SUMMARY
Central arterial wall stiffening driven by a chronic inflammatory milieu accompanies arterial diseases, the leading cause of cardiovascular (CV) morbidity and mortality in Western society. Increase in central arterial wall stiffening, measured as an increase in aortic pulse wave velocity (PWV), is a major risk factor for clinical CV disease events. However, no specific therapies to reduce PWV are presently available. In rhesus monkeys, a two-year diet high in fat and sucrose (HFS) increases not only body weight and cholesterol, but also induces prominent central arterial wall stiffening and increases PWV and inflammation. The observed loss of endothelial cell integrity, lipid and macrophage infiltration, and calcification of the arterial wall were driven by genomic and proteomic signatures of oxidative stress and inflammation. Resveratrol prevented the HFS-induced arterial wall inflammation and the accompanying increase in PWV. Dietary resveratrol may hold promise as a novel therapy to ameliorate increases in PWV.
INTRODUCTION
The incidence of cardiovascular diseases (CVD), mainly arterial diseases of hypertension and atherosclerosis, increases exponentially beyond middle age (Lakatta, 2013). Stiffening of the central arteries is a cardinal feature of advancing age in humans beyond the age of 40 years. Over the last decade numerous epidemiological and longitudinal studies have convincingly demonstrated that carotid-femoral pulse wave velocity (PWV), a direct measure of aortic stiffness, is a highly relevant clinical measure of arterial stiffness. In humans, an increase in PWV shows a strong association with CVD-associated clinical events and all-cause mortality, even after taking other known risk factors into consideration (Najjar et al., 2008). PWV has emerged as an independent predictor for CV disease, morbidity, and mortality. There is also strong evidence to indicate that PWV provides early information about the development/progression of atherosclerosis before macroscopic alterations of the vessel wall occur (Gotschy et al., 2013) and is integral to the retardation of CV events (Reference Values for Arterial Stiffness, 2010). This epidemiologic perspective suggests that the reduction of PWV may carry substantial health benefits. Importantly, metabolic disease in humans accelerates the age-associated increase in PWV (Scuteri et al., 2012). Histological, genomic and proteomic studies provide strong evidence that increased central arterial stiffness occurs in the context of an oxidative stress-driven arterial wall inflammatory profile (for review Wang et al., 2014).
Clinical trials to assess the beneficial effects of pharmacological interventions on vascular health have shown that presently available anti-inflammatory drugs, e.g. statins (Williams et al., 2009) or angiotensin receptor blockers (Hayoz et al., 2012) had only modest effects, if any, at reducing PWV. Thus, at present, there are no effective therapies available to reduce PWV, and novel strategies are required to impact on chronic arterial wall inflammation and stiffening that underlie and accelerate the progression of CV diseases, other than classic regulation of blood pressure. In this regard, vascular protective effects of the polyphenol resveratrol (Resv) have been illustrated in several different animal species (Ramprasath and Jones, 2010). Studies in mice demonstrate that the addition of Resv to a high-fat diet ameliorates arterial wall inflammation and other arterial markers associated with aging (Pearson et al., 2008). Further, in apolipoprotein E-deficient (apo E−/−) mice, a model of atherosclerosis with very high levels of circulating cholesterol, dietary supplementation of Resv leads to improvement of lipid profile, accompanied by the prevention of intimal lesion formation and inhibition of HMG-CoA reductase to decrease cholesterol formation (Do et al., 2008). In pig models, Resv also improves myocardial perfusion, regional contractility, and decreases oxidative stress (Elmadhun et al., 2013). We have recently reported that Resv promotes metabolic and inflammatory adaptations in visceral white adipose tissue (Jimenez-Gomez et al., 2013) and prevents pancreatic β-cell dedifferentiation (Fiori et al., 2013) of rhesus monkeys on a high-fat, high-sucrose (HFS) diet. In the present study, the hypothesis tested that HFS will induce arterial wall inflammation driven by oxidative stress and cause deleterious increase in central arterial wall stiffness, manifest as an increased PWV, and that these effects will be ameliorated by the addition of Resv to the HFS in a clinically relevant nonhuman primate (NHP) model of metabolic disease.
RESULTS AND DISCUSSION
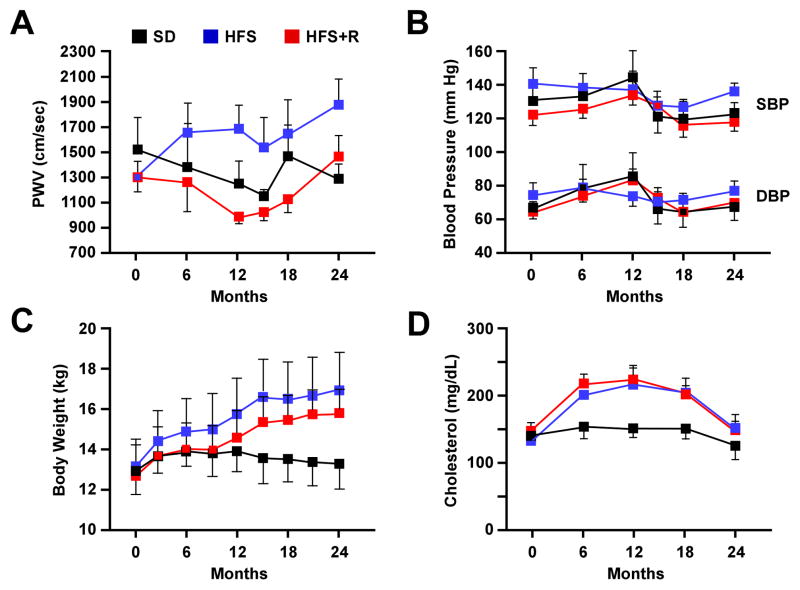
Baseline characteristics of the NHPs comprised in this study while on a standard diet (SD) are detailed in Table S1. A two-year HFS diet in adult (7–13 years) male Macaca mulatta caused an increase in body weight, an elevation in plasma cholesterol, and an approximately 40% increase in aortic PWV –an index of central arterial stiffness. Daily dietary supplementation with Resv (80 mg for first year and 480 mg for the second year) prevented the increase in PWV in HFS-fed monkeys (Figure 1A). The effect on PWV was most profound at 12 months when the differences between HFS-fed and those supplemented with Resv (HFS+R) was 38% (p<0.05). The gradual increase in PWV during the second year could be due to the HF diet overwhelming the system; alternatively, higher dose of Resv may elicit side-effects and offer lower health benefits (Mukherjee et al., 2010). Despite preventing the HFS-induced increase in PWV, Resv had no effect on blood pressure (Figure 1B), body weight (Figure 1C), serum cholesterol (Figure 1D) or total low density lipoprotein (not shown) in linear mixed model analyses of paired data. Serum levels of both intercellular adhesion molecule 1 (ICAM) and macrophage inflammatory protein 1 (MIP-1), markers of arterial wall inflammation, increased during the two-year study in all groups and was not affected by Resv (not shown). Thus, these typical clinically measured risk factors for CV disease did not relate to events occurring within the arterial wall that lead to the reduction in PWV by the addition of Resv. Furthermore, the size and weight of the heart and gross morphometric measurements of aortic remodeling did not vary whether monkeys were fed a HFS or HFS+R diet (Table S2).
Figure 1. Physiological Measurements in Rhesus Monkeys Maintained on HFS, HFS + R, or SD Diet for up to 2 Years.
(A) Pulse wave velocity, (B) Blood pressure, (C) Body weight and (D) serum cholesterol levels. Results are mean ± SEM
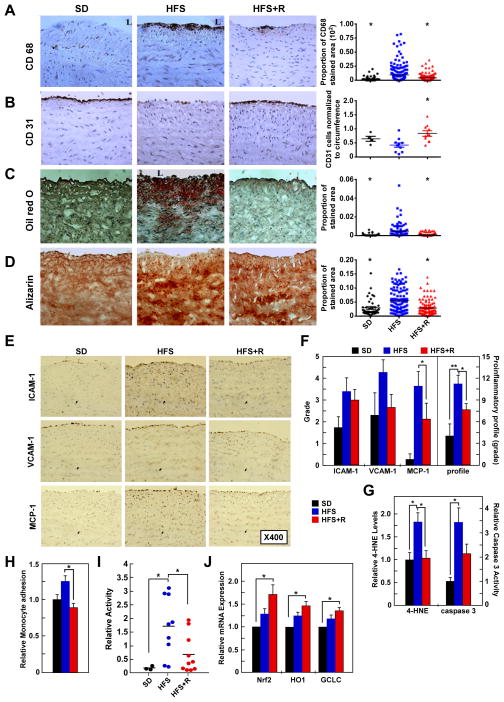
Microscopic morphometric analyses of immunolabeled aortic cross-sections were performed to determine whether Resv could prevent the signature of inflammation within the arterial wall (Figure 2 and Table S2). HFS diet induced monocyte/macrophage infiltration (Figure 2A), endothelial cell loss (Figure 2B) and enhanced staining for fat deposition and calcification (Figure 2C, D); furthermore, ICAM-1, vascular cell adhesion molecule 1 (VCAM-1), and monocyte chemoattractant protein-1 (MCP-1) immunostaining was also increased in aortic cross-sections of HFS-fed animals, but normalized by Resv supplementation (Figure 2E, F) despite no effect on serum lipid profile or circulatory markers of inflammation. When compared to SD-fed controls, aortic homogenates from HFS-fed cohort exhibited significantly higher levels of the stable lipid peroxidation marker, 4-hydroxynonenal (4-HNE), and caspase 3 activity, which were reversed by Resv (Figure 2G). Thus, the antioxidant and antiapoptotic response of Resv could be part of the mechanism used by this polyphenol to preserve vascular health (Ungvari et al., 2007; Kaneko et al., 2011).
Figure 2. Resveratrol Supplementation Retards the Adverse Molecular and Cellular Events within the Aortic Wall of Rhesus Monkeys on a HFS Diet and CAEC Incubation with Serum from HFS+Resv Serum Decreases Monocyte Adhesion.
(A) Photomicrographs (X400) of the paraffin sections after immunostaining for CD68, a marker of monocyte/macrophage cells (left panel) (brown color determined by 3,3′-diaminobenzidine (DAB)); the average density of CD68-stained area/field/cross-section (right panel). (B) Photomicrographs (X400) of paraffin sections after immunostaining for CD31, a marker of endothelial cells (left panel); the number of CD31-stained endothelial cells normalized by the circumference (right panel). (C) Photomicrographs (X400) of frozen sections after Oil O Red staining (left panel); the density of lipid deposition/field/cross-section (right panel). (D) Photomicrographs (X400) of frozen sections after Alizarin red staining (left panel); the density of calcification/field/cross-section (right panel). For A–D, * p < 0.05 versus HFS. (E) Photomicrographs (X400) of frozen sections after immunostaining with antibodies for the proinflammatory molecules ICAM-1, VCAM-1, and MCP-1. (F) Grade average for ICAM-1, VCAM-1, and MCP-1 immunostaining as well as their summation (defined as local proinflammatory profile) of cells plus matrix within the intimal and medial compartment. (For AD, F: SD, n=4; HFS, n=10; HFS+R, n=10). (G) 4-HNE content and caspase 3 activity in aorta (SD, n=3; HFS, n=9; HFS+R, n=9). (H) Monocyte adhesion assay in human coronary artery endothelial cells (CAECs) cultured for 24 h with 10% serum from SD-, HFS- or HFS+R-fed rhesus monkeys. (I) NF-κB reporter assays were initiated after a 24-h incubation of human CAECs with HFS monkey serum. (J) Expression of Nrf2, HO1 and GCLC mRNA in human CAECs was determined by quantitative RT-PCR analysis. The effects of a 24-h incubation with HFS and HFS+R monkey serum were normalized to SD serum (for H–J: SD, n=3; HFS, n=9; HFS+R, n=10), for F–J: *, ** p< 0.05 and <0.01, respectively. Data are mean ± SEM for all graphs.
Monocyte adhesion to human coronary artery endothelial cells (CAECs) is a key initiating event of arterial wall inflammation associated with metabolic disease. To ascertain whether Resv could prevent the HFS-mediated initiation of inflammation at the monocyte-endothelial cell interface, we assessed the leukocyte adhesion properties of CAECs after a 24-h incubation with medium containing 10% serum from monkeys on experimental diets for 2 years. Strikingly, medium supplementation with HFS+R serum significantly reduced HFS serum-mediated increase in PMA-activated human monocytic THP-1 cell adhesion onto confluent monolayer of human CAECs (p<0.01, Figure 2H). As a measure of the anti-inflammatory action of Resv, we determined the activation status of NF-κB in human CAECs treated either with SD, HFS or HFS+R monkey serum (Figure 2I). The increase in NF-κB promoter activity in response to HFS monkey serum (9.28 ± 2.80-fold vs. SD serum, p=0.017) was reduced by ~60% in cells treated with HFS+R serum (p=0.025). These data suggest that Resv suppresses NF-κB transcriptional activity to block induction of endothelial genes implicated in leukocyte recruitment and transmigration as well as tissue remodeling (Csiszar et al., 2012; Tuttolomondo et al., 2012).
Activation of the nuclear factor-E(2)-related factor-2 (Nrf2) pathway improves vascular function in patients with risk of vascular events through upregulated expression of genes that contain antioxidant response elements in their promoters (Carrizzo et al., 2013). Nrf2 mRNA levels were significantly higher in human CAECs incubated with HFS+R serum, but not in HFS serum-treated cells (Figure 2J); moreover, expression of Nrf2 target genes, heme oxygenase-1 and gamma-glutamylcysteine synthetase, was also upregulated by HFS+R serum, consistent with previous reports in the NHP animal model (Ungvari et al., 2011). Thus, a 2-year consumption of Resv may confer atheroprotective effects to the vascular endothelium of HFS-fed monkeys via Nrf2 activation.
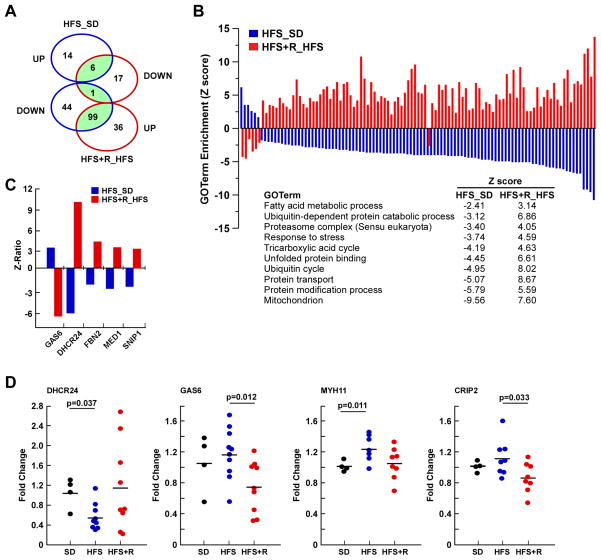
To identify genes that are key components of the anti-oxidative and anti-inflammatory pathways of Resv, we performed cDNA microarray analysis of thoracic aorta. The analysis revealed the expression of 217 gene sets whose levels were significantly altered in HFS and HFS+R cohorts based on unbiased gene ontology (GO) annotations. Of these, 106 gene sets were shared between the two cohorts (Figure 3A) with all, but one gene set, exhibiting a reciprocal pattern of expression (Figure 3B). Among the top upregulated GO annotations for HFS+R versus HFS were ‘fatty acid metabolic process’, ‘protein catabolic processes’, ‘stress response’ and ‘mitochondrion’. The most highly upregulated gene in the aorta of HFS+R with respect to HFS was DHCR24, followed by FBN2, MED1 and SNIP1, while the most downregulated gene was GAS6 (Figure 3C). DHCR24/seladin-1 encodes a multifunctional enzyme that not only scavenges H2O2 but also is involved in cholesterol synthesis (Lu et al., 2008). GAS6 plays a key role in vascular remodeling and calcification of vascular smooth muscle (Konishi et al., 2004; Son et al., 2007). The expression of selected genes from the microarray analyses was verified using quantitative RT-PCR analysis (Figure 3D). Significant changes in GO terms and gene expression levels shared between the HFS and HFS+R cohorts are compiled in Tables S4 and S5. Expression of gene sets relevant to arterial inflammation that were altered by the experimental diets were: the ‘interleukin receptor activity’, ‘activation of NF-κB transcription’, and ‘nitric oxide biosynthetic process’. Of significance, Resv supplementation reversed the HFS-induced reduction in NOS3 expression, which encodes endothelial nitric oxide synthase (eNOS), consistent with recent preclinical studies showing that Resv upregulates eNOS activity to enhance the production/bioavailability of NO and subsequent endothelium-dependent vasodilation (Carrizzo et al., 2013). Moreover, HFS feeding coordinately regulated expression of genes implicated in matrix foundation structure and function and arterial remodeling, which, for the most part, was reversed by Resv supplementation (Figure S1). Thus, Resv may have beneficial effects on CV health by evoking effective protection against diet-induced activation of multiple routes that link inflammation to arterial stiffness. The raw data file and the filtered, normalized results are available online in the Gene Expression Omnibus, accession number GSE45927.
Figure 3. Resveratrol Supplementation Reverses Global Transcriptional Effects of a HFS in Aorta of Rhesus Monkeys.
(A) Venn diagram of GO terms significantly affected by HFS diet vs. SD, and HFS+R vs. HFS. (B) Graphical representation of the significant 106 GO terms shared by HFS– (plotted in blue) and HFS+R –fed animals (plotted in red). Metabolic and catabolic processes, stress response and mitochondrion were among the regulated GO terms in aorta. Full GO term listing is provided in the Supplementary material. (C) Change in expression of select genes between HFS vs. SD and HFS+R vs. HFS is depicted as Z-ratios. List of genes whose expression in the HFS-fed group was reversed significantly by Resv supplementation is provided in the Supplemental material. (D) Validation of the microarray data by quantitative RT-PCR.
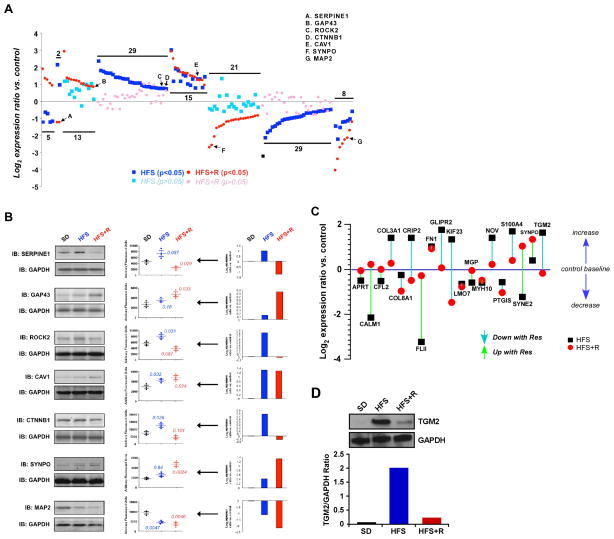
To assess molecular signatures consistent with improved vascular health following Resv supplementation, vascular smooth muscle (VSM) cells from the monkey aorta were isolated, placed in primary culture and then were subjected to quantitative proteomics by stable isotope labeling with amino acids (SILAC) combined with LC-MS/MS, as previously reported (Park et al., 2012). Of the 750 proteins identified, expression of 121 proteins significantly differed in either the HFS and/or HFS+R compared to SD (Table S3C). GO Term annotation and other Bioinformatic tools [e.g., KEGG signaling pathway, WikiPathways, and PathwayCommons analyses] revealed that the differentially altered proteins in VSM cells included several proteins involved in cytoskeletal dynamic regulation, calcium regulation, energy metabolism and vascular functionality (Figure S2A, Tables S7–S10). A representative set of seven proteins showed a complex series of dynamic changes with experimental diets. These included proteins that were upregulated in the HFS-fed animals, but became either downregulated with Resv supplementation (e.g., Serpine1) or normalized to SD controls (e.g., Rock2, β-catenin (Ctnnb1)); proteins whose expression moved in the same direction in HFS and HFS+R groups (e.g., caveolin 1 (Cav1), microtubule-associated protein 2 (MAP2)); and proteins that responded only to Resv (e.g., neuromodulin (GAP43), synaptopodin 2 (SYNPO)) (Table S3C, Figure 4A). Serpine1, Rock2 and β-catenin are related to VSM cell inflammation (Satoh et al., 2011; Vykoukal and Davies, 2011; Xiao et al., 2014) and their expression levels were validated in aortic protein extracts by Western blotting (Figure 4B).
Figure 4. Resveratrol Supplementation Restores the Conversion of ‘Multidimensional’ Protein Network of the Cytoskeletal Dynamics and Vascular Functionality.
(A) Log2-transformed SILAC expression ratio data for proteins extracted and identified in primary cultures of vascular smooth muscle (VSM) from rhesus monkey on SD, HFS and HFS+R diet. These proteins were selected due to their implicit textual association with vascular functionality (see Supplemental material for additional information). The proteins identified demonstrated a significantly altered degree of expression regulation in either the HFS (blue squares) or HFS+R (red circle) cohort (p<0.05). Non-significant expression changes are identified for the HFS cohort with light blue squares and for the HFS+R cohort with pink circles. (B) Western blot validation of several proteins identified with SILAC. Representative Western blot panels display the expression profile from a set of SD, HFS and HFS+R-fed monkeys. The associated scatter-plot histograms indicate the degree of individual animal variation in expression, as well as the significant p value (t-test) for HFS or HFS+R compared to SD. (C) The effects of Resv and HFS diet treatments upon nineteen ‘multidimensional’ proteins identified from multiple bioinformatic interpretation (GO-bp, KEGG, WikiPathways, PathwayCommons). These proteins isolated from VSM extracts were associated with all the interrogator terms. (D) TGM2 expression in primary culture of rhesus monkey VSM cells was measured using Western blot and expression levels were normalized to GAPDH.
Latent Semantic Indexing (LSI) heatmap analysis (Chadwick et al., 2011) of the 121-protein dataset affected by diet demonstrated that nineteen proteins were implicitly involved in inter-related functional networks associated with vascular inflammation and protection, regeneration and development (Figure S2B). Aortic VSM cells exhibited higher levels of NOV (CCN family member 3), among other proteins, when comparing HFS to either SD or HFS+R cohort (Figure 4C). NOV is a potent inhibitor of vascular repair program (Shimoyama et al., 2010). Several protein markers of endothelial dysfunction that were upregulated in response to HFS were found to be normalized to SD levels by Resv supplementation. These included COL3A1, cysteine-rich protein 2 and GLIPR2, the former being associated with the pathogenesis of vascular lesions (Smith et al., 2011). Moreover, the levels of cytoskeletal regulatory protein Nesprin 2 (SYNE2) were downregulated by HFS, but restored by Resv supplementation to levels that were even higher than in VSM from SD controls (Figure 4C). HFS increased expression of transglutaminase 2 (TGM2), an arterial calcification-related protein that is positively associated with hypertension and atherosclerosis (Johnson et al., 2008; Matlung et al., 2012) and cross-linking of extracellular matrix proteins (Bakker et al., 2008; Santhanam et al., 2010). This upregulation of TGM2 protein level and activity in response to HFS was restored to control levels in VSM cells from the HFS+R cohort (Figure 4C and 4D, Figure S3). Consistent with the established effects of NO bioavailability on TGM2 regulation (Santhanam et al., 2010), we propose that Resv supplementation offers endothelial cell protection against diet-induced metabolic stress partly though TGM2 signaling.
In summary, we provide data about functional and morphometric measurements in non-human primates, as well as genomic and quantitative proteomic analyses of arterial wall tissues and cells, which establish that a two-year dietary supplementation of Resv has a marked effect to reduce central arterial wall inflammation that underlie arterial wall stiffening, which accompanies chronic metabolic stress, advancing age and age-associated diseases, i.e. atherosclerosis, hypertension and diabetes, that have become rampant within Western society. Thus, dietary resveratrol at doses achievable in humans can safely reduce many of the negative consequences of excess caloric intake, and it may hold promise as a novel therapy to ameliorate increases in PWV and metabolic stress-induced factors that accelerate CV disease.
EXPERIMENTAL PROCEDURES
Animals and Diets
During baseline assessments, all monkeys were maintained on a commercially available closed formula monkey chow (TestDiet® #5038 Purina Mills, Richmond, IN). After baseline assessment, the twenty-four male rhesus monkeys were quasi-randomized into one of three groups: a HFS diet (n=10); HFS+R (n=10); or, remaining on the healthy SD (n=4). The SD was a purified biscuit consisting of 13% of kcal in fat and less than 5% sucrose by weight. The HFS diet was a specially formulated purified ingredient diet with 42% of kcal in fat and approximately 27% sucrose by weight (Harlan, Teklad, Madison, WI); additional details are provided in the Supplements. The monkeys were gradually switched to the HFS diet over a 3-week period. All groups received 2 meals per day of the specified diet in allotments that represent ad libitum feeding, yet consumption was isocaloric across groups. Resveratrol was supplied by DSM Nutritional Products (Parsippany, NJ). HFS+R monkeys received 40 mg Resv twice a day for the first year which was increased to 240 mg twice a day during the second year. The HFS and SD monkeys received a placebo treat. All procedures were approved by the Animal Care and Use Committee of the NIA Intramural Research Program.
Gene Expression
Microarray and quantitative RT-PCR techniques were carried out according to standard procedures to determine the effects of Resv on gene expression in rhesus monkey aorta. Full methodological details are described in Supplementary Methods. The primer sequences used for quantitative RT-PCR are summarized in Table S5.
Determination of 4-HNE Content and Caspase 3 Activity in Aorta Samples
Frozen aorta samples were pulverized and aliquots were homogeneized in assay-specific lysis buffers. Tissue content in 4-HNE was assessed using the OxiSelect HNE-His Adduct ELISA kits (Cell BioLabs, Inc., San Diego, CA).
Caspase 3 activity, a useful marker of apoptosis, was measured using the Caspase-Glo 3/7 assay system (Promega), as previously described (Ungvari et al., 2007). Luminescence intensity was measured using an Infinite M200 plate reader (Tecan US, Inc., Morrisville, NC) and was normalized to the sample protein concentration.
Effect of Monkey Serum on Human CAECs
Primary human CAECs (Cell Applications, Inc., San Diego, CA) were cultured in MesoEndo Endothelial Cell Growth Medium (Cell Applications) supplemented with 10% fetal calf serum and 1% antibiotics (Gibco, Gaithersburg, MD) until the time of treatment. For treatment, cells (passage 4 and up) were incubated for 24 h in serum-free medium supplemented with serum collected from SD, HFS and HFS+R monkeys (10% final concentration). Monocyte adhesion assay and quantitative RT-PCR analysis were carried out (see Supplemental section for additional details).
Transient Transfection and NF-κB Reporter Gene Assay
Transcriptional activity of NF-κB was tested in monkey serum-treated CAECs by a reporter gene assay as described (Ungvari et al., 2011). A NF-κB reporter comprised of an NF-κB response element upstream of firefly luciferase (NF-κB-Luc, Stratagene) together with a renilla luciferase plasmid under the control of CMV promoter was used in this assay.
Statistical Analyses
PWV, body weight, and serum cholesterol repeated measures data were analyzed using linear mixed-effects models. A group or group*time interaction were considered statistically significant at p≤0.05. For histochemical analysis, a one-way ANOVA was done to compare mean values for the SD, HFS and HFS+R groups. Pairwise comparisons were made using the Bonferroni’s Multiple Comparison Test. Data are presented as mean ± SEM. In each Western blot histogram, data represent the means ± S.E. Statistical analyses (Student’s t test) were performed using GraphPad Prism (GraphPadSoftware, San Diego, CA). p≤0.05 w asconsidered statistically significant.
Supplementary Material
Highlights.
Resveratrol supplementation prevented diet-induced increase in arterial stiffness
Resveratrol reduced macrophage infiltration, lipid deposition, and calcification
Gene expression changes indicate anti-oxidative and anti-inflammatory effects
Primary culture of vascular smooth muscle cells support a role of resveratrol
Acknowledgments
We thank the animal care staff and technicians at the NIHAC, in particular Joe Travis, as well as William Woods III, Elin Lehrmann, Yongqing Zhang, and Kevin G. Becker for microarray analysis. This research was supported by the Intramural Research Program of the NIH, National Institute on Aging and the Office of Dietary Supplements, NIH. SSA was the recipient of a National Heart, Lung, and Blood Institute Grant HL107361. JAB is a New Scholar of the Ellison Medical Foundation. ZU was supported by NIH/NCCAM (AT006526). The resveratrol used in this study was a generous gift from DSM Nutritional Products (resVida).
Footnotes
No potential conflicts of interest relevant to this article were reported.
Supplemental information includes 3 figures, 5 tables, Supplemental Experimental Procedures, and Supplemental references that can be found with this article online at http://xxxxxx.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bakker EN, Pistea A, VanBavel E. Transglutaminases in vascular biology: relevance for vascular remodeling and atherosclerosis. J Vasc Res. 2008;45:271–278. doi: 10.1159/000113599. [DOI] [PubMed] [Google Scholar]
- Carrizzo A, Puca A, Damato A, Marino M, Franco E, Pompeo F, Traficante A, Civitillo F, Santini L, Trimarco V, et al. Resveratrol improves vascular function in patients with hypertension and dyslipidemia by modulating NO metabolism. Hypertension. 2013;62:359–366. doi: 10.1161/HYPERTENSIONAHA.111.01009. [DOI] [PubMed] [Google Scholar]
- Chadwick W, Keselman A, Park SS, Zhou Y, Wang L, Brenneman R, Martin B, Maudsley S. Repetitive peroxide exposure reveals pleiotropic mitogen-activated protein kinase signaling mechanisms. J Signal Transduct. 2011;2011:636951. doi: 10.1155/2011/636951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Sosnowska D, Wang M, Lakatta EG, Sonntag WE, Ungvari Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: reversal by resveratrol treatment. J Gerontol A Biol Sci Med Sci. 2012;67:811–820. doi: 10.1093/gerona/glr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do GM, Kwon EY, Kim HJ, Jeon SM, Ha TY, Park T, Choi MS. Long-term effects of resveratrol supplementation on suppression of atherogenic lesion formation and cholesterol synthesis in apo E-deficient mice. Biochem Biophys Res Commun. 2008;374:55–59. doi: 10.1016/j.bbrc.2008.06.113. [DOI] [PubMed] [Google Scholar]
- Elmadhun NY, Sabe AA, Robich MP, Chu LM, Lassaletta AD, Sellke FW. The pig as a valuable model for testing the effect of resveratrol to prevent cardiovascular disease. Ann NY Acad Sci. 2013;1290:130–135. doi: 10.1111/nyas.12216. [DOI] [PubMed] [Google Scholar]
- Fiori JL, Shin YK, Kim W, Krzysik-Walker SM, Gonzalez-Mariscal I, Carlson OD, Sanghvi M, Moaddel R, Farhang K, Gadkaree SK, et al. Resveratrol Prevents beta-cell Dedifferentiation in Non-Human Primates Given a High Fat/ High Sugar Diet. Diabetes. 2013;62:3500–3513. doi: 10.2337/db13-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschy A, Bauer E, Schrodt C, Lykowsky G, Ye YX, Rommel E, Jakob PM, Bauer WR, Herold V. Local Arterial Stiffening Assessed by MRI Precedes Atherosclerotic Plaque Formation. Circ Cardiovasc Imaging. 2013;6:916–923. doi: 10.1161/CIRCIMAGING.113.000611. [DOI] [PubMed] [Google Scholar]
- Hayoz D, Zappe DH, Meyer MA, Baek I, Kandra A, Joly MP, Mazzolai L, Haesler E, Periard D. Changes in aortic pulse wave velocity in hypertensive postmenopausal women: comparison between a calcium channel blocker vs angiotensin receptor blocker regimen. J Clin Hypertens (Greenwich) 2012;14:773–778. doi: 10.1111/jch.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Gomez Y, Mattison JA, Pearson KJ, Martin-Montalvo A, Palacios HH, Sossong AM, Ward TM, Younts CM, Lewis K, Allard JS, et al. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell Metab. 2013;18:533–545. doi: 10.1016/j.cmet.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Polewski M, Terkeltaub RA. Transglutaminase 2 is central to induction of the arterial calcification program by smooth muscle cells. Circ Res. 2008;102:529–537. doi: 10.1161/CIRCRESAHA.107.154260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko H, Anzai T, Morisawa M, Kohno T, Nagai T, Anzai A, Takahashi T, Shimoda M, Sasaki A, Maekawa Y, et al. Resveratrol prevents the development of abdominal aortic aneurysm through attenuation of inflammation, oxidative stress, and neovascularization. Atherosclerosis. 2011;217:350–357. doi: 10.1016/j.atherosclerosis.2011.03.042. [DOI] [PubMed] [Google Scholar]
- Konishi A, Aizawa T, Mohan A, Korshunov VA, Berk BC. Hydrogen peroxide activates the Gas6-Axl pathway in vascular smooth muscle cells. J Biol Chem. 2004;279:28766–28770. doi: 10.1074/jbc.M401977200. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. The reality of aging viewed from the arterial wall. Artery Res. 2013;7:73–80. doi: 10.1016/j.artres.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Kambe F, Cao X, Kozaki Y, Kaji T, Ishii T, Seo H. 3beta-Hydroxysteroid-delta24 reductase is a hydrogen peroxide scavenger, protecting cells from oxidative stress-induced apoptosis. Endocrinology. 2008;149:3267–3273. doi: 10.1210/en.2008-0024. [DOI] [PubMed] [Google Scholar]
- Matlung HL, Neele AE, Groen HC, van Gaalen K, Tuna BG, van Weert A, de Vos J, Wentzel JJ, Hoogenboezem M, van Buul JD, et al. Transglutaminase activity regulates atherosclerotic plaque composition at locations exposed to oscillatory shear stress. Atherosclerosis. 2012;224:355–362. doi: 10.1016/j.atherosclerosis.2012.07.044. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Dudley JI, Das DK. Dose-Dependency of Resveratrol in Providing Health Benefits. Dose Response. 2010;8:478–500. doi: 10.2203/dose-response.09-015.Mukherjee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najjar SS, Scuteri A, Shetty V, Wright JG, Muller DC, Fleg JL, Spurgeon HP, Ferrucci L, Lakatta EG. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2008;51:1377–1383. doi: 10.1016/j.jacc.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SS, Wu WW, Zhou Y, Shen RF, Martin B, Maudsley S. Effective correction of experimental errors in quantitative proteomics using stable isotope labeling by amino acids in cell culture (SILAC) J Proteomics. 2012;75:3720–3732. doi: 10.1016/j.jprot.2012.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramprasath VR, Jones PJ. Anti-atherogenic effects of resveratrol. Eur J Clin Nutr. 2010;64:660–668. doi: 10.1038/ejcn.2010.77. [DOI] [PubMed] [Google Scholar]
- Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. 2010;31:2338–2350. doi: 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam L, Tuday EC, Webb AK, Dowzicky P, Kim JH, Oh YJ, Sikka G, Kuo M, Halushka MK, Macgregor AM, et al. Decreased S-nitrosylation of tissue transglutaminase contributes to age-related increases in vascular stiffness. Circ Res. 2010;107:117–125. doi: 10.1161/CIRCRESAHA.109.215228. [DOI] [PubMed] [Google Scholar]
- Satoh K, Fukumoto Y, Shimokawa H. Rho-kinase: important new therapeutic target in cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2011;301:H287–H296. doi: 10.1152/ajpheart.00327.2011. [DOI] [PubMed] [Google Scholar]
- Scuteri A, Orru M, Morrell CH, Tarasov K, Schlessinger D, Uda M, Lakatta EG. Associations of large artery structure and function with adiposity: effects of age, gender, and hypertension. The SardiNIA Study. Atherosclerosis. 2012;221:189–197. doi: 10.1016/j.atherosclerosis.2011.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoyama T, Hiraoka S, Takemoto M, Koshizaka M, Tokuyama H, Tokuyama T, Watanabe A, Fujimoto M, Kawamura H, Sato S, et al. CCN3 inhibits neointimal hyperplasia through modulation of smooth muscle cell growth and migration. Arterioscler Thromb Vasc Biol. 2010;30:675–682. doi: 10.1161/ATVBAHA.110.203356. [DOI] [PubMed] [Google Scholar]
- Smith LB, Hadoke PW, Dyer E, Denvir MA, Brownstein D, Miller E, Nelson N, Wells S, Cheeseman M, Greenfield A. Haploinsufficiency of the murine Col3a1 locus causes aortic dissection: a novel model of the vascular type of Ehlers-Danlos syndrome. Cardiovasc Res. 2011;90:182–190. doi: 10.1093/cvr/cvq356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son BK, Kozaki K, Iijima K, Eto M, Nakano T, Akishita M, Ouchi Y. Gas6/Axl-PI3K/Akt pathway plays a central role in the effect of statins on inorganic phosphate-induced calcification of vascular smooth muscle cells. Eur J Pharmacol. 2007;556:1–8. doi: 10.1016/j.ejphar.2006.09.070. [DOI] [PubMed] [Google Scholar]
- Tuttolomondo A, Di Raimondo D, Pecoraro R, Arnao V, Pinto A, Licata G. Atherosclerosis as an inflammatory disease. Curr Pharm Des. 2012;18:4266–4288. doi: 10.2174/138161212802481237. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Bailey-Downs L, Gautam T, Sosnowska D, Wang M, Monticone RE, Telljohann R, Pinto JT, de Cabo R, Sonntag WE, et al. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-{kappa}B activation in the nonhuman primate Macaca mulatta. J Gerontol A Biol Sci Med Sci. 2011;66:866–875. doi: 10.1093/gerona/glr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Orosz Z, Rivera A, Labinskyy N, Xiangmin Z, Olson S, Podlutsky A, Csiszar A. Resveratrol increases vascular oxidative stress resistance. Am J Physiol Heart Circ Physiol. 2007;292:H2417–H2424. doi: 10.1152/ajpheart.01258.2006. [DOI] [PubMed] [Google Scholar]
- Vykoukal D, Davies MG. Vascular biology of metabolic syndrome. J Vasc Surg. 2011;54:819–831. doi: 10.1016/j.jvs.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Jiang L, Monticone RE, Lakatta EG. Proinflammation: the key to arterial aging. Trends Endocrinol Metab. 2014;25:72–79. doi: 10.1016/j.tem.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B, Lacy PS, Cruickshank JK, Collier D, Hughes AD, Stanton A, Thom S, Thurston H. Impact of statin therapy on central aortic pressures and hemodynamics: principal results of the Conduit Artery Function Evaluation-Lipid-Lowering Arm (CAFE-LLA) Study. Circulation. 2009;119:53–61. doi: 10.1161/CIRCULATIONAHA.108.785915. [DOI] [PubMed] [Google Scholar]
- Xiao Q, Zhang F, Grassia G, Hu Y, Zhang Z, Xing Q, Yin X, Maddaluno M, Drung B, Schmidt B, et al. Matrix metalloproteinase-8 promotes vascular smooth muscle cell proliferation and neointima formation. Arterioscler Thromb Vasc Biol. 2014;34:90–98. doi: 10.1161/ATVBAHA.113.301418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.