Abstract
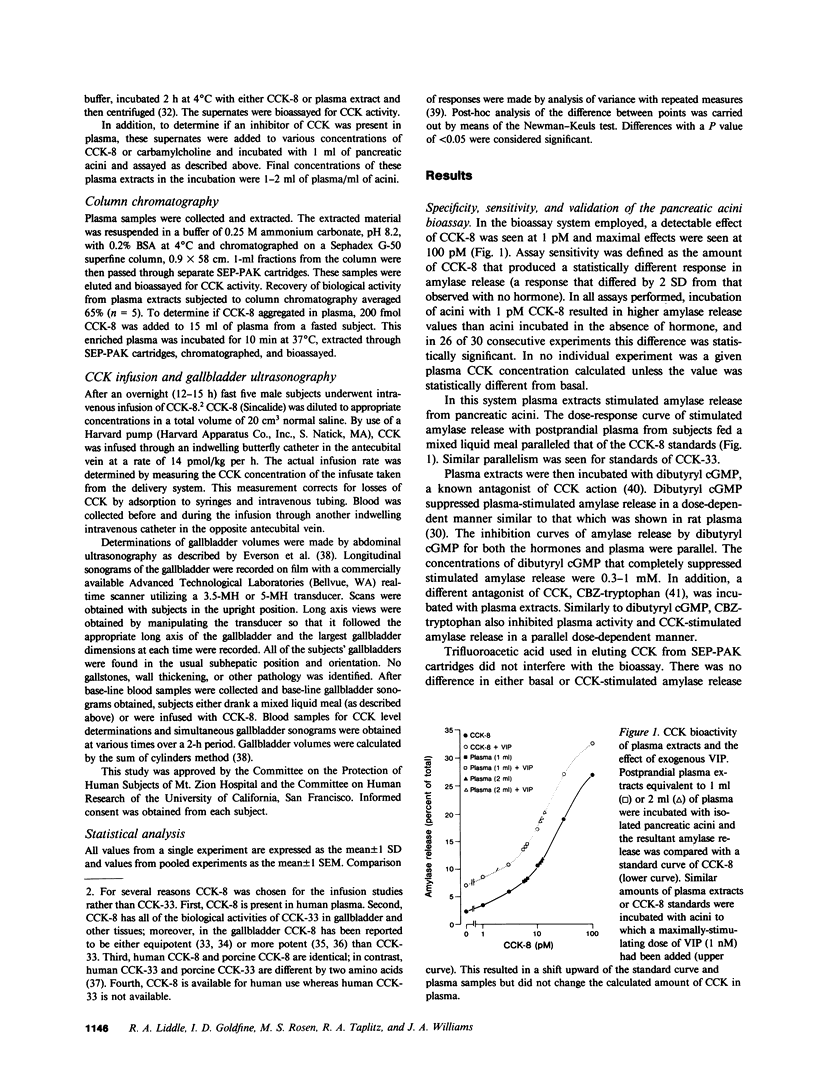
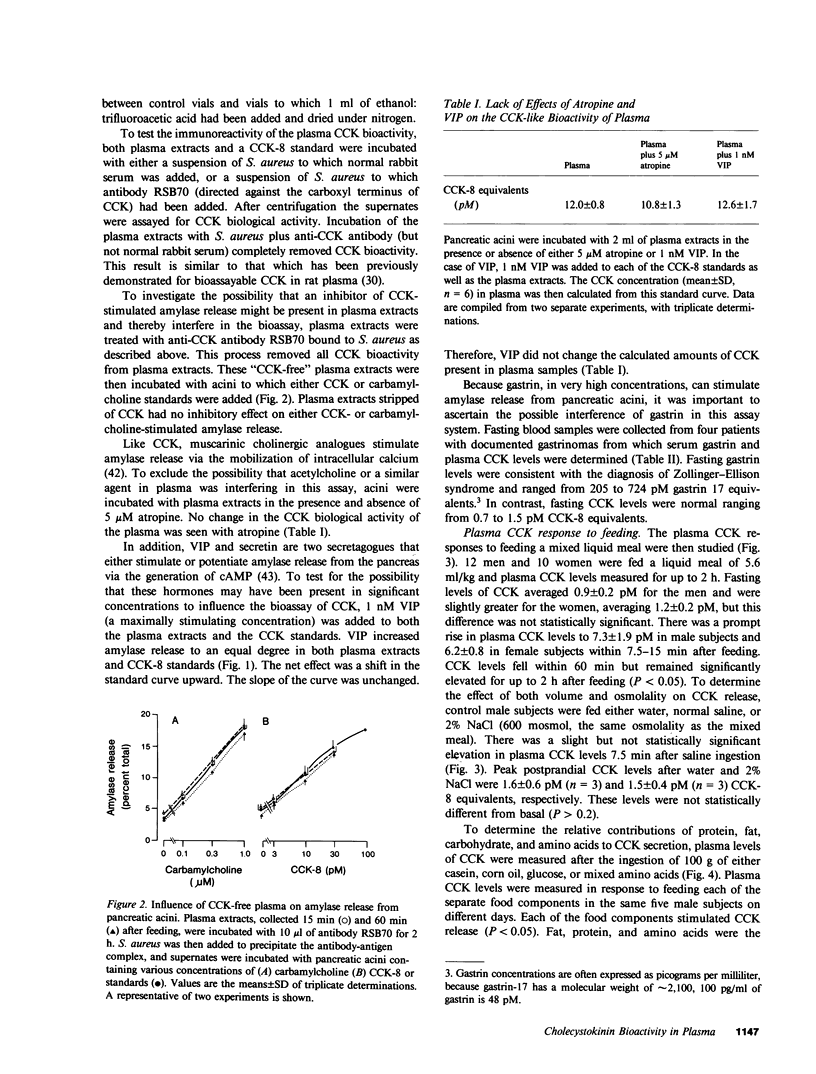
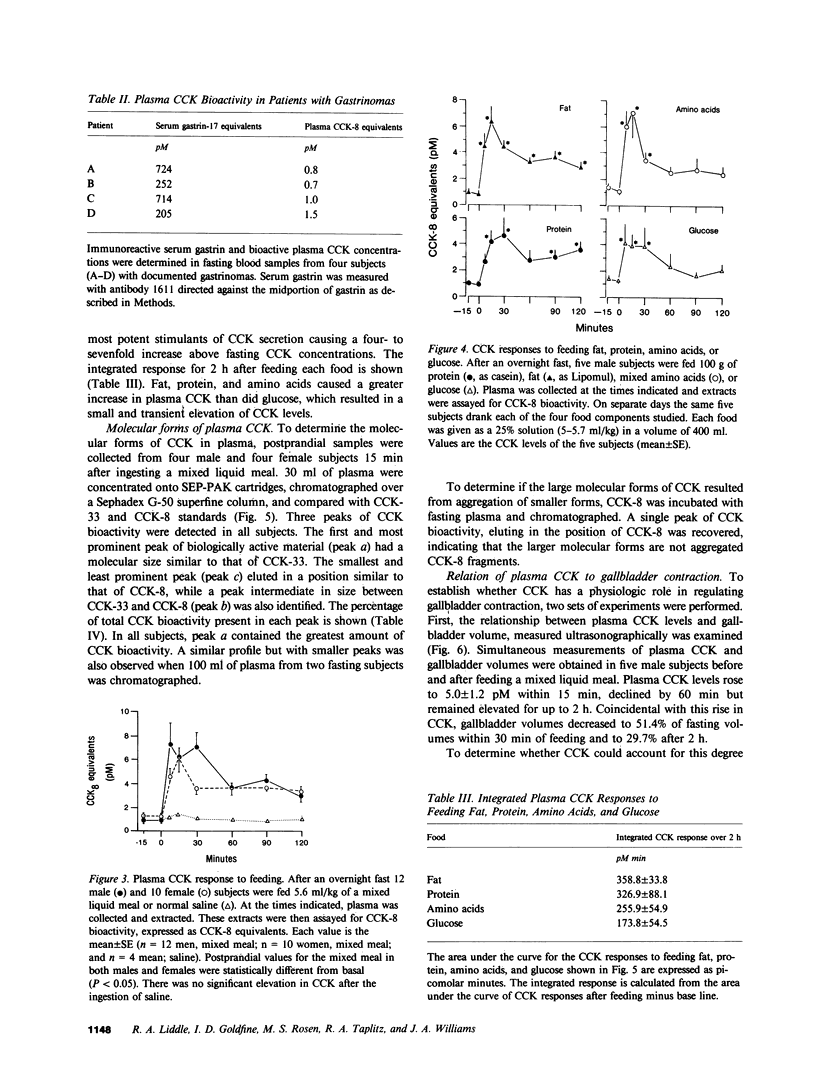
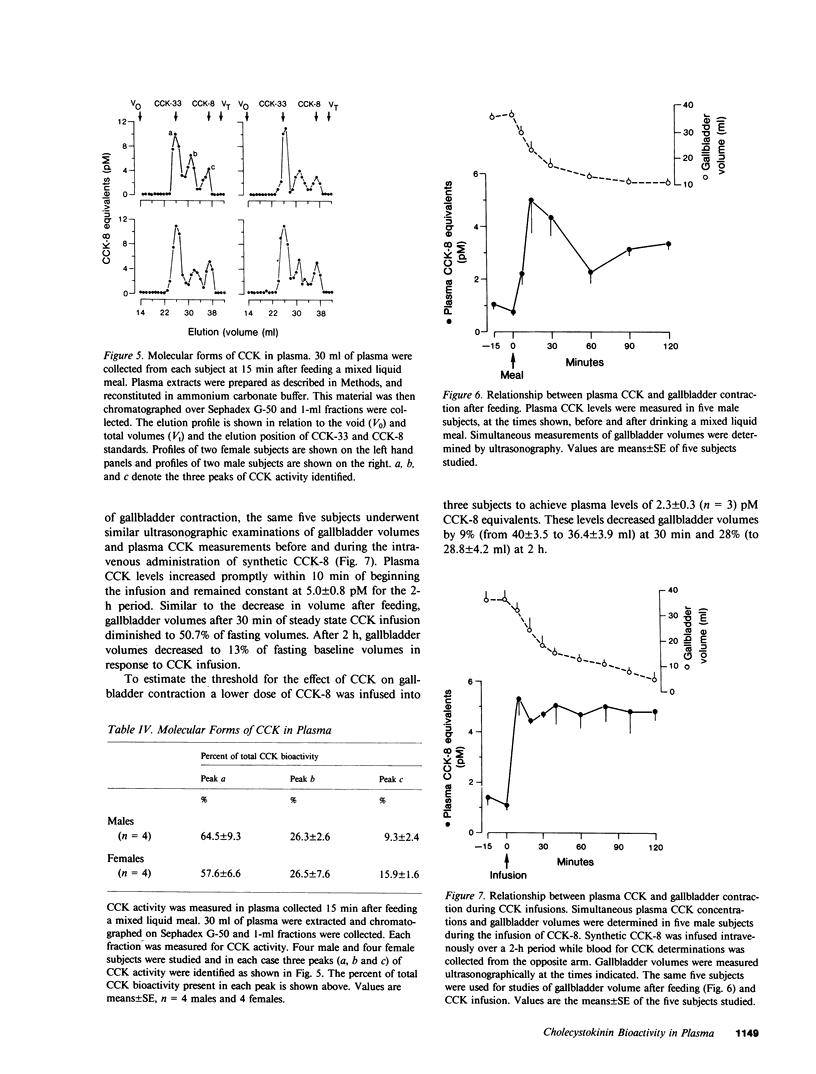
A sensitive and specific bioassay for the measurement of cholecystokinin (CCK) in human plasma was developed to determine the molecular forms of CCK in circulation, CCK responses to feeding, and the physiologic role of CCK in gallbladder contraction. First, plasma was quantitatively extracted and concentrated with octadecylsilylsilica, and the extracts were then assayed for their ability to stimulate amylase release from isolated rat pancreatic acini. Acini were highly sensitive to CCK whereas gastrin reacted only weakly in this system. With the assay, plasma levels of cholecystokinin octapeptide (CCK-8) bioactivity as low as 0.2 pM were detectable. CCK bioactivity in plasma was inhibited by the CCK antagonist, bibutyryl cyclic guanosine monophosphate, and was eliminated by immunoadsorption with an antibody directed against the carboxyl terminus of CCK. Detection of fasting levels of CCK was possible in all individuals tested and averaged 1.0 +/- 0.2 pM (mean +/- SE, n = 22) CCK-8 equivalents. Plasma CCK biological activity was normal in patients with gastrin-secreting tumors. After being fed a mixed liquid meal, CCK levels rose within 15 min to 6.0 +/- 1.6 pM. The individual food components fat, protein, and amino acids were all potent stimulants of CCK secretion; in contrast, glucose caused a significant but smaller elevation in plasma CCK levels. Gel filtration studies identified three major forms of CCK bioactivity in human plasma: an abundant form that eluted with CCK-33, a smaller form that eluted with CCK-8, and an intermediate form that eluted between CCK-33 and CCK-8. Ultrasonic measurements of gallbladder volume indicated that this organ decreased 51% in size 30 min after feeding a mixed liquid meal. This contraction occurred coincidentally with the increase in plasma CCK levels. Next CCK-8 was infused to obtain CCK levels similar to postprandial levels. This infusion caused a decrease in gallbladder volume, similar to that seen with a meal. The present studies indicate, therefore, that CCK can be bioassayed in fasting and postprandial human plasma. These studies also suggest that CCK may be an important regulator of gallbladder contraction.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behar J., Biancani P. Effect of cholecystokinin and the octapeptide of cholecystokinin on the feline sphincter of Oddi and gallbladder. Mechanisms of action. J Clin Invest. 1980 Dec;66(6):1231–1239. doi: 10.1172/JCI109974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes D. J., Borody T., Daskalopoulos G., Boyle M., Benn I. Cholecystokinin and gallbladder contraction: effect of CCK infusion. Peptides. 1981;2 (Suppl 2):259–262. doi: 10.1016/0196-9781(81)90041-3. [DOI] [PubMed] [Google Scholar]
- Byrnes D. J., Henderson L., Borody T., Rehfeld J. F. Radioimmunoassay of cholecystokinin in human plasma. Clin Chim Acta. 1981 Mar 19;111(1):81–89. doi: 10.1016/0009-8981(81)90424-1. [DOI] [PubMed] [Google Scholar]
- Calam J., Ellis A., Dockray G. J. Identification and measurement of molecular variants of cholecystokinin in duodenal mucosa and plasma. Diminished concentrations in patients with celiac disease. J Clin Invest. 1982 Jan;69(1):218–225. doi: 10.1172/JCI110433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T. M., Chey W. Y. Radioimmunoassay of cholecystokinin. Dig Dis Sci. 1983 May;28(5):456–468. doi: 10.1007/BF02430535. [DOI] [PubMed] [Google Scholar]
- Collen M. J., Sutliff V. E., Pan G. Z., Gardner J. D. Postreceptor modulation of action of VIP and secretin on pancreatic enzyme secretion by secretagogues that mobilize cellular calcium. Am J Physiol. 1982 Apr;242(4):G423–G428. doi: 10.1152/ajpgi.1982.242.4.G423. [DOI] [PubMed] [Google Scholar]
- Cox K. L., Rosenquist G. L., Iwahashi-Hosoda C. K. Noncholecystokinin peptides in human serum which cause gallbladder contraction. Life Sci. 1982 Dec 27;31(26):3023–3029. doi: 10.1016/0024-3205(82)90070-4. [DOI] [PubMed] [Google Scholar]
- Dockray G. J. Immunochemical evidence of cholecystokinin-like peptides in brain. Nature. 1976 Dec 9;264(5586):568–570. doi: 10.1038/264568a0. [DOI] [PubMed] [Google Scholar]
- Dockray G. J. Immunoreactive component resembling cholecystokinin octapeptide in intestine. Nature. 1977 Nov 24;270(5635):359–361. doi: 10.1038/270359a0. [DOI] [PubMed] [Google Scholar]
- Everson G. T., Braverman D. Z., Johnson M. L., Kern F., Jr A critical evaluation of real-time ultrasonography for the study of gallbladder volume and contraction. Gastroenterology. 1980 Jul;79(1):40–46. [PubMed] [Google Scholar]
- Eysselein V. E., Reeve J. R., Jr, Shively J. E., Hawke D., Walsh J. H. Partial structure of a large canine cholecystokinin (CCK58): amino acid sequence. Peptides. 1982 Jul-Aug;3(4):687–691. doi: 10.1016/0196-9781(82)90171-1. [DOI] [PubMed] [Google Scholar]
- Gardner J. D. Receptors for gastrointestinal hormones. Gastroenterology. 1979 Jan;76(1):202–214. [PubMed] [Google Scholar]
- Go V. L., Hofmann A. F., Summerskill W. H. Pancreozymin bioassay in man based on pancreatic enzyme secretion: potency of specific amino acids and other digestive products. J Clin Invest. 1970 Aug;49(8):1558–1564. doi: 10.1172/JCI106373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper A. A., Raper H. S. Pancreozymin, a stimulant of the secretion of pancreatic enzymes in extracts of the small intestine. J Physiol. 1943 Jun 30;102(1):115–125. doi: 10.1113/jphysiol.1943.sp004021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. F., Dowsett L., Hartog M., Read A. E. Radioimmunoassay of cholecystokinin-pancreozymin. Gut. 1974 Sep;15(9):690–699. doi: 10.1136/gut.15.9.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himeno S., Tarui S., Kanayama S., Kuroshima T., Shinomura Y., Hayashi C., Tateishi K., Imagawa K., Hashimura E., Hamaoka T. Plasma cholecystokinin responses after ingestion of liquid meal and intraduodenal infusion of fat, amino acids, or hydrochloric acid in man: analysis with region specific radioimmunoassay. Am J Gastroenterol. 1983 Nov;78(11):703–707. [PubMed] [Google Scholar]
- Jansen J. B., Lamers C. B. Radioimmunoassay of cholecystokinin in human tissue and plasma. Clin Chim Acta. 1983 Jul 15;131(3):305–316. doi: 10.1016/0009-8981(83)90100-6. [DOI] [PubMed] [Google Scholar]
- Jensen R. T., Gardner J. D. Interaction of physalaemin, substance P, and eledoisin with specific membrane receptors on pancreatic acinar cells. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5679–5683. doi: 10.1073/pnas.76.11.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. T., Jones S. W., Gardner J. D. Structure-function studies of N-acyl derivatives of tryptophan that function as specific cholecystokinin receptor antagonists. Biochim Biophys Acta. 1983 Dec 27;761(3):269–277. doi: 10.1016/0304-4165(83)90076-4. [DOI] [PubMed] [Google Scholar]
- Johnson A. G., Marshall C. E., Wilson I. A. Effects of some drugs and peptide hormones on the responsiveness of the rabbit isolated gall-bladder to cholecystokinin. J Physiol. 1982 Nov;332:415–425. doi: 10.1113/jphysiol.1982.sp014421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. G., McDermott S. J. Sensitive bioassay of cholecystokinin in human serum. Lancet. 1973 Sep 15;2(7829):589–591. doi: 10.1016/s0140-6736(73)92416-1. [DOI] [PubMed] [Google Scholar]
- Jorpes E., Mutt V. Cholecystokinin and pancreozymin, one single hormone? Acta Physiol Scand. 1966 Jan-Feb;66(1):196–202. doi: 10.1111/j.1748-1716.1966.tb03185.x. [DOI] [PubMed] [Google Scholar]
- Jung D. H. Preparation and application of Procion Yellow starch for amylase assay. Clin Chim Acta. 1980 Jan 1;100(1):7–11. doi: 10.1016/0009-8981(80)90179-5. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Cell membrane antigen isolation with the staphylococcal protein A-antibody adsorbent. J Immunol. 1976 Nov;117(5 Pt 1):1482–1490. [PubMed] [Google Scholar]
- Kothary P. C., Vinik A. I., Owyang C., Fiddian-Green R. G. Immunochemical studies of molecular heterogeneity of cholecystokinin in duodenal perfusates and plasma in humans. J Biol Chem. 1983 Mar 10;258(5):2856–2863. [PubMed] [Google Scholar]
- Liddle R. A., Goldfine I. D., Williams J. A. Bioassay of plasma cholecystokinin in rats: effects of food, trypsin inhibitor, and alcohol. Gastroenterology. 1984 Sep;87(3):542–549. [PubMed] [Google Scholar]
- Marshall C. E., Egberts E. H., Johnson A. G. An improved method for estimating cholecystokinin in human serum. J Endocrinol. 1978 Oct;79(1):17–27. doi: 10.1677/joe.0.0790017. [DOI] [PubMed] [Google Scholar]
- Maton P. N., Selden A. C., Chadwick V. S. Differential distribution of molecular forms of cholecystokinin in human and porcine small intestinal mucosa. Regul Pept. 1984 Jan;8(1):9–19. doi: 10.1016/0167-0115(84)90024-7. [DOI] [PubMed] [Google Scholar]
- Maton P. N., Selden A. C., Chadwick V. S. Large and small forms of cholecystokinin in human plasma: measurement using high pressure liquid chromatography and radioimmunoassay. Regul Pept. 1982 Oct;4(5):251–260. doi: 10.1016/0167-0115(82)90118-5. [DOI] [PubMed] [Google Scholar]
- Muller J. E., Straus E., Yalow R. S. Cholecystokinin and its COOH-terminal octapeptide in the pig brain. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3035–3037. doi: 10.1073/pnas.74.7.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutt V. Further investigations of intestinal hormonal polypeptides. Clin Endocrinol (Oxf) 1976;5 (Suppl):175S–183S. doi: 10.1111/j.1365-2265.1976.tb03825.x. [DOI] [PubMed] [Google Scholar]
- Otsuki M., Williams J. A. Effect of diabetes mellitus on the regulation of enzyme secretion by isolated rat pancreatic acini. J Clin Invest. 1982 Jul;70(1):148–156. doi: 10.1172/JCI110588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peikin S. R., Costenbader C. L., Gardner J. D. Actions of derivatives of cyclic nucleotides on dispersed acini from guinea pig pancreas. Discovery of a competitive antagonist of the action of cholecystokinin. J Biol Chem. 1979 Jun 25;254(12):5321–5327. [PubMed] [Google Scholar]
- Rehfeld J. F. Immunochemical studies on cholecystokinin. I. Development of sequence-specific radioimmunoassays for porcine triacontatriapeptide cholecystokinin. J Biol Chem. 1978 Jun 10;253(11):4016–4021. [PubMed] [Google Scholar]
- Schlegel W., Raptis S., Grube D., Pfeiffer E. F. Estimation of cholecystokinin-pancreozymin (CCK) in human plasma and tissue by a specific radioimmunoassay and the immunohistochemical identification of pancreozymin-producing cells in the duodenum of humans. Clin Chim Acta. 1977 Oct 15;80(2):305–316. doi: 10.1016/0009-8981(77)90038-9. [DOI] [PubMed] [Google Scholar]
- Solomon T. E., Yamada T., Elashoff J., Wood J., Beglinger C. Bioactivity of cholecystokinin analogues: CCK-8 is not more potent than CCK-33. Am J Physiol. 1984 Jul;247(1 Pt 1):G105–G111. doi: 10.1152/ajpgi.1984.247.1.G105. [DOI] [PubMed] [Google Scholar]
- Stadil F., Rehfeld J. F. Determination of gastrin in serum. An evaluation of the reliability of a radioimmunoassay. Scand J Gastroenterol. 1973;8(2):101–112. [PubMed] [Google Scholar]
- Walsh J. H., Lamers C. B., Valenzuela J. E. Cholecystokinin-octapeptidelike immunoreactivity in human plasma. Gastroenterology. 1982 Mar;82(3):438–444. [PubMed] [Google Scholar]
- Wiener I., Inoue K., Fagan C. J., Lilja P., Watson L. C., Thompson J. C. Release of cholecystokinin in man: correlation of blood levels with gallbladder contraction. Ann Surg. 1981 Sep;194(3):321–327. doi: 10.1097/00000658-198109000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. A. Regulation of pancreatic acinar cell function by intracellular calcium. Am J Physiol. 1980 Apr;238(4):G269–G279. doi: 10.1152/ajpgi.1980.238.4.G269. [DOI] [PubMed] [Google Scholar]
- Williams J. A., Sankaran H., Korc M., Goldfine I. D. Receptors for cholecystokinin and insulin in isolated pancreatic acini: hormonal control of secretion and metabolism. Fed Proc. 1981 Aug;40(10):2497–2502. [PubMed] [Google Scholar]



