idiotypically related to the 16/6 system had marked sequence homology with WEA, a Waldenstrom IgM that binds to Klebsiella polysaccharides and expresses the 16/6 idiotype. These results indicate a striking homology in the amino termini of the heavy and light chains of the lupus autoantibodies studied and suggest that the V regions of the heavy and light chains of the 16/6 idiotype-positive DNA-binding lupus auto-antibodies are each encoded by a single germ line gene.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrzejewski C., Jr, Rauch J., Lafer E., Stollar B. D., Schwartz R. S. Antigen-binding diversity and idiotypic cross-reactions among hybridoma autoantibodies to DNA. J Immunol. 1981 Jan;126(1):226–231. [PubMed] [Google Scholar]
- Andrzejewski C., Jr, Stollar B. D., Lalor T. M., Schwartz R. S. Hybridoma autoantibodies to DNA. J Immunol. 1980 Mar;124(3):1499–1502. [PubMed] [Google Scholar]
- André-Schwartz J., Datta S. K., Shoenfeld Y., Isenberg D. A., Stollar B. D., Schwartz R. S. Binding of cytoskeletal proteins by monoclonal anti-DNA lupus autoantibodies. Clin Immunol Immunopathol. 1984 May;31(2):261–271. doi: 10.1016/0090-1229(84)90246-0. [DOI] [PubMed] [Google Scholar]
- Brauer A. W., Margolies M. N., Haber E. The application of 0.1 M quadrol to the microsequence of proteins and the sequence of tryptic peptides. Biochemistry. 1975 Jul;14(13):3029–3035. doi: 10.1021/bi00684a036. [DOI] [PubMed] [Google Scholar]
- Briles D. E., Forman C., Hudak S., Claflin J. L. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J Exp Med. 1982 Oct 1;156(4):1177–1185. doi: 10.1084/jem.156.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHeer D. H., Pages J. M., Bussard A. E. Specificity of antierythrocyte autoantibodies secreted by a NZB-derived hybridoma and NZB peritoneal cells. Cell Immunol. 1980 Jan;49(1):135–141. doi: 10.1016/0008-8749(80)90063-5. [DOI] [PubMed] [Google Scholar]
- Diamond B., Scharff M. D. Somatic mutation of the T15 heavy chain gives rise to an antibody with autoantibody specificity. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5841–5844. doi: 10.1073/pnas.81.18.5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilat D., Asofsky R., Laskov R. A hybridoma from an autoimmune NZB/NZW mouse producing monoclonal antibody to ribosomal-RNA. J Immunol. 1980 Feb;124(2):766–768. [PubMed] [Google Scholar]
- Eilat D., Hochberg M., Pumphrey J., Rudikoff S. Monoclonal antibodies to DNA and RNA from NZB/NZW F1 mice: antigenic specificities and NH2 terminal amino acid sequences. J Immunol. 1984 Jul;133(1):489–494. [PubMed] [Google Scholar]
- Fohlman J., Rask L., Peterson P. A. High-pressure liquid chromatographic identification of phenylthiohydantoin derivatives of all twenty common amino acids. Anal Biochem. 1980 Jul 15;106(1):22–26. doi: 10.1016/0003-2697(80)90113-x. [DOI] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Chemical typing of human kappa light chain subgroups expressed by human hybrid myelomas. Immunology. 1982 Jan;45(1):125–128. [PMC free article] [PubMed] [Google Scholar]
- Goñi F., Frangione B. Amino acid sequence of the Fv region of a human monoclonal IgM (protein WEA) with antibody activity against 3,4-pyruvylated galactose in Klebsiella polysaccharides K30 and K33. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4837–4841. doi: 10.1073/pnas.80.15.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg D. A., Shoenfeld Y., Madaio M. P., Rauch J., Reichlin M., Stollar B. D., Schwartz R. S. Anti-DNA antibody idiotypes in systemic lupus erythematosus. Lancet. 1984 Aug 25;2(8400):417–422. doi: 10.1016/s0140-6736(84)92904-0. [DOI] [PubMed] [Google Scholar]
- Kabat E. A., Liao J., Bretting H., Franklin E. C., Geltner D., Frangione B., Koshland M. E., Shyong J., Osserman E. F. Human monoclonal macroglobulins with specificity for Klebsiella K polysaccharides that contain 3,4-pyruvylated-D-galactose and 4,6-pyruvylated-D-galactose. J Exp Med. 1980 Oct 1;152(4):979–995. doi: 10.1084/jem.152.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapper D. G., Wilde C. E., 3rd, Capra J. D. Automated amino acid sequence of small peptides utilizing Polybrene. Anal Biochem. 1978 Mar;85(1):126–131. doi: 10.1016/0003-2697(78)90282-8. [DOI] [PubMed] [Google Scholar]
- Klotz J. L., Phillips M. L., Miller M. M., Teplitz R. L. Monoclonal autoantibody production by hybrid cell lines. Clin Immunol Immunopathol. 1981 Mar;18(3):368–374. doi: 10.1016/0090-1229(81)90130-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lafer E. M., Rauch J., Andrzejewski C., Jr, Mudd D., Furie B., Furie B., Schwartz R. S., Stollar B. D. Polyspecific monoclonal lupus autoantibodies reactive with both polynucleotides and phospholipids. J Exp Med. 1981 Apr 1;153(4):897–909. doi: 10.1084/jem.153.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Siekevitz M., Margolies M. N., Mudgett-Hunter M., Gefter M. L. Hybridoma proteins expressing the predominant idiotype of the antiazophenylarsonate response of A/J mice. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1120–1124. doi: 10.1073/pnas.77.2.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pages J. M., Bussard A. E. Establishment and characterization of a permanent murine hybridoma secreting monoclonal autoantibodies. Cell Immunol. 1978 Nov;41(1):188–194. doi: 10.1016/s0008-8749(78)80038-0. [DOI] [PubMed] [Google Scholar]
- Rajewsky K., Takemori T. Genetics, expression, and function of idiotypes. Annu Rev Immunol. 1983;1:569–607. doi: 10.1146/annurev.iy.01.040183.003033. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y., Hsu-Lin S. C., Gabriels J. E., Silberstein L. E., Furie B. C., Furie B., Stollar B. D., Schwartz R. S. Production of autoantibodies by human-human hybridomas. J Clin Invest. 1982 Jul;70(1):205–208. doi: 10.1172/JCI110595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoenfeld Y., Isenberg D. A., Rauch J., Madaio M. P., Stollar B. D., Schwartz R. S. Idiotypic cross-reactions of monoclonal human lupus autoantibodies. J Exp Med. 1983 Sep 1;158(3):718–730. doi: 10.1084/jem.158.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoenfeld Y., Rauch J., Massicotte H., Datta S. K., André-Schwartz J., Stollar B. D., Schwartz R. S. Polyspecificity of monoclonal lupus autoantibodies produced by human-human hybridomas. N Engl J Med. 1983 Feb 24;308(8):414–420. doi: 10.1056/NEJM198302243080802. [DOI] [PubMed] [Google Scholar]
- Siekevitz M., Huang S. Y., Gefter M. L. The genetic basis of antibody production: a single heavy chain variable region gene encodes all molecules bearing the dominant anti-arsonate idiotype in the strain A mouse. Eur J Immunol. 1983 Feb;13(2):123–132. doi: 10.1002/eji.1830130207. [DOI] [PubMed] [Google Scholar]
- Tron F., Charron D., Bach J. F., Talal N. Establishment and characterization of a murine hybridoma secreting monoclonal anti-DNA autoantibody. J Immunol. 1980 Dec;125(6):2805–2809. [PubMed] [Google Scholar]



